Abstract
Heavy alcohol consumption is associated with an increased risk of erectile dysfunction (ED); however, the acute effects of ethanol (EtOH) on penile tissue are not fully understood. We sought to investigate the effects of EtOH on corporal tissue tonicity, as well as the intracellular Ca2+ concentration ([Ca2+]i) and potassium channel activity of corporal smooth muscle. Strips of corpus cavernosum (CC) from rabbits were mounted in organ baths for isometric tension studies. Electrical field stimulation (EFS) was applied to strips precontracted with 10 μmol L−1 phenylephrine as a control. EtOH was then added to the organ bath and incubated before EFS. The [Ca2+]i levels were monitored by the ratio of fura-2 fluorescence intensities using the fura-2 loading method. Single-channel and whole-cell currents were recorded by the conventional patch-clamp technique in short-term cultured smooth muscle cells from human CC tissue. The corpus cavernosal relaxant response of EFS was decreased in proportion to the concentration of EtOH. EtOH induced a sustained increase in [Ca2+]i in a dose-dependent manner, Extracellular application of EtOH significantly increased whole-cell K+ currents in a concentration-dependent manner (P < 0.05). EtOH also increased the open probability in cell-attached patches; however, in inside-out patches, the application of EtOH to the intracellular aspect of the patches induced slight inhibition of Ca2+-activated potassium channel (KCa) activity. EtOH caused a dose-dependent increase in cavernosal tension by alterations to [Ca2+]i. Although EtOH did not affect KCa channels directly, it increased the channel activity by increasing [Ca2+]i. The increased corpus cavernosal tone caused by EtOH might be one of the mechanisms of ED after heavy drinking.
Keywords: alcohol, Ca2+-activated potassium channel, corporal smooth muscle, penile erection
Introduction
Erectile dysfunction (ED) is defined as the persistent inability to achieve or maintain an erection sufficient for satisfactory sexual performance 1. Penile erection comprises increased arterial inflow, sinusoidal smooth muscle relaxation and restricted venous outflow from the penis. Decreased penile vascular resistance induced by corporal smooth muscle relaxation is the most important step in penile erection 2. Trouble with any of these vascular mechanisms may lead to ED. That is, the pathophysiology of ED is associated with a decrease in smooth muscle relaxation and/or an increase in penile vasculature contractile tone 3, 4.
Modulation of corporal smooth muscle tone is a complex process, requiring the integration of a host of intracellular events and extracellular signals. Nitric oxide (NO) is one of the major physiological mediators of corporal smooth muscle relaxation in extracellular signals 5, 6, 7. Potassium channels and calcium channels have major roles in the intracellular events of corporal smooth muscle cells, although Cl− channels may also have a significant role 8, 9, 10.
Chronic alcoholism is a well-known risk factor for ED 11. Although there are studies that link ethanol (EtOH) consumption to an alteration in nitric oxide concentration and endothelial function, this relationship is unclear 12, 13. Generally, an adequate volume of alcohol relieves nervousness and enhances sexual desire, but excessive intake of alcohol can induce erectile problems by impairing penile tumescence and reducing sexual performance 14. EtOH consumption causes the contraction of various vascular smooth muscles such as those in the aorta, pulmonary vessels, coronary arteries and cerebral arteries 15, 16, 17, 18, 19, 20, 21, 22. However, there are few reports of its effect on the corpus cavernosum (CC).
Ca2+-activated potassium (KCa) channel activity controls depolarization and contraction in smooth muscle. Activation of KCa channels gives rise to positive outward currents, which then drive the smooth muscle cell membrane potential in a negative direction and counteract contraction 23. EtOH decreases the activity of aortic smooth muscle KCa channels reconstituted into lipid bilayers 24. However, EtOH inhibition of KCa channels is not universal, because both channel activation 25 and refractoriness 26 were observed. Therefore, we hypothesized that acute EtOH-induced enhancement of the intracellular Ca2+ level may be the mechanism underlying the contraction of corpora cavernosal smooth muscles triggered by EtOH. In addition, acute EtOH-induced inhibition of KCa channels may contribute to acute EtOH-induced enhancement of the intracellular Ca2+ level. In the present study, we investigate the effects of high EtOH concentration on the tonicity of corporal tissue and its effects on the intracellular calcium concentration ([Ca2+]i) and potassium channel activity of corporal smooth muscles.
Materials and methods
Electrical field stimulation (EFS)
Strips of CC from male New Zealand White rabbits were mounted in organ baths for isometric tension studies. Six strips of CC were used from three rabbits (mean weight 3.0 kg). The penis was removed at the level of the attachment of the corporal bodies to the ischium. The grossly dissected organ preparation was then placed in Kreb's solution. The CC was sharply dissected, and longitudinal sections of CC (unstretched length about 1 cm) were placed in organ baths containing 20 mL Kreb's buffer at 37°C. After equilibration at 2 g, changes in muscle tension were measured and recorded.
