Abstract
This study was carried out to analyze the vertical transmission of Yq AZFc microdeletions from father to son in infertile Han Chinese families to investigate genetic factors and family background affecting fertility status. The peripheral blood of infertile males in 19 Han families was extracted and screened with modified multiplex polymerase chain reaction (PCR). Family trees were drawn according to fertility status and clinical characteristics of the subjects. The vertical transmission of Yq AZFc microdeletions was detected in six cases of 19 investigated families (31.6%, 6/19). Although both fathers and sons showed a similar type of Yq AZFc deletion, the fathers were fertile, whereas the sons were infertile and showed severe oligozoospermia. The vertical transmission of Yq AZFc microdeletion from fertile fathers to infertile sons over generations is not rare. This has different effects on fertility status in fathers and sons in Han Chinese families. Both genetic factors and family background affect spermatogenetic phenotypes.
Keywords: infertility, microdeletion, vertical transmission, Y chromosome
Introduction
The Y chromosome harbors substantial genetic diversity in the form of polymorphisms in genetic elements that differentially affect the expression of hundreds of X-linked and autosomal genes, including genes on the long arm of the Y chromosome (Yq) that are related to spermatogenesis. New findings provide a mechanism for adaptive phenotypic variation associated with the Y chromosome 1. At least three different azoospermia factor (AZF) regions (AZFa, b and c) exist on the Y chromosome. Microdeletions in these loci may result in azoospermia or severe oligozoospermia 2, 3. In general, men with deletions on Yq are infertile. Therefore, deletions are not transmitted to sons unless either in-vitro fertilization (IVF) or intracytoplasmic sperm injection (ICSI) is performed 4. Assisted reproductive technology may increase clinical mutation detection in male offspring. Feng et al. 5 showed that de novo Y chromosome microdeletions were present in one of 19 (5.3%) IVF offsprings and in three of 18 (16.7%) ICSI offsprings. However, a few rare instances of father-to-son transmission of Y-chromosome microdeletions have been reported 6. In 2002, Rolf et al. 7 described a case of natural transmission of a partial AZFb over three generations. Here, we describe six cases of transmission over two or three generations in which fathers and sons share an apparently identical microdeletion of the AZFc region. This evidence proves that one type of Yq deletion can cause varying phenotypes in different individuals. It is clinically significant that the presence of the Yq AZFc deletion is not an absolute marker for male infertility. The aim of the present study was to analyze the incidence rate of vertical transmission of the Yq AZFc deletion among infertile Han Chinese males who suffer from either nonobstructive azoospermia (NOA) or severe oligozoospermia. Moreover, a family-tracing investigation was carried out to determine the influence of genetic risks and family background on male reproductive function.
Materials and methods
Patients
Cases were constituted of all men suffering from either NOA or severe oligozoospermia. NOA, sometimes called primary spermatogenic failure, was defined as any spermatogenic alteration not caused by hypothalamic-pituitary diseases, as defined in the 2005 Guidelines on Male Infertility from the European Association 8. Typically, semen analysis indicates normal ejaculate volume and azoospermia after several rounds of centrifugation at 600 × g for 10 min and a thorough microscopical examination of the pellet (× 600 magnification). The upper fluid was then re-centrifuged (8 000 × g) for an additional 10 min and examined. All samples were stained and re-examined under the microscope, wherein spermatozoa could not be found. Moreover, no obstructive factors related to azoospermia could be confirmed through diagnostic management such as clinical history, physical examination, hormone check and ultrasonography. Severe oligozoospermia was defined as the semen density of < 1–5 × 106 mL−1 according to the World Health Organization (WHO) Laboratory Manual on Semen Analysis (1999 edition) 9. The subjects attended the Urology Clinic of Renji Hospital, Shanghai Jiao Tong University School of Medicine from January 2005 to March 2007. Altogether, 452 infertile men received molecular screening for Y-chromosome microdeletion, according to the protocol recommended by the European Molecular Genetics Quality Network (EMQN) and the European Academy of Andrology (EAA) 10. The protocol was modified as in our previous screening study 11. All subjects signed an informed consent, and the study was approved by the Renji Hospital Ethics Committee.
