Abstract
Objective measurements are required for computer-aided sperm morphometric analysis (CASMA) machines to distinguish normal from abnormal sperm heads. The morphometric characteristics of spermatozoa in 72 samples of semen and of spermatozoa from 72 other semen samples after swim-up were quantified by the semi-automated Integrated Sperm Analysis System (ISAS) computer-aided system, which measured the sperm head parameters length (L), width (W), area (A), perimeter (P), acrosomal area (Ac), and the derived values L/W and P/A. For each man a homogeneous population of distributions characterized seminal spermatozoa (7 942 cells: median values L 4.4 μm, W 2.8 μm, A 9.8 μm2, P 12.5 μm, Ac 47.5%, L/W 1.57, P/A 1.27), and there was no significant difference in within- and among-individual variation. Different men could have spermatozoa of significantly different dimensions. Head dimensions for swim-up spermatozoa from different men (4 812 cells) were similar to those in semen, differing only by 2%–5%. The values of L, W and L/W fell within the limits given by the World Health Organization (WHO). Although these samples were not biologically matched, linear mixed-effects statistical analyses permitted valid comparison of the groups. A subpopulation of 404 spermatozoa considered to fit the stringent criteria of WHO 'normal' seminal spermatozoa from both semen and swim-up were characterized by median values (and 95% confidence intervals) of L, 4.3 μm (3.8–4.9), W, 2.9 μm (2.6–3.3), A, 10.2 μm2 (8.5–12.2), P, 12.4 μm (11.3–13.9), Ac, 49% (36–60), L/W, 1.49 (1.32–1.67) and P/A, 1.22 (1.11–1.35). These median values fall within the 95th centile confidence limits given by WHO, but the confidence intervals for L and W were larger. Although these differences in head dimensions among men and after swim-up could be detected by CASMA, the small differences make it unlikely that technicians would be able to distinguish them. The values could be used as default sperm head values for the CASMA machine used here.
Keywords: male infertility, semen, sperm head
Introduction
The assessment of sperm morphology remains a controversial topic: should purely 'normal/ideal' forms be assessed; how should normal forms be defined 1, 2; should abnormal forms be monitored; should multiple anomalies' indices be calculated? Previous editions of the World Health Organization (WHO) handbook for semen analysis 3, 4, 5 recommended assessing 'normal' (or 'ideal') forms, as being representative of potentially fertilizing spermatozoa. These are defined as having no obvious defects in the head, midpiece or principal piece and are based on the form of spermatozoa found in the endocervical mucus after intercourse 6. Similar defect-free spermatozoa are found attached to the zona pellucida after in vitro fertilisation (IVF) 7, 8.
The problem of deciding when to classify a spermatozoon as normal depends on many factors, including the technician's concept of the definition of normality and their personal, subjective categorization of spermatozoa. Visual methods used in the analysis of human sperm morphology usually lead to significant variations between observers and laboratories. For a good visualization of spermatozoa, the Papanicolaou stain is recommended, as some smears stained by rapid procedures, such as the Diff-Quik, have high background staining 9 and the heads of spermatozoa appear larger than those stained by Papanicolaou 4. By contrast, the Shorr stain gives similar results to those of the Papanicolaou stain for the percentages of normal forms 10. A recent study 11 confirmed that sperm head measurements in dried smears vary with fixative and stain and that SpermBlue stain and fixative provide head measurements of fixed spermatozoa close to those measured for living spermatozoa in semen. The normal use of reference values is to compare them with patient's values; if the measurements fall outside, there may be a problem with fertility. This requires that categorization as 'normal' be as accurate as possible. However, subjective analysis by technicians may be influenced by the appearance of other spermatozoa in the sample; for example, a 'normal' spermatozoon in one semen sample containing other spermatozoa of larger size may be considered to be abnormally small and not counted as normal, whereas in another sample a similar spermatozoon, with smaller neighbouring spermatozoa, may be discounted as abnormally large. Although this can be checked by ocular measurements of sperm head size, it emphasizes the importance of accurate sperm head measurements 1.
To obviate this problem, the size of sperm heads has been suggested to define normal cells. As reported in the fifth WHO semen manual 3, a spermatozoon is defined as normal when it presents a normal head, neck, midpiece and principal piece. In previous editions different values have been given: length 4.0–5.5 μm (1992) 5 and 3–5 μm (1987) 12, and width 2–3 μm (1987) 12, but for none of the values is the source of the 'reference' spermatozoa given (within semen or cervical mucus post-coitum) or the validity of the data (from how many men and whether fertile or donors). Garrett and Baker 13 have provided sizes of the potentially fertilizing spermatozoa attached to the zona pellucid. The fifth edition of the WHO manual 3 provides measurements obtained from a computerized system of magnified digitized images of head categorized as 'normal': the heads were oval, with length (5th and 95th centiles) 3.7–4.7 μm; width 2.5–3.2 μm; length/width ratio 1.3–1.8, the acrosomal region comprising 40%–70% of the head area.
