Abstract
Successful spermatogonial transplantation requires depletion of the host germ cells to allow efficient colonization of the donor spermatogonial stem cells. Although a sterilizing drug, busulfan (Myleran), is commonly used for preparing a recipient mouse before transplantation, the optimal dose of this drug has not yet been defined. The present study investigated the effects of different doses of busulfan (10–50 mg per kg body weight) on survival rate, testicular mass and histomorphology, and on the haploid spermatids and spermatozoa of male BALB/c mice. The results suggest that a dosage of 30 mg kg−1 is optimal for the ablative treatment with busulfan used to prepare the recipient mice. This dose results in an adequate depletion of the host germ cells for colonization of donor-derived spermatogonial stem cells and causes the lowest death rate of the animals.
Keywords: busulfan, germ cells, infertility, mice, myleran, spermatogonial transplantation, testis
Introduction
Spermatogenesis is a complex process by which stem cells in the seminiferous tubules of the testis develop into male gametes (spermatozoa). Studies of spermatogenesis were long hampered because of a lack of powerful in vitro and in vivo assay systems until an exciting new technique, a method for the transplantation of germ cells from one animal to another, was established 1. It was shown that germ cells from hamsters, rabbits, dogs, sheep or bulls can be heterotransplanted into the mouse, which then survive and result in long-lasting spermatogenesis in the host testes, proliferate and differentiate into spermatozoa or elongated spermatids 2, 3, 4, 5. Germ-cell transplantation has also been successfully performed in cat, goat, pig, monkey and human testes 6, 7, 8, 9. During the past decade, spermatogonial transplantation has become a powerful tool for investigators to gain insight into the biology of male germline stem cells and their niche, to restore fertility in infertile animals and to produce transgenic animals with genetically modified germline cells. In addition, spermatogonial transplantation is a potentially powerful technique for assisted reproductive strategies 10, 11.
It is well documented that efficient colonization of seminiferous tubules by the donor cells is required for the transplantation to achieve its potential. Shinohara et al. 12 suggested that colonization depends on how well the transplanted cells compete with the endogenous spermatogonia for available stem cell niches, which is determined by the relative size of the donor and host spermatogonia populations and the access of the transplanted cells to the basal lamina of the seminiferous tubules. The efficiency of donor engraftment is increased by ablative treatments that remove endogenous stem cells and increase niche accessibility 12.
Germ-cell-deficient mutant W mice (a transgenic mouse strain with a mutated c-kit receptor tyrosine kinase) are sterile and serve as a valuable recipient model, but commercially available W animals are very expensive and complex breeding strategies are required to generate homozygous mutants 13. Thus, a more commonly used method involves the depletion of the germ cells of the recipient animal before transplantation to help the donor spermatogonial stem cells to get attached to the basal lamina of the seminiferous tubules.
Several techniques have been developed to reduce the number of germ cells in the testes, including irradiation 6, 14, experimental cryptorchidism 15, heat treatment 16, cold ischemia 17, gonadotropin-releasing hormone (GnRH) antagonist treatment 9 and sterilizing drug administration 1, 2, 3, 13. Among the sterilizing drugs, busulfan is the most commonly used drug. Busulfan is a DNA alkylating agent that can deplete germ cells 18 and disrupt the junctions between Sertoli cells, thus promoting the immigration of transplanted spermatogonia into the basal lamina 1.
Busulfan can be easily administered by a single intraperitoneal (i.p.) injection. The required sterilizing dose reported in the literature varies widely between different species. For example, doses of 40–100 mg per kg body weight were used in pigs 19 and 4–12 mg kg−1 doses were used in coyotes 20. Recipient rats were usually prepared by administering two injections of 10 or 15 mg kg−1 of busulfan 21, 22, whereas 40–44 mg kg−1 of busulfan was the most commonly used dose for mice 1, 3, 12, 23. To date, there has not yet been a systematic study, in particular there is no quantitative data, in which the optimal dose for experimental animals such as the mouse has been determined.