DNA isolation and multiplex PCR for Y-chromosome microdeletion analysis
Peripheral intravenous blood (3 mL) was sampled from each patient/donor and mixed with anti-coagulant in a 6:1 ratio. DNA was extracted from white blood cells using a Blood Genomic DNA Extraction kit (Shanghai Sangon Company, Shanghai, China) according to the manufacturer's instructions. Multiplex polymerase chain reaction (PCR) was performed according to the previous protocol recommended by EAA and EMQN 11. Both a female control sample and a blank (water) control were included in each multiplex reaction to detect reagent contamination. In addition, a normal male control was employed to detect the sensitivity and specificity of the experiment. Both the SRY gene located on the short arm of the Y chromosome and the ZFX/ZFY gene found on the short arms of both the X and the Y chromosomes served as internal controls for PCR.
Multiplex PCR primers sY14 (SRY), ZFX/ZFY, sY84, sY86, sY127, sY134, sY254 and sY255 were described in our previous report 11. Two multiplex reactions (A and B) were designed for all DNA samples. Both multiplex PCR reactions contained AZFa, b and c loci, along with internal control fragments. In multiplex A, primers ZFY, SRY, sY254, sY86 and sY127 were used; primers of ZFY, SRY, sY284, sY134 and sY255 were used in multiplex B.
The PCR reagents (Qiagen Multiplex PCR Kit, Qiagen Corp., Köln, Germany) used for 100 × 50 μL multiplex PCR reactions were 2 × Qiagen Multiplex PCR Master Mix (providing a final concentration of 3 mmol L-1 MgCl2, 3 × 0.85 mL); 5 × Q-Solution (1 × 2.0 mL); and distilled water (2 × 1.7 mL). Amplification conditions were as follows: an initial activation step of 30 min at 95°C, 35 cycles of 30 s denaturation (95°C), 30 s annealing (59°C), 30 s elongation (72°C) and a final elongation step of 10 min at 72°C. PCR reaction products were separated by electrophoresis for 40 min at 98 V on a 3% agarose gel containing ethidium bromide.
Analyses of subjects' clinical characteristics
The clinical characteristics of the patients were collected and analyzed, including information about male family members. Data included ages of both the husband and the wife, medical history (marriage and childbearing history), testicular volume, results of semen analysis and Yq AZF microdeletion type.
Results
Y-chromosome microdeletion screening and analysis of vertical transmission from father to son
Of 452 patients, 44 were found to carry the AZF microdeletion. A family-tracing investigation was conducted in 19 cases with Yq AZFc microdeletion in our trial and six families (31.6%, 6/19) were found to have undergone vertical transmission of Yq AZFc microdeletion (Table 1). The six family trees and results of modified PCR for AZF microdeletion screening are shown in Figures 1, 2 3, 4, 5, 6, 7.
Table 1. Deletion types and clinical characteristics of six families.