Our technicians' subjective opinion is that semen samples from different men contain spermatozoa of different size. This could reflect the stresses affecting spermatozoa during smearing and air drying of the semen sample that are known to produce swelling of immature sperm heads 14, 15, loss of cytoplasmic droplets 16 and cell shrinkage 11, 17. If men do have spermatozoa of different size, the detection of large sperm heads may be indicative of the presence of less mature spermatozoa that may have a lower fertilizing potential. Detection of these may thus be of potential value in diagnosing epididymal dysfunction.
It is known that in subfertile couples, the swim-up technique is an easy, reliable and effective sperm-processing method for insemination purposes 18. Originally described by Mahadevan and Baker 19, the method is still used largely in IVF laboratories around the world. Although its use among the male factor infertility group is limited, the swim-up technique is the standard technique for patients with normozoospermia and female infertility 20. It is likely that the swim-up methodology would increase the percentage of morphologically normal spermatozoa in the selected population, since spermatozoa with morphologically normal heads are also likely to have normal tails and thus be effective at progressing forwards.
Over the past 15 years, many authors have suggested the use of computer-aided techniques for appraising human sperm morphology (computer-aided sperm morphometric analysis, CASMA systems) in order to avoid errors due to subjectivity 21, 22. When used both with standardized methods and with variables for analysis 21, 23, 24, these machines provide high repeatability and precision compared with subjective morphological evaluation 25, 26. However, problems of cell recognition, image digitization 2, and the effects of staining procedures 11 cannot be avoided and the process is time-consuming.
As CASMA machines can measure dimensions accurately, the purpose of this study was three-fold: to determine whether different men have spermatozoa of different size, to compare the morphometric characteristics of heads of seminal spermatozoa with those of post-swim-up cells and to determine the size of 'normal' spermatozoa observed in semen and swim-up samples. The latter values could serve as default values for CASMA machines' categorization of normal sperm heads.
Materials and methods
Spermatozoa
Seventy-two stored, fixed and stained slides of seminal smears were selected from those of patients attending the Clinic (Centre of Reproductive Medicine and Andrology, Münster). Samples had been prepared for sperm morphology by smearing 10–20 μL semen according to the WHO method 4, fixing in ether-ethanol 50:50 (v/v) and staining with Papanicolaou and mounting in Eukitt for long-term storage of the samples. Spermatozoa from another 72, unmatched, semen samples from men whose partners were undergoing IVF were prepared by swim-up by layering 0.5–2.0 mL of semen over 3.5–2.0 mL IVF medium (Sperm Preparation Medium, Medicult, Berlin, Germany), depending on the sperm concentration in semen, centrifuging for 10 min at 390 × g. After the supernatant was removed, the pellet was washed in 2.0 mL IVF medium, centrifuged again and the supernatant removed again. The pellet was gently overlaid by 1.0 mL IVF medium and incubated for 60 min at 37°C. At this time the top 0.7 mL was removed and 20 μL taken for motility assessment and 10–20 μL for morphological smears.
The Papanicolaou staining procedure used here was satisfactory for spermatozoa in semen for technicians and the CASMA system. However, staining of several swim-up samples proved inadequate for the CASMA: whereas technicians were able to assess the samples by focusing through the slide, the contrast and depth of staining was often inadequate for the CASMA system to digitize the image satisfactorily. Therefore only well-stained samples were used in this study, and so they could not be matched with native semen samples from the same man. Assessable samples were collected until there were 72 of each in each group. An account of the possible differences between the populations providing semen and swim-up samples was included in the statistical analysis.
CASMA
The Integrated Sperm Analysis System (ISAS®v1.2, Proiser R+D, Valencia, Spain) was used to analyse the head of 200 spermatozoa for each sample (giving a sampling error of 14% 3). The final resolution of the images was of 0.083 μm per pixel in both horizontal and vertical axes and the system has been validated 21. This system uses a Balser monochrome camera (Basler AG, Ahrensberg, Germany) to record images of the sperm head that are digitized and false-colour-coded to indicate the acrosomal area in yellow, the post-acrosomal area in blue and the midpiece in green (Figure 1). All images were examined for correct digitization (by comparing the black and white sperm head image with the adjacent false-colour-coded image) and eliminating images that were out of focus, that did not contain a midpiece (the programme forces green colour [assessed as midpiece] onto the head if no obvious midpiece image is captured) or that included extracellular material (proteinacious background adherent to the sperm head, sperm tails, debris) as sperm structures. Incorrectly digitized images could sometimes be amended by the program by switching the colour coding of midpiece and acrosomal areas; when not, they were eliminated. The morphometric parameters generated were head length (L: major axis), width (W: minor axis), perimeter (P), area (A) and percentage of the head occupied by the acrosome (Ac) (Figure 2). The ratios L/W and A/P were derived subsequently.