To address this question, the present study examined the dose-dependent response of BALB/c mice to busulfan and provides detailed quantitative (rather than descriptive) data for the determination of an optimal dose of busulfan to maximally deplete germ cells in the seminiferous tubules while maintaining the lowest possible death rate.
Materials and methods
Animals
The BALB/c mice were obtained from Wuhan University Laboratory Animal Center (Wuhan, China), and the protocol for animal use in the present investigation was approved by the university's institutional animal care and use committee. The mice were maintained in a germ-free isolation facility at 22 ± 1°C with 70% humidity under a light:dark cycle of 10 h:14 h. The food and water were autoclaved and were available ad libitum.
Assays for dose-related responses of BALB/c mice to busulfan
Busulfan administration
A total of 208 male mice aged 4–6 weeks were randomly allocated to seven groups. The control animals received a single i.p. injection of vehicle (0.2 mL) (a 1:1 mixture of dimethyl sulfoxide and distilled water). The treatment groups were given different doses of busulfan (B-2635; Sigma-Aldrich, St. Louis, MO, USA). The mice either received a single i.p. injection of 10, 20, 30, 40 or 50 mg kg−1 busulfan, or two injections of 10 mg kg−1 at an interval of 7 days. At 4, 8 and 12 weeks after the (last) injection, the mice were killed for analyses. The survival rates of the animals were calculated at the end of the fourth week after treatment.
Testicular mass and histomorphology
The animals were fixed by perfusion with 4% (w/v) paraformaldehyde in 0.1 mol L−1 phosphate buffer after anesthetization. Testes were collected, weighed after removing the epididymis and surrounding fat and then post-fixed in the same fixative overnight. The testes from each mouse were embedded in paraffin and 4-μm thick sections were cut perpendicular to the long axis of the testes. Three non-adjacent sections were selected from each testis at an interval of no less than 120 μm and stained with hematoxylin-eosin. A total of 12 testes from six mice in each group were analyzed for each time point. The sections were examined blindly by three independent observers, and 20 seminiferous tubules were scored per section. The seminiferous epithelia of the tubules were classified as: no spermatogenesis (no evidence of regeneration of a seminiferous epithelium), partial spermatogenesis (showing spermatogonial proliferation and spermatocytes, but no later stages of spermatogenesis) or full spermatogenesis (showing a complete seminiferous epithelium with spermatids and spermatozoa) 24.
Flow cytometry analysis for spermatids and spermatozoa
The animals were killed by cervical dislocation after administering anesthesia. Single testicular cell suspensions were prepared according to the method described by Brinster and Avarbock 23. The cells were fixed in precooled 70% alcohol for 2 h, resuspended and washed twice in staining buffer (0.01 mol L−1 PBS, pH 7.2), incubated in 1 g L−1 RNase at 37°C for 30 min and stained in 100 mg L−1 propidium iodide (PI) at 4°C for 1 h. The samples were then diluted to 1 × 109 cells per L and analyzed with a flow cytometer (EPICS ALTRA II, Beckman Coulter, Los Angeles, CA, USA) at a wavelength of 488 nm. The red PI fluorescence was detected at 610 nm. Six testicular samples from three mice in each group were analyzed for each time point with a minimum of 1 × 104 events for each sample.
Statistical analysis
The results were analyzed by performing ANOVA (analysis of variance) and χ2-tests (SPSS, version 11.5; Chicago, IL, USA). A P < 0.05 was considered statistically significant.
Results
Survival rate
The survival rates of the mice 4 weeks after the administration of different doses of busulfan are shown in Figure 1. Generally, if the mice did not die in the first week after the injection, they survived throughout the whole experimental period. The doses of 10–30 mg kg−1, including the double injections of 10 mg kg−1, had no significant effects on survival, although the 30 mg kg−1 group had a slightly decreased survival rate (92%; P > 0.05, compared with the control animals). However, injection of busulfan at doses higher than 30 mg kg−1 led to a clearly decreased survival rate, and dose of 50 mg kg−1 was 100% lethal. Owing to the unacceptable high death rate, the mice treated with 40 and 50 mg kg−1 busulfan were omitted from further studies.