For the control group, the response to field stimulation was observed after the 10 μmol L−1 phenylephrine-induced tissue contraction reached its plateau. EFS was performed by biphasic square wave pulses of 80 V, at 0.5 ms and 1 ms duration and various frequencies from 1 to 4 Hz. The EFS setting was confirmed by organ bath exams of each voltage, duration and frequency. The interval between stimulations was at least 2 min. After the tissues were washed three times, 50 mmol L−1 EtOH was added to each chamber for 30 min, after which the tissues were precontracted with phenylephrine and field stimulation was recorded. The same procedure was repeated at 100 and 200 mmol L−1 EtOH. Finally, tissue viability was confirmed by the existence of a contractile response to phenylephrine.
The contractile responses are expressed in gram tension and relaxation is expressed as percentage relaxation of total tonic tension.
Explant cell cultures
All studies were performed according to a protocol approved by the internal review board of the Sungkyunkwan University School of Medicine/Samsung Medical Center. Human erectile tissue was obtained from the CC of patients undergoing surgery for penile prosthesis implantation or penectomy for penile cancer. Homogeneous explant cell cultures of human corporal smooth muscle cells were prepared as follows. Briefly, radial sections of approximately 3 × 3 × 10 mm3 in size were excised from the mid-penile shaft of each patient. These specimens consisted exclusively of smooth muscle, endothelium and connective tissue, with occasional nerve fibers. The tissue was washed, cut into 1–2 mm pieces, and placed in tissue culture dishes with a minimal volume of Dulbecco's medium (DMEM; GIBCO) with 20% fetal calf serum. After the tissue was allowed to attach to the plate (usually 1–2 days), additional medium was added. Smooth muscle cells migrated from the explant and underwent division. Cells were subsequently detached using a trypsin/EDTA protocol to establish a secondary culture from the explants. These cultures were morphologically homogeneous and did not display the cobblestone morphologies characteristic of endothelial cells or the very flattened and spread out shapes characteristic of fibroblasts. Cellular homogeneity was further verified by the presence of smooth muscle-specific α-actin immunoreactivity. Cultures were maintained for no more than four passages; importantly, during this time all measured pharmacological and molecular properties observed in the intact tissue are retained in culture, such as cAMP formation, calcium mobilization, expression or function of the gap junction protein, connexin 43 27, 28, 29, 30, 31.
Intracellular Ca2+ measurement
The cultured corporal myocytes were loaded with the acetoxymethyl ester form of fura-2 (2 μmol L−1) in Tyrode's solution for 30 min at room temperature. To improve the loading efficiency, 0.12% pluronic F-127 was used and then washed three times with HEPES (4-[2-hydroxyethyl]-1-piperazineethanesulfonic acid) buffer solution. The recording of [Ca2+]i was performed using a microfluorimetric system consisting of an inverted fluorescence microscope (IX-70; Olympus, Nagano, Japan) with a dry-type fluorescence objective lens (× 40, NA 0.85), a photomultiplier tube (type R 1527, Hamamatsu, Shizuoka, Japan) and a Deltascan illuminator (Photon Technology International Inc, Birmingham, NJ, USA). Light was provided by a 75-W xenon lamp (Ushino, Japan). A chopper wheel alternated the light path to monochromators (340 and 380 nm) with a frequency of 5 Hz, and the intensity of emitted light at 510 nm was measured. The fluorescence emission ratio at 340/380 nm excitation (F340/380) is presented as a measure of [Ca2+]i. The measurements were performed in the presence of the L-type calcium channel blocker nifedipine (1 μmol L−1).
