Abstract
Sterols are essential components of the cell membrane lipid bilayer that include molecules such as cholesterol and desmosterol, which are significantly found in the spermatozoa of several animal species. However, the presence of desmosterol in rabbit semen has never been investigated. The aims of this study were to characterize the sterol composition of subfractions of ejaculated rabbit semen and evaluate the in vitro effects of sterol on the spermatozoa acrosome reaction and motility. Two sterols, occurring prevalently in the free form (94.3%), were identified in whole semen collected from 10 fertile New Zealand White rabbits, specifically desmosterol (58.5% of total sterols) and cholesterol (35.9% of total sterols). Desmosterol was the predominant sterol found in all subfractions of rabbit semen, varying from 56.7% (in the prostatic secretory granules, PSGs) to 63.8% (in the seminal plasma). Spermatozoa contained an intermediate proportion of desmosterol (59.8%), which was asymmetrically distributed between the heads (52.0% of the total content of sterols) and the tails (81.8%). Results showed that both desmosterol and cholesterol can be transferred from the PSGs to the spermatozoa and are equally effective in inhibiting in vitro spermatozoa capacitation at a concentration higher than 1 mg L−1. In contrast, neither desmosterol nor cholesterol had a significant effect on spermatozoa motility. Thus, it was concluded that, the various fractions of rabbit seminal fluid differ from each other in sterol composition and quantity, probably due to their different functional properties, and these fractions may undergo significant sterol changes depending on the stage of spermatozoa capacitation.
Keywords: acrosome reaction, cholesterol, desmosterol, motility, prostatic secretory granules, rabbit, seminal plasma, spermatozoa head, spermatozoa tail
Introduction
Ejaculated rabbit semen is a heterogeneous mixture consisting of seminal plasma in which the mature spermatozoa are suspended along with numerous membrane-surrounded granules. These vesicular formations, which have a predominantly round shape and a diameter ranging from 0.5 to 4 μm, are produced and secreted by the anterior part of the prostate (namely pro-prostate) as shown by their presence in vasectomised rabbit bucks 1. Similar multi-lamellar granules have also been isolated from human semen and from the semen of other animal species as prostasomes and prostasome-like vesicles, respectively 2, 3, 4, 5. The physiological roles of these prostatic secretory granules (PSGs) have been thoroughly debated and several hypotheses have been put forward 3. In rabbits, PSGs are able to inhibit spermatozoa capacitation, delaying the acrosome reaction, and they promote spermatozoa motility during the first few hours after mating 1, 6.
Both spermatozoa and PSGs are characterized by a unique lipid and fatty acid composition 7, 8. The lipid bilayer of the rabbit spermatozoa membrane is mainly composed of phospholipids (69% of the total amount of lipids) 7, with phosphatidylcholine being the major one, followed by phosphatidyl-ethanolamine and lyso-phosphatidylcholine. Sterols are the second major component of rabbit spermatozoa and are considered to be important modulators of membrane fluidity and the acrosome reaction 9. However, there is no information about the sterol composition of rabbit spermatozoa or on how these lipids are distributed between the spermatozoa head and tail. Unlike phospholipid composition, which is determined by chromatographic techniques, sterols have been characterized in rabbit semen by spectrophotometry, which is a limited technique.
PSGs have a dissimilar lipid composition compared with the spermatozoa plasma membrane 7. In particular, they contain twice as many sterols as phospholipids and have a considerable amount of sphingomyelin. The unusual abundance of these two lipids is thought to be responsible for the ordered structure of the PSG membrane and also for the role of PSGs in reversing or blocking spermatozoa capacitation 1, 6, 7. However, there are no experimental data demonstrating a role for PSG-related sterols in this function. Apart from cholesterol, the presence of other sterols in PSGs has not been studied.
On the basis of these considerations and the crucial role of lipids in spermatozoa biology, the present study seeks to characterize the sterol composition in subfractions of rabbit seminal fluid and in the major functional parts of the spermatozoa (head and tail). A potential transfer of sterols from PSGs to spermatozoa is investigated here, and the effects of these compounds on the in vitro spermatozoa acrosome reaction and motility are evaluated.
Materials and methods
Reagents
Unless otherwise noted, all of the chemicals were of analytical grade or high-performance liquid chromatography (HPLC) grade and were purchased from the Sigma Chemical Company (St. Louis, MO, USA). The protein dye reagent was obtained from BioRad Laboratories (Hercules, CA, USA).
Animals, semen collection and determination of basic semen characteristics
The trial was carried out at the farm of the Animal Science Section of Perugia University (Italy) according to the National Research Council's guidelines. Ten fertile New Zealand White rabbits (10 months of age) were housed in individual cages with a photoperiod of 16 h light per day, at an intensity of 40 lux and temperatures ranging from 16 °C to 25 °C 10. Fresh water was constantly supplied. All the animals were fed ad libitum on a standard diet composed of dehydrated alfa–alfa meal (40%), soybean meal (18%), barley (30%), wheat bran (10%), mineral and vitamins (2%) 11. The results of chemical analysis of the diet (expressed as % dry matter) were crude protein (17.5%), crude fibre (15.5%), fat (2.5%), polysaccharides (58.3%) and ash (6.2%).
