Abstract
This paper describes the effect of various pathologies on power reflectance (PR) and absorbance measured in human adults. The pathologies studied include those affecting the tympanic membrane, the middle-ear ossicles, the middle-ear cavity, the inner ear, and intracranial pressure. Interesting pathology-induced changes in PR that are statistically significant have been reported. Nevertheless, because measurements of PR obtained from normal-hearing subjects have large variations and some pathology-induced changes are small, it can be difficult to use PR alone for differential diagnosis. There are, however, common clinical situations without reliable diagnostic methods that can benefit from PR measurements. These conditions include ears with a normal-appearing tympanic membrane, aerated middle-ear cavity and unknown etiology of conductive hearing loss. PR measurements in conjunction with audiometric measurements of air-bone gap have promise in differentiating among stapes fixation, ossicular discontinuity and superior semicircular canal dehiscence. Another possible application is to monitor an individual for possible changes in intracranial pressure. Descriptions of mechanisms affecting PR change and utilization of PR measurements in clinical scenarios are presented.
Introduction
In an earlier paper in this volume, Rosowski et al. define: acoustic immittance, reflectance, absorbance and power reflectance (PR) and describe their measurement over a wide band of frequency (Rosowski, this issue, pp. XXXX). Also detailed in Rosowski et al.’s article, the advantage of PR measurements clinically is that PR is less sensitive than other measurements to variations in ear-canal length, but at the cost of losing information such as phase data that fully characterize the impedance of the system.
A number of studies have measured wideband acoustic immittance (WAI) – with devices such as the Mimosa Acoustics system and Reflwin Interacoustics system – and investigated the effects of various disorders on PR in humans. Because published studies on the effect of ear disorders on WAI report PR (as opposed to absorbance), we will mostly focus on PR analyses in this chapter. Most of the disorders we discuss significantly affect PR. However, the range of PR varies greatly within “normal” ears, i.e. free of disease and with normal hearing (see Shahnaz et al. in this supplement), which limits the utility of PR measurements as a diagnostic tool (Shahnaz, this issues, pp. XXXX). For example, although the mean of PR measured in many ears with stapes fixation are statistically different from the mean of normal ears, the pathological change in PR in any individual pathological ear may be insufficient to reliably separate it from normal. However, if we combine measurements of air-bone gap and PR we can more clearly differentiate between the possible pathologies.
In this paper, we both provide an explanation of how disease can affect PR measurements, and provide information regarding the useful application of PR measurements for the clinical setting.
Disease affecting the tympanic membrane
Tympanic membrane perforation
The effect of tympanic membrane (TM) perforation has been studied extensively (Lerut et al. 2012; Mehta et al. 2006; Voss et al. 2001a, 2001b, 2001c). Transduction of sound from the ear canal towards the cochlea has been shown to decrease with the increase in perforation size at lower frequencies, while the location of the perforation does not affect the transduction. Importantly, these findings disproved a long-held belief that the location of the perforation was an important determinant of sound transduction, a belief that sometimes affected surgical decisions.
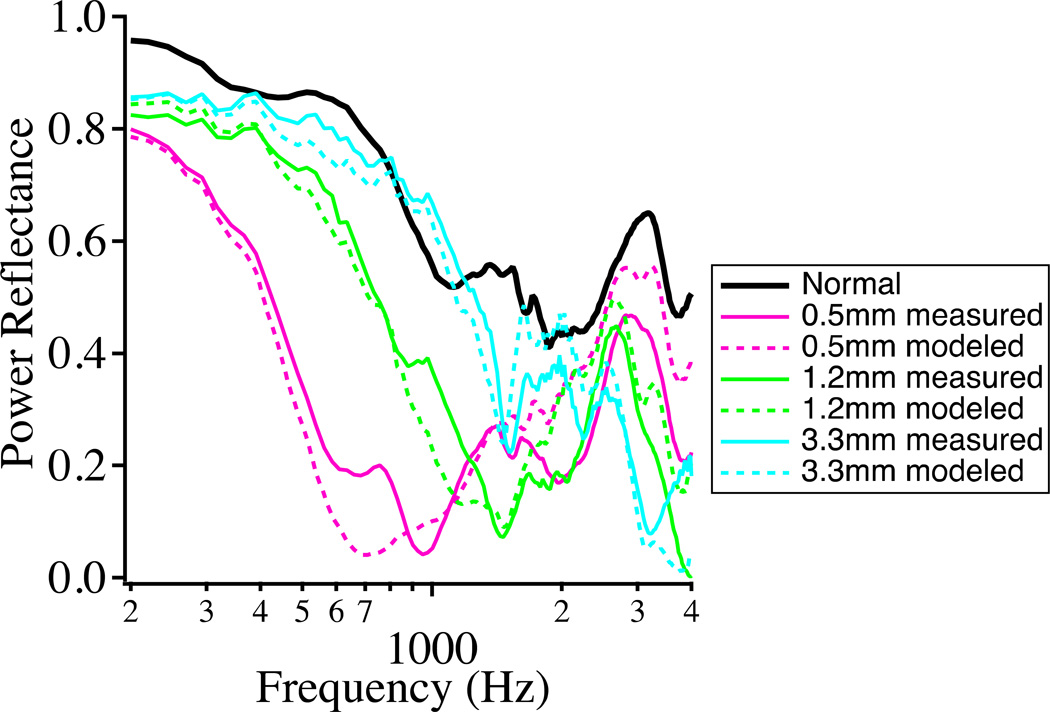
More recently, the detailed effect of various sized perforations on PR measurements was studied by Voss et al. (2012) utilizing cadaveric specimens. PR was shown to decrease with TM perforation at low frequencies, where surprisingly, the smallest perforations had the largest effect (Fig. 1). Explanation of this effect for frequencies below 1 kHz is as follows: With no TM perforation, the compliance of the TM and ossicles dominates the impedance, resulting in large impedance and “normal” PR. A small TM perforation introduces a low-frequency mass term due to the air channeled in the perforation, which forms a resonance with the middle-ear cavity compliance. Because of the mass term, the low frequency middle-ear input impedance is small, with even smaller impedance in the middle frequency ranges where the resonance occurs and PR is decreased at low frequencies. As the perforation diameter increases, the impedance at the TM is dominated by the compliant impedance of the cavity and the resonance moves to higher frequencies. At higher frequencies (>2.5 kHz), the impedance at the TM with a perforation approximates the normal impedance, and the reflectance with both larger and smaller perforations approximates the normal case.