Figure 1.
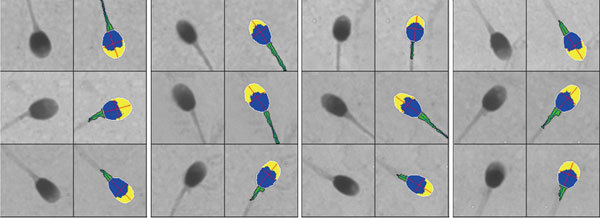
Paired original (left panel) and false-colour-coded digitized (right panel) images, showing the sperm midpiece (green with axis) and head subdivided into acrosomal area (yellow) and post-acrosomal region (blue). The major (length) and minor (width) axes are the red lines and perimeter is in white.
Figure 2.
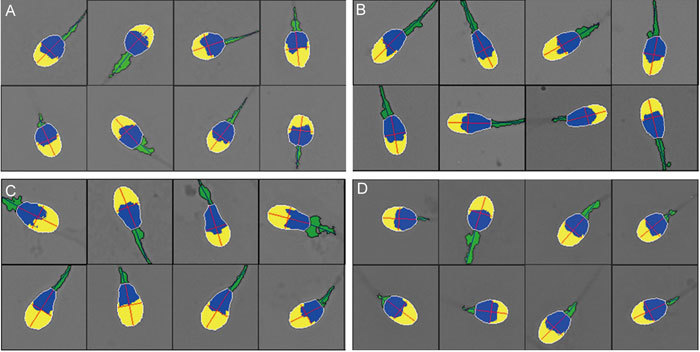
Selected images of sperm heads from men with predominantly round cells (A), elongated forms (B), expanded acrosomal areas (C) and normal forms (D).
Morphological data from a subset of 404 spermatozoa identified as 'normal' were selected from correctly digitized images from a total of 94 samples (53 semen samples and 41 swim-up samples). These had the characteristics described by the Tygerberg criteria 1 of smooth oval outlines, not too round or long, no more than two vacuoles in the acrosome region covering no more than 20% of the head area and no vacuoles in the post-acrosomal area with insertion of the midpiece along the long axis of the head. To eliminate between-observer bias, only one observer (conversant with the criteria of the form of sperm head cell considered 'normal' in the new WHO manual 3) selected these cells from the monochrome images.
Statistics
For each semen and swim-up sample descriptive statistics were performed and the values of each parameter were ranked in decreasing order (Sigma Stat v. 3.5; Systat, Erkrath, Germany). Descriptive statistics are given of the morphometric variables of spermatozoa in neat semen and in the swim-up preparations from each of the 144 samples, and the individual parameters of the entire population of spermatozoa (7 942 cells), as well as 'normal' spermatozoa selected from semen (252) and swim-up (152) from the 72 men. To account for the fact that the semen and swim-up samples were not from the same men, a linear mixed-effects ANOVA was used to describe variability within and between men 27. Tests were performed for analysing differences in the morphometric parameters between seminal and swim-up samples (fixed effect), and the large number of individuals from whom semen samples (random effect) were obtained.
Results
Capture of acceptable images
The success of capturing images was low. Of the 200 cells routinely analysed for each sample, only 48% of spermatozoa in semen and 31% of spermatozoa in swim-up samples were considered to have been correctly assessed. More images were collected until 200 had been captured. Many semen samples presented heavy background staining that was mistaken for spermatozoa and many swim-up samples were faintly stained so that false colour was incorrectly superimposed on the cell or patchy colour under-represented the sperm head domains. Although this possibly biases the spermatozoa selected, it no way affects the analysis of the selected cells.
Morphometric characteristics of spermatozoa in semen and spermatozoa after swim-up
In addition to the pleiomorphic character of their spermatozoa, some men were characterized by a plurality of spermatozoa with specific head forms, for example, tending towards round (Figure 2A), elongated (Figure 2B) or wider acrosomal regions (tapering forms: Figure 2C). There were no significant differences between the median values for spermatozoa in semen and in the swim-up preparations of head lengths, L/W ratios or acrosomal areas, but median sperm head widths, perimeters and areas were significantly larger in swim-up spermatozoa; the P/A ratio was significantly lower in the swim-up samples (P < 0.05) (Table 1). Although significantly different, the percentage differences were small, ranging from 1.8% to 5.1%. Figure 3 displays histograms of several sperm head parameters of 123 and 149 spermatozoa from two men whose sperm values were towards each end of the distribution of median values of all men. Whereas sperm head length was not so different between these men, sperm width, area and the extent of acrosomal coverage were markedly different.
