Abstract
The occurrence of tyrosine phosphorylation (TP) in the sperm head during capacitation has been poorly investigated, and no data exist on the relationship of its dynamics with the acquisition of sperm fertilizing ability. This study localized TP of head proteins in human spermatozoa during capacitation and explored its relationship with acquisition of the ability to display progesterone (P)-stimulated acrosome reactions (ARs) and to penetrate zona-free hamster oocytes. By immunofluorescence, TP immunoreactivity was revealed in the acrosomal region of formaldehyde-fixed/unpermeabilized samples, whereas it was abolished in fixed/permeabilized samples, in which TP immunoreactivity was high in the principal piece. No TP immunoreactivity was detectable in unfixed spermatozoa. Head TP immunoreactivity was localized externally to the acrosome, close to the cytoplasmic membrane, as assessed by transmission electron microscopy. The increase in head TP was an early event during capacitation, occurring within 1 h in capacitating conditions. At this time, the P-stimulated ARs were also increased, whereas egg penetration was as poor as in uncapacitated spermatozoa. At 5 h of capacitation, the extent of neither head TP nor the P-induced ARs were greater than that at 1 h, whereas egg penetration had significantly increased. Seminal plasma inhibited head TP, P-induced ARs and egg penetration. None of these inhibitory effects, unlike those on tail TP, were prevented by the cAMP analogue dbcAMP (N,2-O-dibutyryladenosine 3′,5′-cyclic monophosphate). In conclusion, head TP is a subsurface event occurring early during capacitation and is closely related to acquisition of the ability to display P-stimulated ARs, whereas the ability to fuse with oolemma and to decondense is a later capacitation-related event.
Keywords: acrosome reaction, capacitation, human spermatozoa, sperm–oocyte fusion, tyrosine phosphorylation
Introduction
Mammalian spermatozoa must undergo capacitation in the female reproductive tract to acquire the ability to fertilize oocytes. Capacitation enables spermatozoa to gain hyperactive motility, adhere to the zona pellucida, respond to physiological inducers of the acrosome reaction (AR) and initiate fusion with the oocyte 1. The increase in tyrosine phosphorylation (TP) of sperm proteins is a major event occurring during capacitation 2, and some studies have correlated the level of TP with the capacitated state of mammalian spermatozoa 3, 4. Indeed, factors with a role in regulating capacitation also regulate TP. In particular, both sperm TP and capacitation are stimulated by cAMP analogues and phosphodiesterase inhibitors and are inhibited by protein kinase A (PKA) inhibitors, thereby suggesting that cAMP/PKA signalling pathways are involved in the two processes 5. Furthermore, seminal plasma, which prevents capacitation 6, also prevents sperm TP 7. Nevertheless, the suggestion that TP could be a marker of a fully capacitated state is contradicted by the observation of an increase in TP of a 32-kDa protein in porcine spermatozoa during incubation in non-capacitating media (depleted of bicarbonate 8 and under PKA pathway inhibition 9).
In humans, the relationship between increase in TP and acquisition of sperm fertilizing ability during capacitation has been poorly investigated. In a recent study, we explored the relationship between the capacitation-related increase in global TP, quantified by a flow cytometric assay, and the acquisition of human sperm fertilizing ability, evaluated by the progesterone (P)-enhanced hamster egg penetration test (HEPT) 7. An increase in global TP seemed to be an early event in the capacitation process, whereas the P-enhanced sperm–oocyte fusion required a longer capacitation time. Furthermore, it was possible to dissociate the increase in TP and the P-enhanced egg penetration under different experimental conditions, suggesting that sperm fertilizing ability is always associated with an increase in global TP, whereas TP does not necessarily reflect the acquisition of sperm fertilizing ability. However, global TP, quantified in fixed and permeabilized sperm suspensions, reflected the TP of sperm proteins that were mainly distributed along the flagellum, as evaluated by immunofluorescence. Indeed, most of the rare studies that have explored the link between the phosphorylation status of mammalian spermatozoa and their fertilizing ability have focused on TP of sperm flagellar proteins, because the flagellum seems to be the major sperm compartment undergoing TP in a number of species 10, 11, 12, 13, 14, 15. In particular, in human spermatozoa, PKA-anchoring proteins (AKAPs) localized on the fibrous sheath, namely, AKAP82, its precursor pro-AKAP82 and FSP95, are the most prominent tyrosine-phosphorylated proteins during capacitation 10, 14. Although it has been reported that a low percentage of human spermatozoa with phosphotyrosine residues on the principal piece is associated with reduced sperm–zona pellucida binding 16 and reduced in vitro fertilization 17, the link between the increase in tail TP and the acquisition of hyperactivated motility is better established 11, 12, 14, 15.
Spermatozoa are highly polarized cells, with the head performing functions related to oocyte interaction and the tail being involved in energy production and motility. Therefore, the increase in TP of the flagellum could be related to the onset of hyperactivated motility during capacitation 11, 12, but it cannot directly account for the acquisition of the ability to interact with the oocyte, where the sperm head is involved.