Figure 1.
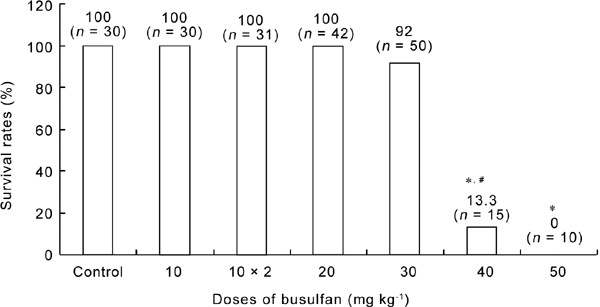
The survival rates of BALB/c mice 4weeks after the administration of busulfan. “10 × 2” represents two injections of 10 mg kg−1 busulfan. *P < 0.05, compared with control group; #P < 0.05, compared with the previous adjacent dose.
Body weight
The body weights of both the control and the busulfan-treated mice slightly and steadily increased with time during the 12 weeks after injection. In general, the doses of 10–30 mg kg−1 busulfan had no obvious effects on the time-dependent body weight increase (Table 1).
Table 1. Changes in body weight (g) of BALB/c mice with time after injection of different doses of busulfan (mean ± SD).
| Group | 0 week | 4 weeks | 8 weeks | 12 weeks |
|---|---|---|---|---|
| Control | 20.0 ± 1.9 | 24.5 ± 2.3* | 25.3 ± 2.3 | 27.6 ± 2.4 |
| 10 mg kg−1 | 21.0 ± 1.3 | 24.5 ± 2.1* | 26.0 ± 0.7 | 26.2 ± 0.4 |
| 10 mg kg−1 × 2 | 21.3 ± 2.1 | 26.5 ± 2.3* | 27.6 ± 0.5 | 28.7 ± 2.2 |
| 20 mg kg−1 | 21.4 ± 1.2 | 24.8 ± 2.0* | 25.3 ± 1.9 | 26.3 ± 2.7 |
| 30 mg kg−1 | 20.9 ± 2.5 | 22.6 ± 2.6* | 24.3 ± 1.1 | 24.4 ± 0.5 |
P < 0.05, compared with the previous adjacent time-point. There are no statistical differences (P > 0.05) between adjacent doses. “10 mg kg−1 × 2” represents two injections of 10 mg kg−1 busulfan.
Mass of testes
Testicular mass did not change significantly in the control animals from 4 to 12 weeks after injection (Figure 2). However, a sharp decrease in the testicular mass (60.5%–29.2% of the control value) appeared in all animals treated with different doses of busulfan (including double injections of 10 mg kg−1) at the end of week 4. The decreased testicular mass gradually restored to or near the control level in the groups treated with 10–20 mg kg−1 busulfan during the subsequent 8 weeks (8–12 weeks after injection), but it did not reverse in the group of animals treated with 30 mg kg−1 busulfan.
Figure 2.
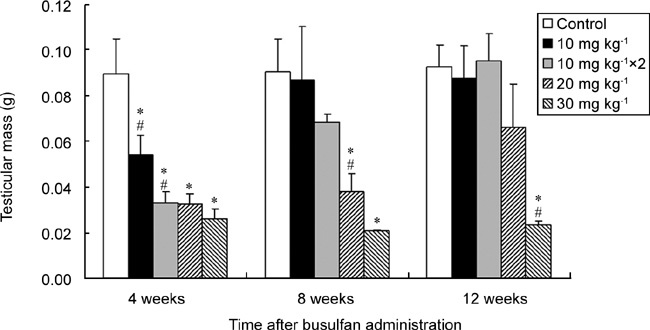
Changes in testicular mass of BALB/c mice over time after injection of different doses of busulfan. “10 mg kg−1 × 2” represents two injections of 10 mg kg−1 busulfan. *P < 0.05, compared with the control group; #P < 0.05, compared with the previous adjacent dose; n = 6.