Semen collection was performed twice a week for a period of 2 weeks (total semen samples, n = 40) by means of an artificial vagina that was kept at 37°C and filled up with heated water at 39°C–40°C at the moment of semen collection. After collection, the semen samples were immediately transferred to the Laboratory of Animal Science, University of Perugia, Italy, for further processing. The volume and spermatozoa concentration of the ejaculates were recorded using a graduated tube and a Thoma–Zeiss cell counting chamber (BRAND, Wertheim, Germany) fitted with a light microscope (Olympus CH-2; Olympus Optical Co. Ltd., Tokyo, Japan).
The number of live and mobile cells, as well as the spermatozoa motion patterns, was evaluated using a computer-assisted sperm analyser (CASA, model ISAS®; Proiser, Valencia, Spain) as previously described, by setting the acquisition rate at 100 Hz 12. For each sample, two drops (the volume of two drops is 10 μL per drop and the depth was 10 microns as reported for an ordinary Makler cell chamber) and three microscopic fields were analysed by the same person (about 500 spermatozoa). The recorded parameters of spermatozoa motion were curvilinear (VCL) and straight linear velocities (VSL), linearity (LIN) and amplitude lateral head displacement (ALH). The VSL and LIN are indicators of spermatozoa progression, whereas VCL and ALH are measures of spermatozoa vigour.
Seminal plasma was separated from spermatozoa and PSGs by centrifugation (700 × g for 15 min). The resulting pellet, containing the remaining semen subfractions, was subjected to colloidal silica Percoll® gradient-density centrifugation (300 × g for 20 min) to separate the spermatozoa (pellet) from the PSGs 13. The band of supernatant containing the PSGs was removed and centrifuged at 700 × g to pellet the granules. The resulting pellets were re-suspended in a suitable volume of phosphate buffered saline (PBS) and the amount of PSGs in ejaculated semen was determined by measuring the protein content after a reaction with Coomassie Brilliant Blue G-250 using bovine serum albumin (BSA) as a standard 14. The values were expressed as mg protein per mL of ejaculate. To obtain enough material for the purposes of the study, semen samples (from 3 to 5 rabbit bucks) were pooled.
The acrosome states (the spontaneous acrosome reaction [SAR] and the induced acrosome reaction [IAR]) of the spermatozoa were evaluated using fluorescein isothiocyanate (FITC)-conjugated Pisum sativum agglutinin (PSA) staining, which was analysed on an epifluorescence microscope (Olympus CH-2 excitation filter 335–425 nm) 15. A minimum of 200 cells per sample were scored according to the following staining patterns observed: (a) bright, homogeneous fluorescence in the anterior spermatozoa head region and (b) patchy fluorescence or staining limited to the equatorial segment, indicating an intact acrosome and a partially or totally reacted spermatozoa, respectively. The percentage of capacitated spermatozoa was calculated by taking the difference between spermatozoon with IAR and those with SAR.
Spermatozoa fractionation
Aliquots of Percoll-separated spermatozoa (100 × 106 cells) were gently suspended in 500 μL of PBS containing EDTA (2 mmol L−1), 2-mercaptoethanol (1 mmol L−1) and phenylmethylsulfonyl fluoride (1 mmol L−1) 16. To dissociate spermatozoa tails from heads, the spermatozoa were subjected to ultra-sonication at an intensity of 30% for 2 min (IKASONIC, model U50 IKA®, Staufen, Germany) and then mixed with an equal volume of 1.8 mol L−1 sucrose. Fractionated samples were loaded onto a discontinuous sucrose gradient from 2.0 to 2.2 mol L−1 and centrifuged at 104 000 × g for 60 min at 4°C with the aid of a Beckman SW 28.1 rotor (Beckman Coulter Inc., Brea, CA, USA). After stratification, each band (the higher band contained the tails, while the heads were contained in the pellet 16) was suspended in PBS and further ultracentrifuged (60 000 × g for 30 min) to obtain the purified spermatozoa heads or tails. These were stored at −80 °C until the day of analysis.
Extraction of sterols from spermatozoa subfractions, seminal plasma and PSGs
Sterols were extracted from the intact and fractionated spermatozoa specimens (100 × 106 cells), PSGs (1 mg protein) and seminal plasma devoid of spermatozoa and PSGs (200 μL) using the method of Folch et al. 17 adapted for semen analysis 18 with slight modifications. Briefly, all samples were mixed with 8 mL chloroform–methanol (2:1, v/v) and sonicated at 30% intensity for 10 s (model IKA® U50). The mixture was vortexed and filtered through Whatman paper (No.1) to eliminate cell debris. Next, 1.5 mL of distilled water containing 0.6% NaCl was added and the mixture was vortexed and allowed to stand at room temperature for 1 h before centrifugation at 500 × g for 10 min. The upper layer was resuspended in 8 mL chloroform–methanol–water containing NaCl (86:16:1, v/v) and centrifuged at 500 × g for 10 min. The chloroform extracts were pooled and dried under a flow of nitrogen. The residue was dissolved in 200 μL mobile phase and filtered through the regenerated cellulose syringe disposals (RC Phenex 4 mm, 0.26 μm pore size; Phenomenex SRL., Bologna, Italy) prior to injection into the HPLC system.
