Abstract
Intracellular cAMP and Ca2+ are involved in the regulation of steroidogenic activity in Leydig cells, which coordinate responses to luteinizing hormone (LH) and human chorionic gonadotropin (hCG). However, the identification of Ca2+ entry implicated in Leydig cell steroidogenesis is not well defined. The objective of this study was to identify the type of Ca2+ channel that affects Leydig cell steroidogenesis. In vitro steroidogenesis in the freshly dissociated Leydig cells of mice was induced by hCG incubation. The effects of mibefradil (a putative T-type Ca2+ channel blocker) on steroidogenesis were assessed using reverse transcription (RT)-polymerase chain reaction analysis for the steroidogenic acute regulatory protein (StAR) mRNA expression and testosterone production using radioimmunoassay. In the presence of 1.0 mmol L−1 extracellular Ca2+, hCG at 1 to 100 IU noticeably elevated both StAR mRNA level and testosterone secretion (P < 0.05), and the stimulatory effects of hCG were markedly diminished by mibefradil in a dose-dependent manner (P < 0.05). Moreover, the hCG-induced increase in testosterone production was completely removed when external Ca2+ was omitted, implying that Ca2+ entry is needed for hCG-induced steroidogenesis. Furthermore, a patch-clamp study revealed the presence of mibefradil-sensitive Ca2+ currents seen at a concentration range that nearly paralleled those inhibiting steroidogenesis. Collectively, our data provide evidence that hCG-stimulated steroidogenesis is mediated at least in part by Ca2+ entry carried out by the T-type Ca2+ channel in the Leydig cells of mice.
Keywords: Leydig cells, mibefradil, StAR, steroidogenesis, T-type Ca2+ channel
Introduction
Leydig cell production of testicular androgens is tightly controlled by endocrine interactions among the hypothalamus, the pituitary gland and the testis, as well as through the paracrine and autocrine regulation within the testis 1, 2, 3. Leydig cells secrete testosterone responsible for the onset of both spermatogenesis and male sexual development. Endocrine control of Leydig cell steroidogenic activity by luteinizing hormone (LH), follicle-releasing hormone (FSH) or human chorionic gonadotropin (hCG) has been exerted through their respective receptors coupled to the cAMP- or the Ca2+-mediated signalling pathway 4, 5, 6.
Ca2+ is one of the most common signal transduction elements in cells. Ca2+ helps regulate a variety of cellular functions in many different cells, including germ cells and somatic cells in the testis, as well as spermatozoa, in response to endocrine hormones and local regulators 7, 8, 9, 10, 11. Moreover, alteration of the Ca2+ signalling pathway has a drastic impact on many cellular physiologies 12, 13, 14, 15. Cytosolic free Ca2+ or [Ca2+]i is replenished from two Ca+2 sources: intracellular Ca2+ storage in both the endoplasmic reticulum and the mitochondria and extracellular Ca2+. The extracellular Ca2+ must enter the cell via Ca2+ channels in the cell membrane before having an effect. Membrane potential depolarization or ligand binding causes the membrane Ca2+ channels to open; the former is known as 'voltage-gated (dependent) Ca2+ channel' and the latter is known as 'ligand-gated (dependent) Ca2+ channel'. Different types of voltage-gated Ca2+ channels that open their pores in response to transmembrane potential changes have been classified based on biophysical properties such as the voltage dependence of the channel gating and other pharmacological criteria 16.
Although an increase in [Ca2+]i is a prerequisite to the onset of Leydig cell steroidogenesis, an unequivocal pathway identity for Ca2+ entry remains elusive. Some studies have suggested that cytosolic Ca2+ changes may result only from intracellular Ca2+ release 5, 6, 17, while other studies have argued that voltage-dependent Ca2+ channels (VDCCs) are also involved 18, 19, 20. Recently, mibefradil, a new nondihydropyridine calcium antagonist, has been shown to block the T-type Ca2+ channel with a high affinity and selectivity in a variety of cell preparations 19, 20, 21, 22. For instance, mibefradil was about 30 times more potent in blocking the T-type Ca2+ channel in cardiac muscle cells compared with the L-type channel, whereas other available calcium antagonists such as nifedipine did not block the T-type channel at biologically relevant concentrations 23.
Therefore, the present study evaluated the potential role of Ca2+ entry via the T-type calcium channel in hCG-stimulated steroidogenesis probed with mibefradil in the Leydig cells of mice.