Histological changes of testes
In the control group at week 4 after injection, most of the seminiferous tubules showed a thick wall with a very limited lumen (Figure 3A). The wall consists of several layers of seminiferous epithelial cells, with the spermatogonia in the outer layer, the spermatocytes in the middle and the spermatozoa and spermatids protruding toward the lumen (Figure 3B), which we defined in the present study as the 'fully spermatogenic' seminiferous tubule. After administration of lower doses of busulfan (10 mg kg−1 or 10 mg kg−1 ×2), the walls of the majority of the seminiferous tubules became thinner at the end of week 4 because of the depletion of the spermatids and the spermatozoa in the innermost layer, a typical feature of 'partial spermatogenesis' (Figures 3C, D). In animals treated with higher doses of busulfan (20 or 30 mg kg−1), even thinner walls with only one layer of spermatogonia ('non-spermatogenic' epithelium) were found in most of the seminiferous tubules (Figures 3E, F).
Figure 3.
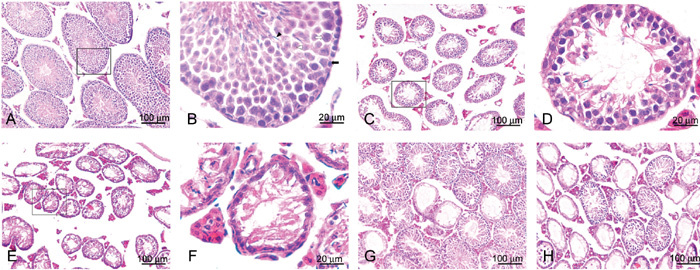
Representative micrographs of testes of BALB/c mice at different time points after the injection of different doses of busulfan. (A): Testis of control animals 4 weeks after injection. (B): An amplified local region of A, showing a typical 'fully spermatogenic' seminiferous tubule with spermatogonia (black arrow), spermatocytes (white arrow), spermatids (Δ) and spermatozoa (▴). (C): Testis of the animals 4 weeks after double injections of 10 mg kg−1 busulfan; (D): An amplified local region of C showing a typical 'partially spermatogenic' seminiferous tubule. (E): Testis of the animals 4 weeks after injection of 30 mg kg−1 busulfan. (F): An amplified local region of E showing a typical 'non-spermatogenic' seminiferous tubule. (G) and (H): Testes from the animals 12 weeks after the injection of 20 mg kg−1 and 30 mg kg−1 busulfan, respectively. Please note that there are numerous seminiferous tubules restored to full spermatogenesis in G, but only a few in H.
During the following 8 weeks (8–12 weeks after injection), most of the seminiferous tubules were restored to full spermatogenesis in the groups treated with 10–20 mg kg−1 busulfan, but in the group treated with 30 mg kg−1 busulfan, only a few of the non-spermatogenic seminiferous tubules reversed and became fully spermatogenic (Figures 3G, H). A quantitative analysis of the three types of seminiferous tubules in the animals treated with different doses of busulfan is shown in Table 2.
Table 2. A quantitative analysis of full-, part- and non-spermatogenic seminiferous tubules in the testes of BALB/c mice with time after injection of different doses of busulfan (mean ± SD).