Electrophysiological recordings
The conventional tight seal method was used in the perforated patch study, in the cell-attached or the inside-out patch mode. The patch electrodes were made from borosilicate glass capillary tubing (World Precision Instruments, Sarasota, FL, USA) and had resistance of 2.5–5 MΩ. The cell suspension was placed into a small chamber (0.6 mL) on the stage of an inverted microscope (TMD Diaphot; Nikon, Tokyo, Japan). Membrane currents in smooth muscle cells were then recorded using a patch-clamp amplifier (Axopatch-lD; Axon Instruments, Union City, CA, USA). The liquid junctional potential between the pipette solution and bath solution was only about 3 mV, and it was not corrected. Series resistance (about 6–10 MΩ) and capacitative currents were also not compensated for because the cell size and measured currents were relatively small. The membrane capacitance was determined from the current amplitude elicited in response to hyperpolarizing voltage ramp pulses from a holding potential of 0 to −5 mV (duration 25 ms at 0.2 V s−1). This procedure avoided interference from any time-dependent ionic currents. The average cell capacitance was 35.3 ± 2.6 pF (n = 44). PCLAMP software v.9.2 and Digidata-1322A (both from Axon Instruments) were used for data acquisition and the application of command pulses. Membrane currents were measured during voltage ramps and filtered at 5 kHz (−3 dB frequency). Current signals were filtered at 5 kHz and then digitized and analyzed on a personal computer using the PCLAMP (v.9.2, Axon Instruments) and Origin v.7.0 (Microcal Software Inc., Northampton, MA, USA) softwares. Single-channel activities were recorded at 10 kHz in cell-attached and inside-out configurations. The voltage and current data were low-pass filtered at 1 kHz and stored. The data were analyzed to obtain an amplitude histogram and the open probability (Po).
Statistics
Data are expressed as mean ± SEM. For statistical analysis, one-way analysis of variance (ANOVA) was used, followed by the Bonferroni's post hoc test or paired t-test. Normal distribution of data was tested with Shapiro–Wilk test. P-values less than 0.05 were considered significant.
Drugs and solutions
For the perforated patch experiment, the external bath solution contained 134 mmol L−1 NaCl, 6 mmol L−1 KCl, 1 mmol L−1 MgCl2, 2 mmol L−1 CaC12, 5 mmol L−1 glucose and 10 mmol L−1 HEPES, set to pH 7.4 with NaOH. The internal electrode solution contained 110 mmol L−1 K-gluconate, 30 mmol L−1 KCl, 10 mmol L−1 NaCl, 10 mmol L−1 HEPES, 1 mmol L−1 MgCl2 and 0.05 mmol L−1 EGTA (ethylene glycol-bis-[2-aminoethyl ether]-N, N′-tetraacetic acid [pH 7.2]), and a final concentration of 0.2 mg mL−1 Nystatin (diluted from 50 mg mL−1 stock in dimethyl sulfoxide) was added. The following solutions were used for single-channel recordings (cell-attached configuration and inside-out patches).
For single-channel recordings of the cell-attached configuration, the bath solution contained 140 mmol L−1 KCl, 0.1 mmol L−1 CaCl2, 1 mmol L−1 MgCl2, 5 mmol L−1 glucose and 10 mmol L−1 HEPES (pH 7.4). For the single-channel recordings of the inside-out patches, the external bath solution contained 140 mmol L−1 KCl, 1 mmol L−1 MgCl2, 3.84 mmol L−1 CaC12, 5 mmol L−1 glucose, 5 mmol L−1 EGTA and 15 mmol L−1 HEPES (pH 7.4), with KOH (≈ pCa 300 nm). The pipette solution used for both cell-attached and inside-out recordings contained 140 mmol L−1 KCl, 1 mmol L−1 MgCl2, 1.8 mmol L−1 CaC12 and 10 mmol L−1 HEPES (pH 7.4), with NaOH. EtOH (Sigma) was freshly diluted in bath solution immediately before use. All chemicals and drugs were purchased from Sigma (St. Louis, MO, USA) except the acetoxymethyl (AM) form of fura-2 and F-127 (Molecular Probes, Eugene, OR, USA).
Results
Effect of EtOH on corporal tissue
When corporal tissue strips were pre-contracted by PE (10 μmol L−1), EFS applied to the plateau phase caused a frequency-dependent relaxation (from 1 to 4 Hz). The relaxant response by EFS was decreased by EtOH in concentration-dependent manner (P < 0.05). When the concentration of EtOH was 200 mmol L−1, the blocking effect after EFS (0.5 ms) was 9.40% ± 4.60%, 15.34% ± 4.60% and 21.30% ± 5.90% at 1, 2 and 4 Hz. The concentration of EtOH 200 mmoL−1 significantly inhibited the relaxant response by EFS at 2, 4 Hz, respectively (P < 0.05) (Figure 1A). Also when the duration of EFS was 1 ms, the concentration of EtOH 100 mmol L−1 decreased the EFS-induced relaxant response by 5.90% ± 2.50%, 12.80% ± 5.40% and 15.30% ± 6.10% at 1, 2 and 4 Hz and the concentration of EtOH 200 mmol L−1 decreased the EFS-induced relaxant response by 21.00% ± 5.80%, 23.50% ± 7.50% and 25.50% ± 9.70% at 1, 2 and 4 Hz. The concentration of EtOH 100 mmol L−1 and EtOH 200 mmol L−1 significantly inhibited the relaxant response by EFS at 1, 2 and 4 Hz, respectively (P < 0.05) (Figure 1B).