For the quantitative evaluation of rabbit semen's esterified sterols, total lipid extracts from the intact and fractionated semen samples were plated on silica gel G-25 thin layer chromatography (TLC) plates (Macherey-Nagel, PA, USA). The mobile phase was composed of hexane–ethyl ether–acetic acid (90:10:1, v/v). After elution, the plates were dried and stained with a solution containing Cu-acetate (3%) in H3PO4 (8%) prior to densitometric analysis. Images were acquired using the VersaDoc Imaging System (Bio-Rad Laboratories Ltd., Hertfordshire, UK) and signals were quantified using quantity one software (Bio-Rad, Milan, Italy). Pure cholesterol and cholesteryl palmitate standards were run on the same plates as the samples to construct calibration curves. To further establish the esterified fraction's sterol composition, the corresponding band was scraped from the TLC plates and sterol esters were twice extracted with chloroform prior to saponification by methanolic KOH (20% in 50% methanol) for 30 min at 37 °C. Free sterols were extracted twice with petroleum ether and the organic solvent was evaporated under a stream of nitrogen. The residue was recovered in HPLC mobile phase and injected into the HPLC system.
HPLC evaluation of sterol content in semen subfractions and spermatozoa subcellular fractions
Extracted sterols from the spermatozoa subcellular fractions, PSGs and seminal plasma were fractionated using a Jasco HPLC system (pump model PU-1 580, equipped with an autosampler model AS 950-10, Tokyo, Japan) on a Waters spherisorb C18 reversed-phase analytical column (ODS-2, 5 μm particle size, 250.0 × 4.6 mm internal diameter; CPS analitica, Milan, Italy). The mobile phase was composed of acetonitrile–isopropanol (70/30, v/v) and released at a flow rate of 1.5 mL min−1. Sterols were identified using a UV detector (model Jasco 2075 Plus, Tokyo, Japan) set at 210 nm and were quantified by using external calibration curves prepared with increasing amounts of pure desmosterol and cholesterol standards in isopropanol. The volume of injection was 20 μL.
Incubation of spermatozoa with PSGs and pure single sterols
The potential transfer of sterols from PSGs to spermatozoa was investigated by measuring sterol levels of spermatozoa before and after incubation of spermatozoa (100 × 108 cells) with a specific amount of PSGs (0.2 mg of protein) for 6 h at 37 °C (in 5% CO2) in buffered Tyrode's medium (1 mL, TALP 12) containing BSA (0.3%), an inducer of spermatozoa capacitation. The number of PSGs was previously established, taking into account the average concentration of PSGs in rabbit ejaculate (1.0 ± 0.3 g protein L−1) 1.
To evaluate the in vitro effects of single sterols, prepared in pure ethanol, on the spermatozoa acrosome reaction, spermatozoa aliquots (10 × 106 cells) were incubated for 6 h at 37°C (in 5% CO2) with increasing amounts of either cholesterol or desmosterol (0–10 mg L−1, final concentration) in TALP/BSA. The final concentration of ethanol in incubating medium was 0.1%. The rate of SAR and IAR as well as the percentage of capacitated spermatozoa was evaluated as described in Section 2.2 and were compared with the corresponding rates obtained after incubation of spermatozoa with PSGs, as described above. Changes in spermatozoa kinetic characteristics were also evaluated. These experiments were repeated 10 times.
Statistical analyses
The results are presented as mean ± SD. Significant differences in sterol proportions and/or sterol molar ratios among the spermatozoa subfractions were evaluated by one-way ANOVA using the post hoc Bonferroni test at P < 0.05. Student's t-test was used for the comparison of mean sterol values between spermatozoa subcellular parts. A paired t-test was used for the evaluation of changes in spermatozoa parameters before and after treatment with PSGs or pure sterols. All statistical analyses were performed using the Stata® statistical package (release 10.0, StataCorp LP, Texas, TX, USA).
Results
Sterol composition of subfractions of rabbit semen and subcellular fractions of spermatozoa
The main characteristics of rabbit semen are summarized in Table 1. These values are in agreement with those reported for New Zealand White rabbits and confirm good semen quality 10, 12. The percentages of spontaneous and induced acrosome-reacted spermatozoa were low, and this was responsible for the fairly low spermatozoa capacitation rate (37.0% ± 4.2%), which was also consistent with our previous findings 1, 6.
Table 1. Main characteristics of rabbit semen.
| Characteristics | Values (mean ± SD, n = 40) |
|---|---|
| Ejaculate volume, mL | 0.6 ± 0.1 |
| Sperm concentration, 108 mL−1 | 4.1 ± 0.4 |
| PSG concentration, mg protein mL−1 | 1.2 ± 0.1 |
| Live cells, % | 74.9 ± 5.0 |
| Motile cells, % | 72.4 ± 3.8 |
| VCL, μm s−1 | 199.8 ± 13.2 |
| LIN, % | 48.1 ± 0.2 |
| ALH, μm | 2.6 ± 1.0 |
| SAR, % | 20.4 ± 2.6 |
| IAR, % | 57.4 ± 3.0 |
| Capacitation, % | 37.0 ± 4.2 |
Abbreviations: ALH, amplitude of lateral head displacement; LIN, linearity (defined as the percentage of VSL/VCL ratio, where VSL is the straight linear velocity); PSG, prostatic secretory granules; SAR and IAR, spontaneous and induced acrosome reaction; VCL, curvilinear velocity.
Chromatographic analysis revealed the presence of two main sterols in ejaculated rabbit semen: desmosterol and cholesterol, which were effectively eluted from the reversed phase column, demonstrating two well-separated peaks. No other cholesterol precursors were identified.