| Group | 4 weeks (%) |
8 weeks (%) |
12 weeks (%) |
||||||
|---|---|---|---|---|---|---|---|---|---|
| Full- | Part- | Non- | Full- | Part- | Non- | Full- | Part- | Non- | |
| Control | 99.2 ± 0.8 | 0.6 ± 0.5 | 0.3 ± 0.5 | 98.9 ± 1.0 | 0.8 ± 0.8 | 0.3 ± 0.5 | 98.9 ± 1.0 | 0.3 ± 0.5 | 0.8 ± 0.8 |
| 10 mg kg−1 | 92.8 ± 2.7* | 6.1 ± 2.4* | 1.1 ± 0.5 | 97.5 ± 2.2 | 1.7 ± 1.7 | 0.8 ± 0.8 | 96.7 ± 0.8 | 3.3 ± 0.8 | 0.0 ± 0.0 |
| 10 mg kg−1 × 2 | 5.8 ± 2.4*, # | 67.9 ± 17.1*, # | 26.3 ± 19.5*, # | 96.7 ± 3.0 | 2.2 ± 2.1 | 1.1 ± 1.0 | 98.1 ± 1.3 | 1.7 ± 1.4 | 0.3±0.5 |
| 20 mg kg−1 | 0.0 ± 0.0*, # | 12.8 ± 1.7*, # | 87.2 ± 1.7*, # | 78.1 ± 10.4* | 9.4 ± 3.2* | 12.5 ± 7.4* | 92.9 ± 3.0* | 5.0 ± 2.4 | 2.1 ± 0.6* |
| 30 mg kg−1 | 0.0 ± 0.0* | 0.3 ± 0.5# | 99.7 ± 0.5*, # | 4.6 ± 3.0*, # | 9.6 ± 7.7* | 85.8 ± 10.6*, # | 7.1 ± 0.6*, # | 5.0 ± 1.2* | 87.9 ± 0.6*, # |
P < 0.05, compared with the control group
P < 0.05, compared with the previous adjacent dose. “10 mg kg−1 × 2” represents two injections of 10 mg kg−1 busulfan.
Flow cytometry
The seminiferous tubules contain three distinct cell populations, each having a different DNA content. The spermatogonial G2 cells and primary spermatocytes are tetraploid, the secondary spermatocytes and spermatogonial G1 cells are diploid and the spermatids and spermatozoa are haploid. In the control animals, the haploid cells (the spermatids and spermatozoa) consisted of more than half of the testicular cell population (Figure 4; Table 3). This finding is similar to the previously reported data of normal eugamic male BALB/c mice 25. At the end of week 4 after treatment, the percentage of these cells sharply declined in all busulfan-treated groups in a dose-dependent manner. During the following 8 weeks (8–12 weeks after injection), the haploid cell population was gradually restored to or near the normal level in the busulfan-treated animals, except for the group treated with 30 mg kg−1 busulfan, in which the haploid cell percentage (13.5% ± 2.6%) was still much lower than that in the control group by the end of week 12 (P < 0.05). These changes in the haploid cell population coincided with the results of the histomorphological examinations.
Figure 4.
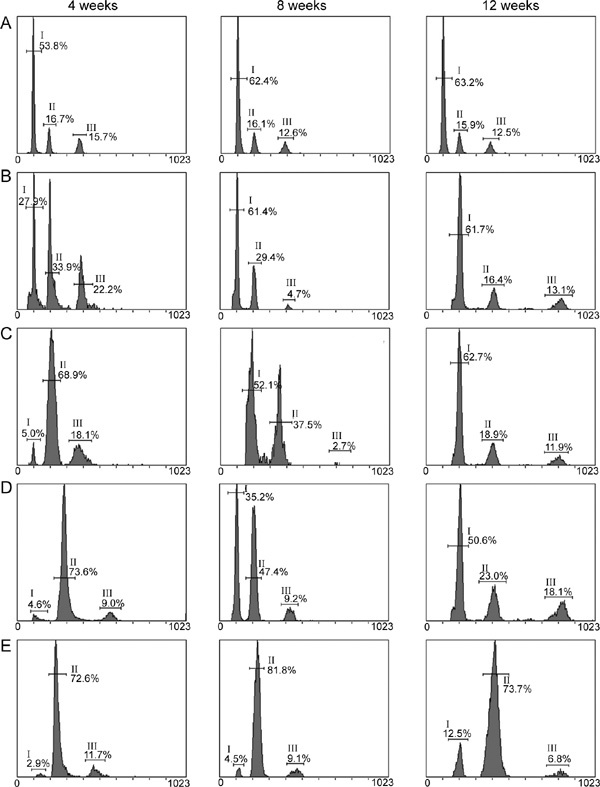
Representative flow cytometric plots for three types of testicular cells in BALB/c mice at different time points after administration of different doses of busulfan.The plots of the haploid, diploid and tetraploid cells are indicated by I, II, and III, respectively. (A): Control; (B): Group treated with 10 mg kg−1 busulfan; (C): Group treated with two injections of 10 mg kg−1 busulfan; (D): Group treated with 20 mg kg−1 busulfan; (E): Group treated with 30 mg kg−1 busulfan.