Table 1. Distributions of head length, width, area, perimeter, acrosomal head coverage, and length/width (L/W) and perimeter/area (P/A) ratios for spermatozoa in semen samples and after swim-up: all samples considered.
| Median | Mean | Max | Min | Range | 5% centiles | 95% centiles | |
|---|---|---|---|---|---|---|---|
| Spermatozoa in semen (central tendency of 72 samples) | |||||||
| Length (μm) | 4.39 | 4.37 | 5.00 | 3.66 | 1.33 | 3.85 | 4.88 |
| Width (μm) | 2.79* | 2.75 | 3.15 | 2.09 | 1.06 | 2.39 | 3.08 |
| Area (μm2) | 9.77* | 9.86 | 12.05 | 7.09 | 4.97 | 8.46 | 11.20 |
| Perimeter (μm) | 12.52* | 12.48 | 13.80 | 11.06 | 2.74 | 11.27 | 13.49 |
| Acrosome (%) | 47.50 | 47.60 | 59.40 | 38.00 | 21.40 | 39.40 | 56.10 |
| L/W | 1.57 | 1.59 | 2.10 | 1.34 | 0.76 | 1.40 | 1.88 |
| P/A | 1.28* | 1.27 | 1.61 | 0.82 | 0.79 | 1.18 | 1.40 |
| Spermatozoa after swim-up (central tendency of 72 samples) | |||||||
| Length (μm) | 4.42 | 4.40 | 5.03 | 3.78 | 1.26 | 3.96 | 486 |
| Width (μm) | 2.84 | 2.82 | 3.27 | 2.44 | 0.83 | 2.53 | 3.14 |
| Area (μm2) | 10.29 | 10.20 | 12.15 | 8.06 | 4.09 | 8.57 | 11.60 |
| Perimeter (μm) | 12.90 | 14.04 | 22.08 | 11.07 | 11.01 | 11.49 | 21.35 |
| Acrosome (%) | 48.20 | 48.20 | 57.20 | 35.40 | 21.80 | 3840 | 55.30 |
| L/W | 1.56 | 1.56 | 1.88 | 1.34 | 0.54 | 1.37 | 1.78 |
| P/A | 1.25 | 1.38 | 1.43 | 1.11 | 0.32 | 1.15 | 1.35 |
P < 0.05, compared with sources of spermatozoa after swim-up.
Figure 3.
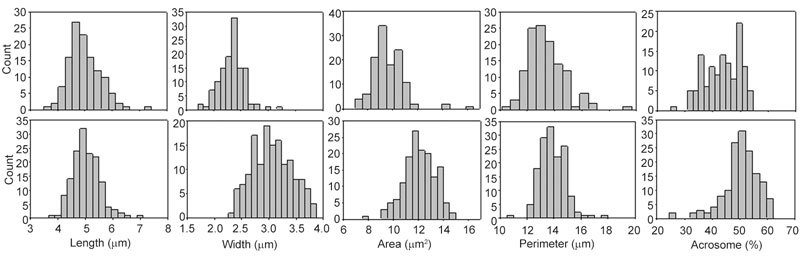
Histograms of the values of sperm head parameters for spermatozoa in semen from a man with narrower spermatozoa (n = 123 spermatozoa, upper panel) and a man with wider spermatozoa (n = 149 spermatozoa, lower panel).
There were no significant differences between head lengths of all the spermatozoa evaluated in semen and those after swim-up, but sperm head widths, areas, perimeters and acrosomal areas were significantly greater in the swim-up samples (P < 0.05), and L/W ratios and P/A ratios were significantly greater for spermatozoa in semen (P < 0.05) (Table 2). Although significantly different, the percentage differences were small, from 0.5% to 3.3%. Histograms of several head parameters of all the spermatozoa evaluated in semen (n = 7 942) and after swim-up (n = 4 812) were similar (data not shown) to the pooled data (Figure 4). The upper panel in Figure 4 displays the within-individual variation in values for each parameter for spermatozoa in semen (light grey) and after swim-up (dark grey). The lack of difference permitted pooling of data and the between-sample variation for pooled samples is given in Figure 4 middle panel.