Materials and methods
Preparation of mouse Leydig cells
ICR male mice (35–40 days old) were sacrificed by cervical dislocation following the guidelines established by the Ajou University Medical School-Institutional Animal Care and Use Committee, South Korea. Testes were excised and decapsulated under sterile condition before the testis cell suspension was washed twice in DMEM/F12 medium (Gibco BRL, Grand Island, NY, USA). Leydig cells were prepared mechanically as described in a previous study 24. The testis cell suspension was pipetted gently to isolate cells from the interstitial tissues of the testes. After centrifugation at 10 000 × g for 5 min, the pellet was washed twice and resuspended in DMEM/F12 medium. The purified Leydig cells were incubated in DMEM/F12 medium containing 1.0 mmol L−1 Ca2+ supplemented with 10% bovine serum (Gibco BRL) and cultured at 31 °C in 5% CO2 before use. We made Ca2+-free medium by iso-osmotically substituting Ca2+ with Na+ supplemented with 4 mmol L−1 Ca2+ chelator EGTA. Leydig cell purity was determined immunocytochemically by immunostaining with anti-3-β-hydroxysteroid dehydrogenase (3-β-HSD) antibodies 25 (kindly provided by Dr Mason, University of Edinburgh, UK).
hCG-induced steroidogenesis
Three days after mouse Leydig cells were seeded, the culture medium was replaced with a fresh medium containing hCG (LG Life Science R & D, Daejon, South Korea) at a concentration of 1, 10 or 100 IU. Cells were incubated for 30 min or 1 h to induce steroidogenesis. To block steroidogenesis, mibefradil (Sigma, St. Louis, MO, USA) was added to the culture medium at concentrations of 0.01, 0.1 or 1.0 mmol L−1.
Radioimmunoassay (RIA) for testosterone
The concentration of testosterone in the Leydig cell-conditioned medium was determined by RIA by using specific testosterone assay kits (Orion Diagnostica, Espoo, Finland) according to the manufacturer's protocol.
Reverse transcription-polymerase chain reaction (RT-PCR) analysis of steroidogenic acute regulatory protein (StAR) mRNA
Total RNA was isolated from the mouse testes using TRIZOL™ reagent (Invitrogen, Carlsbad, CA, USA) according to the manufacturer's instructions. Approximately 5 μg of total RNA was subjected to reverse transcription using Superscript™ II reverse transcriptase (Invitrogen) according to the manufacturer's protocol. The primers for StAR mRNA were 5′-GACCTTGAAAGGCTCAGGAAGAAC-3′ (forward) and 5′-TAGCTGAAGATGGACAGA CTTGC-3′ (reverse), designed on a personal computer according to the mouse StAR cDNA sequence (GenBank Access No. BC060970). The primers for β-actin were 5′-GACCTTGAAAGGCTCAGGAAGAAC-3′ (forward) and 5′-TAGCTGAAGATGGAC AGACTTGC-3′ (reverse) and were based on the mouse β-actin cDNA sequence (GenBank Access No. NM007393). Primers were custom-synthesised and purified (Genotech, Daejorn, South Korea). These primers should create StAR and β-actin cDNA fragments of 980 and 228 bp, respectively. PCR was performed using Ex Taq™ polymerase (Takara, Otsu, Japan) according to the manufacturer's protocol. PCR mixtures were subjected to 25–28 amplification cycles at 95 °C for 1 min, 55 °C for 1 min and 72 °C for 3 min. PCR products were resolved on a 2% agarose gel and visualized under UV illumination after ethidium bromide staining. Band intensities of the RT-PCR products were quantified with Bioprofil software (Vilber Lourmat, France), and the band intensity of the target gene relative to the internal control was calculated.