Table 3. Flow cytometric analysis for three types of testicular cells in BALB/c mice at different time-points after administration of different doses of busulfan (mean ± SD).
| Group | 4 weeks (%) |
8 weeks (%) |
12 weeks (%) |
||||||
|---|---|---|---|---|---|---|---|---|---|
| Haploid | Diploid | Tetraploid | Haploid | Diploid | Tetraploid | Haploid | Diploid | Tetraploid | |
| Control | 54.7 ± 1.9 | 17.0 ± 0.4 | 14.9 ± 1.4 | 62.4 ± 1.8 | 16.1 ± 0.6 | 12.6 ± 0.6 | 63.2 ± 0.8 | 15.9 ± 0.2 | 12.7 ± 0.3 |
| 10 mg kg−1 | 25.1 ± 3.6* | 34.8 ± 9.5* | 23.3 ± 5.9* | 59.5 ± 6.7 | 27.7 ± 2.6* | 5.6 ± 1.7* | 62.4 ± 2.3 | 17.5 ± 1.0 | 10.3 ± 2.3 |
| 10 mg kg−1× 2 | 4.8 ± 1.7*# | 67.1 ± 5.4*# | 18.5 ± 2.5# | 51.6 ± 2.7* | 34.8 ± 3.9* | 3.1 ± 0.6* | 63.2 ± 1.2 | 21.0 ± 3.4 | 9.0 ± 2.7 |
| 20 mg kg−1 | 4.3 ± 1.0* | 72.9 ± 5.2* | 10.9 ± 3.5# | 36.4 ± 1.3*# | 47.3 ± 0.3*# | 8.7 ± 0.5*# | 51.8 ± 3.4*# | 22.9 ± 4.3* | 17.1 ± 0.9* |
| 30 mg kg−1 | 2.9 ± 0.8* | 77.0 ± 4.1* | 10.3 ± 1.6 | 5.1 ± 0.7*# | 81.1 ± 2.6*# | 8.9 ± 0.8* | 13.5 ± 2.6*# | 73.2 ± 2.7*# | 6.5 ± 0.4*# |
P < 0.05, compared with the control group
P < 0.05, compared with the previous adjacent dose. “10 mg kg−1 × 2” represents two injections of 10 mg kg−1 busulfan.
Discussion
Although a dose of 40–44 mg kg−1 of busulfan is commonly used for the ablative treatment used to prepare recipient mice before spermatogonial transplantation, it is still unknown whether this dose is optimal, that is, whether it causes a maximal elimination of germ cells with minimal adverse effects.
The present study showed that the doses of 10–30 mg kg−1, including the double injections of 10 mg kg−1, had no significant effects on the survival of the young (4–6-week-old) BALB/c mice, but an injection of 40 mg kg−1 busulfan caused a death rate of 87%, indicating that the dose of 40 mg kg−1 is higher than the 50% lethal dose of busulfan for mice. The mice that received high doses of busulfan usually died during the first week after injection, suggesting that death was the result of lethal suffering from acute damage.
Histological examination and flow cytometric analysis in the present study showed that at 4 weeks after injection, more than 99% of the seminiferous tubules had become non-spermatogenic, with the depletion of ∼95% of the haploid cells (the spermatids and spermatozoa) in the animals treated with 30 mg kg−1 busulfan. In addition, at the end of week 12 after injection, 87.9% ± 0.6% of the seminiferous tubules remained non-spermatogenic with only 13.5% ± 2.6% of the testicular cells being haploid. This result indicates that the restoration of the non-spermatogenic seminiferous tubules to partially or fully spermatogenic tubules within 12 weeks is very limited in the animals treated with 30 mg kg−1 busulfan. Previous research has shown that it is not until ∼4 weeks after busulfan treatment that the cells in the process of differentiation are cleared from the lumen of the seminiferous tubules 26. Accordingly, transplantation is usually performed 4–6 weeks after treatment. The donor cells usually require ∼4 weeks after transplantation to attach to the basal lamina and proliferate 26, and an additional 4 weeks to produce complete seminiferous epithelium with spermatids and spermatozoa. Thus, the present study suggests that the dosage of 30 mg kg−1 is optimal for the ablative treatment with busulfan to prepare the recipient mice. This dosage can create sufficient niches and maintains the microenvironment for sufficiently longer time periods so that colonization of donor-derived spermatogonial stem cells can take place.