In all of the semen subfractions, both the lipids were present in free form (Table 2), while sterol esters accounted for around 6% of the total sterols. Desmosterol was predominant in whole semen (62.0% of total sterols) and in all semen subfractions, varying from 56.7% (in the PSGs) to 63.8% (in the seminal plasma). In contrast, cholesterol was higher (P < 0.05) in the spermatozoa fraction (40.2%) than in seminal plasma (36.2%). As a consequence, the molar ratio of desmosterol to cholesterol was higher in seminal plasma than in spermatozoa (1.8 ± 0.1 vs. 1.5 ± 0.1, P < 0.05). The PSGs contained intermediate amounts of both desmosterol and cholesterol, with the latter varying greatly from 35.2 to 339.2 nmol per mg protein. Despite the fact that it was much higher, the desmosterol–cholesterol molar ratio did not differ significantly from the sterol molar ratios of the other subfractions.
Table 2. Levels of desmosterol and cholesterol in subfractions of rabbit semen, particularly spermatozoa, prostatic secretory granules (PSGs) and seminal plasma.
| Sterols | Spermatozoa (nmol per 108 cells) | PSGs (nmol per mg protein) | Seminal plasma (nmol mL−1) | Whole semen (nmol per 108 cells) |
|---|---|---|---|---|
| Desmosterol (D) | 142.8 ± 14.6≅ (59.8) | 245.6 ± 24.8 (56.7) | 760.0 ± 27.3≅ (63.8) | 212.1 ± 14.4 (62.0) |
| Cholesterol (C) | 95.8 ± 13.2 (40.2) | 187.2 ± 152.0 (43.3) | 432.0 ± 15.8 (36.2) | 130.0 ± 26.6 (38.0) |
| Total level of free sterols | 238.6 ± 27.8 (93.8) | 432.8 ± 131.9 (93.5) | 1 192.0 ± 42.8 (94.7) | 342.0 ± 41.0 (94.3) |
| Total level of sterol esters | 15.7 ± 1.0 (6.2) | 30.3 ± 1.1 (6.5) | 66.6 ± 1.3 (5.3) | 20.5 ± 1.0 (5.7) |
| Total level of sterols | 254.3 ± 27.8 | 463.1 ± 131.9 | 1 258.6 ± 42.8 | 362.5 ± 41.1 |
| D/C ratio | 1.5 ± 0.1a | 2.7 ± 2.3ab | 1.8 ± 0.1b | 1.6 ± 0.1 |
Total free sterols are the sum of desmosterol and cholesterol (both in the free form as determined by HPLC); Total sterol esters are the sum of esterified demosterol and cholesterol determined as a whole by TLC; Total sterols are the sum of total free sterols and total sterol esters. Data are mean ± SD (n = 10, pooled semen samples). Data shown in parentheses are the percent of total sterols.
Values in the same row with different letters are significantly different, P < 0.05.
P < 0.05, compared with cholesterol in the same column.
The levels of desmosterol esters were slightly lower than the cholesteryl ester concentration (9.1 ± 1.2 vs. 11.4 ± 1.4 nmol per 108 cells) in whole semen, and the proportion of these sterol esters, taken together, varied moderately in the semen subfractions, from 5.3% (in the seminal plasma) to 6.5% (in the PSGs) (Table 2).
The distribution of free sterols in the subcellular fractions of rabbit spermatozoa (Table 3) revealed that approximately 72.9% of desmosterol and cholesteryl were located in the head region, while the tail contained fewer compounds (P < 0.05), mostly represented by desmosterol (81.8% of total sterols). Moreover, the head contained almost equal amounts of these two sterols and maintained a molar desmosterol–cholesterol ratio 4 times lower (P < 0.05) than the tail.
Table 3. Distribution of free desmosterol and cholesterol in subcellular fractions of rabbit spermatozoa.
| Sterols (nmol per 108 cells) | Sperm subcellular fractions |
Sperma (H + T) | |
|---|---|---|---|
| Head (H) | Tail (T) | ||
| Desmosterol (D) | 90.8 ± 8.4* | 53.2 ± 12.9≅ | 143.9 ± 15.4≅ |
| (52.0) | (81.8) | (60.1) | |
| Cholesterol (C) | 83.8 ± 8.1* | 11.8 ± 0.4 | 95.6 ± 13.7 |
| (48.0) | (18.2) | (39.9) | |
| Total level of free sterols | 174.6 ± 15.1* | 65.0 ± 12.9 | 239.5 ± 20.6 |
| D/C ratio | 1.1 ± 0.1 | 4.5 ± 1.1* | 1.5 ± 0.2 |
Data are mean ± SD (n = 10, pooled semen samples). Data shown in parentheses are the percent of total sterols.
Calculated values obtained by summing values related to the head and the tail of rabbit spermatozoa.
P < 0.05, compared with tails.
P < 0.05, compared with cholesterol in the same column.
Changes in the sterol composition of spermatozoa after incubation with PSGs
A 6-h incubation of control spermatozoa in the capacitating medium resulted in a drastic reduction (P < 0.05) of total free sterols, suggesting a considerable sterol efflux from the spermatozoa during the development of acrosomal responsiveness (Table 4). In addition, the reduction in concentration was almost the same for each sterol (44.3% and 43.0%). When spermatozoa were incubated with PSGs, the loss in the total sterols was much lower compared with that observed for the control spermatozoa (24.3% vs. 43.8%, P < 0.05). This was most likely due to the 0.3-fold increase (P < 0.05) of total sterols in the spermatozoa membrane after treatment with PSGs.