Figure 1.
Effect of TM perforations of various diameters on PR. The smallest perforations produced the largest change at low frequencies. The largest perforation size resulted in a PR more similar to the normal ear at frequencies less than 2.5 kHz. Dashed lines correspond to model results. This figure is similar to that published in Voss et al. (2012) (Voss, S., et al., Ear and Hearing, 33, 195–208. Copyright (2012)).
Our acousto-mechanical interpretation of the results of perforation on PR relies on other measurements of the magnitude and angle of the complex middle ear input immittance in perforated ears (Voss et al. 2001b). We must emphasize that PR data alone cannot provide information regarding the effects of the pathology on the acoustics and mechanics of the middle ear because important phase information has been discarded in favor of obtaining lower sensitivity to ear-canal length effects (as described by Rosowski et al. in this volume). In essence, how pathological changes in acousto-mechanical systems, such as the ear, affect PR can be quite complicated and additional information is usually required to understand the mechanisms behind observed changes in PR.
Compliant and stiff tympanic membrane
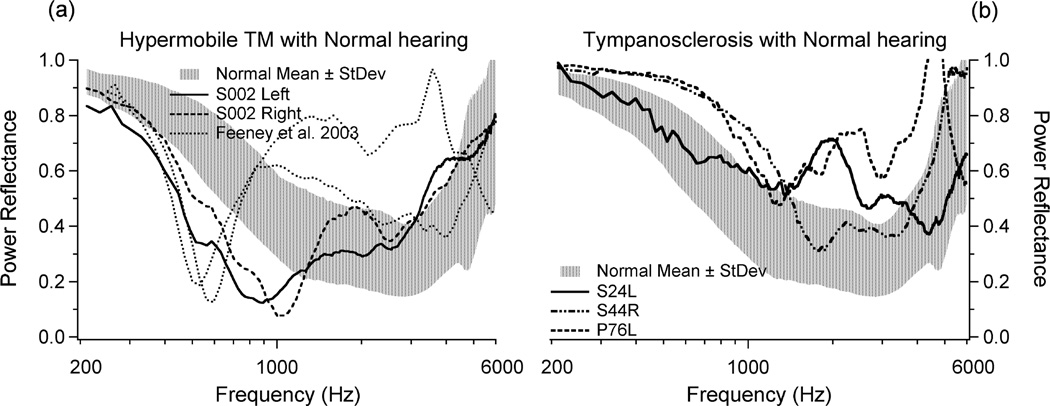
Measurements of PR can be highly influenced by the mechanical characteristics of the intact TM – so much so that the effect due to an abnormal TM can dominate the effect produced by changes in the ossicles or inner ear. This is problematic in the diagnosis of ossicular and inner-ear disease. Figure 2A shows a comparison of the PR for ears with normal hearing, normal TMs and normal tympanograms (shaded area representing one standard deviation above and below the mean), with PR for four ears that had normal audiograms but with abnormally high compliances detected by conventional tympanometry obtained at 226 Hz probe tone (Feeney et al. 2003; Rosowski et al. 2012). All four high-compliance ears have a PR that is significantly lower than the PR in the normal population between 400–1000 Hz. Figure 2B compares the mean +/− one standard deviation of PR for normal ears (shaded) with 3 ears that had tympanosclerosis and normal audiograms. Conventional tympanometry was normal (static compliance of 0.3 ml, volume 1.2 ml) for S024L, near flat (static compliance of 0. 5 ml, volume 0.5 ml) for S044R, and a sharp peak (normal static compliance of 0.7 ml, volume 0.9 ml) for P076L. All three ears show higher than normal PR at low or mid frequency regions. Therefore, the coupling of tympanometry with PR measurements played a significant role in interpreting the PR results, where tympanometry identified the TM abnormality in 6 of the 7 cases of abnormal PR with normal hearing in Fig. 2A and 2B.
Figure 2.
(A) Right and left ears from one subject (Rosowski et al. 2012) (Reprinted with permission from Rosowski, J.J., et al., Ear and Hearing, 33, 19–34. Copyright (2012)) and 2 ears presented by Feeney et al. (2003) (Reprinted with permission from Feeney, M.P., et al., Journal of Speech, Language and Hearing Research, 46, 901–911. Copyright (2003). These ears had normal audiograms but abnormally compliant tympanograms. (B) Three ears with tympanosclerosis and normal audiograms. In both plots, comparison is made to the shaded area representing one standard deviation above and below the mean PR of 58 normal ears. This figure was published in Rosowski et al. (2012) (Reprinted with permission from Rosowski, J.J., et al., Ear and Hearing, 33, 19–34. Copyright (2012).
Disease affecting the middle-ear cavity
Static pressure changes in the middle-ear cavity
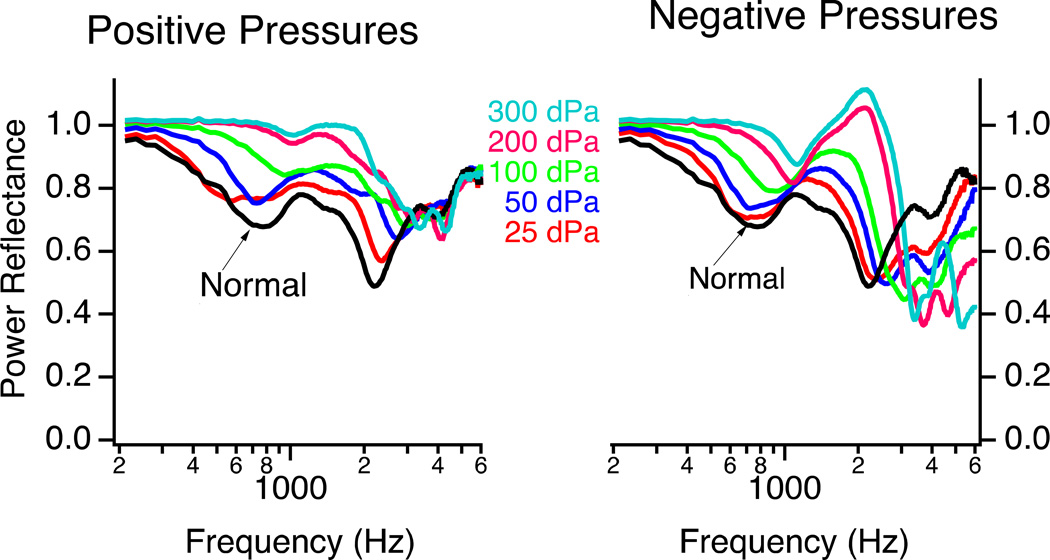
Both positive and negative static pressures in the middle ear cavity result in increased stiffness of the tympanic membrane and increased PR, especially at low frequencies. Static negative pressure of the middle ear was shown to increase PR for a wide frequency band (250–6300 Hz) in 5–7 year-old children by Beers et al. (2010). However, Voss et al. (2012) showed that PR is affected by middle-ear pressure changes in a frequency-dependent manner. Below 2 kHz, incrementally increasing positive or negative middle-ear cavity pressure (between ±300 daPa) in cadaveric preparations generally resulted in incremental increases in PR (i.e. Fig. 3) (Voss et al. 2012). Negative middle-ear pressure tended to have lower PR than positive pressure, especially around 1–3 kHz. Above 3 kHz, negative pressure tended to result in PR that was lower than normal while positive pressure had little effect on PR.