Figure 1.
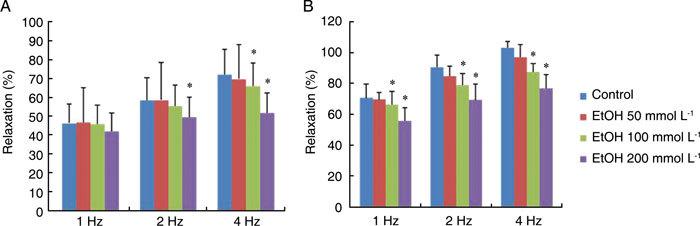
Effect of ethanol (EtOH) on the rabbit corpus cavernosum (CC) in organ baths. The relaxant response by electrical field stimulation (EFS) was decreased in proportion to the concentration of EtOH. EFS was applied to strips that were precontracted with phenylephrine. The effect of EtOH-induced inhibition of relaxation was calculated for the 50 mmol L−1, 100 mmol L−1 and 200 mmol L−1 EtOH groups, respectively. (A): EFS setting (80 V, 0.5 ms duration, 1–4 Hz). (B): EFS setting (80 V, 1 ms duration, 1–4 Hz), *P < 0.05, compared with the control.
Effect of EtOH on intracellular [Ca2+]i
The [Ca2+]i was monitored by the ratio of fura-2 fluorescence using the fura-2 loading method. Cumulative application of EtOH significantly increased the [Ca2+]i in human corporal smooth muscle cells (n = 5, P < 0.05 vs. control) (Figure 2).
Figure 2.
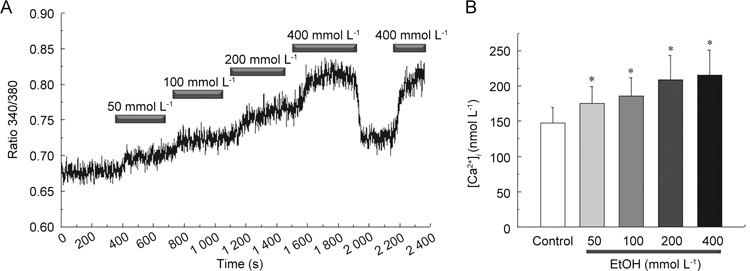
Effect of ethanol (EtOH) on intracellular Ca2+ concentration [Ca2+]i in cultured human corporal smooth muscle cells. EtOH dose dependently increased [Ca2+]i. The cell was loaded with a Ca2+ indicator fura-2/AM. (A): A representative tracing of [Ca2+]i response evoked by various concentrations of EtOH. (B): Summary of the means ± SD amplitudes of EtOH-induced Ca2+ influx. *P < 0.05, compared with the control.
Effect of EtOH on potassium channel
Whole-cell KCa channel currents were recorded at +60 mV with 5 mmol L−1 external and 140 mmol L−1 internal K+ solutions (Figure 3A). Extracellular application of EtOH significantly increased whole-cell K+ currents in a concentration-dependent fashion (0, 50, 100 and 200 mmol L−1 EtOH yielded 236.42 ± 13.50 pA [n = 7] and 361.85 ± 26.54 pA [n = 7] and 475.84 ± 12.98 pA [n = 4] and 727.29 ± 95.60 pA [n = 4], respectively), (P < 0.05 vs. control) (Figure 3B).
Figure 3.
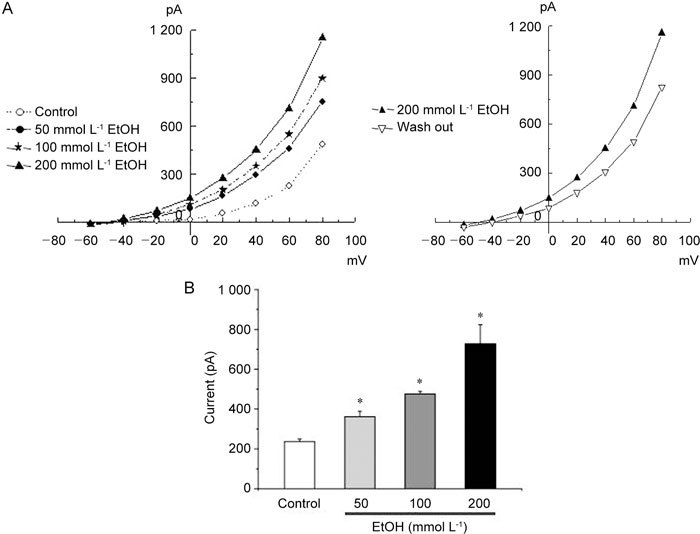
(A): Effect of various concentrations of ethanol (EtOH) on whole-cell currents. Current–voltage relationships obtained using a 500-ms ramp pulse from −60 to +80 mV. Membrane currents were recorded before (control) and after addition of EtOH. (B): Summary of the peak outward current at +60 mV. Values are presented as the mean ± SD of 10 experiments (at +60 mV). *P < 0.05, compared with the control.