A superficial TP immunoreactivity was reported in the head of live mouse spermatozoa by the use of immunomagnetic beads 18. In that study, capacitation promoted the appearance of tyrosine-phosphorylated chaperone proteins on the sperm surface overlying the acrosome, which is thought to facilitate sperm–zona binding. Unfortunately, the same authors failed to confirm these results in humans 19. Nevertheless, previous reports have described a reduction in zona pellucida binding 20 and sperm–oocyte fusion 21 when human spermatozoa were pre-incubated with monoclonal anti-phosphotyrosine antibodies. Scanty and conflicting results exist on the occurrence of or increase in subsurface head TP during capacitation 13, 17, 21, 22, 23.
In the present study, which used fixed un-permeabilized human sperm suspensions and immunofluorescence, we revealed a capacitation-dependent TP of head proteins that had a subsurface localization. We also explored the relationship between head TP and capacitation-dependent acquisition of the ability to undergo P-dependent ARs and egg penetration.
Materials and methods
The study was approved by the Ethics Committee of the Azienda Sanitaria Locale of L'Aquila and all subjects signed an informed consent statement.
Chemicals
All reagents were purchased from Sigma Chemical (St. Louis, MO, USA) unless stated otherwise. P was prepared as a stock solution in dimethyl sulfoxide (DMSO).
P, N,2-O-dibutyryladenosine 3′,5′-cyclic monophosphate (dbcAMP) and H89 were diluted in Biggers, Whitten and Wittingham (BWW) medium to give the final working concentration before use. O-phospho-L-tyrosine was diluted in NH4OH (4 mol L−1).
Sperm processing
Semen samples were collected according to the World Health Organization-recommended procedure 24 by masturbation from healthy normozoospermic donors. All samples were collected in sterile containers and left for at least 30 min to liquefy at 37°C before processing. Motile sperm suspensions were obtained by a swim up procedure. Briefly, spermatozoa were washed twice (700 × g, 7 min) in BWW medium (pH 7.4). After the second centrifugation, supernatants were removed by aspiration, leaving 0.5 mL on the pellet, and after 30 min the supernatants, containing highly concentrated motile spermatozoa, were carefully aspirated, and the sperm concentration was adjusted to 7 × 106 spermatozoa per mL. To evaluate the effect of seminal plasma, 50% seminal plasma/50% BWW medium (pH 7.4) (v/v) was used for washing, the swim up procedure and incubation in capacitating conditions. Seminal plasma was recovered by centrifugation (700 × g, 7 min) of ejaculates from five normozoospermic subjects, sterilized by filtration (Millex GV filters [0.22 μm], Millipore, Bedford, MA, USA), aliquoted, stored at −80°C and thawed before use. Motile sperm suspensions were incubated under capacitation conditions for 1 h and 5 h at 37°C in an atmosphere of 5% CO2/95% air (v/v) in the presence of 1% (w/v) human serum albumin fraction V in the capacitation medium (BWW). Sperm viability, determined by eosin–nigrosin exclusion staining 24, remained unchanged during 5-h capacitation, and always exceeded 90%.
Immunocytochemistry
Immunocytochemistry was performed on (1) live motile sperm suspensions; (2) fixed unpermeabilized motile sperm suspensions; and (3) fixed and permeabilized motile sperm suspensions. Fixation was performed by adding either ice-cold 1% (v/v) formaldehyde in phosphate-buffered saline (PBS, pH 7.4) or ice-cold absolute methanol for 30 min at 4°C. Permeabilization of fixed spermatozoa was performed by using 0.1% (v/v) Triton X-100 in PBS for 10 min at room temperature (RT). Tyrosine phosphoproteins were recognized by a fluorescein isothiocyanate-labelled monoclonal antibody (mAb) against human phosphotyrosine (clone pY20). The optimal working concentration of the anti-phosphotyrosine mAb (10 μg mL−1) and the time of co-incubation with spermatozoa (1 h) were chosen after preliminary experiments. To evaluate the immunostaining specificity, pY20 mAbs were pre-incubated with a saturated solution of 50 mmol L−1 O-phospho-L-tyrosine for 1 h at RT before their addition to motile sperm suspensions incubated under capacitation conditions for 5 h. After three centrifugations (700 × g, 7 min), sperm suspensions were smeared on slides, mounted in PBS–glycerol and observed with a fluorescence microscope (Leica DMLB, Wetzlar, Germany) at × 100 magnification.