Acknowledgments
We thank Prof. Jian Song for his critical reading and revision of the manuscript. We are grateful to Dr Jin Sun and Mr Sjogren Jonas for the statistical analyses and helpful suggestions regarding the manuscript. Financial support for the work was received from the Population and Family Planning Commission of Hubei Province, China (No. 301130994).
References
- Brinster RL, Zimmermann JW. Spermatogenesis following male germ-cell transplantion. Proc Natl Acad Sci USA. 1994;91:11298–302. doi: 10.1073/pnas.91.24.11298. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ogawa X, Bobrinski I, Avarbock MR, Brinster RL. Xenogeneic spermatogenesis following transplantation of hamster germ cells to mouse testes. Biol Reprod. 1999;60:515–21. doi: 10.1095/biolreprod60.2.515. [DOI] [PubMed] [Google Scholar]
- Dobrinski I, Avarbock MR, Brinster RL. Transplantation of germ cells from rabbits and dogs into mouse testes. Biol Reprod. 1999;61:1331–9. doi: 10.1095/biolreprod61.5.1331. [DOI] [PubMed] [Google Scholar]
- Arregui L, Rathi R, Megee SO, Honaramooz A, Comendio M, et al. Xenografting of sheep testis tissue and isolated cells as a model for preservation of genetic material from endangered ungulates. Reproduction. 2008;136:85–93. doi: 10.1530/REP-07-0433. [DOI] [PubMed] [Google Scholar]
- Rathi R, Honaramooz A, Zeng W, Schlatt S, Dobrinski I. Germ cell fate and seminiferous tubule development in bovine testis xenografts. Reproduction. 2005;130:923–9. doi: 10.1530/rep.1.00912. [DOI] [PubMed] [Google Scholar]
- Kim Y, Selvaraj V, Dobrinski I, Lee H, Mcentee MC, et al. Recipient preparation and mixed germ cell isolation for spermatogonial stem cell transplantation in domestic cats. J Androl. 2006;27:248–56. doi: 10.2164/jandrol.05034. [DOI] [PubMed] [Google Scholar]
- Honaramooz A, Behboodi E, Megee SO, Overton SA, Galantino-Homer H, et al. Fertility and germline transmission of donor haplotype following germ cell transplantation in immunocompetent goats. Biol Reprod. 2003;69:1260–4. doi: 10.1095/biolreprod.103.018788. [DOI] [PubMed] [Google Scholar]
- Homaramooz A, Megee SO, Dobrinski I. Germ cell transplantation in pigs. Biol Reprod. 2002;66:21–8. doi: 10.1095/biolreprod66.1.21. [DOI] [PubMed] [Google Scholar]
- Schlatt S, Rosiepen G, Weinbauer GF, Rolf C, Brook PF, et al. Germ cell transfer into rat, bovine, monkey and human testes. Hum Reprod. 1999;14:144–50. doi: 10.1093/humrep/14.1.144. [DOI] [PubMed] [Google Scholar]
- Brinster RL. Germline stem cell transplantation and transgenesis. Science. 2002;296:2174–6. doi: 10.1126/science.1071607. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rodriguez-Sosa JR, Dobrinski I. Recent developments in testis tissue xenografting. Reproduction. 2009;138:187–94. doi: 10.1530/REP-09-0012. [DOI] [PubMed] [Google Scholar]
- Shinohara T, Orwig KE, Avarbock MR, Brinster RL. Germ line stem cell competition in postnatal mouse testes. Biol Reprod. 2002;66:1491–7. doi: 10.1095/biolreprod66.5.1491. [DOI] [PubMed] [Google Scholar]