Figure 3.
The effect of incremental positive or negative pressure in the middle-ear cavity on PR. Generally, PR increases as the absolute value of the middle-ear static pressure increases. An exception to this rule occurs with negative pressures, which decrease PR at frequencies above 3 kHz. This figure is similar to that published in Voss et al. (2012) (Reprinted with permission from Voss, S., et al., Ear and Hearing, 33, 195–208. Copyright (2012).
Fluid in the middle-ear cavity
The middle-ear cavity (including the continuous mastoid air space) is generally filled with compressible air, which allows sound-induced motion of the TM and the middle-ear transduction of sound. Loss of middle-ear cavity aeration results from the accumulation of serous or mucoid fluids as well as the proliferation of mucosal and other tissues within the ear. A non-aerated middle-ear cavity loses compressible air, which increases the cavity’s impedance. Such an increase produces an increase and flattening of PR. Much research has focused on middle-ear fluid effects on PR, and how PR can be used to determine conductive loss due to middle-ear fluid in the pediatric population, where diagnosis can be challenging by conventional methods (see Prieve et al. this volume).
Feeney (2003) showed that PR increases with replacement of middle-ear cavity air with fluid (Fig. 4a). The effect of incremental increase in middle-ear fluid on PR has been studied in cadaveric preparations by Voss et al. (2012). As shown in Fig. 4b, PR generally increases with incremental increase of middle-ear fluid, most significant after at least 50% of the middle-ear space was filled with fluid, and mostly below 3 kHz. The high frequencies (>2 kHz) PR values changed less as fluid replaced air. Similar findings have been reported in live humans (Allen et al. 2005; Beers et al. 2010; Feeney et al. 2003; Piskorski et al. 1999). Gan et al. (2006) also showed that after about 50% of the middle-ear cavity air was reduced by fluid, umbo velocity decreased. Similar experiments were performed by Ravicz et al. (2004), and they found that the low-frequency (<1 kHz) decrease in umbo velocity was due to decreased middle-ear volume, while the high-frequency (>2 kHz) decrease in umbo velocity was more related to fluid and tissue loading of the TM. These and other studies also found that the effect of fluid viscosity was insignificant.
Figure 4.
(A) The effect of otitis media with effusion on PR (also called energy reflectance) in adults (Feeney et al. 2003) (Reprinted with permission from Feeney, M.P., et al., Journal of Speech, Language, and Hearing Research, 46, 901–911. Copyright (2003)). The shaded area represents 5–95% of normal ears. (B) PR measured in a cadaveric temporal bone for incremental increase in fluid volume replacing air in the middle-ear cavity (Voss et al. 2012) (Reprinted with permission from Voss, S., et al., Ear and Hearing, 33, 195–208. Copyright (2012)). The black thick line represents the initial value with normal air volume.
An interesting characteristic noted with incremental decrease of compressible air in the middle-ear space is that a sharp minimum in PR occurs at varying frequencies (Fig. 4b), but that PR generally reaches a high value at all frequencies when all or most of the middle-ear air is replaced by fluid. Ravicz et al. (2004) also found sharp minima and rapid fluctuations with frequency in umbo velocity measurements as the air in the middle-ear cavity was replaced with fluid; as in Voss et al. (2012) these minima disappeared when all of the air was replaced by fluid. These rapid changes with frequency may result from resonating bubbles of air in the partially fluid filled middle ears.
Disease affecting the ossicles
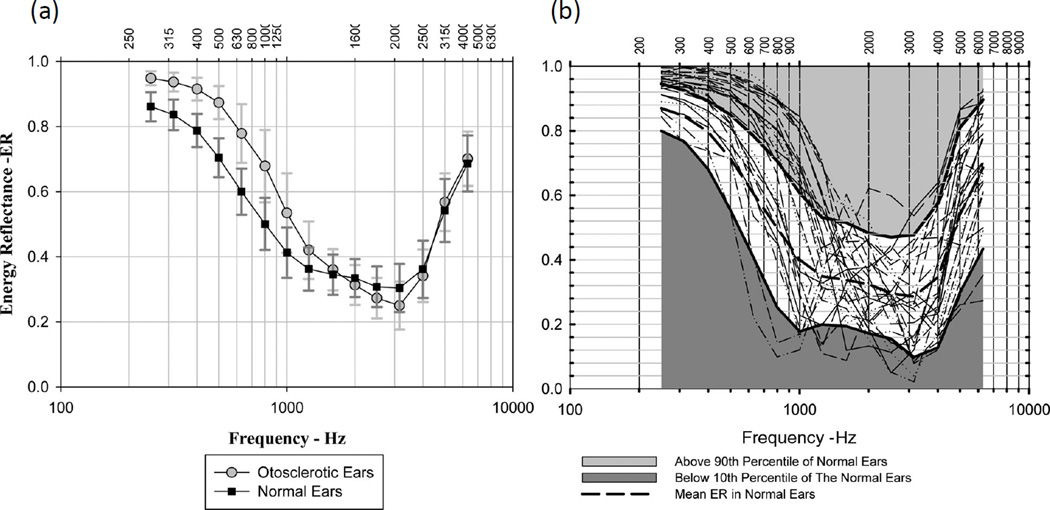
Stapes fixation is most commonly caused by otosclerosis, and has been shown to increase PR at frequencies of 1 kHz and lower (Allen et al. 2005; Nakajima et al. 2012; Shahnaz, Bork, et al. 2009; Shahnaz, Longridge, et al. 2009; Voss et al. 2012). Shahnaz et al. (2009) showed that there is a statistically significant increase in PR below 1 kHz among patients with surgically confirmed stapes fixation, as compared to normal ears (Fig. 5 left). However, the range of PR for stapes fixation has considerable overlap with the range of PR of normal ears (Fig. 5 right). Therefore, even if a particular PR measurement shows a slightly higher PR magnitude as compared to the normal mean, it is difficult to determine with great certainty, by PR measurement alone, whether a measurement is indeed indicative of stapes fixation or a normal ear.