Table 2. Distributions of all values of head length, width, area, perimeter, acrosomal head coverage, and length/width (L/W) and perimeter/area (P/A) ratios for spermatozoa in semen and after swim-up: all spermatozoa considered.
| Median | Mean | Max | Min | Range | 5% centiles | 95% centiles | |
|---|---|---|---|---|---|---|---|
| Spermatozoa in semen (central tendency of 7 942 cells) | |||||||
| Length (μm) | 4.38 | 4.42 | 8.21 | 2.74 | 5.47 | 3.58 | 5.38 |
| Width (μm) | 2.79* | 2.70 | 4.38 | 1.17 | 3.21 | 2.29 | 3.29 |
| Area (μm2) | 9.77* | 10.03 | 19.10 | 5.26 | 13.84 | 7.57 | 12.62 |
| Perimeter (μm) | 12.53* | 12.60 | 23.1 | 8.88 | 14.22 | 10.68 | 14.61 |
| Acrosome (%) | 46.7* | 45.90 | 74.30 | 12.00 | 62.30 | 29.90 | 59.30 |
| L/W | 1.57* | 1.64 | 6.39 | 0.97 | 5.32 | 1.25 | 2.12 |
| P/A | 1.28* | 1.26 | 2.22 | 0.01 | 2.21 | 1.09 | 1.45 |
| Spermatozoa after swim-up (central tendency of 4 812 cells) | |||||||
| Length (μm) | 4.39 | 4.42 | 6.67 | 3.15 | 3.52 | 3.71 | 5.21 |
| Width (μm) | 2.86 | 2.87 | 4.64 | 2.07 | 2.57 | 2.40 | 3.35 |
| Area (μm2) | 10.31 | 10.33 | 20.74 | 6.01 | 14.72 | 8.04 | 12.65 |
| Perimeter (μm) | 12.61 | 12.61 | 18.03 | 0.97 | 17.01 | 11.00 | 14.22 |
| Acrosome (%) | 48.30 | 47.50 | 66.50 | 14.50 | 52.00 | 33.40 | 59.10 |
| L/W | 1.53 | 1.54 | 3.05 | 1.06 | 2.00 | 1.26 | 1.95 |
| P/A | 1.22 | 1.22 | 1.79 | 0.09 | 1.71 | 1.10 | 1.40 |
P < 0.05, compared with sources of spermatozoa after swim-up.
Figure 4.
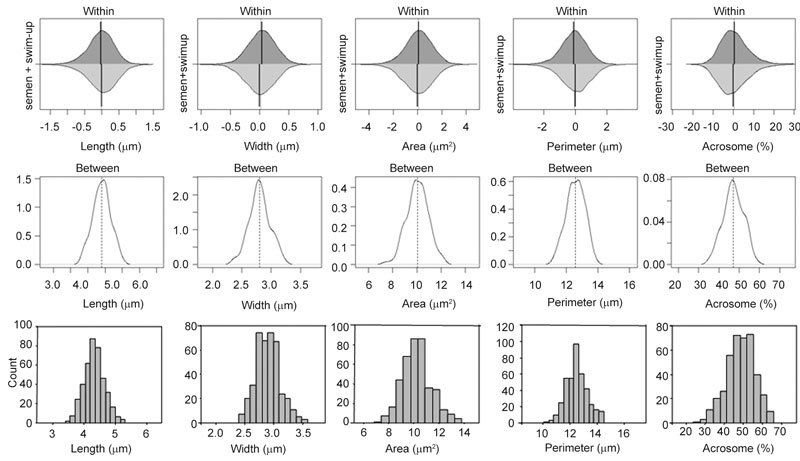
The upper panel shows within-individual variation from the median value (vertical line) for spermatozoa both in semen (light grey) and swim-up (dark grey) expressed as differences from the median. As no significant differences between sources of spermatozoa were observed, all data were pooled to show the inter-individual variability (middle panel) expressed in absolute measures (length, width and perimeter in microns, area in square microns and acrosome as percentage) on the same scale as that in the other graphs. The lower panel depicts frequency histograms of the morphometric data from spermatozoa selected as being 'normal' in semen or swim-up samples.
Morphometric characteristics of 'normal' spermatozoa
The number of spermatozoa considered to be 'normal' in this study (Figure 2D) was very low: 252 (3.1%) of correctly digitized spermatozoa in semen and 152 (2.3%) of swim-up cells. The morphometric parameters of such normal spermatozoa, whether observed in semen or in the swim-up preparations, were similar; only the perimeter was significantly higher in the normal spermatozoa from those in semen, but the difference, 0.2 μm, was small (1.8%). The data from all the normal cells are given in Table 3; they reveal similar median values to all the correctly digitized spermatozoa (Table 2).