Transmission electron microscopy
A pre-embedding procedure and a sensitive peroxidase system were used to detect the subcellular localization of tyrosine-phosphorylated sperm head proteins. Motile sperm suspensions incubated under capacitation conditions for 5 h were washed by centrifugation (1 000 × g, 4 min) in PBS (pH 7.2), and pellets were resuspended in 2.5% (v/v) glutaraldehyde (AGAR Scientific Ltd, Essex, UK) in sodium cacodylate buffer (pH 7.2), for 1 h at 4°C. The immunostaining procedure was performed according to the protocol from the Envision + Dual Link System-HRP kit (DakoCytomation, Carpentaria, CA, USA). The high sensitivity of this system is based on a horseradish peroxidase-labelled polymer that is conjugated to the secondary antibodies. Briefly, after centrifugation, endogenous peroxidase was blocked with 0.3% (w/v) hydrogen peroxide containing sodium azide (1 mmol L−1), and after further centrifugation as above, spermatozoa were incubated overnight at RT with pY20 mAbs or MOPC-21 mAbs as a control, both diluted in the ratio 1:50 in PBS with 2% (w/v) bovine serum albumin. After three centrifugations as above in PBS, pellets were resuspended in a peroxidase-labelled polymer conjugated to goat anti-mouse F(ab′)2 immunoglobulins for 1 h at RT, followed by a washing step in PBS. Immunoreactivity was revealed with 3′,3′-diaminobenzidine (DAB) as the chromogen. After repeated washes by centrifugation, samples were post-fixed in 1% (w/v) osmium tetroxide in distilled water, dehydrated in graded ethanol and embedded in Epon 812 (AGAR Scientific Ltd., Stansted, UK). Ultrathin sections (70 nm) were cut on a ultramicrotome (Reichert, Depew, NY, USA) equipped with a diamond knife, then contrasted briefly with uranyl acetate and lead citrate (AGAR Scientific Ltd.) and viewed in a Philips CM100 transmission electron microscope (Philips Electronics, Eindhoven, the Netherlands).
Hamster egg penetration test
The P-enhanced HEPT was performed as previously described 7. Briefly, motile sperm suspensions were incubated under capacitating conditions for 1 and 5 h and then exposed to P (5 μmol L−1) for 15 min or to the same volume of DMSO (as a control) before incubating with oocytes.
In one set of experiments, spermatozoa treated with 50% (v/v) seminal plasma were incubated under capacitating conditions for 5 h in the presence or absence of the cell-permeable cAMP analogue dbcAMP (5 mmol L−1) and then exposed to P (5 μmol L−1) or DMSO before incubating with oocytes.
Standard procedures were used for the recruitment of oocytes from mature golden Syrian hamsters of the species Mesocricetus auratus 24. Between 15 and 20 zona-free oocytes were added to each 100-μL droplet containing 0.7 × 106 motile spermatozoa. After 3 h co-incubation at 37°C in an atmosphere of 5% CO2/95% air (v/v), oocytes were recovered from the droplets, washed free of loosely adherent spermatozoa and labelled with SYBR 14 (2 μmol L−1). SYBR 14 is a nuclear membrane-permeable DNA-specific fluorochrome. It stains sperm nuclei and emits fluorescence at 515 nm. To determine sperm penetration, oocytes were examined for the evidence of swollen sperm heads in the cytoplasm with a microscope equipped with epifluorescence (Leica DMLB). The number of spermatozoa penetrating each egg was assessed and expressed as the total number of penetrations/total number of oocytes (penetration index).
AR assessment
Assessment of P (15 μmol L−1)-induced ARs was performed as previously described 25 in the same experimental conditions used for the HEPT. Briefly, sperm suspensions were centrifuged and resuspended in hypoosmotic solution for 1 h to check for sperm viability 24. After centrifugation, sperm suspensions were smeared, fixed in methanol, incubated with fluoresceinated Pisum sativum agglutinin at 100 μg mL−1 in PBS (pH 7.2) for 2 h, washed and observed under a fluorescence microscope (Leica DMLB). At least 200 spermatozoa were counted in each smear, and the percentage of spermatozoa not uniformly fluorescing at the anterior region of the head (reacted spermatozoa) was evaluated. Only spermatozoa with coiled tails were considered viable and thus scored for true (non-degenerative) ARs 26. P-induced ARs were calculated as increase in the AR rate after exposure to P.
Statistical analysis
Statistical analysis was performed using the SAS statistical software (version 9.1; SAS Institute Inc., Cary, NC, USA). Immunocytochemistry and AR data were analysed by analysis of variance (ANOVA). The HEPT results were subjected to two-way analysis of variance to separate replicate from treatment variations (general linear model procedure, PROC GLM). Post hoc comparisons between pairs of groups were performed using the Tukey's studentized range (HSD) test and statistical significance was set at P ≤ 0.05. Results were expressed as mean ± SEM.
Results
Immunodetection of sperm head TP
Using immunofluorescence, head TP immunoreactivity was revealed only in formaldehyde-fixed/unpermeabilized samples. A fluorescent signal distributed in the anterior region of the head was observed for the majority of 5-h-capacitated spermatozoa (Figure 1A). A minority of spermatozoa also exhibited fluorescence along the principal piece of the sperm tail (Figure 1A). Both methanol fixation and permeabilization of formaldehyde-fixed sperm suspensions completely abolished head TP immunoreactivity, although they increased TP immunoreactivity of the principal piece (Figures 1B and C). No TP immunoreactivity could be detected in viable unfixed 5-h-capacitated spermatozoa (Figure 1D). Pre-incubation of the anti-phosphotyrosine mAb with a saturated solution of 50 mmol L−1 O-phospho-L-tyrosine, its specific antigen, abolished all TP immunoreactivity in formaldehyde-fixed/unpermeabilized (Figure 1E) and fixed/permeabilized 5-h-capacitated spermatozoa (Figure 1F).
Figure 1.