| Husband's age (years) | Wife's age (years) | Semen concentration (× 106 mL-1) | Marriage and childbearing history | Deletion type | Testicular volume (mL) | ||
|---|---|---|---|---|---|---|---|
| Left | Right | ||||||
| Family 1 | |||||||
| Proband | 34 | 31 | 0.21 | 7 years, no children | AZFc | 10 | 8 |
| Brother | 27 | 26 | 0.65 | 3 years, no children | AZFc | 15 | 12 |
| Father | 68 | 65 | — | One daughter at 28 years old, one son at 34 years old and one son at 41 years old | AZFc | 15 | 15 |
| Family 2 | |||||||
| Proband | 29 | 28 | 0.13 | 3 years, no children | AZFc | 20 | 20 |
| Brother | 31 | 27 | — | One son at 27 years old | AZFc | 15 | 15 |
| Father | 55 | 53 | — | One son at 24 years old and one son at 26 years old | AZFc | 15 | 15 |
| Nephew | 4 | — | — | — | AZFc | — | — |
| Family 3 | |||||||
| Proband | 28 | 28 | 0.14 | 1 year, no children | AZFc | 10 | 15 |
| Father | 60 | 57 | — | Miscarriage at 30 years and one son at 32 years old | AZFc | 15 | 15 |
| Family 4 | |||||||
| Proband | 29 | 27 | 0.09 | 1 year, no children | AZFc | 20 | 20 |
| Father | 60 | 58 | 0.84 | One son at 31 years old | AZFc | 20 | 10 |
| Family 5 | |||||||
| Proband | 27 | 27 | 0.13 | 3 years, no children | AZFc | 15 | 15 |
| Brother | 40 | 40 | — | 14 years, no children | AZFc | 15 | 10 |
| Father | 68 | 66 | — | One son at 28 years old, one daughter at 30 years old, miscarriage at 33 years and one son at 41 years old | AZFc | 15 | 15 |
| Family 6 | |||||||
| Proband | 32 | 27 | 0.15 | 1.5 years, no children | AZFc | 15 | 15 |
| Father | 61 | 58 | 0.10 | One son at 29 years old | AZFc | 5 | 5 |
Figure 1.
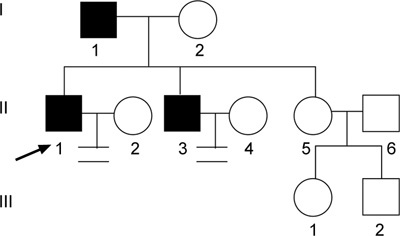
Pedigree of family 1. Proband 1 (↗), his father and brother were all found to have AZFc microdeletions that were transmitted between two generations.
Figure 2.
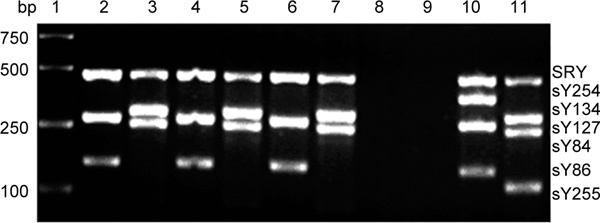
Multiplex PCR products analysis of STS on Yq in family 1. Lane 1, Marker (DL2000); Lane 2, PCR A products from proband 1; Lane 3, PCR B products from proband 1; Lane 4, PCR A products from the father of proband 1; Lane 5, PCR B products from the father of proband 1; Lane 6, PCR A products from the elder brother of proband 1; Lane 7, PCR B products from the elder brother of proband 1; Lane 8, Control in PCR A; Lane 9, Control in PCR B; Lane 10, PCR A products from a sperm donor; Lane 11, PCR B products from a sperm donor. No sY254 STS products are seen in lanes 2, 4 and 6, and no sY255 STS products are observed in lanes 3, 5 and 7, which indicates AZFc deletion.
Figure 3.
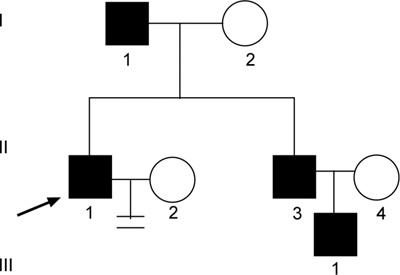
Pedigree of family 2. Proband 2 (↗), his father, elder brother and nephew were all found to have an AZFc microdeletion that was transmitted across three generations.
Figure 4.
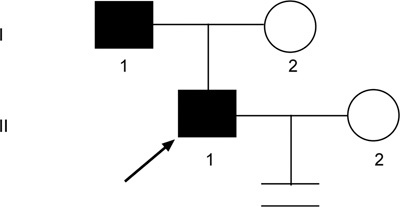
Pedigree of family 3. Proband 3 (↗) and his father were found to have an AZFc microdeletion, which was transmitted across two generations.
Figure 5.