Table 3. Distributions of values of head length, width, area, perimeter, acrosomal head coverage, and length/width (L/W) and perimeter/area (P/A) ratios for spermatozoa in semen and after swim-up selected as being 'normal' by Tygerberg criteria.
| Median | Mean | Max | Min | Range | 5% centiles | 95% centiles | |
|---|---|---|---|---|---|---|---|
| Spermatozoa in semen and after swim-up (central tendency of 404 cells) | |||||||
| Length (μm) | 4.33 | 4.33 | 5.32 | 3.40 | 1.92 | 3.82 | 4.88 |
| Width (μm) | 2.90 | 2.90 | 3.59 | 2.40 | 1.19 | 2.59 | 3.25 |
| Area (μm2) | 10.21 | 10.26 | 13.83 | 6.84 | 6.96 | 8.54 | 12.24 |
| Perimeter (μm) | 12.43 | 12.49 | 14.51 | 10.03 | 4.48 | 11.26 | 13.88 |
| Acrosome (%) | 49.00 | 48.76 | 65.86 | 23.88 | 41.98 | 35.84 | 60.15 |
| L/W | 1.49 | 1.50 | 1.93 | 1.19 | 0.73 | 1.32 | 1.67 |
| P/A | 1.22 | 1.22 | 1.54 | 1.03 | 0.51 | 1.11 | 1.35 |
Discussion
The WHO laboratory manual for the examination of human semen 3 suggests measuring sperm head size with an ocular micrometer in order to determine whether a questionable spermatozoon is normal, and provides ranges of acceptable values. Discerning normal spermatozoa is fraught with difficulties, despite the ability to focus through a preparation, as the assessment of 'oval', 'smooth', 'irregular' and 'asymmetric' is extremely subjective 1, 2. Our technicians' impression has been that semen samples from different men contain spermatozoa of different size. This could reflect the stresses affecting spermatozoa during smearing and air drying of the semen sample that are known to produce swelling of immature sperm heads 14, 15, apparent loss of cytoplasmic droplets 16 and cell shrinkage 17. The response of the cells to these stresses may be characteristic of each man, and spermatozoa with expanded post-acrosomal regions are indeed detected in human semen 28; if these are less mature spermatozoa, detecting them would be of value in diagnosing epididymal dysfunction.
Computer-aided methods are better able than technicians to distinguish normal from abnormal forms 2 and the objective measurements could be used to categorize them if precise parameters defining the normal state were available. CASMA methods are not without problems, albeit of a different nature. The optimal cell staining for a computer may not be that preferred by the human eye, pattern recognition and separation of the cell from background (particularly in neat semen) is problematical, and only one optical section of the head is examined. This means that many images are rejected so that the analysable and thus correctly digitized images may represent a biased population. In this study, a computer-aided technique was used on the analysable images to measure sperm head length, width, area, perimeter and length/width ratios, but between 50% and 70% of recorded images were not captured and analysed. The high rejection rate could be explained by the use of the Papanicolaou staining technique, recommended by the WHO manual for analysis by technicians 4 and not the DiffQuik stain, recommended to be used with the ISAS®. When the CASMA technique is used with this stain, the proportion of digitized and analysed cells is about 95% 29.
A wide range of values for these sperm head parameters was evident, both within samples and among men, with some men having spermatozoa differing considerably in, for example, head width. Differences in values both within samples and among individuals are also present in species with homomorphic spermatozoa such as deer 29 and stallions 30. Despite this variability among men, the mean values for the sperm head dimensions length (4.3 μm), width (2.9 μm), area (10.3 μm2) and perimeter (12.5 μm) are close to those reported by Maree et al. 11 for spermatozoa in normozoospermic semen smears stained with SpermBlue and analysed by another CASMA system (4.7 μm, 2.8 μm, 10.5 μm2 and 13.0 μm, respectively). The median length (4.3 μm), width (2.9 μm) and L/W ratio (1.5) are close to those given by WHO 3 for normal forms (4.1 μm, 2.8 μm and 1.5, respectively).
The admittedly small, but significant, differences found between spermatozoa in semen and spermatozoa after swim-up included greater sperm head widths (and associated areas and perimeters) and acrosomal area in the swim-up preparations and greater length/width and perimeter/area ratios in native semen. That there was no difference in head length between treatments may indicate that the changes observed reflect the well-known lateral 'explosion' artefacts caused by the smearing procedure 14, 15 on spermatozoa that would have already been subjected to osmotic stress during liquefaction and in the swim-up medium. In future, better-designed experiments on matched samples should be performed by the use of stains that are equally good for technicians and computerized assessment.
Selecting a subpopulation of spermatozoa with normal heads is difficult. The percentages of normal forms selected here was very low (2%–3% of the correctly digitized, analysable forms), reflecting the quality of the semen from the patients attending our clinic. There was no major difference in the morphometric parameters of 'normal' spermatozoa selected from the semen or swim-up samples. Dominguez et al. 31 examined 52 'normal' spermatozoa from 15 semen samples with a manual method and found a greater sperm head width after swim-up, as confirmed here. Their increase in W/L ratio (a decrease in L/W) and decrease in head length were not confirmed in this study and this may reflect the fact that the cells measured were those assessed to be live 31. The median values of the 'normal' spermatozoa fell within the intervals given by WHO 3 for length, width, acrosomal area and L/W ratio. The 95% confidence limits for the length and width of sperm heads selected as normal in this study were marginally higher or lower than those currently given in the current WHO edition of the semen analysis manual, which were based on another digitizing system.