Table 4. Changes in sterol composition after incubation of spermatozoa with prostatic secretory granules (PSGs) for 6 h at 37°C.
| Sterols (nmol per 108 cells) | Control sperm |
Spermatozoa challenged with PSGsa | |
|---|---|---|---|
| 0 h | 6 h | ||
| Desmosterol (D) | 140.9 ± 32.6 | 78.5 ± 14.3* | 109.3 ± 2.9** |
| Cholesterol (C) | 97.6 ± 11.8 | 55.6 ± 9.7* | 71.3 ± 5.6** |
| Total free sterols | 238.6 ± 20.8 | 134.1 ± 3.6* | 180.6 ± 2.7** |
| D/C ratio | 1.5 ± 0.5 | 1.4 ± 1.5 | 1.6 ± 0.4 |
Data are mean ± SD (n = 5, pooled semen samples per group).
The amount of PSGs used for the treatment of 108 sperm cells was equal to 0.2 mg of protein.
P < 0.05, compared with the sterol values of control spermatozoa at baseline (0-h).
P < 0.05, compared with the sterol values of control spermatozoa after a 6-h incubation.
In vitro effects of PSGs and pure sterols on spermatozoa acrosome reaction and motility
The in vitro effects of PSGs and pure sterols on the spermatozoa acrosome reaction and motility are reported in Figure 1. During the 6-h incubation, the incidence of spontaneously reacted spermatozoa increased by 48% (P < 0.05) in comparison with the initial value (20.4% ± 2.6%), consistent with sterol changes in the spermatozoa membrane. Alternatively, spermatozoa incubated with PSGs had a lower incidence of induced acrosome reaction (11% ± 1% vs. 54% ± 4%, P < 0.05) and thus a lower capacitation rate compared with control spermatozoa (Figure 1A). Adding increasing amounts of desmosterol or cholesterol to the incubation medium noticeably inhibited in vitro spermatozoa capacitation, even at a low concentration (1 mg L−1). Interestingly, the amount of cholesterol necessary to produce the same effect on spermatozoa capacitation as PSGs was approximately 5 mg L−1 (a concentration corresponding to the point of intersection between the concentration–effect curve of cholesterol and the line referring to the effect of PSGs on spermatozoa capacitation). Regarding the desmosterol inhibiting activity on spermatozoa capacitation, concentration higher than 8 mg L−1 should be released by PSGs to the spermatozoa to achieve a comparable effect. These data were quite consistent with the amount of sterols transferred from PSGs to spermatozoa.
Figure 1.
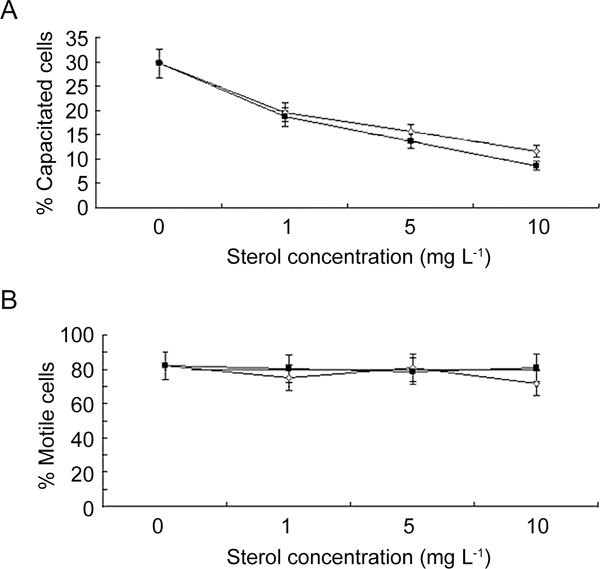
Concentration–effect curves of desmosterol (—Δ—) and cholesterol (—▪—) on sperm capacitation (A) and motility (B) after incubation for 6 h at 37°C. The fragmented line (— —) represents the effect of PSGs (0.2 mg, see materials and methods) on sperm capacitation or motility (y axis) and is independent on sterol concentration reported in the x axis. The intersection points of this line with the dose-curves of sterols represent the presumed quantities of each sterol necessary for the PSGs to affect sperm capacitation or motility. Sample number = 5 pooled semen samples/treatments. Control group is referred to the values of capacitated cells at 0 mg L−1 of sterols added in the medium.
In contrast, spermatozoa motility was not affected by the presence of sterols in the incubation medium, suggesting that these compounds have no role in the promotion of spermatozoa motility observed for PSGs (Figure 1B). The other kinetic parameters were also not affected by PSGs or the sterols studied. In addition, the use of ethanol (0.1% final concentration) to dissolve pure sterols had no effect on spermatozoa capacitation or on the kinetic characteristics (data not shown).
Discussion
Sterols and phospholipids are essential constituents of mammalian semen and have an important role in the fertilizing capacity of sperm cells. Besides cholesterol, our data showed for the first time that rabbit semen also contains considerable amounts of desmosterol, which is the immediate precursor of cholesterol in the Bloch biochemical pathway. Various proportions of this unusual sterol have also been reported in human semen 19 and in the semen of other mammalian species such as the rhesus monkey 20, hamster 21 and boar 22. In particular, we found that this molecule is more abundant than the cholesterol present in rabbit spermatozoa, seminal plasma and PSGs. Monkey spermatozoa is also richer in desmosterol than in cholesterol 20, while the opposite has been observed for human 18 and boar spermatozoa 22. It is speculated that these differences depend on the levels of Δ-24 reductase and/or on the presence or absence of an inhibitor of this enzyme among the various species, as suggested by Lin et al. 20.