Electrophysiological recording
Fresh mouse Leydig cells were grown attached on a 13-mm round coverslip for 1 to 2 days before being transferred to a recording chamber equipped on the stage of an inverted microscope (Diaphot 200; Nikon, Tokyo, Japan). The whole-cell patch-clamp mode was used to measure the membrane current 27. Patch micropipettes with a resistance of 1.2–2.5 MΩ were pulled from borosilicate glass capillaries (TW150F-4; World Precision Instruments, Sarasota, FL, USA) using a puller (P87; Sutter Instruments, Novato, CA, USA) and fire-polished with a microforge (MF-79; Narishige, Tokyo, Japan). The pipette solution contained the following: 130 mmol L−1 CsCl, 10 mmol L−1 EGTA, 25 mmol L−1 HEPES, 3 mmol L−1 ATP (Mg) and 0.5 mmol L−1 GTP (Na), pH 7.2 adjusted with CsOH (290–300 mOsm). Cells were recorded in a bath solution containing: 10 mmol L−1 CaCl2, 5 mmol L−1 4-aminopyridine, 130 mmol L−1 TEA-Cl, 1 mmol L−1 MgCl2, 25 mmol L−1 HEPES and 20 mmol L−1 glucose (pH 7.4 adjusted with TEAOH; 290–310 mOsm). Membrane currents were recorded at room temperature (∼20°C) using an Axopatch-1D amplifier (Axon Instruments, Foster City, CA, USA) and digitized using a 12-bit analogue-to-digital interface (Digidata 1200, Axon Instruments). The depolarization-induced currents were filtered at 1 or 2 kHz and sampled at 10 kHz using pClamp6.0 (Axon Instruments). Capacitative and leakage currents were subtracted using the P-P/4 procedure. We applied either the bathing solution or the solution containing drugs to the recording chamber via a gravity-fed perfusion system. The T-type Ca2+ channel current (ICaT) was recorded at a test pulse of −30 mV delivered from a holding potential of −100 mV. As in previous studies, ICaT was obtained as the difference between the maximal inward current amplitude and the zero current level 16, 20, 23.
Statistical analyses
The data are presented means ± SD. We used a one-way ANOVA to analyse the data. The level of significance used was P < 0.05.
Results
Isolation and identification of mouse Leydig cells
Leydig cell-rich fractions obtained by dissociation of mouse testicular tissues after centrifugal elutriation were viewed microscopically to assess their purity. Leydig cells were easily recognized by their size and shape, as illustrated in Figure 1A (arrows). The purity of Leydig cells was also immunocytochemically confirmed. Approximately 70%–80% of cells were positive for 3-β-HSD (arrows), a cellular marker for Leydig cells (Figure 1B).
Figure 1.
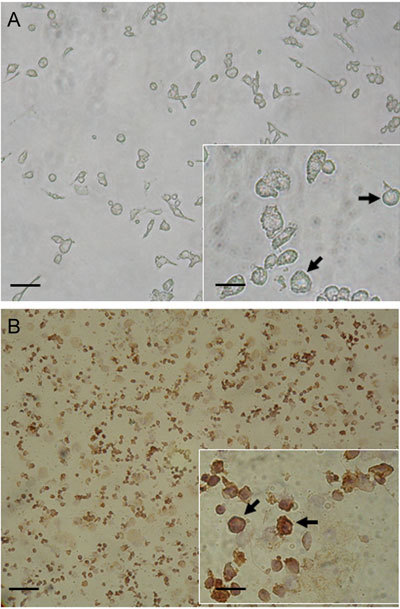
Microphotographs of Leydig cells after acute isolation from interstitial tissues of mouse testis. (A): Mouse Leydig cells were mechanically isolated and viewed microscopically as indicated by the arrows. (B): Acutely dissociated mouse Leydig cells were subjected to immunostaining by 3-β-hydroxysteroid dehydrogenase (3-β-HSD), a cellular marker for Leydig cells, and viewed microscopically as indicated by the arrows. Bars = 100 μm, bars = 25 μm in inset panels.
hCG-stimulated testosterone production is Ca2+-dependent and inhibited by mibefradil
The onset of steroidogenesis in mouse Leydig cells was triggered by adding hCG at 1 to 100 IU to the 1.0 mmol L−1 Ca2+-containing culture medium in which the cells were incubated for 30 min or 1 h. The amount of testosterone secretion into the culture medium was quantified by RIA. hCG caused a dose-dependent increase in testosterone production of mouse Leydig cells (Figure 2A). On the contrary, replacing Ca2+ iso-osmotically from the medium and chelating with 4 mmol L−1 EGTA (Ca2+-free medium) completely abolished the hCG-stimulated testosterone production to the basal level (P < 0.05; Figure 2B), which implies that the absolute requirement of extracellular Ca2+ for testosterone production, secretion or both by mouse Leydig cells occurs in vitro. The fact that testis somatic and germ cells might possess the T-type Ca2+ channel 10 possibly implicates them in spermatogenesis 27; thus, we sought to examine the T-type Ca2+ channel's role in hCG-stimulated testosterone production by incubating Leydig cells in the presence of mibefradil, a putative T-type Ca2+ channel blocker. Testosterone production was suppressed by mibefradil in a dose-dependent manner with the highest amount of suppression (for example, > 60%) manifested at 1 mmol L−1 (P < 0.05; Figure 2C).