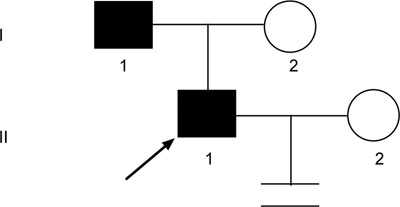
Pedigree of family 4. Proband 4 (↗) and his father were found to have an AZFc microdeletion that was transmitted across two generations.
Figure 6.
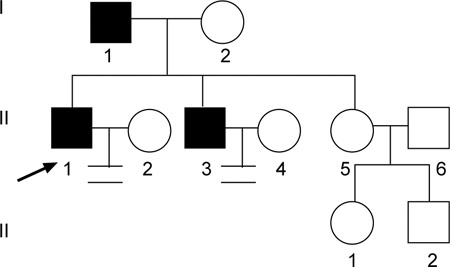
Pedigree of family 5. Proband 5 (↗), his father and brother were all found to have an AZFc microdeletion that was transmitted across two generations.
Figure 7.
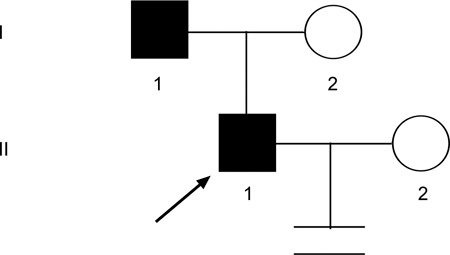
Pedigree of family 6. Proband 6 (↗) and his father were found to have an AZFc microdeletion that was transmitted across two generations.
Analysis of clinical characteristics
The clinical characteristics of patients and male family members were taken in six families found to have vertical transmission of a Yq AZFc microdeletion (Table 1). In family 1, proband 1, his father and his elder brother were found to have the AZFc deletion. Proband 1 had been married for over 3 years, but produced no offspring. However, the elder sister of proband 1 had conceived two daughters aged 15 and 7 years old, respectively. Figure 1 shows the family tree and Figure 2 shows PCR products from the tested members of family 1.
Proband 2 (from family 2) suffered from infertility despite normal karyotype and hormone levels. His semen analysis showed severe oligozoospermia. Microdeletion screening by sequence-tagged site (STS)-based PCR revealed the Yq AZFc deletion. The proband's brother had fathered a 4-year-old son, but was known to have severe oligozoospermia. The proband's father, elder brother and nephew were all found to carry AZFc deletions. The family tree is shown in Figure 3.
In family 3, proband 3, who showed severe oligozoospermia and had been married for 1 year without any child. The subject's mother experienced a miscarriage when her husband, the patient's father, was 30 years of age. Two years later, the couple had a child. The family tree is shown in Figure 4.
Both proband 4 and his father (from family 4) exhibited severe oligozoospermia. Although the father produced a child (proband 4) when he was 31 years old, his son had not conceived a child after 1 year of marriage. This family tree is shown in Figure 5.
In family 5, 27-year-old proband 5 exhibits severe oligozoospermia and has been infertile for 3 years after marriage. His elder brother, whose semen quality is unknown, has been unable to have a child for 14 years. Proband 5 also has an elder sister. Their father produced his last child at the age of 41 years. The family tree is shown in Figure 6.
In family 6, both proband 6 and his father have severe oligozoospermia. The latter was 29 years old when he fathered this son, whereas proband 6 has been married for one and a half years without any progeny. Figure 7 shows this family tree.