Figure 5.
(A) Mean and standard deviation of PR (called energy reflectance in these plots) for otosclerotic ears (stapes fixation) and normal ears. (B) Individual PR for otosclerotic ears plotted with white area representing 10–90% and dashed line the mean of normal ears. These plots were published in Shahnaz et al. (2009) (Reprinted with permission from Shahnaz, N., et al., Ear and Hearing, 30, 219–233. Copyright (2009)).
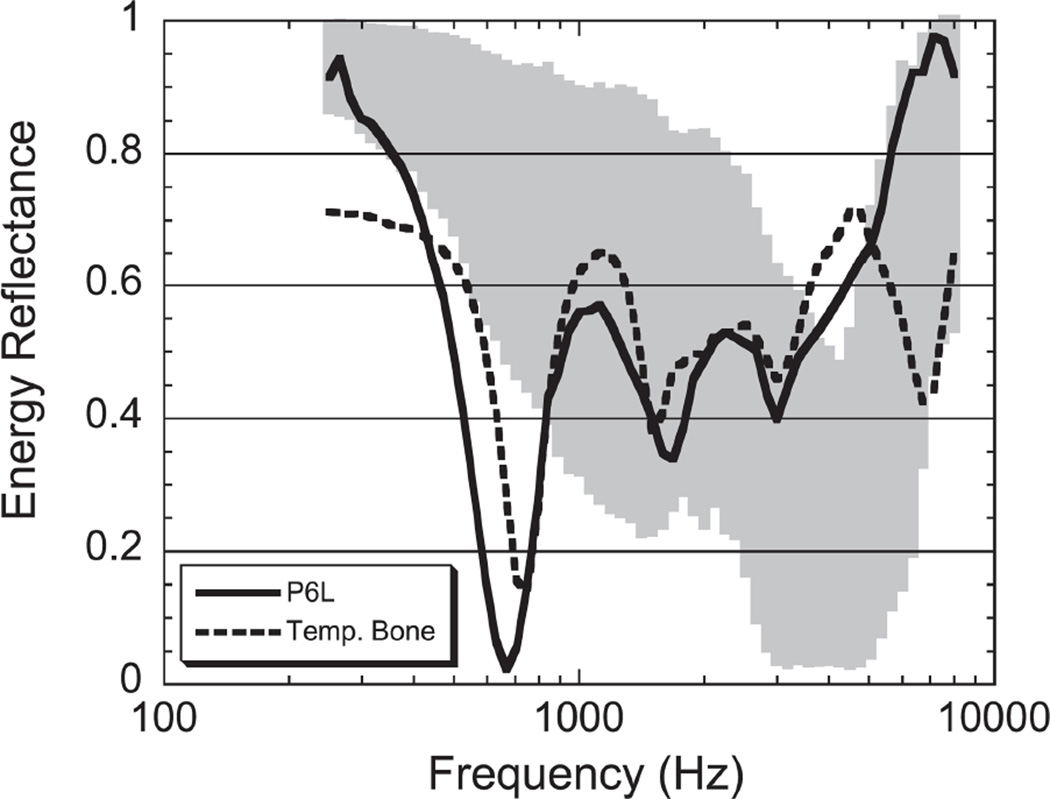
Ossicular discontinuity can occur anywhere in the ossicular chain. Clinically, common areas include the incudo-stapedial joint, stapes crura, and the malleus neck. Ossicular discontinuity has been shown to result in a prominent notch in PR around 400–800 Hz (Feeney et al. 2003; Feeney et al. 2009; Nakajima et al. 2012; Voss et al. 2012). Figure 6 shows examples of PR measurements taken from a patient and temporal bone with ossicular discontinuity (Feeney et al. 2003). The sharp notch-like minimum in PR around 400–800 Hz is generally well below the PR value in normal ears.
Figure 6.
Figure from Feeney et al. (2003) (Reprinted with permission from Feeney, M.P., et al., Journal of Speech, Language, and Hearing Research, 46, 901–911. Copyright (2003)), showing PR (also called energy reflectance) from a patient and temporal bone with ossicular discontinuity. The shaded gray area represents 5–95% of normal ears.