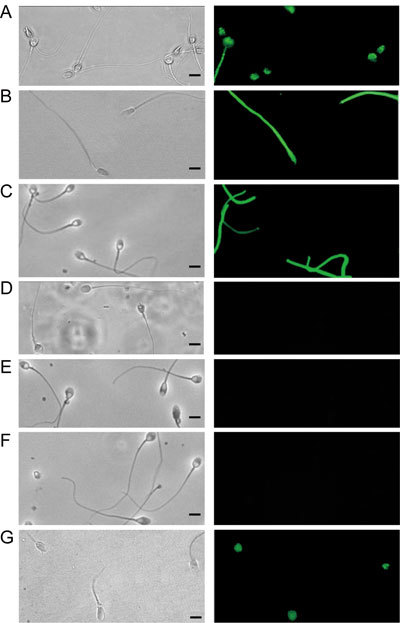
Immunoreactivity of monoclonal antibody pY20 by an immunofluorescence test on 5-h-capacitated human spermatozoa using (A) formaldehyde-fixed/unpermeabilized, (B) methanol-fixed and (C) formaldehyde-fixed/permeabilized spermatozoa. No fluorescent labelling was detected with unfixed spermatozoa (D). There was no fluorescent labelling when formaldehyde-fixed/unpermeabilized (E) and fixed/permeabilized (F) spermatozoa were exposed to pY20 mAb pre-incubated with a saturated solution of O-phospho-L-tyrosine. (G): Immunoreactivity of formaldehyde-fixed/unpermeabilized spermatozoa was unchanged after 5 h of incubation under capacitating conditions in the presence of the protein kinase A inhibitor H89 (50 μmmol L−1). Phase contrast (left) and corresponding immunofluorescence photographs (right) (Bars = 5 μm).
Head TP immunoreactivity was localized externally to the acrosome, close to the cytoplasmic membrane, as assessed by transmission electron microscopy (Figure 2).
Figure 2.
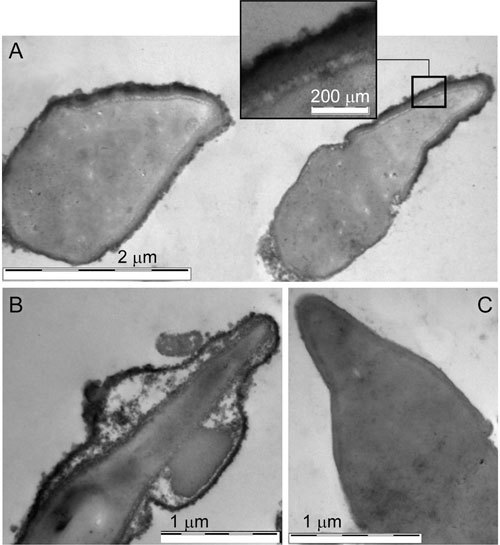
Transmission electron micrographs of longitudinal sections of human sperm heads after incubation for 5 h under capacitating conditions. (A): Immunoelectron microscopic peroxidase labelling on fixed/unpermeabilized spermatozoa incubated with monoclonal antibody pY20. Tyrosine phosphorylation (TP) immunoreactivity is localized in the anterior region of the head (detail is showed in the upper panel), external to the acrosome. (B): A swollen acrosome in which phosphotyrosine immunoreactivity was retained along the swollen membranes. (C): No immunoperoxidase labelling was detected on capacitated spermatozoa when the MOPC-21 antibody was used as a control for non-specific binding.
Time course of sperm head TP, ARs and oocyte penetration during capacitation
The increase in head TP, as evaluated in formaldehyde-fixed/unpermeabilized samples, seemed to be an early event during capacitation. In five experiments on spermatozoa from different donors, the percentage of TP-positive spermatozoa increased significantly from zero time to 1 h of capacitation (Figure 3A). A further increase after 5 h of capacitation was not significant (Figure 3A). To determine the relationship between TP of the sperm head and acquisition of sperm-fertilizing ability, we assessed P-induced ARs and the P-enhanced HEPT under the same experimental conditions used to evaluate TP.
Figure 3.
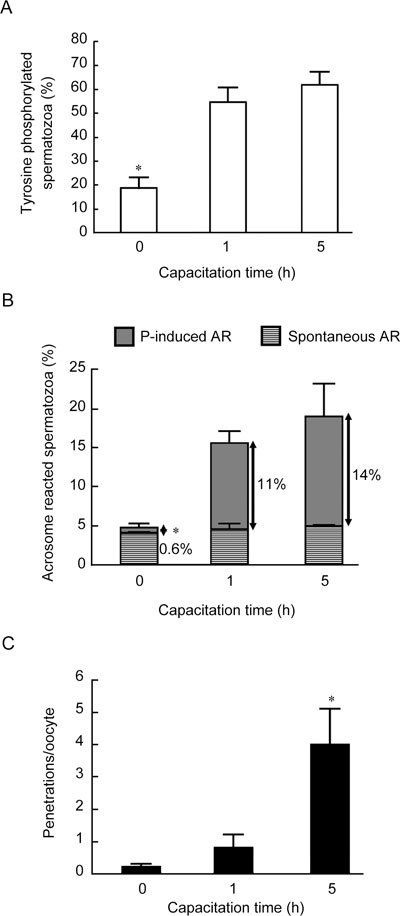
Time course of tyrosine phosphorylation (TP) of head proteins in human spermatozoa (A), spontaneous and progesterone (P)-induced acrosome reactions (ARs) (B) and P-stimulated sperm–oocyte fusion during capacitation (C). Results are from five experiments with different donor semen. (A): Overall significance: P < 0.0001 by ANOVA; *P < 0.05, compared with 1 h and 5 h. (B): Overall significance: P < 0.0001 with ANOVA; *P < 0.05, compared with 1 h and 5 h. (C): Overall significance: P < 0.0001 with PROC GLM. For this experiment 215 oocytes were used; *P < 0.05, compared with 0 h and 1 h.