Figure 2.
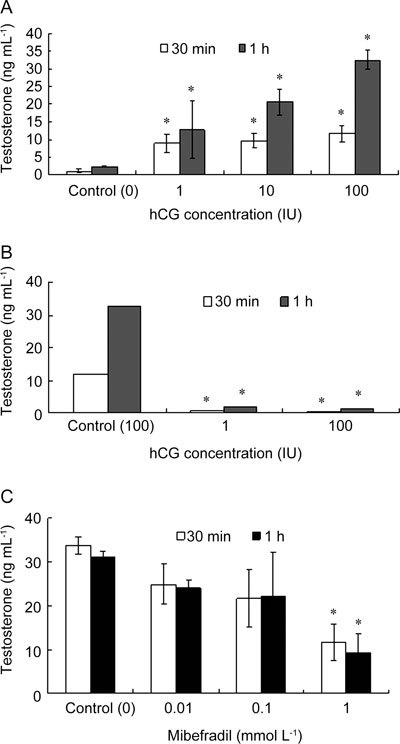
Inhibitory effects of mibefradil on hCG-stimulated testosterone production in mouse Leydig cells. Purified mouse Leydig cells were incubated in DMEM/F12 medium in the presence (A, C) or absence of 1.0 mmol L−1 Ca2+ (B). (A): The onset of steroidogenesis was induced by adding hCG at a concentration ranging from 1 to 100 IU for 30 min or for 1 h. (B): the control experiment is done with Ca2+ in the presence of 100 IU hCG whereas the other two are done without Ca2+. (C): The cells were all treated with 100 IU hCG to induce testosterone production in the presence of mibefradil, ranging from at 0.01 to 1.00 mmol L−1. All data are means ± SD (n = 5) compared with controls respectively ([0 hCG, A]; [100 IU hCG with Ca2+, B]; [0 mibefradil, C]). *P < 0.05, compared with corresponding control.
hCG-stimulated StAR mRNA expression is inhibited by mibefradil
The hCG-stimulated expression of StAR mRNA, a key regulator of steroid biosynthesis, has been closely correlated with steroidogenesis in Leydig cells 28, 29. Consistently, our RT-PCR analysis revealed that Leydig cell stimulation with hCG at 100 IU gave rise to StAR mRNA expression detectable by RT-PCR and that mibefradil diminished hCG-stimulated StAR mRNA expression in a dose-dependent manner with a significant decrease seen at 1 mmol L−1 (P < 0.05; Figure 3A and 3B).
Figure 3.
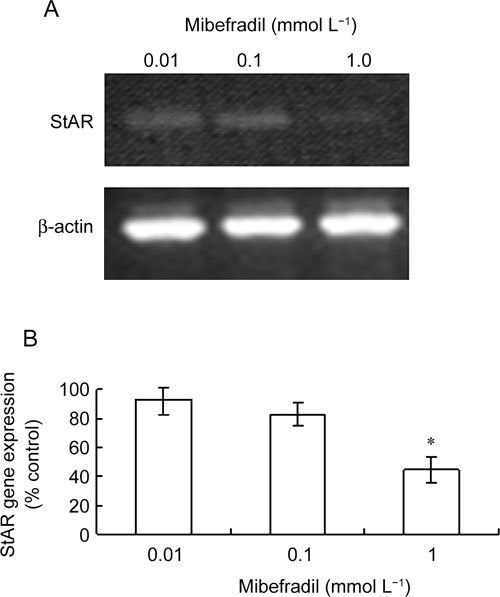
Expression of StAR mRNA in mouse Leydig cells is inhibited by mibefradil. Acutely purified mouse Leydig cells were incubated in 1.0 mmol L−1 Ca2+-containing DMEM/F12 medium and stimulated by 100 IU hCG treatment in the presence of mibefradil at 0.01, 0.1 or 1.0 mmol L−1 for 1 h before StAR mRNAs were semi-quantitatively amplified in RT-PCR. (A) A representative RT-PCR profile of StAR mRNA expression. (B): The band intensities of StAR mRNA products were quantified densitometrically and expressed relative to control (that is, no mibefradil). Data are means ± SD of results from five independent experiments. *P < 0.05 compared with control (100%).