Despite confirming technicians' impressions that spermatozoa may differ in size among men, the small changes in absolute and relative terms of differences in dimensions of seminal and swim-up spermatozoa, and the overlap in dimensions of normal spermatozoa with all those in semen and the swim-up fraction makes visual assessment by a technician difficult and use of CASMA mandatory. In a previous paper it was observed that small cells were better for successful prognosis in cases of intrauterine insemination and intra cytoplasmic sperm injection 32. So, not only the percentage of normal forms, even if established by a computer-aided system, but also the morphometric data must be used in future for estimation of the morphological quality of a semen sample 33. The present data should be implemented in the ISAS® system for the automatic evaluation and classification of normal cells.
Conflict of interest
The authors declare no conflicts of interest.
Acknowledgments
We thank Dr Francisco Javier Barón Lòpez, Bioestadística, Facultad de Medicina, Málaga, Spain for help with the statistical analysis.
References
- Menkveld R. Clinical significance of the low normal sperm morphology value as proposed in the fifth edition of the WHO Laboratory Manual for the Examination and Processing of Human Semen. Asian J Androl. 2010;12:47–58. doi: 10.1038/aja.2009.14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Auger J. Assessing human sperm morphology: top models, underdogs or biometrics. Asian J Androl. 2010;12:36–46. doi: 10.1038/aja.2009.8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- World Health Organization. WHO Laboratory Manual for the Examination and Processing of Human SemenGeneva: World Health Organization; 2010 . http://www.who.int/reproductivehealth/publications/infertility/Examination_ and_processing_of_human_semen.pdf
- World Health Organization. WHO Laboratory Manual for the Examination of Human Semen and Sperm-Cervical Mucus InteractionCambridge: Cambridge University Press; 1999
- World Health Organization. WHO Laboratory Manual for the Examination of Human Semen and Sperm-Cervical Mucus InteractionCambridge: Cambridge University Press; 1992
- Menkveld R, Stander FSH, Kotze T JvW, Kruger TF, van Zyl JA. The evaluation of morphological characteristics of human spermatozoa according to stricter criteria. Hum Reprod. 1990;5:586–92. doi: 10.1093/oxfordjournals.humrep.a137150. [DOI] [PubMed] [Google Scholar]
- Menkveld R, Franken DR, Kruger TF, Oehninger S, Hodgen GD. Sperm selection capacity of the human zona pellucida. Mol Reprod Dev. 1991;30:346–52. doi: 10.1002/mrd.1080300409. [DOI] [PubMed] [Google Scholar]
- Liu DY, Baker HWG. Morphology of spermatozoa bound to the zona pellucida of human oocytes that failed to fertilize in vitro. J Reprod Fertil. 1992;94:71–84. doi: 10.1530/jrf.0.0940071. [DOI] [PubMed] [Google Scholar]
- Kruger TF, Ackerman SB, Simmons KF, Swanson RJ, Brugo SS, et al. A quick, reliable staining technique for human sperm morphology. Arch Androl. 1987;18:275–7. doi: 10.3109/01485018708988493. [DOI] [PubMed] [Google Scholar]
- Meschede D, Keck C, Zander M, Cooper TG, Yeung CH, et al. Influence of three different preparation techniques on the results of human sperm morphology analysis. Int J Androl. 1993;16:362–9. doi: 10.1111/j.1365-2605.1993.tb01363.x. [DOI] [PubMed] [Google Scholar]
- Maree L, du Plessis SS, Menkveld R, van der Horst G. Morphometric dimensions of the human perm head depend on the staining method used. Human Reprod. 2010;25:1369–82. doi: 10.1093/humrep/deq075. [DOI] [PubMed] [Google Scholar]
- World Health Organization. WHO Laboratory Manual for the Examination of Human Semen and Semen-Cervical Mucus InteractionNew York: Cambridge University Press; 1987
- Garrett C, Baker HW. A new fully automated system for the morphometric analysis of human sperm heads. Fertil Steril. 1995;63:1306–17. [PubMed] [Google Scholar]
- Yeung CH, Pérez-Sánchez F, Soler C, Poser D, Kliesch S, et al. Maturation of human epididymal spermatozoa (from selected epididymides of prostatic carcinoma patients) with respect to their morphology and ability to undergo the acrosome reaction. Human Reprod Update. 1997;3:205–13. doi: 10.1093/humupd/3.3.205. [DOI] [PubMed] [Google Scholar]
- Soler C, Pérez-Sánchez F, Schulze H, Bergmann M, Oberpenning F, et al. Objective evaluation of the morphology of human epididymal sperm heads. Int J Androl. 2000;23:77–84. doi: 10.1046/j.1365-2605.2000.00211.x. [DOI] [PubMed] [Google Scholar]
- Cooper TG, Yeung CH, Fetic S, Sobhani A, Nieschlag E. Cytoplasmic droplets are normal structures of human spermatozoa but are not well preserved by routine procedures for assessing sperm morphology. Human Reprod. 2004;19:2283–8. doi: 10.1093/humrep/deh410. [DOI] [PubMed] [Google Scholar]
- Katz DF, Overstreet JW, Samuels SJ, Niswander PW, Bloom TD, et al. Morphometric analysis of spermatozoa in the assessment of human male fertility. J Androl. 1986;7:203–10. doi: 10.1002/j.1939-4640.1986.tb00913.x. [DOI] [PubMed] [Google Scholar]
- Jameel T. Sperm swim-up: a simple and effective technique of semen processing for intrauterine insemination. J Pak Med Assoc. 2008;58:71–4. [PubMed] [Google Scholar]
- Mahadevan M, Baker G.Assessment and preparation of semen for in vitro fertilizationIn: Wood C, Trounson A, editors. Clinical In Vitro Fertilization. Berlin: Springer-Verlag; 198483–97.