Analogous to the early increase in TP, a significant increase in P-induced ARs over the spontaneous rate was observed at 1 h of capacitation (Figure 3B); In contrast, at this time, sperm–oocyte fusion was as poor as in uncapacitated samples, but it was significantly increased after 5 h of capacitation (Figure 3C).
Effect of seminal plasma and a cAMP analogue
As shown in Figure 4A, in five experiments with different donors, the exposure of spermatozoa to 50% (v/v) seminal plasma during 5 h of capacitation prevented the development of the capacitation-related increase in sperm head TP in formaldehyde-fixed/unpermeabilized samples. Seminal plasma also inhibited the increase in tail TP during the 5-h capacitation, as evaluated after fixation and permeabilization. The inhibition of head TP exerted by seminal plasma was not abolished by the concomitant addition of 5 mmol L−1 dbcAMP, which prevented seminal plasma inhibition of tail TP, as expected. In accordance with the cAMP/PKA pathway playing a minor role in head TP, the PKA inhibitor H89 (50 μmol L−1) did not affect head TP immunoreactivity during a 5-h incubation under capacitating conditions (Figure 1G).
Figure 4.
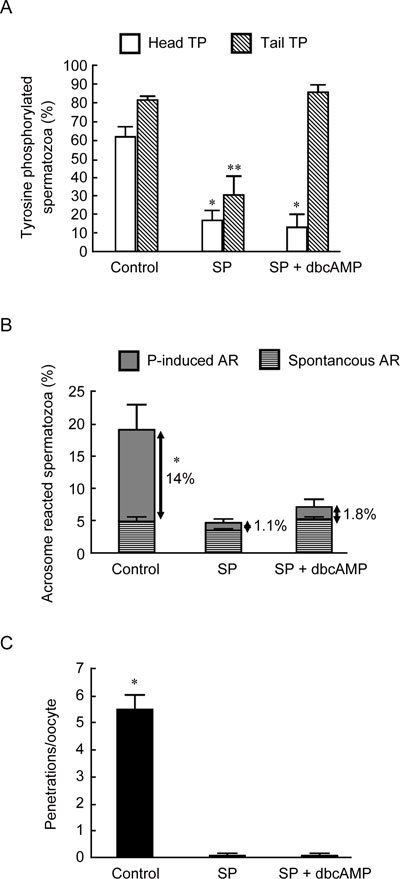
The effect of the presence of 50% (v/v) seminal plasma (SP) and SP with the cAMP analogue, dbcAMP (5 mmol L−1), during sperm processing and during a 5-h capacitation on sperm head tyrosine phosphorylation (TP) (A), spontaneous and progesterone (P)-induced acrosome reactions (ARs) (B) and P-stimulated sperm–oocyte fusion (C). The results are from five experiments using different donor semen. (A): Overall significance: P < 0.0001 with ANOVA; *P < 0.05, compared with the control and **P < 0.05 compared with the control and SP + dbcAMP. (B): Overall significance: P < 0.0001 with ANOVA; *P < 0.05, compared with SP and SP + dbcAMP. (C): Overall significance: P < 0.0001 with PROC GLM. For this experiment, 220 oocytes were used; *P < 0.05, compared with SP and SP + dbcAMP.
Seminal plasma inhibited both P-induced ARs (Figure 4B) and the number of penetrations per oocyte (Figure 4C). None of these effects was prevented by the concomitant addition of 5 mmol L−1 dbcAMP (Figures 4B and C).
Discussion
Although the capacitation-dependent tail TP in mammalian spermatozoa is a well-recognized event, TP of the sperm head is not as well characterized. TP of tail proteins accounts for the almost global TP occurring during capacitation, as detected by immunofluorescence assays 7 and immunoblotting 15. However, as spermatozoa are highly polarized cells, the increase in TP of the sperm flagellum may have a role in the acquisition of hyperactivated motility during capacitation 11, 12, but not in the head events involved in fertilization.