Effects of mibefradil on the T-type Ca2+ current (ICaT)
To further test the relevance of the inhibitory effects of mibefradil on steroidogenesis, we sought to study the effect of mibefradil on ICaT in freshly isolated Leydig cells from young mouse testes. As previous studies have shown, ICaT was recorded at depolarization (−30 mV) in complete isolation from the L-type Ca2+ current 30. Not surprisingly, ICaT was highly sensitive to mibefradil. Mibefradil (1.0 μmol L−1) applied from outside the cell blocked ICaT by as much as 70% (Figure 4). Immunostaining studies confirmed that the Leydig cells exhibiting ICaT were also 3-β-HSD-positive (10 of 13 cells, data not shown). These data strongly suggest that the T-type Ca2+ channel might be a primary target through which mibefradil binds and thereby exerts its inhibitory actions on Leydig cell steroidogenesis.
Figure 4.
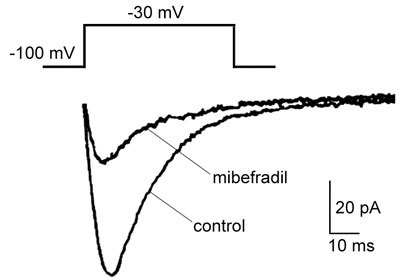
The blockade of T-type Ca2+ current (ICaT) by mibefradil in mouse Leydig cells. Acutely dissociated mouse Leydig cells were bathed in a bath solution containing 2 mmol L−1 CaCl2, 5 mmol L−1 4-aminopyridine, 136 mmol L−1 TEA-Cl, 1.1 mmol L−1 MgCl2, 25 mmol L−1 HEPES and 22 mmol L−1 glucose, pH 7.4, and held at −100 mV. A command pulse was applied to −30 mV for 40 msec. Representative ICaT traces were generated in the presence or absence of 1.0 μmol L−1 mibefradil. The capacitive transients were suppressed from the traces for the clarity.
Discussion
The present study supports a close functional correlation between hCG-induced steroidogenesis and Ca2+ entry through the T-type VDCC in mouse Leydig cells in many respects: (i) mibefradil was highly effective in both blocking the T-type Ca2+ currents and attenuating hCG-induced testosterone production and StAR expression; (ii) mibefradil's inhibitory actions occurred in comparable concentration ranges (that is, micromolar range); (iii) hCG-induced testosterone production is dependent on extracellular Ca2+ entry.
The rate-limiting step in steroid biosynthesis is the transport of cholesterol to the inner mitochondrial membrane, a process that is dependent on the actions of StAR 28, 29, 31. Two different stimulation protocols have routinely induced steroidogenesis in Leydig cells: tropic hormonal stimulation with LH/hCG and direct treatment with cAMP—a downstream second messenger—or an analogue 3, 31. In the present study, hCG receptor stimulation was preferred (i) to preserve Ca2+-mediated signalling activated by hCG and (ii) because a functional correlation between an hCG-induced increase in [Ca2+]i and StAR expression has been well documented in Leydig cells 28, 32. Therefore, our current working hypothesis is that the binding of LH/hCG to its specific receptors on the Leydig cell membranes is followed by a sequence of intracellular events that lead to an increase in [Ca2+]i that is responsible, at least in part, for StAR expression (possibly through transcription factor-mediated gene expression regulation effects). Given the importance and the close association of StAR in this process, our observation that StAR mRNA expression increased in association with hCG-induced steroidogenesis was expected. In keeping with this expectation, LH/hCG receptor may be coupled with both adenylate cyclase and the protein kinase C signalling pathway in mouse Leydig cells 33.