The increase in whole-cell K+ currents showed marked outward rectification at potentials greater than +40 mV, and they were almost abolished by 1 mmol L−1 of the KCa channel blocker tetraethylammonium (data not shown). Consistent with the whole-cell results, EtOH increased the open probability in the cell-attached patch. Figure 4 shows an original recording of single-channel activity before and after 10 min (or 20 min) of 50–200 mmol L−1 EtOH exposure. However, in inside-out patches, the application of EtOH to the intracellular aspect of the patches indicated slight inhibition of BKCa channel activity (Figure 5) in average changes in NPo induced by EtOH (at +60 mV, 0.67 ± 0.07 for control [n = 5]; 0.61 ± 0.05 for 50 mmol L−1 EtOH [n = 4]; however, there was no statistical difference (P > 0.05).
Figure 4.
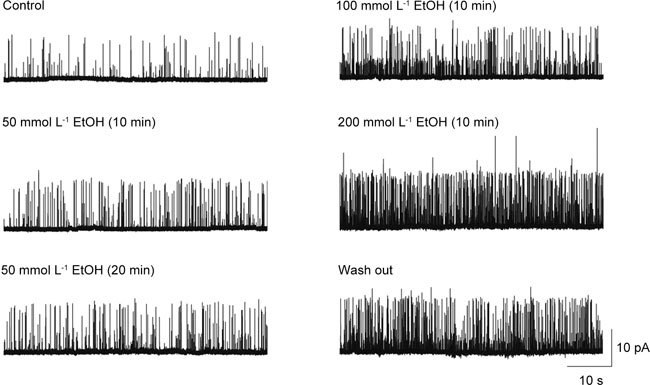
Effect of ethanol (EtOH) on cultured human corporal smooth muscle cells (HCC) in cell-attached patches. Continuous recordings from the cell-attached patch before (control) and after application of EtOH. The holding potential was +60 mV.
Figure 5.
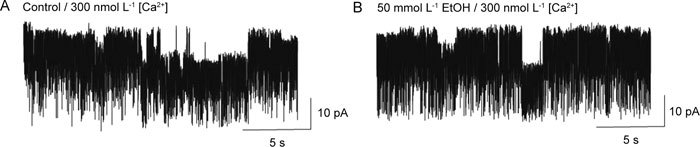
Effect of ethanol (EtOH) on cultured human corporal smooth muscle cells in inside-out patches. The application of EtOH to the intracellular aspect of the patches indicated slight inhibition of BKCa channel activity. Channel activity was recorded the same inside-out patch before (A: control) and after addition of EtOH (B). The concentration of Ca2+ was lowered to 300 nmol L−1.
Discussion
The modulation of corporal smooth muscle tone is a complex process. In the corporal smooth muscle of the penis, potassium channels are important in determining smooth muscle tone and serve as targets for neurotransmitters and other messengers that act on smooth muscle. Several studies have shown at least four potassium channel subtypes in corporal smooth muscle: (i) the KCa channel, (ii) the metabolically regulated potassium channel (KATP), (iii) the delayed rectifier and (iv) the fast transient A current (IA). Among the several subtypes of potassium channels, the KCa and ATP-sensitive (KATP) channel subtypes are thought to be the most physiologically relevant in human corpora cavernosal smooth muscle 3, 8.
The KCa channel is very pronounced in corporal smooth muscle cells and has a large unitary conductance value similar to that seen in other smooth muscle cells. The KCa channel is an important modulator of resting potential in the corpora. Moreover, because increases in intracellular calcium levels provide the trigger for smooth muscle contraction, activation of the KCa channels during contraction may be an important modulator of smooth muscle tone and may also be involved in hyperpolarization and reestablishment of membrane potential on removal of the contractile stimulus. Thus, any significant alteration in KCa channel activity could have a profound impact on smooth muscle tone 8.