An increase in the proportion of mouse and human spermatozoa undergoing TP of head proteins during capacitation has been reported by some authors 21, 22, 23 and denied by others 13, 17. These conflicting reports could be explained by differences in the immunocytochemical methods used. In the present study, head TP immunoreactivity could be revealed only in 1% formaldehyde-fixed/unpermeabilized sperm suspensions. Aldehyde fixation ensures antibody access inside the cell by partially permeabilizing the sperm plasma membrane while still preserving membrane integrity, thereby avoiding the loss of internal membrane-bound antigens 27. The permeabilization procedure is required for the consistent observation of TP immunoreactivity of the principal piece proteins, namely, the AKAPs, which are deeply localized, being the major structural fibrous sheath proteins of the principal piece 28. Nevertheless, permeabilization and methanol fixation completely abolished head TP immunoreactivity, owing to their disruptive effects on sperm membranes and the loss of membrane-bound antigens. No TP immunoreactivity could be detected in unfixed capacitated human spermatozoa, which rules out any surface expression of phosphotyrosine residues. Reports have described a reduction in zona pellucida binding 20 and sperm–oocyte fusion 21 in live human spermatozoa pre-incubated with monoclonal anti-phosphotyrosine antibodies. More recently, a superficial TP immunoreactivity could be revealed in the head of live mouse spermatozoa using immunomagnetic beads 18. Nevertheless, the authors, using the same method, failed to confirm any expression of phosphotyrosine residues in the human sperm head 19.
In the present study, head TP immunoreactivity was localized external to the acrosome, close to the cytoplasmic membrane when assessed by transmission electron microscopy that used a pre-embedding procedure and a sensitive peroxidase system. To our knowledge, this is the first report showing the application of the sensitive Envision + Dual Link Peroxidase System (DakoCytomation) for immunoelectron microscopy. A subsurface localization is inferred from the electron microscopy and the lack of immunoreactivity in unfixed, live spermatozoa.
We recently reported that flow cytometry is a rapid, simple and reliable technique to assess and quantify the levels of TP in fixed and permeabilized human spermatozoa 7. As the use of fixed unpermeabilized human spermatozoa did not provide a clear dissociation between head and tail phosphotyrosine immunoreactivity, the interference of flagellar immunoreactivity prevented us from monitoring the dynamics of head TP during capacitation by flow cytometry. Immunofluorescence, however, did reveal an increase in TP of the human sperm head, similar to that reported for the flagellum 7, which seems to be an early event in the capacitation process with an ∼ threefold mean increase within 1 h of capacitation.
Some interesting observations on the relationship between head TP and acquisition of sperm fertilizing ability arise from this study. Similar to the increase in head TP, the ability to display P-induced ARs was an early event during capacitation, whereas egg penetration occurred later, as expected 29. These observations strongly suggest a direct link between the increase in head TP during capacitation and AR inducibility. In streptolysin O-permeabilized human spermatozoa, a relationship between head TP and acrosomal exocytosis had been reported, as the inhibition of tyrosine kinase abolished both 30. Intriguingly, a valosin-containing protein, known as p97 (VCP/p97), has been identified in the acrosomal region of human spermatozoa 23. As in other cell types, tyrosine-phosphorylated VCP/p97 mediates the fusion of Golgi membranes and seems to be implicated in exocytosis processes 31, and its involvement in sperm preparation for ARs (a form of regulated exocytosis) has been hypothesized 23.
Seminal plasma inhibited the increase in head TP during capacitation, as well as P-induced ARs and oocyte penetration. None of these effects was prevented by the cell-permeable cAMP analogue dbcAMP. The inhibitory effect exerted by seminal plasma on capacitation-related events was expected, as many decapacitating factors from seminal plasma have been partially or completely characterized 6. As seminal plasma is rich in cholesterol, it inhibits plasma membrane cholesterol efflux, thereby preventing an increase in sperm membrane fluidity, HCO3− influx and all other mechanisms involved in the activation of the cAMP/PKA pathway, a key event for capacitation and TP of the flagellum. Accordingly, on the basis of immunofluorescence, dbcAMP overcame the seminal plasma inhibition of tail TP, paralleling our recently published flow cytometric data of the whole sperm cell 7. The involvement of the cAMP/PKA pathway in key functions of the flagellum is well known. In mammalian spermatozoa, cAMP/PKA-dependent phosphorylation of flagellar proteins is involved in the initiation and maintenance of sperm motility 32. Early TP of AKAPs recruits the ubiquitous PKA to the fibrous sheath and facilitates additional local phosphorylation to enhance sperm motility 14.
The inability of dbcAMP to prevent the inhibition of head TP exerted by seminal plasma is a novel and interesting observation in the present study. In line with the cAMP/PKA pathway having a minor role in the phosphorylation of head proteins during capacitation, incubating sperm under capacitating conditions in the presence of the PKA inhibitor H89 did not affect the occurrence of head TP. Spermatozoa are highly polarized cells, and the cAMP/PKA pathway could have different regulatory roles in each subcellular sperm compartment. Sperm membrane receptors involved in the sperm–oocyte interaction undergo autophosphorylation, owing to an intrinsic tyrosine kinase activity triggered by receptor aggregation 21, 22, thereby making this process independent of the cAMP/PKA pathway. Indeed, functional aggregates could also occur spontaneously as a result of increased fluidity of sperm membranes during capacitation 22, thereby explaining the inhibitory effect of seminal plasma on head TP.
In conclusion, in human spermatozoa, head TP is a subsurface event that occurs early during capacitation, reflecting the dynamics of tail TP, the main determinant of global sperm TP, although a minor role is suggested for the cAMP/PKA pathway. Head TP also seems to be tightly related to acquisition of the ability of spermatozoa to display P-stimulated ARs, whereas the ability to fuse with oolemma and to decondense is a later 29 and more comprehensive capacitation-related event 33. In this light, the occurrence of TP may be responsible for events occurring early during capacitation both in the tail (for example, hyperactivated motility) and in the head (for example, inducibility of ARs). However, it does not reflect the acquisition of the full capacitation-dependent sperm fertilizing ability.