- Brinster CJ, Ryu BY, Avarbock MR, Karagenc L, Brinster RL, et al. Restoration of fertility by germ cell transplantation requires effective recipient preparation. Biol Reprod. 2003;69:412–20. doi: 10.1095/biolreprod.103.016519. [DOI] [PubMed] [Google Scholar]
- Zhang Z, Shao S, Meistrich ML. The radiation-induced block in spermatogonial differentiation is due to damage to the somatic environment, not the germ cells. J Cell Physiol. 2007;211:149–58. doi: 10.1002/jcp.20910. [DOI] [PubMed] [Google Scholar]
- Shinohara T, Avarbock MR, Brinster RL. Functional analysis of spermatogonial stem cells in steel and cryptorchid infertile mouse models. Dev Biol. 2000;220:401–11. doi: 10.1006/dbio.2000.9655. [DOI] [PubMed] [Google Scholar]
- Jégou B, Laws AO, Kretser DM. Changes in testicular function induced by short-term exposure of the rat testis to heat: further evidence for interaction of germ cells, Sertoli cells and Leydig cells. Int J Androl. 1984;7:244–57. doi: 10.1111/j.1365-2605.1984.tb00781.x. [DOI] [PubMed] [Google Scholar]
- Young GP, Goldstein M, Philips DM, Sundaram K, Gunsalus GL, et al. Sertoli cell-only syndrome produced by cold testicular ischemia. Endocrinology. 1988;122:1074–82. doi: 10.1210/endo-122-3-1074. [DOI] [PubMed] [Google Scholar]
- Bucci LR, Meistrich ML. Effects of busulfan on murine spermatogenesis: cytotoxicity, sterility, sperm abnormalities, and dominant lethal mutations. Mutat Res. 1987;176:259–68. doi: 10.1016/0027-5107(87)90057-1. [DOI] [PubMed] [Google Scholar]
- Kim JH, Jung-Ha HS, Lee HT, Chung KS. Development of a positive method for male stem cell-mediated gene transfer in mouse and pig. Mol Reprod Dev. 1997;46:515–26. doi: 10.1002/(SICI)1098-2795(199704)46:4<515::AID-MRD10>3.0.CO;2-V. [DOI] [PubMed] [Google Scholar]
- Stellflug J, Green JS, Leathers CW. Antifertility effect of busulfan and procarbazine in male and female coyotes. Biol Reprod. 1985;33:1237–43. doi: 10.1095/biolreprod33.5.1237. [DOI] [PubMed] [Google Scholar]
- Ogawa T, Dobrinski I, Brinster RL. Recipient preparation is critical for spermatogonial transplantation in the rat. Tissue Cell. 1999;31:461–72. doi: 10.1054/tice.1999.0060. [DOI] [PubMed] [Google Scholar]
- Jiang FX. Behaviour of spermatogonia following recovery from busulfan treatment in the rat. Anat Embryol. 1998;198:53–61. doi: 10.1007/s004290050164. [DOI] [PubMed] [Google Scholar]
- Brinster RL, Avarbock MR. Germline transmission of donor haplotype following spermatogonial transplantation. Proc Natl Acad Sci USA. 1994;91:11303–7. doi: 10.1073/pnas.91.24.11303. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moisan AE, Foster RA, Betteridge KJ, Hahnel AC. Dose-response of RAG2−/−/γc−/− mice to busulfan in preparation for spermatogonial transplantation. Reproduction. 2003;126:205–16. doi: 10.1530/rep.0.1260205. [DOI] [PubMed] [Google Scholar]
- Deitch AD, Goldberg SD, White RW. Flow cytometric analysis of testicular tissue: detection of injury and recovery. World J Urol. 1986;4:71–6. [Google Scholar]
- Nagano A, Avarbock MR, Brinster RL. Pattern and kinetics of mouse donor spermatogonial stem cell colonization in recipient testes. Biol Reprod. 1999;60:1429–36. doi: 10.1095/biolreprod60.6.1429. [DOI] [PMC free article] [PubMed] [Google Scholar]