Modeling middle-ear disease and PR
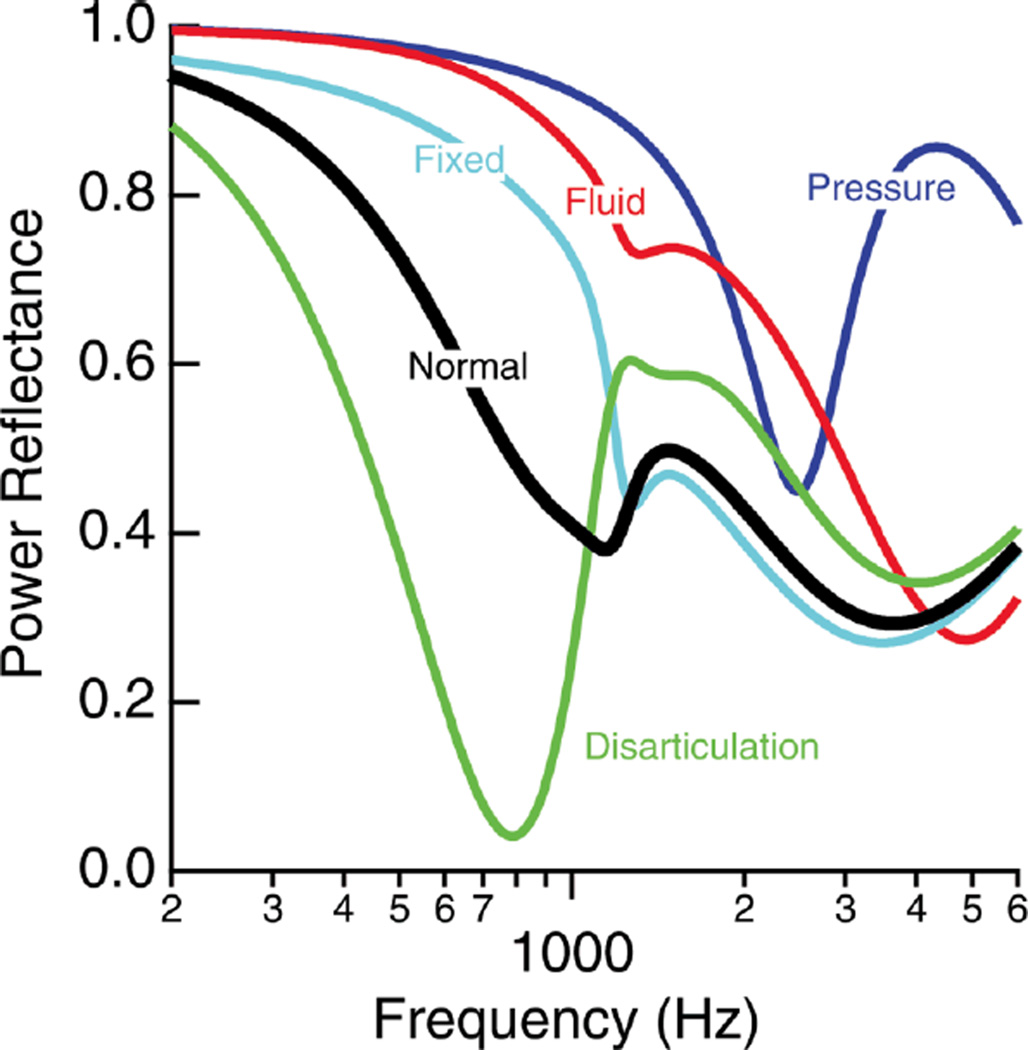
How middle-ear diseases affect the middle-ear system can be understood by analyzing and studying the impedance and sound-transfer characteristics of the system. Measurements comparing normal and diseased responses of the magnitude and phase (or real and imaginary parts) of transfer functions or input impedances obtained by various types of measurements have been used to develop and test quantitative models of the ear. Voss et al. (2012) used modifications of a model developed by Kringlebotn (1988) to simulate PR measurements under various conditions. This exercise demonstrated that simple changes in model values were able to simulate different diseased conditions. Figure 7 shows the PR model results for middle-ear pressure change, middle-ear fluid, and fixed and disarticulated ossicles that are generally similar to the PR measurements obtained for different pathologies by various investigators. In regards to middle-ear pressure change, the model does not differentiate between positive and negative pressures, but the temporal-bone measurements showed differences between positive and negative pressures especially above about 2 kHz. Figure 1 (dashed lines) also plots PR modeling results for various TM perforation sizes.
Figure 7.
Simulation results of PR for normal and various diseased states. The model does not differentiate between positive and negative middle-ear pressure. Figures were published in Voss et al. (2012) (Reprinted with permission from Voss, S., et al., Ear and Hearing, 33, 195–208. Copyright (2012)).
Third window lesion of the inner ear
Superior semicircular canal dehiscence (SCD) is the introduction of an abnormal ‘third-window’ into the inner ear due to the development of an opening in the osseous labyrinth of the superior semicircular canal. Symptoms vary considerably, ranging from classic third-window or perilymphatic fistula symptoms to vague vestibular and/or auditory manifestations. Thus SCD is known as a great mimicker of other disorders. For example, some patients have only an air-bone gap similar to otosclerosis, while others only complain of ear blockage similar to eustachian-tube dysfunction, or autophony similar to patulous eustachian tube. Others have intermittent vague dizziness without clear inciting stimuli, similar to many vestibular and neurologic diseases, either in combination or without auditory symptoms.
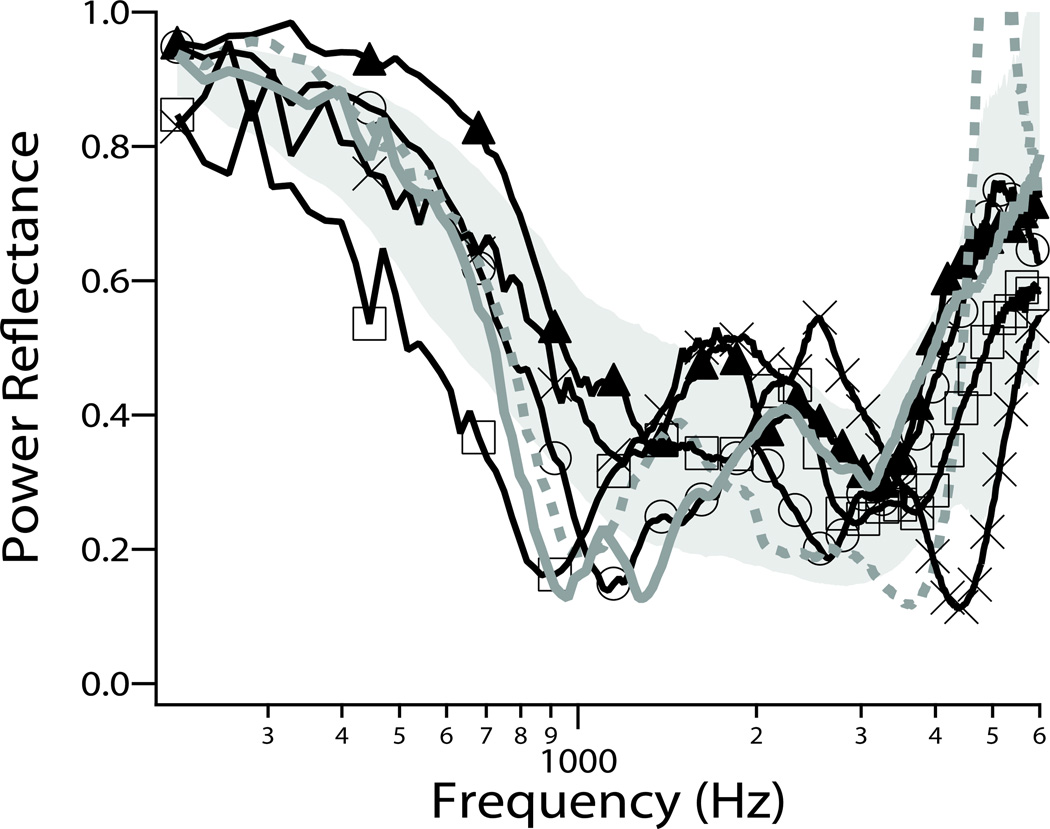
Because sound energy can leave the inner ear via the dehiscence, SCD can produce a decrease in the impedance sensed by the stapes at the oval window during sound stimulation. PR measurements of ears with SCD have shown a distinct local minimum or notch around 1 kHz. This ‘SCD notch’ differs from the notch seen in ears with ossicular discontinuity in that the SCD notch is at 1 kHz (instead of around 400–800 Hz with ossicular discontinuity), and the SCD notch is smaller and not as sharp as the notch due to ossicular discontinuity. Figure 8 compares PR measurements taken from six ears with SCD with the normal range of PR response (Nakajima et al. 2012).