- Henkel RR, Schill WB. Sperm preparation for ART. Reprod Biol Endocrinol. 2003;1:108. doi: 10.1186/1477-7827-1-108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Soler C, de Monserrat JJ, Gutiérrez R, Núñez J, Núñez M, et al. Use of the Sperm-Class Analyser® for objective assessment of human sperm morphology. Optimisation of sperm preparation. Int J Androl. 2003;26:262–70. doi: 10.1046/j.1365-2605.2003.00422.x. [DOI] [PubMed] [Google Scholar]
- Schmassmann A, Mikuz G, Bartsch G, Rohr H. Spermiometrics: objective and reproducible methods for evaluating sperm morphology. Eur Urol. 1982;8:274–9. doi: 10.1159/000473535. [DOI] [PubMed] [Google Scholar]
- Davis RO, Gravance CG. Standardisation of specimen preparation, and sampling methods improves automated sperm head morphometry analysis. Fertil Steril. 1993;59:412–7. doi: 10.1016/s0015-0282(16)55686-6. [DOI] [PubMed] [Google Scholar]
- Lacquet FA, Kruger TF, Du Toit TC, Lombard CJ, Sanchez Sarmiento CA, et al. Slide preparation and staining procedures for reliable results using computerized morphology. Arch Androl. 1996;36:133–8. doi: 10.3109/01485019608987089. [DOI] [PubMed] [Google Scholar]
- Wang C, Leung A, Tsoi WL, Leung J, Ng V, et al. Computer-assisted assessment of human sperm morphology: usefulness in predicting fertilizing capacity of human spermatozoa. Fertil Steril. 1991;55:989–93. doi: 10.1016/s0015-0282(16)54311-8. [DOI] [PubMed] [Google Scholar]
- Coetzee K, Kruger TF, Lombard CJ. Predictive value of normal sperm morphology: a structured literature review. Hum Reprod Update. 1998;4:73–82. doi: 10.1093/humupd/4.1.73. [DOI] [PubMed] [Google Scholar]
- Bates D, Maechler M.Linear mixed-effects models using S4 classesR package version 0.999375-33. 2010 . http://CRAN.R-project.org/package=lme4
- Ludwig L, Frick J.Spermatology: An Atlas and ManualBerlin: Springer Verlag; 1990
- Soler C, Gadea B, Soler AJ, Fernández-Santos MR, Esteso MC, et al. Comparison of three different staining methods for the assessment of epididymal red deer sperm morphometry by computerized analysis with ISAS®. Theriogenology. 2005;64:1236–43. doi: 10.1016/j.theriogenology.2005.02.018. [DOI] [PubMed] [Google Scholar]
- Hidalgo M, Molina I, Dorado J, Soler C. Morphometric classification of Spanish thoroughbred stallion sperm heads. Anim Reprod Sci. 2008;103:374–8. doi: 10.1016/j.anireprosci.2007.06.001. [DOI] [PubMed] [Google Scholar]
- Domínguez LA, Burgos MH, Fornés MW. Morphometrical comparison of human spermatozoa obtained from semen and swim-up methodology. Andrologia. 1999;31:23–6. [PubMed] [Google Scholar]
- Soler C, Gassner P, Nieschlag E, de Montseratt JJ, Gutiérrez R, et al. Use of the integrated semen analysis system (ISAS®) for morphometric analysis and its role in assisted reproduction technologies (in Spanish) Rev Int Androl. 2005;3:12–9. [Google Scholar]
- Davis RO, Bain DE, Siemers RJ, Thal DM, Andrew JB, et al. Accuracy and precision of the CellForm-Human automated sperm morphometry instrument. Fertil Steril. 1992;58:763–9. [PubMed] [Google Scholar]