The present study was designed to determine the acute effects of EtOH on corporal tissue tonicity, [Ca2+]i levels and potassium channel activity of corporal smooth muscle. In the strip study of rabbit cavernosal smooth muscles, we could see that EtOH dose-dependently inhibited the relaxant response to the electrical stimulations. This organ bath study is limited by the use of EtOH at high concentration. The EtOH blood level constituting legal intoxication is 20 mmol L−1, and ≥ 100 mmol L−1 is usually lethal in naïve humans 32. Hence, we focused on ≤ 100 mmol L−1 (especially 50 mmol L−1) EtOH to address the mechanistic aspects of EtOH action. The EtOH concentration used in our study falls within the range obtained in the previous in vitro studies concerning the acute effect of EtOH on smooth muscle cells 19, 20, 21, 33, 34.
Saito et al.33, 35 reported that acute exposure of the CC to EtOH inhibited relaxation induced by EFS and enhanced relaxation induced by ATP, but chronic EtOH consumption augmented both contractile and relaxation responses. In addition, Briner et al.36 reported that acute EtOH exposure in cultured vascular smooth muscle cells inhibited calcium uptake into vascular cells mediated by vasopressin and KCl, but chronic EtOH exposure increased calcium uptake through both voltage-sensitive and receptor-operated calcium channels. Acute exposure to a clinically relevant concentration of EtOH causes contraction of peripheral, cerebral and umbilical arteries, as well as that of the CC 14, 16, 37. In addition, EtOH inhibition of KCa channel activity may be the mechanism underlying, or at least contributing to, EtOH-induced contraction of vascular smooth muscles 21, 24, 38, 39. Our data show that acute exposure to EtOH decreases the corporal smooth muscle relaxation response induced by EFS. The intracellular calcium level was increased by EtOH in a dose-dependent fashion. EtOH also increased the open probability of KCa channels in the cell-attached patch, consistent with the increased whole-cell current. However, in inside-out patches, exposure of EtOH to the intracellular aspect of the patch caused a slight inhibition of KCa channel activity. However, these results were not statistically significant (P > 0.05). The data from this patch-clamp research indicate that the activation of BKCa channels seen in the cell-attached patch study and the whole-cell study may be caused secondarily by EtOH-induced increment in intracellular [Ca2+]i. On the basis of these results, we suggest that erectile problems caused by heavy alcohol consumption are due to an increase in intracellular calcium rather than a direct effect on KCa channels.
The limitation of this study is that we were unable to elucidate whether the increase in the intracellular calcium level induced by EtOH was related to calcium channels. EtOH did not directly increase voltage-dependent Ca2+ currents in isolated myocytes of the cerebral artery 21. As there are few reports concerning the relationship between the EtOH-induced increase in intracellular calcium levels and calcium channels in the CC, more investigations are needed.
Our studies show that EtOH concentration dose-dependently increases tissue tension through an increasing [Ca2+]i. EtOH did not directly affect KCa channels but it did increase the channel activity by increasing [Ca2+]i. The increased [Ca2+]i caused by EtOH could be one of the mechanisms underlying ED after heavy alcohol drinking.
Acknowledgments
This study was supported by a grant of the Korea Healthcare Technology R&D Project, Ministry for Health, Welfare & Family Affairs, Korea (No. A060043).
References
- NIH Consensus Conference. Impotence. NIH Consensus Development Panel on Impotence JAMA 199327083–90. [PubMed] [Google Scholar]
- Lue TF, Tanagho EA. Physiology of erection and pharmacological management of impotence. J Urol. 1987;137:829–36. doi: 10.1016/s0022-5347(17)44267-4. [DOI] [PubMed] [Google Scholar]
- Fan SF, Brink PR, Melman A, Christ GJ. An analysis of the Maxi-K+ (KCa) channel in cultured human corporal smooth muscle cells. J Urol. 1995;153:818–25. [PubMed] [Google Scholar]
- Christ GJ. The penis as a vascular organ. The importance of corporal smooth muscle tone in the control of erection. Urol Clin North Am. 1995;22:727–45. [PubMed] [Google Scholar]
- Kim N, Azadzoi KM, Goldstein I, Saenz de Tejada I. A nitric oxide-like factor mediates nonadrenergic-noncholinergic neurogenic relaxation of penile corpus cavernosum smooth muscle. J Clin Invest. 1991;88:112–8. doi: 10.1172/JCI115266. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rajfer J, Aronson WJ, Bush PA, Dorey FJ, Ignarro LJ. Nitric oxide as a mediator of relaxation of the corpus cavernosum in response to nonadrenergic, noncholinergic neurotransmission. N Engl J Med. 1992;326:90–4. doi: 10.1056/NEJM199201093260203. [DOI] [PubMed] [Google Scholar]
- Knispel HH, Goessl C, Beckmann R. Nitric oxide mediates neurogenic relaxation induced in rabbit cavernous smooth muscle by electric field stimulation. Urology. 1992;40:471–6. doi: 10.1016/0090-4295(92)90469-d. [DOI] [PubMed] [Google Scholar]
- Christ GJ, Spray DC, Brink PR. Characterization of K currents in cultured human corporal smooth muscle cells. J Androl. 1993;14:319–28. [PubMed] [Google Scholar]
- Kuriyama H, Kitamura K, Nabata H. Pharmacological and physiological significance of ion channels and factors that modulate them in vascular tissues. Pharmacol Rev. 1995;47:387–573. [PubMed] [Google Scholar]
- Nelson MT, Quayle JM. Physiological roles and properties of potassium channels in arterial smooth muscle. Am J Physiol. 1995;268:799–822. doi: 10.1152/ajpcell.1995.268.4.C799. [DOI] [PubMed] [Google Scholar]
- Benson GS BM.The penis: sexual function and dysfunctionIn: Gillenwater JY, Grayhack JT, Howards SS, Duckett JW, editors. Adult and Pediatric Urology2nd edn. Philadelphia: Mosby; 19911599–642.