As Ca2+ is required for several steps of steroidogenesis, mibefradil, as a specific T-type Ca2+ channels blocker, would be expected to have an effect on steroidogenesis. For example, when the primary culture of rodent Leydig cells was incubated with Ni2+, nifedipine, or nimodipine, decreases in LH-induced and lactate-stimulated testosterone production might result from a blockade of VDCC type Ca2+ channels 24, 27. In addition, the inhibitory actions of putative L-type Ca2+ channel blockers on hCG- or cAMP-stimulated steroidogenesis are mediated by transcriptional repression of the StAR gene in mouse Leydig tumour cells 28, 34. Collectively, these results clearly suggest that Ca2+ entry is needed for steroidogenesis in Leydig cells. Despite previous research, which type of VDCC (L-type Ca2+ channel, T-type Ca2+ channel or both) is involved in steroidogenesis remains unanswered. This uncertainty may arise from tissue specificity or developmental differentiation; nonetheless, the present data support the hypothesis that T-type Ca2+ channels have a major role in Ca2+ entry needed for the hCG-induced steroidogenesis in mouse Leydig cells. At the same time, the present data raise an interesting question regarding how Ca2+ entry through the T-type Ca2+ channel is coupled to the hCG-triggered intracellular signalling, thus modulating testosterone production. As previously suggested by others, store-operated Ca2+ influx is one possibility 12, 13. The other possibility is that surface density or Ca+2 channel membrane activity is subjected to hCG signalling pathway effector proteins 28, possibly through phosphorylation, membrane trafficking or both. In fact, evidence has accumulated in favour of the involvement of protein phosphorylation, which facilitates Leydig cell steroidogenesis 35.
Our interpretation of the present study has some limitations that need to be addressed. Primarily, are the mibefradil concentrations used physiologically relevant? In this study, the concentration–response for testosterone production and StAR gene expression revealed that at 1 mmol L−1 or higher, mibefradil exerted a significant effect on inhibition (P < 0.05), whereas a similar inhibitory effect occurred at 1.0 μmol L−1 in ICaT; seemingly, there are approximately 3 orders of magnitude between them. There are two possible explanations for this. First, steroidogenesis as assessed by testosterone secretion or StAR expression may reflect a massive cellular event evident only downstream of the hCG-induced signal cascade. The mibefradil-induced suppression in the membrane Ca2+ current, however, reflects an early event that would subsequently lead to hCG-evoked cascade inhibition. An accumulation of many early events may lead to massive functional changes, such as testosterone secretion inhibition. On the contrary, ICaT inhibition is an immediate early response elicited immediately after mibefradil binds to the T-type Ca2+ channel. Therefore, the mibefradil concentration that evokes this change actually reflects the concentration in the vicinity of its receptor. However, StAR gene expression or secretion of testosterone could be manifested only after an accumulation of many early events. Consequently, the mibefradil concentration that triggers StAR gene expression or testosterone secretion may not reflect what happens at the receptor level. In addition, biophysical calcium current measurement techniques are more sensitive than biochemical measurements for gene expression or steroidogenesis detection, which could also contribute to the observed difference in mibefradil sensitivity.
Acknowledgments
We would like to thank Dr Mason at the University of Edinburgh, UK, for providing us with 3-β-HSD antibodies. This study was supported by the National Research Foundation of Korea Grant, funded by the Korean Government MEST (KRF-2009-0070606) to Dr Churl K. Min.
References
- Dufau ML. Endocrine regulation and communicating functions of the Leydig cell. Ann Rev Physiol. 1988;50:483–508. doi: 10.1146/annurev.ph.50.030188.002411. [DOI] [PubMed] [Google Scholar]