Discussion
The Y chromosome evolved from a pair of autosomes, accumulating male-specific genes including SRY and other spermatogenesis-related genes. The structure of the Y chromosome is distinct from that of autosomes and consists of multiple gene copies, DNA sequences and haplotype polymorphisms. Many gene rearrangements have occurred during its evolution. One subset of Y-chromosome microdeletions is a major cause of male infertility 3. This study showed vertical transmission from father to son over two or three generations in six Han Chinese families, with 19 cases exhibiting the AZFc deletion. Only rare cases of this deletion have been reported in other countries 6, 7, 12. In our previous study of 12 Han Chinese families 13, infertile sons had inherited the Yq AZFc microdeletion from their fathers. Semen analyses from families chosen for their father-to-son vertical transmission relationships showed that two probands were severe oligozoospermic, eight probands were azoospermic and two others were severe oligozoospermic with de novo AZFc deletions. We speculated that the vertical transmission from fertile father to infertile son may not be rare in Han Chinese if the proband with the AZFc deletion is severe oligozoospermic. In this study, we systematically analyzed a total of 19 cases, including 12 families from our previous investigation, all with Yq AZF deletion. We found that vertical transmission of AZF deletion was not accidental. Of the 19 Han Chinese families screened for AZF microdeletions, six (31.6%, 6/19) had vertically transmitted the microdeletion from father to son over two or three generations. Interestingly, the semen analysis of six probands with the vertically transmitted Yq AZFc deletion showed severe oligozoospermia. Of our 19 subjects, eight cases exhibited severe oligozoospermia, whereas six probands (75%, 6/8) inherited the Yq AZFc deletion from their fathers.
The fathers from families 1, 2, 3 and 5 in our study produced two or more children. In families 1 and 5, each father's latest childbearing age was 41 years. As both fathers refused to provide semen samples, hence no data were available regarding whether their sperm concentration or motility declined gradually with age. However, in families 1 and 5, the fathers did sire children while between the ages of 28 and 41 years. We speculated that ageing did not significantly affect their sperm quality. Semen analysis showed that the fathers in both families 4 and 6 experienced severe oligozoospermia. The father in family 6 had been infertile for 5 years after marriage and then fathered a son at 29 years old. The father in family 4, who produced a son after being married for 1 year, had been taking contraceptive measures until our visit. Semen analysis proved that he suffered from severe oligozoospermia. All the fathers and sons in these six cases inherited the same Yq AZFc microdeletion. Y microdeletions are specific to spermatogenic failure, that is, no deletions have been found in sperm donors. Although fertility can be compatible with Y deletions, this study revealed that natural fertilization may occur even with relatively low sperm counts, depending on the female partner's fertility status. For this reason, it is more appropriate to consider Y deletions a cause of oligo- or azoospermia rather than a source of infertility, specifically.
The set of PCR primers used in our multiplex PCR reactions was recommended by EAA/EMQN guidelines as the best choice for diagnosing the microdeletion of the AZFa, AZFb and AZFc region, namely sY14 (SRY), ZFX/ZFY, sY84, sY86, sY127, sY134, sY254 and sY255. This primer set is sufficient for routine diagnostic use, enabling the detection of almost all clinically relevant deletions and of over 95% of the deletions in the three AZF regions reported in the literature. Application of this set of primers by all laboratories is strongly recommended, as it requires minimal optimization and allows a comparison of laboratory performance and inter-laboratory variability 10. The EAA/EMQN guidelines should be followed for routine molecular diagnosis of Y-chromosome microdeletions. To clarify genotype/phenotype correlations, it will be necessary to screen more STS markers of Yq through DNA microarray experiments to discover additional mutation in the Y or other chromosomes. It is possible that different Yq deletions exist in different families and in different members of the same family.
The gene families in the AZFc region of the Y chromosome are functionally important for human spermatogenesis. Deletions of the AZFc region (b2/b4) are associated with variable clinical and histological phenotypic expression. In general, AZFc deletions are compatible with residual spermatogenesis and can be found in men with azoospermia or severe oligozoospermia 10. Yq AZFc microdeletion affects spermatogenesis and gives rise to infertility, which has been proven by our previous studies in Han Chinese as well as various investigations in other ethnic populations 11, 14, 15.