- Persson MG, Gustafsson LE. Ethanol can inhibit nitric oxide production. Eur J Pharmacol. 1992;224:99–100. doi: 10.1016/0014-2999(92)94826-h. [DOI] [PubMed] [Google Scholar]
- Criscione L, Powell JR, Burdet R, Engesser S, Schlager F, et al. Alcohol suppresses endothelium-dependent relaxation in rat mesenteric vascular beds. Hypertension. 1989;13:964–7. doi: 10.1161/01.hyp.13.6.964. [DOI] [PubMed] [Google Scholar]
- Rubin HB, Henson DE. Effects of alcohol on male sexual responding. Psychopharmacologia. 1976;47:123–34. doi: 10.1007/BF00735810. [DOI] [PubMed] [Google Scholar]
- Tirapelli CR, Al-Khoury J, Bkaily G, D'Orleans-Juste P, Lanchote VL, et al. Chronic ethanol consumption enhances phenylephrine-induced contraction in the isolated rat aorta. J Pharmacol Exp Ther. 2006;316:233–41. doi: 10.1124/jpet.105.092999. [DOI] [PubMed] [Google Scholar]
- Altura BM, Altura BT. Alcohol, the cerebral circulation and strokes. Alcohol. 1984;1:325–31. doi: 10.1016/0741-8329(84)90056-9. [DOI] [PubMed] [Google Scholar]
- Gordon EL, Nguyen TS, Ngai AC, Winn HR. Differential effects of alcohols on intracerebral arterioles. Ethanol alone causes vasoconstriction. J Cereb Blood Flow Metab. 1995;15:532–8. doi: 10.1038/jcbfm.1995.66. [DOI] [PubMed] [Google Scholar]
- Yang ZW, Wang J, Zheng T, Altura BT, Altura BM. Importance of PKC and PI3Ks in ethanol-induced contraction of cerebral arterial smooth muscle. Am J Physiol Heart Circ Physiol. 2001;280:2144–52. doi: 10.1152/ajpheart.2001.280.5.H2144. [DOI] [PubMed] [Google Scholar]
- Rogers PJ, Bove AA. Epicardial coronary artery constriction with intravenous ethanol. Int J Cardiol. 1989;22:301–10. doi: 10.1016/0167-5273(89)90271-4. [DOI] [PubMed] [Google Scholar]
- Drummond WH, Gause GE, Polak MJ, Lyles D, Cassin S. Ethanol induces acute pulmonary vasoconstriction in salt-perfused rat lungs. Exp Lung Res. 1989;15:447–58. doi: 10.3109/01902148909087870. [DOI] [PubMed] [Google Scholar]
- Liu P, Xi Q, Ahmed A, Jaggar JH, Dopico AM. Essential role for smooth muscle BK channels in alcohol-induced cerebrovascular constriction. Proc Natl Acad Sci USA. 2004;101:18217–22. doi: 10.1073/pnas.0406096102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yang Z, Wang J, Zheng T, Altura BT, Altura BM. Importance of extracellular Ca2+ and intracellular Ca2+ release in ethanol-induced contraction of cerebral arterial smooth muscle. Alcohol. 2001;24:145–53. doi: 10.1016/s0741-8329(01)00145-8. [DOI] [PubMed] [Google Scholar]
- Jaggar JH, Porter VA, Lederer WJ, Nelson MT. Calcium sparks in smooth muscle. Am J Physiol Cell Physiol. 2000;278:235–56. doi: 10.1152/ajpcell.2000.278.2.C235. [DOI] [PubMed] [Google Scholar]
- Walters FS, Covarrubias M, Ellingson JS. Potent inhibition of the aortic smooth muscle maxi-K channel by clinical doses of ethanol. Am J Physiol Cell Physiol. 2000;279:1107–15. doi: 10.1152/ajpcell.2000.279.4.C1107. [DOI] [PubMed] [Google Scholar]
- Crowley JJ, Treistman SN, Dopico AM. Cholesterol antagonizes ethanol potentiation of human brain BKCa channels reconstituted into phospholipid bilayers. Mol Pharmacol. 2003;64:365–72. doi: 10.1124/mol.64.2.365. [DOI] [PubMed] [Google Scholar]