Figure 8.
Examples of PR measurements in six ears with SCD. Four of the ears show notch-like local minima in PR near 1 kHz. The gray area represents ±1 SD around the mean of normal PR. Similar to plot published in Nakajima et al. (2012) (Reprinted with permission from Nakajima, H.H., et al., Ear and Hearing, 33, 35–43. Copyright (2012)).
Clinical applications of PR measurements
Differentiating among etiologies that cause conductive hearing loss
As explained above, the near-flat high level PR measurements seen with fluid and significant middle-ear pressures are generally easily recognizable. However, when the TM is aerated and intact, it is difficult to differentiate between a normal ear and a diseased ear using only PR, perhaps with the exception of ossicular discontinuity. Both stapes fixation and SCD affect PR, but the magnitude of this effect may not result in a PR value that falls outside of the normal range. However, PR measurements can aid in differential diagnoses in these cases.
In the otology clinic, conventional air and bone audiometry does a very good job of identifying those ears with conductive hearing loss. On the other hand, it is not easy to differentiate the different causes of conductive hearing loss in patients with intact, healthy tympanic membranes and aerated middle-ear cavities. Usually, the presumed pathology is ossicular fixation because it is the most common etiology. However, the disorder could be an ossicular discontinuity or a third window lesion such as SCD. A simple non-invasive diagnostic test that differentiates among these disorders would be of clinical value. It would help preoperative counseling of patients regarding surgical risks and preoperative planning, and most importantly prevent unnecessary surgery. Patients still undergo unnecessary middle-ear exploration and even stapedotomy/stapdectomy surgery because they were not correctly diagnosed with SCD.
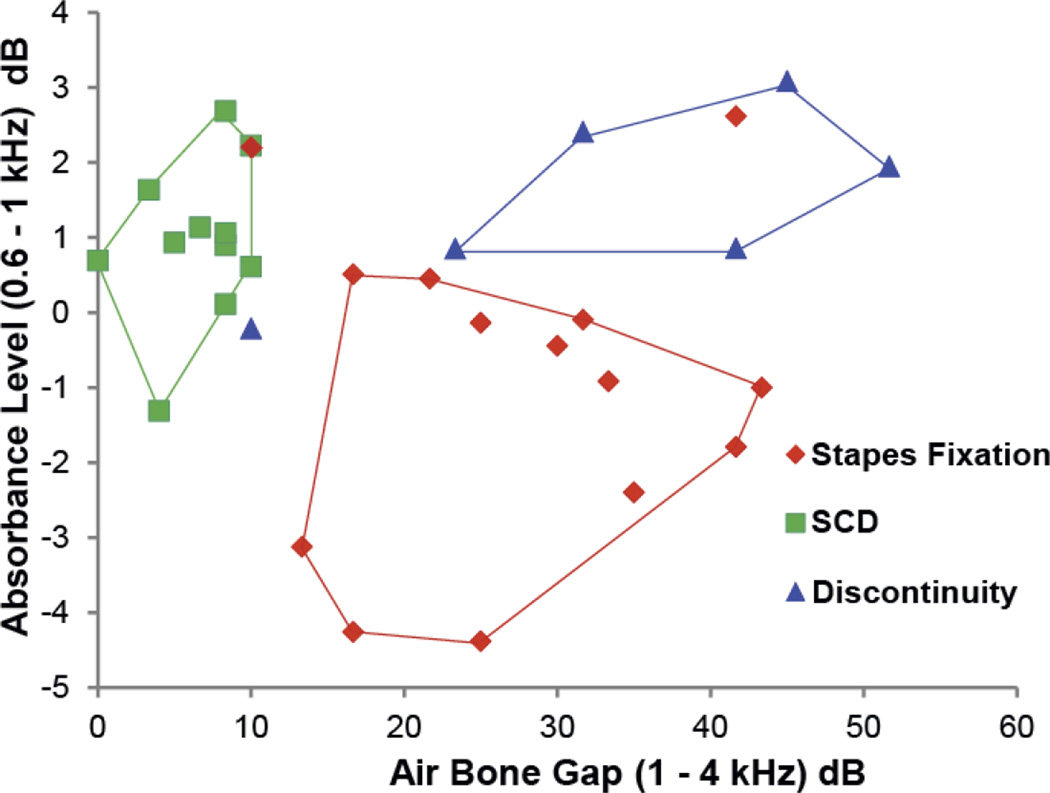
Preliminary results show the combination of PR measurement and air-bone gap, can generally differentiate between stapes fixation, ossicular discontinuity and SCD (Nakajima et al. 2012). Figure 9 shows a plot of absorbance level (absorbance level = 1×log10[1–PR]) averaged between 0.6–1 kHz, versus air-bone gap averaged between 1–4 kHz. Most of the resultant measurements have unique areas in the plot for the different pathologies. Both the sensitivity and specificity to determine the pathological condition among the three conditions were over 80% (average of 93%), as shown in table 1.