As found for monkey spermatozoa 24, we found that the desmosterol–cholesterol ratio was higher in the tail than in the head of rabbit spermatozoa. This asymmetric desmosterol–cholesterol composition between the spermatozoa head and tail has been suggested to be responsible for the different functions of these two spermatozoa subcellular parts. In particular, desmosterol has an additional double bond with respect to cholesterol and adequately modulates the membrane fluidity, which is necessary for spermatozoa motility 24. In addition, there is experimental evidence that spermatozoa suspensions with a higher desmosterol–cholesterol molar ratio are characterized by a greater percentage of motile cells and a higher forward progression speed, further corroborating this assumption 23.
It is worth noting that both the sterols were present in the free form in all rabbit semen subfractions, while sterol esters were less abundant in the seminal plasma, most likely due to the higher affinity of these hydrophobic compounds for the less polar membrane-surrounded components of the seminal fluid. Unlike the semen from other mammals, rabbit semen contains discrete amounts of sterol esters and the presence of desmosterol esters (representing about 44% of total sterol esters) is a unique biological finding. Sterol esterification occurs during spermatozoa transit through the epididymis in the ram 25, and this could also be true for the rabbit. In hamster and monkey spermatozoa, desmosterol is also present chiefly in the free form, and only a minor proportion is conjugated with a thiol group, exhibiting several structural (spermatozoa membrane stabilizer) and functional (modulator of capacitation, acrosin inhibitor) roles 21, 24. The presence of desmosterol and/or cholesteryl sulphate in rabbit semen remains to be verified.
Desmosterol has not been found in significant quantities elsewhere in the body except in the brain 26 and testis, and it has been proposed that it is a biochemical marker of puberty in the monkey 27. Although there is no direct evidence, the presence of this sterol in the PSGs of rabbit semen, which are specifically produced by the prostate, is a unique biological finding and may indicate an active desmosterol synthesis in this accessory sex gland. This latter aspect is currently under investigation in our laboratories. Whether desmosterol is present in human prostasomes or in prostasome-like vesicles from other mammalian species remains to be clarified yet.
Characterization of the basal sterol composition of rabbit semen is useful for future intervention studies intended to evaluate dietary or pharmaceutical influences on cholesterol metabolism in the testis and/or prostate.
In the present study, we also investigated the changes in rabbit sterol composition after inducing the acrosome reaction in the presence or absence of PSGs, which are recognized as spermatozoa capacitation inhibitors 6. To our knowledge, this is the first study addressing these issues in this animal species and we found that (1) both sterols drastically decrease after the incubation of spermatozoa in the absence of PSGs, and (2) either desmosterol or cholesterol can be transferred from the PSGs to spermatozoa when the PSGs are added to the incubation medium.
It is worth noting that, in both cases, there is no evident spermatozoa preference for a specific sterol, suggesting that desmosterol and cholesterol can be equally involved in the initial mandatory step (that of sterol depletion from the spermatozoa plasma membrane 28) of rabbit spermatozoa capacitation and probably share the same carriers and molecular mechanisms for transfer from PSGs to spermatozoa. This could be due to the very similar chemical structures of both the sterols. Xu et al. 29 have demonstrated that desmosterol may efficiently replace cholesterol in the cell membrane without compromising the functional properties because there are compensatory changes in membrane composition.
In vitro trafficking of lipophilic compounds, such as vitamin E homologues, from PSGs to spermatozoa has been previously reported, and the seminal albumin was hypothesized to be the potential carrier 30. This protein was also present in the incubating medium, making the transfer of sterols from the PSGs to spermatozoa feasible. However, the spermatozoa–PSG interaction is a more complex phenomenon involving both the lipid and the protein constituents 31, 32, which warrants a more detailed investigation.
Once sterol transfer from PSGs to spermatozoa was established, the role of single sterols in the regulation of the in vitro spermatozoa capacitation by PSGs was further investigated by adding increasing amounts of desmosterol or cholesterol to the incubating medium and scoring the spermatozoa with a spontaneous and induced acrosome reaction. Specifically, our data demonstrate that both sterols are able to reverse or inhibit in vitro spermatozoa capacitation at a concentration ≥ 1 mg L−1. Nimmo and Cross 33 also found that either desmosterol or cholesterol can prevent the in vitro acrosome reaction of human spermatozoa.
Comparing the effects of PSGs and pure sterols on this spermatozoa function, we hypothesize that PSGs may suppress in vitro spermatozoa acrosome responsiveness by releasing or transferring approximately 5 mg L−1 of cholesterol and/or 8 mg L−1 of desmosterol to the spermatozoa. These values were similar to those measured in spermatozoa after incubation with PSGs, further confirming this assumption. Minelli et al. 34 also demonstrated that sterol-rich vesicles from stallion prostate can effectively block sterol efflux from the spermatozoa plasma membrane. The slightly higher amounts of desmosterol necessary for the PSG-related blocking activity may partially justify the abundance of this sterol in PSGs. On the other hand, we observed a surprisingly large variation in the cholesterol content of PSGs and believe that it is due to the heterogeneous vesicles constituting the PSG population 6. Taken together, these findings confirm and expand our previous observations of the role of PSGs in spermatozoa capacitation by providing a biochemical explanation 1, 6.