Yq AZFc microdeletion is a key genetic risk for poor sperm production. However, this same microdeletion may lead to different impacts on spermatogenesis in fathers and in sons. New findings on partial AZFc deletion and the Y haplogroup showed that, although Yq AZFc microdeletion does affect male sperm production and lead to spermatogenic failure in some people, not all men with this microdeletion are infertile 16, 17. This was also proven by our previous study in the Han Chinese 18, 19. We have shown that the partial AZFc deletion (gr/gr deletion) is not associated with an increased risk of sperm production or spermatogenic failure in Han Chinese. In fact, in our survey of 886 East Asians from eight ethnic groups, the gr/gr deletion appeared frequently (about 8%). Furthermore, the DAZ1/DAZ2 deletion has been detected as the primary subtype of the gr/gr deletion in East Asians, although this doublet has been considered crucial for normal spermatogenesis in Europeans.
Failure of spermatogenesis may be related to either genetic background or environment and is actually controlled by a network of genes that may be located on the Y chromosome, autosomes or even on the X-chromosome. AZFc microdeletion is not a unique marker that predicts infertility in male offspring. In the present study, we found that subjects with vertical transmission of the AZFc deletion may present special characteristics in the clinical phenotype, including smaller or normal testicular volume, as well as normal or abnormal sex hormone levels. Male fertility is reliant not only on genes regulating the male germ line but also on genes in the networks responsible for developing the male gonads 20, 21. All these phenotypes are related to environmental and/or genetic backgrounds. When beginning screening for causes underlying idiopathic male infertility, we should consult with their family members and investigate their family and ethnic backgrounds to judge their genetic risks.
In summary, Yq AZF microdeletion is a critical risk factor for primary spermatogenic failure not only in European and North American populations but also in Han Chinese people. However, vertical transmission of the Yq AZFc microdeletion from fertile fathers to infertile sons over two or three generations is not rare and has different effects on the fertility status of fathers and of sons in Han Chinese families. Further studies of a greater variety of STS markers on Yq using fluorescence in situ hybridization or microarray analyses are needed to further the understanding of the effects of Yq microdeletion and ultimately of the molecular and genetic mechanisms leading to spermatogenic failure.
Acknowledgments
This article was supported by the Project of 'Basic Research and Application on Male Reproduction' (Science and Technology Commission of Shanghai Municipality NO. 08410701700, China). We appreciate Drs Jin-Yi Jiang and Yi-Fang Wang for their critical reading and revisions of our manuscript.
References
- Lemos B, Araripe LO, Hartl DL. Polymorphic Y chromosomes harbor cryptic variation with manifold functional consequences. Science. 2008;319:91–3. doi: 10.1126/science.1148861. [DOI] [PubMed] [Google Scholar]
- Ravel C, Chantot-Bastaraud S, El Houate B, Rouba H, Legendre M, et al. Y-chromosome AZFc structural architecture and relationship to male fertility Fertil Steril 2009921924–33.Epub 2008 Nov 6. [DOI] [PubMed] [Google Scholar]
- Li Z, Haines CJ, Han Y. 'Micro-deletions' of the human Y chromosome and their relationship with male infertility. J Genet Genomics. 2008;35:193–9. doi: 10.1016/S1673-8527(08)60027-2. [DOI] [PubMed] [Google Scholar]
- Minor A, Wong EC, Harmer K, Ma S. Molecular and cytogenetic investigation of Y chromosome deletions over three generations facilitated by intracytoplasmic sperm injection. Prenat Diagn. 2007;27:743–7. doi: 10.1002/pd.1772. [DOI] [PubMed] [Google Scholar]