Figure 9.
Absorbance level averaged between 0.6–1 kHz plotted against air-bone gap averaged between 1–4 kHz. The area designated with red border encloses 12 or 14 cases of surgically confirmed stapes fixation, the blue border encloses 5 of 6 cases of surgically confirmed ossicular discontinuity, and the green border encloses 12 of 12 cases of radiologically confirmed SCD. A similar plot was published in Nakajima et al. (2012) (Reprinted with permission from Nakajima, H. H., et al., Ear and Hearing, 33, 35–43. Copyright (2012)).
Table 1. Absorbance Level with Air-Bone Gap Measurements.
Sensitivity and Specificity of Absorbance Level Measurements (from Nakajima et al. 2012)
| Pathology | Sensitivity | Specificity |
|---|---|---|
| Stapes Fixation | 86% | 100% |
| Ossicular Discontinuity | 83% | 96% |
| SCD | 100% | 95% |
Monitoring Intracranial Pressure
Many neurological conditions can result in the elevation of intracranial pressure (ICP) which can be devastating to patient’s outcome. To monitor intracranial pressure, it is presently necessary to measure it invasively by direct intracranial transducers. A non-invasive method for monitoring ICP would be of great benefit.
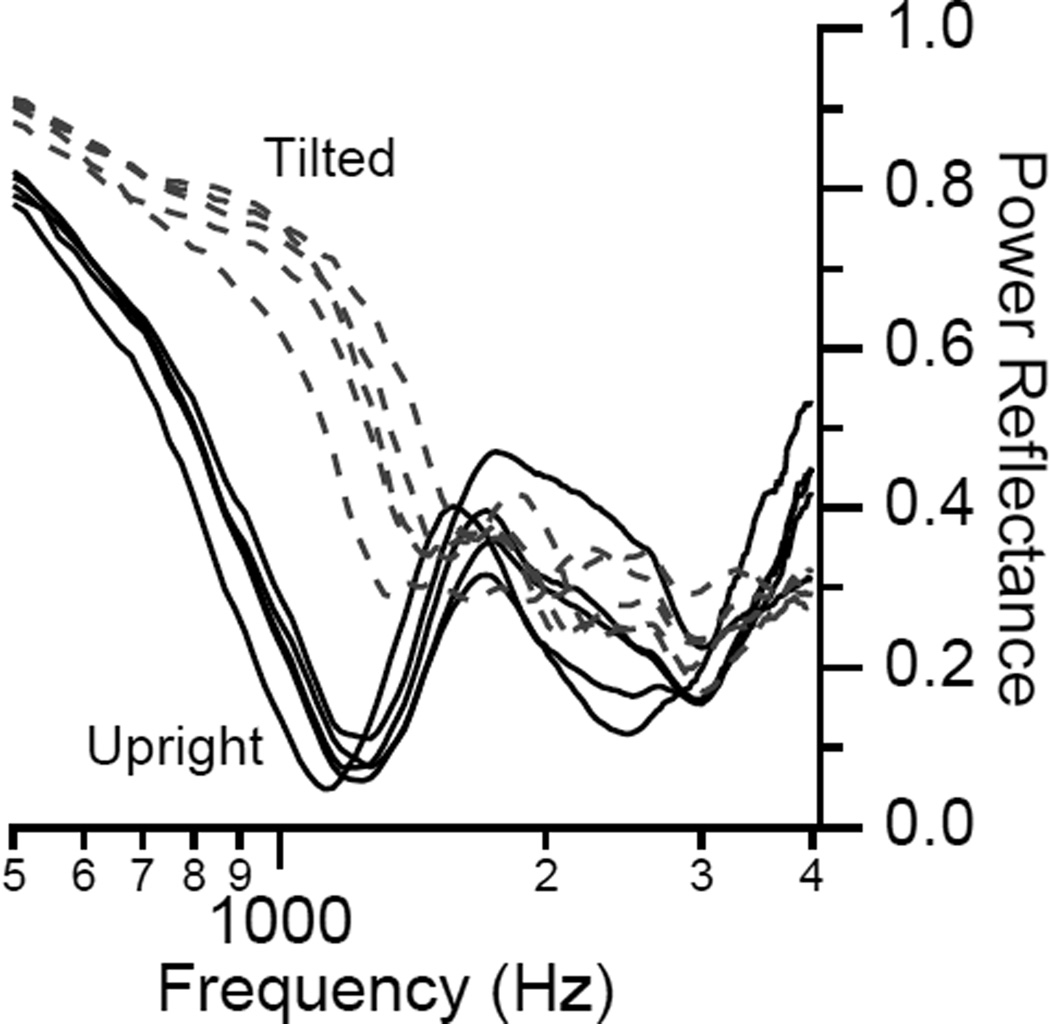
Voss et al. (2010) showed that increases in ICP can result in changes of PR measurements in subjects on a tilting table (Fig. 10). Although increases in ICP can be sensed by PR measurement, the range of PR measurements among subjects with normal ICP can greatly vary. Thus PR measurements will not be useful in indicating absolute ICP level. However, PR measurements may be useful in the clinical scenarios where serial measurements are made to monitor for changes in ICP.
Figure 10.
Increase in low-frequency PR due to subject tilted with head lower than the heart (dashed lines). The multiple lines represent repeated measurements. Similar plots were published in Voss et al. (2010) (Reprinted with permission from Voss, S., et al., Hearing Research, 263, 43–51. Copyright (2010)).
Future work for WAI measurements in the clinical setting
Future work utilizing WAI measurements will require building upon the understanding we have of how this measurement relates to the transduction of sound and is affected by disease. Below are some key areas of focus for the future:
Continue collecting data from subjects and patients with various pathologies in addition to measurements on fresh cadaveric temporal bone specimens with simulated pathologies. This is important to our understanding the effects of pathologies as well as the mechanisms responsible for the effects on WAI measurements.
Analyze various aspects of WAI measurements (e.g. impedance, phase, time response) taken from patients and temporal bones.
Improve the use of WAI for detecting pathological conditions.