We found that neither desmosterol (the proposed spermatozoa motility modulator) nor cholesterol affected spermatozoa motility in vitro. Alternatively, the PSGs were able to modulate this spermatozoa function, which is in agreement with our previous findings 6. Perhaps molecules other than sterols are implicated in this PSG-related property. However, to better understand the role of sterols in spermatozoa motility, further studies on the sterol composition of spermatozoa heads and tails, before and after incubation with the PSGs, will be helpful.
In conclusion, desmosterol and cholesterol are the main sterol constituents of rabbit spermatozoa. They are asymmetrically distributed between the heads and tails, and their relative abundance in these cells is partially regulated by the presence of PSGs in the seminal fluid. In particular, we found that as long these sterol-rich vesicles remain in contact with the spermatozoa, they may prevent the spermatozoa acrosome reaction by donating a part of their endogenous sterols. This is an important issue for the fertilizing capacity of rabbit spermatozoa because these cells have to wait for long time (16–18 h) for the eggs to become available in the oviduct without losing their membrane integrities 6. In addition, the role of the seminal plasma-derived sterols in the spermatozoa acrosome reaction is currently under investigation in our laboratories.
Acknowledgments
We thank Dr Paolo Lattaioli, Mr Carlo Ricci and Mrs Stefania Diarena for technical assistance. We are also grateful to Sister Mary A Traynor for her helpful linguistic suggestions.
References
- Cardinali R, Dal Bosco A, Mourvaki E, Del Vecchio MT, Sartini B, et al. Rabbit semen particles: secretion pattern and main effect on the sperm functions. J Submicrosc Cytol Pathol. 2007;39:3–10. [Google Scholar]
- Arienti G, Carlini E, De Cosmo AM, Di Profio P, Palmerini CA. Prostasome-like particles in stallion semen. Biol Reprod. 1998;59:309–13. doi: 10.1095/biolreprod59.2.309. [DOI] [PubMed] [Google Scholar]
- Kravets FG, Lee J, Singh B, Trocchia A, Pentyala SN, et al. Prostasomes: current concepts. Prostate. 2000;43:169–74. doi: 10.1002/(sici)1097-0045(20000515)43:3<169::aid-pros2>3.0.co;2-d. [DOI] [PubMed] [Google Scholar]
- Ghaoui Rel-H, Thomson PC, Evans G, Maxwell WM. Characterization and localization of membrane vesicles in ejaculate fractions from the ram, boar and stallion. Reprod Domestic Anim. 2004;39:173–80. doi: 10.1111/j.1439-0531.2004.00499.x. [DOI] [PubMed] [Google Scholar]
- Siciliano L, Marcianò V, Carpino A. Prostasome-like vesicles stimulate acrosome reaction of pig spermatozoa. Reprod Biol Endocrinol. 2008;6:5. doi: 10.1186/1477-7827-6-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Castellini C, Mourvaki E, Cardinali R, Del Vecchio MT.Rabbit seminal granules: secretion site and main roleIn: Collodel G, Moretti E, editors. Sperm Morphology and PathologyKerala: Research Signpost; 2008p45–59.
- Castellini C, Cardinali R, Dal Bosco A, Minelli A, Camici O. Lipid composition of the main fractions of rabbit semen. Theriogenology. 2006;65:703–12. doi: 10.1016/j.theriogenology.2005.05.053. [DOI] [PubMed] [Google Scholar]
- Mourvaki E, Cardinal R, Dal Bosco A, Corazzi L, Castellini C. Effects of flaxseed dietary supplementation on sperm quality and on lipid composition of sperm subfractions and prostatic granules in rabbit. Theriogenology. 2010;73:629–37. doi: 10.1016/j.theriogenology.2009.10.019. [DOI] [PubMed] [Google Scholar]
- Go KJ, Wolf DP. The role of sterols in sperm capacitation. Adv Lipid Res. 1983;20:317–30. [PubMed] [Google Scholar]
- Besenfelder U, Theau-Clément M, Sabbioni E, Castellini C, Renieri T, et al. Effects of different light intensities on quality of spermatozoa in rabbits. World Rabbit Sci. 2004;12:227–34. [Google Scholar]
- De Blas C, Wiseman J.The nutrition of the rabbitWallingford: CABI Publishing/CAB International; 1998p241–53.