Acknowledgments
This work was supported by the Ministero dell'Istruzione, dell'Università e della Ricerca, Italy.
References
- Yanagimachi R.Mammalian fertilization. In: Knobil E, Neill JD, editors. The Physiology of Reproduction2nd edn. New York: Raven Press; 1994pp189–96.
- Naz RK, Rajesh PB. Role of tyrosine phosphorylation in sperm capacitation/acrosome reaction. Reprod Biol Endocrinol. 2004;2:75–86. doi: 10.1186/1477-7827-2-75. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Visconti PE, Bailey JL, Moore GD, Pan D, Olds-Clarke P, et al. Capacitation of mouse spermatozoa. I. Correlation between the capacitation state and protein tyrosine phosphorylation. Development. 1995;121:1129–37. doi: 10.1242/dev.121.4.1129. [DOI] [PubMed] [Google Scholar]
- Tardif S, Dubé C, Chevalier S, Bailey JL. Capacitation is associated with tyrosine phosphorylation and tyrosine kinase-like activity of pig sperm proteins. Biol Reprod. 2001;65:784–92. doi: 10.1095/biolreprod65.3.784. [DOI] [PubMed] [Google Scholar]
- Baldi E, Luconi M, Bonaccorsi L, Krausz C, Forti G. Human sperm activation during capacitation and acrosome reaction: role of calcium, protein phosphorylation and lipid remodelling pathways. Front Biosci. 1996;1:d189–205. doi: 10.2741/a125. [DOI] [PubMed] [Google Scholar]
- Calvete JJ, Sanz L. Insights into structure-function correlations of ungulate seminal plasma proteins. Soc Reprod Fertil Suppl. 2007;65:201–15. [PubMed] [Google Scholar]
- Barbonetti A, Vassallo MR, Cinque B, Antonangelo C, Sciarretta F, et al. Dynamics of the global tyrosine phosphorylation during capacitation and acquisition of the ability to fuse with oocytes in human spermatozoa. Biol Reprod. 2008;79:649–56. doi: 10.1095/biolreprod.108.068254. [DOI] [PubMed] [Google Scholar]
- Tardif S, Dubé C, Bailey JL. Porcine sperm capacitation and tyrosine kinase activity are dependent on bicarbonate and calcium but protein tyrosine phosphorylation is only associated with calcium. Biol Reprod. 2003;68:207–13. doi: 10.1095/biolreprod.102.005082. [DOI] [PubMed] [Google Scholar]
- Tardif S, Lefièvre L, Gagnon C, Bailey JL. Implication of cAMP during porcine sperm capacitation and protein tyrosine phosphorylation. Mol Reprod Dev. 2004;69:428–35. doi: 10.1002/mrd.20178. [DOI] [PubMed] [Google Scholar]
- Mandal A, Naaby-Hansen S, Wolkowicz MJ, Klotz K, Shetty J, et al. FSP95, a testis-specific 95-kilodalton fibrous sheath antigen that undergoes tyrosine phosphorylation in capacitated human spermatozoa. Biol Reprod. 1999;61:1184–97. doi: 10.1095/biolreprod61.5.1184. [DOI] [PubMed] [Google Scholar]
- Nassar A, Mahony M, Morshedi M, Lin MH, Srisombut C, et al. Modulation of sperm tail protein tyrosine phosphorylation by pentoxifylline and its correlation with hyperactivated motility. Fertil Steril. 1999;71:919–23. doi: 10.1016/s0015-0282(99)00013-8. [DOI] [PubMed] [Google Scholar]
- Si Y, Okuno M. Role of tyrosine phosphorylation of flagellar proteins in hamster sperm hyperactivation. Biol Reprod. 1999;61:240–6. doi: 10.1095/biolreprod61.1.240. [DOI] [PubMed] [Google Scholar]
- Urner F, Leppens-Luisier G, Sakkas D. Protein tyrosine phosphorylation in sperm during gamete interaction in the mouse: the influence of glucose. Biol Reprod. 2001;64:1350–7. doi: 10.1095/biolreprod64.5.1350. [DOI] [PubMed] [Google Scholar]
- Luconi M, Carloni V, Marra F, Ferruzzi P, Forti G, et al. Increased phosphorylation of AKAP by inhibition of phosphatidylinositol 3-kinase enhances human sperm motility through tail recruitment of protein kinase A. J Cell Sci. 2004;117:1235–46. doi: 10.1242/jcs.00931. [DOI] [PubMed] [Google Scholar]
- Luconi M, Porazzi I, Ferruzzi P, Marchiani S, Forti G, et al. Tyrosine phosphorylation of the A kinase anchoring protein 3 (AKAP3) and soluble adenylate cyclase are involved in the increase of human sperm motility by bicarbonate. Biol Reprod. 2005;72:22–32. doi: 10.1095/biolreprod.104.032490. [DOI] [PubMed] [Google Scholar]