Determine various clinical applications that would benefit from WAI measurements. For example, an investigation is in progress to determine whether PR is a viable screening tool for SCD (a third window lesion of the inner ear) that is sometimes challenging to diagnose because the symptoms can be varied and common. As a screening tool, it would be used prior to more expensive or invasive diagnostic procedures.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Contributor Information
Hideko Heidi Nakajima, Department of Otology & Laryngology, Harvard Medical School, Boston, Massachusetts; Eaton-Peabody Laboratory, Massachusetts Eye & Ear Infirmary, Boston, Massachusetts.
John J. Rosowski, Department of Otology & Laryngology, Harvard Medical School, Boston, Massachusetts Eaton-Peabody Laboratory, Massachusetts Eye & Ear Infirmary, Boston, Massachusetts.
Navid Shahnaz, School of Audiology & Speech Sciences, University of British Columbia, Vancouver, British Columbia, Canada.
Susan E. Voss, Picker Engineering Program, Smith College, Northampton, Massachusetts
References
- Allen JB, Jeng PS, Levitt H. Evaluation of human middle ear function via an acoustic power assessment. Journal of rehabilitation research and development. 2005;42:63–78. doi: 10.1682/jrrd.2005.04.0064. [DOI] [PubMed] [Google Scholar]
- Beers AN, Shahnaz N, Westerberg BD, et al. Wideband reflectance in normal Caucasian and Chinese school-aged children and in children with otitis media with effusion. Ear and hearing. 2010;31:221–233. doi: 10.1097/AUD.0b013e3181c00eae. [DOI] [PubMed] [Google Scholar]
- Feeney MP, Grant IL, Marryott LP. Wideband energy reflectance measurements in adults with middle-ear disorders. Journal of speech, language, and hearing research : JSLHR. 2003;46:901–911. doi: 10.1044/1092-4388(2003/070). [DOI] [PubMed] [Google Scholar]
- Feeney MP, Grant IL, Mills DM. Wideband energy reflectance measurements of ossicular chain discontinuity and repair in human temporal bone. Ear and hearing. 2009;30:391–400. doi: 10.1097/AUD.0b013e3181a283ed. [DOI] [PubMed] [Google Scholar]
- Gan RZ, Dai C, Wood MW. Laser interferometry measurements of middle ear fluid and pressure effects on sound transmission. The Journal of the Acoustical Society of America. 2006;120:3799–3810. doi: 10.1121/1.2372454. [DOI] [PubMed] [Google Scholar]
- Kringlebotn M. Network model for the human middle ear. Scandinavian audiology. 1988;17:75–85. doi: 10.3109/01050398809070695. [DOI] [PubMed] [Google Scholar]
- Lerut B, Pfammatter A, Moons J, et al. Functional correlations of tympanic membrane perforation size. Otology & neurotology : official publication of the American Otological Society, American Neurotology Society [and] European Academy of Otology and Neurotology. 2012;33:379–386. doi: 10.1097/MAO.0b013e318245cea5. [DOI] [PubMed] [Google Scholar]
- Mehta RP, Rosowski JJ, Voss SE, et al. Determinants of hearing loss in perforations of the tympanic membrane. Otology & neurotology : official publication of the American Otological Society, American Neurotology Society [and] European Academy of Otology and Neurotology. 2006;27:136–143. doi: 10.1097/01.mao.0000176177.17636.53. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nakajima HH, Pisano DV, Roosli C, et al. Comparison of ear-canal reflectance and umbo velocity in patients with conductive hearing loss: a preliminary study. Ear and hearing. 2012;33:35–43. doi: 10.1097/AUD.0b013e31822ccba0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Piskorski P, Keefe DH, Simmons JL, et al. Prediction of conductive hearing loss based on acoustic ear-canal response using a multivariate clinical decision theory. The Journal of the Acoustical Society of America. 1999;105:1749–1764. doi: 10.1121/1.426713. [DOI] [PubMed] [Google Scholar]
- Ravicz ME, Rosowski JJ, Merchant SN. Mechanisms of hearing loss resulting from middle-ear fluid. Hearing research. 2004;195:103–130. doi: 10.1016/j.heares.2004.05.010. [DOI] [PubMed] [Google Scholar]
- Rosowski JJ, Nakajima HH, Hamade MA, et al. Ear-canal reflectance, umbo velocity, and tympanometry in normal-hearing adults. Ear and hearing. 2012;33:19–34. doi: 10.1097/AUD.0b013e31822ccb76. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shahnaz N, Bork K, Polka L, et al. Energy reflectance and tympanometry in normal and otosclerotic ears. Ear and hearing. 2009;30:219–233. doi: 10.1097/AUD.0b013e3181976a14. [DOI] [PubMed] [Google Scholar]
- Shahnaz N, Longridge N, Bell D. Wideband energy reflectance patterns in preoperative and post-operative otosclerotic ears. International journal of audiology. 2009;48:240–247. doi: 10.1080/14992020802635317. [DOI] [PubMed] [Google Scholar]
- Voss SE, Adegoke MF, Horton NJ, et al. Posture systematically alters ear-canal reflectance and DPOAE properties. Hearing research. 2010;263:43–51. doi: 10.1016/j.heares.2010.03.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Voss SE, Merchant GR, Horton NJ. Effects of middle-ear disorders on power reflectance measured in cadaveric ear canals. Ear and hearing. 2012;33:195–208. doi: 10.1097/AUD.0b013e31823235b5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Voss SE, Rosowski JJ, Merchant SN, et al. How do tympanic-membrane perforations affect human middle-ear sound transmission? Acta otolaryngologica. 2001a;121:169–173. doi: 10.1080/000164801300043343. [DOI] [PubMed] [Google Scholar]
- Voss SE, Rosowski JJ, Merchant SN, et al. Middle-ear function with tympanic-membrane perforations. I. Measurements and mechanisms. The Journal of the Acoustical Society of America. 2001b;110:1432–1444. doi: 10.1121/1.1394195. [DOI] [PubMed] [Google Scholar]
- Voss SE, Rosowski JJ, Merchant SN, et al. Middle-ear function with tympanic-membrane perforations. II. A simple model. The Journal of the Acoustical Society of America. 2001c;110:1445–1452. doi: 10.1121/1.1394196. [DOI] [PubMed] [Google Scholar]