- Feng C, Wang LQ, Dong MY, Huang HF. Assisted reproductive technology may increase clinical mutation detection in male offspring. Fertil Steril. 2008;90:92–6. doi: 10.1016/j.fertnstert.2007.06.004. [DOI] [PubMed] [Google Scholar]
- Gatta V, Stuppia L, Calabrese G, Morizio E, Guanciali-Franchi P, Palka G. A new case of Yq microdeletion transmitted from a normal father to two infertile sons. J Med Genet. 2002;E27.;39 doi: 10.1136/jmg.39.6.e27. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rolf C, Gromoll J, Simoni M, Nieschlag E. Natural transmission of a partial AZFb deletion of the Y chromosome over three generations: case report. Hum Reprod. 2002;17:2267–71. doi: 10.1093/humrep/17.9.2267. [DOI] [PubMed] [Google Scholar]
- Dohle GR, Colpi GM, Hargreave TB, Papp GK, Jungwirth A, et al. EAU Working Group on Male Infertility. EAU guidelines on male infertility. Eur Urol. 2005;48(5):703–11. doi: 10.1016/j.eururo.2005.06.002. [DOI] [PubMed] [Google Scholar]
- WHO Laboratory Manual for the Examination of Human Semen and Sperm-Cervical Mucus Interaction, 4th Edition, New York: Cambridge University Press, 1999
- Simoni M, Bakker E, Krausz C. EAA/EMQN best practice guidelines for molecular diagnosis of Y-chromosome microdeletions. State of the art 2004. Int J Androl. 2004;27:240–9. doi: 10.1111/j.1365-2605.2004.00495.x. [DOI] [PubMed] [Google Scholar]
- Zhu XB, Guo AL, Cao XR, Liu Y, Sun XX, et al. Screening for Y chromosomal microdeletions in AZF region with modified multiplex PCR. Zhonghua Nan Ke Xue. 2006;12:199–201. [PubMed] [Google Scholar]
- Samli H, Murat Samli M, Solak M. Natural transmission of AZFb Y-chromosomal microdeletion from father to his three sons. Arch Androl. 2006;52:423–6. doi: 10.1080/01485010600822655. [DOI] [PubMed] [Google Scholar]
- Zhu XB, Li Z, Guo AL, Cao XR, Liu Y, Gong DM, et al. Study on the vertical transmission of Y chromosome microdeletions from father to son. Zhonghua Yi Xue Yi Chuan Xue Za Zhi. 2007;24:203–5. [PubMed] [Google Scholar]
- El Awady MK, El Shater SF, Ragaa E, Atef K, Shaheen IM, et al. Molecular study on Y chromosome microdeletions in Egyptian males with idiopathic infertility. Asian J Androl. 2004;6:53–7. [PubMed] [Google Scholar]
- Babu SR, Swarna M, Padmavathi P, Reddy PP. PCR analysis of Yq microdeletions in infertile males, a study from South India. Asian J Androl. 2002;4:265–8. [PubMed] [Google Scholar]
- Ferlin A, Tessari A, Ganz F, Marchina E, Barlati S, et al. Association of partial AZFc region deletions with spermatogenic impairment and male infertility. J Med Genet. 2005;42:209–13. doi: 10.1136/jmg.2004.025833. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yang Y, Ma M, Li L, Zhang W, Chen P, et al. Y chromosome haplogroups may confer susceptibility to partial AZFc deletions and deletion effect on spermatogenesis impairment. Hum Reprod. 2008;23:2167–72. doi: 10.1093/humrep/den229. [DOI] [PubMed] [Google Scholar]
- Zhang F, Li Z, Wen B, Jiang J, Shao M, et al. A frequent partial AZFc deletion does not render an increased risk of spermatogenic impairment in East Asians Ann Hum Genet 200670(Pt 3): 304–13. [DOI] [PubMed] [Google Scholar]
- Zhang F, Lu C, Li Z, Xie P, Xia Y, et al. Partial deletions are associated with an increased risk of complete deletion in AZFc: a new insight into the role of partial AZFc deletions in male infertility. J Med Genet. 2007;44:437–44. doi: 10.1136/jmg.2007.049056. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reyes-Vallejo L, Lazarou S, Morgentaler A. Y chromosome microdeletions and male infertility: who should be tested and why. BJU Int. 2006;97:441–3. doi: 10.1111/j.1464-410X.2006.06057.x. [DOI] [PubMed] [Google Scholar]
- Vogt PH. Molecular genetic of human male infertility: from genes to new therapeutic perspectives. Curr Pharm Des. 2004;10:471–500. doi: 10.2174/1381612043453261. [DOI] [PubMed] [Google Scholar]