- Castellini C, Mourvaki E, Sartini B, Cardinali R, Moretti E, et al. In vitro toxic effects of metal compounds on kinetic traits and ultrastructure of rabbit spermatozoa. Reprod Toxicol. 2009;27:46–54. doi: 10.1016/j.reprotox.2008.12.003. [DOI] [PubMed] [Google Scholar]
- Gliozzi TM, Luzi F, Cerolini S. Assessment of sperm viability in boar, rabbit and rooster: a modification the fluorometric ethidium bromide exclusion procedure. Theriogenology. 2003;60:635–45. doi: 10.1016/s0093-691x(03)00082-7. [DOI] [PubMed] [Google Scholar]
- Bradford MM. A rapid and sensitive method for the quantification of microgram quantities of protein utilizing the principle of protein dye binding. Anal Biochem. 1976;72:248–54. doi: 10.1006/abio.1976.9999. [DOI] [PubMed] [Google Scholar]
- Mendoza C, Carreras A, Moos J, Tesarik J. Distinction between true acrosome reaction and degenerative acrosome loss by a one-step staining method using Pisum sativum agglutinin. J Reprod Fertil. 1992;95:755–63. doi: 10.1530/jrf.0.0950755. [DOI] [PubMed] [Google Scholar]
- Furland NE, Oresti GM, Antollini SS, Venturino A, Maldonado EN. Very long-chain polyunsaturated fatty acids are the major acyl groups of sphingomyelins and ceramides in the head of mammalian spermatozoa. J Biol Chem. 2007;282:18151–61. doi: 10.1074/jbc.M700709200. [DOI] [PubMed] [Google Scholar]
- Folch J, Lees M, Sloane-Stanley H. A simple method for the isolation and purification of total lipids from animal tissues. J Biol Chem. 1957;226:497–502. [PubMed] [Google Scholar]
- Sion B, Grizard G, Boucher D. Quantitative analysis of desmosterol, cholesterol and cholesterol sulfate in semen by high-performance liquid chromatography. J Chromatogr A. 2001;935:259–65. doi: 10.1016/s0021-9673(01)01105-0. [DOI] [PubMed] [Google Scholar]
- Lalumiere G, Bleau G, Chapdelaine A, Roberts KD. Cholesteryl sulfate and sterol sulfatase in the human reproductive tract. Steroids. 1976;27:247–59. doi: 10.1016/0039-128x(76)90101-x. [DOI] [PubMed] [Google Scholar]
- Lin DS, Connor WE, Wolf DP, Neuringer M, Hachey DL. Unique lipids of primate spermatozoa: desmosterol and docosahexaenoic acid. J Lipid Res. 1993;34:491–99. [PubMed] [Google Scholar]
- Bleau G, Vandenheuvel WJ. Desmosteryl sulfate and desmosterol in hamster epididymal spermatozoa. Steroids. 1974;24:549–55. doi: 10.1016/0039-128x(74)90135-4. [DOI] [PubMed] [Google Scholar]
- Nikolopoulou M, Soucek DA, Vary JC. Lipid composition of the membrane released after in vitro acrosome reaction of epididymal boar sperm. Lipids. 1986;21:566–9. doi: 10.1007/BF02534053. [DOI] [PubMed] [Google Scholar]
- Connor WE, Weleber RG, Defrancesco CA, Lin DS, Wolf DP. Sperm abnormalities in retinitis pigmentosa. Invest Ophthalmol Vis Sci. 1997;38:2619–28. [PubMed] [Google Scholar]
- Connor WE, Lin DS, Wolf DP, Alexander M. Uneven distribution of desmosterol and docosahexaenoic acid in the heads and tails of monkey sperm. J Lipid Res. 1998;39:1404–11. [PubMed] [Google Scholar]
- Quinn PJ, White IG. Phospholipid and cholesterol content of epididymal and ejaculated ram spermatozoa and seminal plasma in relation to cold shock. Aust J Biol Sci. 1967;20:1205–15. doi: 10.1071/bi9671205. [DOI] [PubMed] [Google Scholar]
- Edmond JR, Korsak A, Morrow JW, Torok-Both G, Catlin TH. The origin of cholesterol in brain of developing rat. FASEB J. 1990;4:A532. doi: 10.1093/jn/121.9.1323. [DOI] [PubMed] [Google Scholar]
- Connor WE, Lin DS, Neuringer M. Biochemical markers for puberty in the monkey testis: desmosterol and docosahexaenoic acid. J Clin Endocrinol Metab. 1997;82:1911–6. doi: 10.1210/jcem.82.6.4001. [DOI] [PubMed] [Google Scholar]
- Cross NL. Effect of cholesterol and other sterols on human sperm acrosomal responsiveness. Mol Reprod Dev. 1996;45:212–7. doi: 10.1002/(SICI)1098-2795(199610)45:2<212::AID-MRD14>3.0.CO;2-2. [DOI] [PubMed] [Google Scholar]
- Xu F, Rychnovsky SD, Belani JD, Hobbs HH, Cohen JC, et al. Dual roles for cholesterol in mammalian cells. Proc Natl Acad Sci USA. 2005;102:14551–6. doi: 10.1073/pnas.0503590102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mourvaki E, Cardinali R, Dal Bosco A, Castellini C. In vitro antioxidant activity of the prostatic secretory granules in rabbit semen after exposure to organic peroxides. Reprod Biol Endocrinol. 2010;8:16. doi: 10.1186/1477-7827-8-16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Arienti G, Carlini E, Verdacchi R, Cosmi EV, Palmerini CA. Prostasome to sperm transfer of CD13/aminopeptidase N (EC 3.4.11.2) Biochim Biophys Acta. 1997;1336:533–8. doi: 10.1016/s0304-4165(97)00071-8. [DOI] [PubMed] [Google Scholar]
- Palmerini CA, Cametti C, Sennato S, Gaudino D, Carlini E, et al. Role of cholesterol, DOTAP, and DPPC in prostasome/spermatozoa interaction and fusion. J Membr Biol. 2006;211:185–90. doi: 10.1007/s00232-006-0009-2. [DOI] [PubMed] [Google Scholar]
- Nimmo MR, Cross NL. Structural features of sterols required to inhibit human sperm capacitation. Biol Reprod. 2003;68:1308–17. doi: 10.1095/biolreprod.102.008607. [DOI] [PubMed] [Google Scholar]
- Minelli A, Moroni M, Martinez E, Mezzasoma I, Ronquist G. Occurrence of prostasome-like membrane vesicles in equine seminal plasma. J Reprod Fertil. 1998;114:237–43. doi: 10.1530/jrf.0.1140237. [DOI] [PubMed] [Google Scholar]