- Dopico AM, Widmer H, Wang G, Lemos JR, Treistman SN. Rat supraoptic magnocellular neurones show distinct large conductance, Ca2+-activated K+ channel subtypes in cell bodies versus nerve endings. J Physiol. 1999;519:101–14. doi: 10.1111/j.1469-7793.1999.0101o.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Abeysinghe HR, Clancy J, Qiu Y. Comparison of endothelin-1-mediated tissue tension and calcium mobilization effects in isolated rabbit corpus cavernosum. Urology. 2002;60:925–30. doi: 10.1016/s0090-4295(02)01845-9. [DOI] [PubMed] [Google Scholar]
- Insuk SO, Chae MR, Choi JW, Yang DK, Sim JH, et al. Molecular basis and characteristics of KATP channel in human corporal smooth muscle cells. Int J Impot Res. 2003;15:258–66. doi: 10.1038/sj.ijir.3901013. [DOI] [PubMed] [Google Scholar]
- Palmer LS, Valcic M, Melman A, Giraldi A, Wagner G, et al. Characterization of cyclic AMP accumulation in cultured human corpus cavernosum smooth muscle cells. J Urol. 1994;152:1308–14. doi: 10.1016/s0022-5347(17)32573-9. [DOI] [PubMed] [Google Scholar]
- Lee SW, Wang HZ, Christ GJ. Characterization of ATP-sensitive potassium channels in human corporal smooth muscle cells. Int J Impot Res. 1999;11:179–88. doi: 10.1038/sj.ijir.3900398. [DOI] [PubMed] [Google Scholar]
- Han DH, Chae MR, So I, Park JK, Lee SW. The effects of dopamine receptor agonists on BKCa channels and signal transduction mechanism in corpus cavernosal smooth muscle cells. Int J Impot Res. 2008;20:53–9. doi: 10.1038/sj.ijir.3901623. [DOI] [PubMed] [Google Scholar]
- Diamond I.Alcoholism and alcohol abuseIn: Wyngaarden JB SL, Bennet JC, editors. Cecil Textbook of MedicinePhiladelphia: Saunders; 1992p44–7.
- Saito M, Broderick GA, Hypolite JA, Levin RM. Pharmacological effect of ethanol on the function of rabbit corporal cavernosal tissue. Pharmacology. 1994;48:335–40. doi: 10.1159/000139197. [DOI] [PubMed] [Google Scholar]
- Dopico AM. Ethanol sensitivity of BK(Ca) channels from arterial smooth muscle does not require the presence of the beta 1-subunit. Am J Physiol Cell Physiol. 2003;284:1468–80. doi: 10.1152/ajpcell.00421.2002. [DOI] [PubMed] [Google Scholar]
- Saito M, Broderick GA, Wein AJ, Levin RM. Effect of chronic ethanol consumption on the pharmacological response of the rabbit corpus cavernosum. Pharmacology. 1994;49:386–91. doi: 10.1159/000139257. [DOI] [PubMed] [Google Scholar]
- Briner VA, Tsai P, Wang X, Schrier RW. Divergent effects of acute and chronic ethanol exposure on contraction and Ca2+ mobilization in cultured vascular smooth muscle cells. Am J Hypertens. 1993;6:268–75. doi: 10.1093/ajh/6.4.268. [DOI] [PubMed] [Google Scholar]
- Farago M, Szabo C, Horvath I, Dora E, Kovach AG. Differential vascular actions of ethanol in feline middle cerebral and mesenteric artery. Acta Physiol Hung. 1991;78:119–25. [PubMed] [Google Scholar]
- Bukiya AN, Liu J, Dopico AM. The BK channel accessory beta1 subunit determines alcohol-induced cerebrovascular constriction. FEBS Lett. 2009;583:2779–84. doi: 10.1016/j.febslet.2009.07.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Werber AH, Morgan RA, Zhou P, Yang C. Intracellular mechanisms of constriction of rat aorta by ethanol. Alcohol. 1997;14:351–60. doi: 10.1016/s0741-8329(96)00183-8. [DOI] [PubMed] [Google Scholar]