- Liu DY, Klarke GN, Baker HWG. Tyrosine phosphorylation on capacitated human sperm tail detected by immunofluorescence correlates strongly with sperm–zona pellucida (ZP) binding but not with the ZP-induced acrosome reaction. Hum Reprod. 2006;21:1002–8. doi: 10.1093/humrep/dei435. [DOI] [PubMed] [Google Scholar]
- Sakkas D, Leppens-Luisier G, Lucas H, Chardonnens D, Campana A, et al. Localization of tyrosine phosphorylated proteins in human sperm and relation to capacitation and zona pellucida binding. Biol Reprod. 2003;68:1463–9. doi: 10.1095/biolreprod.102.011023. [DOI] [PubMed] [Google Scholar]
- Asquith KL, Baleato RM, McLaughlin EA, Nixon B, Aitken RJ. Tyrosine phosphorylation activates surface chaperones facilitating sperm-zona recognition. J Cell Sci. 2004;117:3645–57. doi: 10.1242/jcs.01214. [DOI] [PubMed] [Google Scholar]
- Mitchell LA, Nixon B, Aitken RJ. Analysis of chaperone proteins associated with human spermatozoa during capacitation. Mol Hum Reprod. 2007;13:605–13. doi: 10.1093/molehr/gam043. [DOI] [PubMed] [Google Scholar]
- Kadam AL, Fateh M, Naz RK. Fertilization antigen (FA-1) completely blocks human sperm binding to human zona pellucida: FA-1 antigen may be a sperm receptor for zona pellucida in humans. J Reprod Immunol. 1995;29:19–30. doi: 10.1016/0165-0378(95)00928-e. [DOI] [PubMed] [Google Scholar]
- Naz RK, Ahmad K, Kumar R. Role of membrane phosphotyrosine proteins in human spermatozoal function. J Cell Sci. 1991;99:157–65. doi: 10.1242/jcs.99.1.157. [DOI] [PubMed] [Google Scholar]
- Leyton L, Saling P. 95 kd sperm protein bind ZP3 and serve as tyrosine kinase substrates in response to zona binding. Cell. 1989;57:1123–30. doi: 10.1016/0092-8674(89)90049-4. [DOI] [PubMed] [Google Scholar]
- Ficarro S, Chertihin O, Westbrook VA, White F, Jayes F, et al. Phosphoproteome analysis of capacitated human sperm. Evidence of tyrosine phosphorylation of a kinase-anchoring protein 3 and valosin-containing protein/p97 during capacitation. J Biol Chem. 2003;278:11579–89. doi: 10.1074/jbc.M202325200. [DOI] [PubMed] [Google Scholar]
- World Health Organization. WHO Laboratory Manual for the Examination of Human Semen and Semen-Cervical Mucus Interaction4th edn. London: Cambridge University Press; 1999pp4–33.
- Barbonetti A, Vassallo MR, Antonangelo C, Nuccetelli V, D'Angeli A, et al. RANTES and human sperm fertilizing ability: effect on acrosome reaction and sperm/oocyte fusion. Mol Hum Reprod. 2008;14:387–91. doi: 10.1093/molehr/gan031. [DOI] [PubMed] [Google Scholar]
- Aitken RJ, Buckingham DW, Fang HG. Analysis of response of human spermatozoa to A23187 employing a novel technique for assessing acrosome reaction. J Androl. 1993;14:132–41. [PubMed] [Google Scholar]
- Gabriel LK, Franken DR, van der Horst G, Kruger TF. Localization of wheat germ agglutinin lectin receptors on human sperm by fluorescence microscopy: utilization of different fixatives. Arch Androl. 1994;33:77–85. doi: 10.3109/01485019408987807. [DOI] [PubMed] [Google Scholar]
- Eddy EM, Toshimori K, O'Brian DA. Fibrous sheath of mammalian spermatozoa. Microsc Res Tech. 2003;61:103–15. doi: 10.1002/jemt.10320. [DOI] [PubMed] [Google Scholar]
- Francavilla F, Romano R, Santucci R, Macerola B, Ruvolo G, et al. Effect of human sperm exposure to progesterone on sperm/oocyte fusion and sperm-zona pellucida binding under various experimental conditions. Int J Androl. 2002;25:106–12. doi: 10.1046/j.1365-2605.2002.00333.x. [DOI] [PubMed] [Google Scholar]
- Tomes CN, Roggero CM, De Blas G, Saling PM, Mayorga LS. Requirement of protein tyrosine kinase and phosphatase activities for human sperm exocytosis. Dev Biol. 2004;15265:399–415. doi: 10.1016/j.ydbio.2003.09.032. [DOI] [PubMed] [Google Scholar]
- Kondo H, Rabouille C, Newman R, Levine TP, Pappin D, et al. p47 is a cofactor for p97-mediated membrane fusion. Nature. 1997;388:75–8. doi: 10.1038/40411. [DOI] [PubMed] [Google Scholar]
- Carr DW, Newell AE. The role of A-kinase anchoring proteins (AKaps) in regulating sperm function. Soc Reprod Fertil Suppl. 2007;63:135–41. [PubMed] [Google Scholar]
- Irvine DS, Aitken RJ. Seminal fluid analysis and sperm function testing. Endocrinol Metab Clin North Am. 1994;23:725–48. [PubMed] [Google Scholar]