- Saez JM. Leydig cells: endocrine, paracrine, and autocrine regulation. Endocr Rev. 1994;15:574–626. doi: 10.1210/edrv-15-5-574. [DOI] [PubMed] [Google Scholar]
- Chen YC, Nagpal ML, Stocco DM, Lin T. Effects of genistein, resveratrol, and quercetin on steroidogenesis and proliferation of MA-10 mouse Leydig tumor cells. J Endocrinol. 2007;192:527–37. doi: 10.1677/JOE-06-0087. [DOI] [PubMed] [Google Scholar]
- Medelson C, Dufau ML, Catt KJ. Gonadotropin binding and stimulation of cAMP and testosterone production in isolated Leydig cells. J Biol Chem. 1975;250:8818–23. [PubMed] [Google Scholar]
- Sullivan MH, Cooke BA. The role of Ca2+ in steroidogenesis in Leydig cells: stimulation of intracellular free Ca2+ by lutropin (LH), luliberin (LHRH) agonist and cyclic AMP. Biochem J. 1986;236:45–51. doi: 10.1042/bj2360045. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tomić M, Dufau ML, Catt KJ, Stojilkovic SS. Calcium signalling in single rat Leydig cells. Endocrinology. 1995;136:3422–29. doi: 10.1210/endo.136.8.7628378. [DOI] [PubMed] [Google Scholar]
- Steele GL, Leung PC. Intragonadal signalling mechanisms in the control of steroid hormone production. J Steroid Biochem Mol Biol. 1992;41:515–22. doi: 10.1016/0960-0760(92)90376-t. [DOI] [PubMed] [Google Scholar]
- Li LH, Wine RN, Miller DS, Reece JM, Smith M, et al. Protection against methoxyacetic-acid-induced spermatocyte apoptosis with calcium channel blockers in cultured rat seminiferous tubules: possible mechanisms. Toxicol Appl Pharmacol. 1997;144:105–19. doi: 10.1006/taap.1997.8129. [DOI] [PubMed] [Google Scholar]
- Publicover SJ, Barratt CLR. Voltage-operated Ca2+ channels and the acrosome reaction: which channels are present and what do they do. Hum Reprod. 1999;14:873–9. doi: 10.1093/humrep/14.4.873. [DOI] [PubMed] [Google Scholar]
- Berridge MJ, Lipp P, Bootman MD. The versatility and universality of calcium signalling. Nat Rev Mol Cell Biol. 2000;1:11–21. doi: 10.1038/35036035. [DOI] [PubMed] [Google Scholar]
- Rommerts FF, Lyng FM, von Ledebur E, Quinlan L, Jones GR, et al. Calcium confusion-is the variability in calcium response by Sertoli cells to specific hormones meaningful or simply redundant. J Endocrinol. 2000;167:1–5. doi: 10.1677/joe.0.1670001. [DOI] [PubMed] [Google Scholar]
- Rossato M, Nogara A, Merico M, Ferlin A, Garolla A, et al. Store-operated calcium influx and stimulation of steroidogenesis in rat Leydig cells: role of Ca(2+)-activated K(+) channels. Endocrinology. 2001;142:3865–72. doi: 10.1210/endo.142.9.8373. [DOI] [PubMed] [Google Scholar]
- Machaca K. Ca2+-calmodulin-dependent protein kinase II potentiates store-operated Ca2+current. J Biol Chem. 2003;278:33730–7. doi: 10.1074/jbc.M305023200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bergh JJ, Xu Y, Farach-Carson MC. Osteoprotegerin expression and secretion are regulated by calcium influx through the L-type voltage-sensitive calcium channel. Endocrinology. 2004;145:426–36. doi: 10.1210/en.2003-0319. [DOI] [PubMed] [Google Scholar]
- Yamaguchi M. Role of regucalcin in maintaining cell homeostasis and function. Int J Mol Med. 2005;15:371–89. [PubMed] [Google Scholar]
- Hille B.Calcium channelsIn: Hille B, ed. Ionic Channels of Excitable Membranes, 2nd ednSunderland, MA: Sinauer; 1992. p83
- Adebanjo OA, Igietseme J, Huang CL, Zaidi M. The effect of extracellularly applied divalent cations on cytosolic Ca2+ in murine Leydig cells: evidence for a Ca2+-sensing receptor. J Physiol. 1998;513:399–410. doi: 10.1111/j.1469-7793.1998.399bb.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kawa K. Existence of calcium channels and intercellular coupling in the testosterone-secreting cells of the mouse. J Physiol. 1987;393:647–66. doi: 10.1113/jphysiol.1987.sp016846. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Arnoult C, Villaz M, Florman HM. Pharmacological properties of the T-type Ca2+ current of mouse spermatogenic cells. Mol Pharmacol. 1998;53:1104–11. [PubMed] [Google Scholar]
- Costa RR, Varanda WA. Intracellular calcium changes in mice Leydig cells are dependent on calcium entry through T-type calcium channels. J Physiol. 2007;585:339–49. doi: 10.1113/jphysiol.2007.137950. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lalevee N, Pluciennik F, Joffre M. Voltage-dependent calcium current with properties of T-type current in Sertoli cells from immature rat testis in primary cultures. Biol Reprod. 1997;56:680–7. doi: 10.1095/biolreprod56.3.680. [DOI] [PubMed] [Google Scholar]
- De Paoli P, Cerbai E, Koidl B, Kirchengast M, Sartiani L, et al. Selectivity of different calcium antagonists on T- and L-type calcium currents in guinea-pig ventricular myocytes. Pharmacol Res. 2002;46:491–7. doi: 10.1016/s1043661802002360. [DOI] [PubMed] [Google Scholar]
- Leuranguer V, Mangoni ME, Nargeot J, Richard S. Inhibition of T-type and L-type calcium channels by mibefradil: physiologic and pharmacologic bases of cardiovascular effects. J Cardiovasc Pharmacol. 2001;37:649–61. doi: 10.1097/00005344-200106000-00002. [DOI] [PubMed] [Google Scholar]
- Lin H, Wang SW, Wang RY, Wang PS. Stimulatory effect of lactate on testosterone production by rat Leydig cells. J Cell Biochem. 2001;83:147–54. doi: 10.1002/jcb.1213. [DOI] [PubMed] [Google Scholar]
- Ge R, Hardy MP. Protein kinase C increases 11β-hydroxyasteroid dehydrogenase oxidation and inhibits reduction in rat Leydig cells. J Androl. 2002;23:135–43. doi: 10.1002/j.1939-4640.2002.tb02606.x. [DOI] [PubMed] [Google Scholar]
- Hamill OP, Marty E, Neher E, Sakman B, Sigworth FJ. Improved patch-clamp techniques for high-resolution current recording from cells and cell-free membrane patches. Pflügers Arch. 1981;391:85–100. doi: 10.1007/BF00656997. [DOI] [PubMed] [Google Scholar]
- Forgacs Z, Paksy K, Lazar P, Tatrai E. Effect of Ni2+ on the testosterone production of mouse primary Leydig cell culture. J Toxicol Environ Health. 1998;55:213–24. doi: 10.1080/009841098158502. [DOI] [PubMed] [Google Scholar]
- Manna PR, Pakarinen P, El-Hefnawy T, Huhtaniemi IT. Functional assessment of the calcium messenger system in cultured mouse Leydig tumor cells: regulation of human chorionic gonadotropin-induced expression of the steroidogenic acute regulatory protein. Endocrinology. 1999;140:1739–51. doi: 10.1210/endo.140.4.6650. [DOI] [PubMed] [Google Scholar]
- Stocco DM. StAR protein and the regulation of steroid hormone biosynthesis. Annu Rev Physiol. 2001;63:193–213. doi: 10.1146/annurev.physiol.63.1.193. [DOI] [PubMed] [Google Scholar]
- Hollywood MA, Woolsey S, Walsh IK, Keane PF, McHale NG, et al. T- and L-type Ca2+ currents in freshly dispersed smooth muscle cells from the human proximal urethra. J Physiol. 2003;550:753–64. doi: 10.1113/jphysiol.2003.043695. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Manna PR, Huhtaniemi IT, Stocco DM. Detection of hCG responsive expression of the steroidogenic acute regulatory protein in mouse Leydig cells. Biol Proc Online. 2004;6:83–93. doi: 10.1251/bpo76. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kumar S, Blumberg DL, Canas JA, Maddaiah VT. Human chorionic gonadotropin (hCG) increases cytosolic free calcium in adult rat Leydig cells. Cell Calcium. 1994;15:349–55. doi: 10.1016/0143-4160(94)90010-8. [DOI] [PubMed] [Google Scholar]
- Wűrthner JU, Kistler M, Kratzmeier M, Mukhopadhyay AK. LH/hCG-receptor is coupled to both adenylate cyclase and protein kinase C signaling pathways in isolated mouse Leydig cells. Endocrine. 1995;3:579–84. doi: 10.1007/BF02953022. [DOI] [PubMed] [Google Scholar]
- Pandey AK, Li W, Yin X, Stocco DM, Grammas P, et al. Blocking L-type calcium channels reduced the threshold of cAMP-induced steroidogenic acute regulatory gene expression in MA-10 mouse Leydig cells. J Endocrinol. 2010;204:67–74. doi: 10.1677/JOE-09-0206. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dyson MT, Kowalewski MP, Manna PR, Stocco DM. The differential regulation of steroidogenic acute regulatory protein-mediated steroidogenesis by type I and type II PKA in MA-10 cells. Mol Cell Endocrinol. 2009;300:94–103. doi: 10.1016/j.mce.2008.11.029. [DOI] [PMC free article] [PubMed] [Google Scholar]


