Abstract
To evaluate the role of high-dose dietary zinc in the process of prostate malignancy, 60 Sprague-Dawley rats were randomly divided into four groups: tumor induction with carcinogen and hormone (group 1), oral zinc administration without tumor induction (group 2), oral zinc administration with tumor induction (group 3) and a control without zinc administration or tumor induction (group 4). Zinc was supplied orally in the form of zinc sulfate heptahydrate dissolved in drinking water to groups 2 and 3 for 20 weeks. Although the serum level of zinc measured at 20 weeks was maintained similarly in each group (P = 0.082), intraprostatic zinc concentrations were statistically different. Group 1 prostates contained the least amount of zinc in both the dorsolateral and ventral lobes at levels of 36.3 and 4.8 μg g−1, respectively. However, in group 3, zinc levels increased in both lobes to 59.3 and 12.1 μg g−1, respectively, comparable with that of group 4 (54.5 ± 14.6 and 14.1 ± 2.4 μg g−1). In spite of these increases in zinc concentration, the prevalence of prostate intraepithelial neoplasm was rather increased in group 3 (53.3% and 46.7%) compared with group 1 (33.3% and 33.3%) in both dorsolateral and ventral prostate lobes. Although prostate intraepithelial neoplasm did not develop in any prostate in group 4, zinc administration did induce prostate intraepithelial neoplasm in group 2 (46.7% and 40.0%). Thus, although high dietary zinc increased intraprostatic zinc concentrations, it promoted, instead of preventing, prostate intraepithelial neoplasm in a murine prostate malignancy induction model.
Keywords: experimental animal model, prostatic cancer, prostatic intraepithelial neoplasia, zinc
Introduction
Prostate cancer is a complex and heterogeneous disease, the etiology of which is poorly understood 1. Genetic predisposition and numerous environmental factors, including diet and hormonal changes, exert important effects in the pathogenesis of this disease 2. Owing to the clinically significant incidence rate 3, as well as slow disease progression requiring a long precancerous period and the known effect of diet on cancer development 1, prostate cancer has been highlighted in the field of chemoprevention. Several studies have shown that the concentration of zinc and citrate is lower in prostate cancer cells than in normal prostate cells; moreover, in prostate cells of benign hyperplasia 4, the theory that the inability of cells to accumulate intracellular zinc leads to development of prostate cancer has gained attention. In addition, further studies that reported that maintaining supraphysiological concentrations of zinc was effective in the treatment and prevention of prostate cancer also helped to highlight zinc as a possible chemopreventive agent against prostate cancer 5, 6.
However, large-scale epidemiological studies produced results that were in disagreement with those of in vitro studies. Although initial studies reported that zinc supplementation lowered the risk of prostate cancer development 7, 8, this effect was not reproducible by other researchers 9, 10, 11. On the contrary, recent study reported that excessive intake increased the risk of progressing to advanced prostate cancer 12. However, these epidemiological studies had common inherent limitations in terms of questionnaire-based investigation and the complexity of the human diet. Thus, to validate whether the role of dietary zinc in the development of prostate malignancy is preventive or promotive, an in vivo study built around a prostate adenocarcinoma induction model with controlled diet is imperative. At present, there are no published reports regarding the effect of dietary zinc using this model system, as one of the main obstacles is the prolonged experimental period of over a year for the induction of malignancy. Herein, preliminarily, to evaluate the role of high dietary zinc in the development of premalignant lesion instead of prostate adenocarcinoma to evade this time obstacle, we investigated prostate histology, serum and intraprostatic zinc concentration after each treatment of tumor induction, high-zinc diet, a combination of these and in normal control.
Materials and methods
Animals
Healthy, 50-day-old adult male Sprague-Dawley rats weighing between 150 g and 180 g were used in this study. The animals were housed in clean polypropylene metabolic cases (Jeung Do Bio & Plant Co., Seoul, Korea) and maintained in an air-conditioned animal house with an alternating 12 h: 12 h light : dark diurnal schedule. All animals were fed a standard rat pellet diet (AIN-93G Purified Rodent Diet; Dyets, Bethlehem, PA, USA; containing 1.65 g kg−1 zinc carbonate). All animal experiments were conducted under the Guiding Principles for the Care and Use of Animals, approved by the American Physiological Society 13.
Treatment for each animal group
After adaptation to diet and diurnal cycles for 1 week, 60 rats were randomly assigned to one of four groups (15 rats per group), and then treated. In group 1, prostate malignancies were induced using cyproterone acetate, testosterone propionate (Sigma Chemicals, St. Louis, MO, USA) and methylnitrosourea (MNU; Ashe Stevens, Detroit, MI, USA), as previously published 14. Group 2 was treated with oral zinc administration without tumor induction, and group 3 was treated with both oral zinc administration and tumor induction. In groups 2 and 3, rats received zinc in the form of zinc sulfate heptahydrate (ZnSO4·7H2O; Sigma Chemicals), dissolved in drinking water at a dose of 227 mg Zn per liter for 20 weeks 15, 16, 17, together with the standard rat pellet diet. Rats were permitted access to drinking water ad libitum, and the amount of water imbibed and the body weight of each animal were recorded daily. Group 4 served as the control without zinc administration or tumor induction.
Histological examination
At the 20th week of treatment, rats from all groups were killed and prostate tissue was excised. Adhering connective tissue around the prostate was removed, and the prostate was divided into ventral and dorsolateral lobes. Each prostate lobe was embedded in paraffin and stained with hematoxylin–eosin for histological evaluation. Two investigators blindly and independently evaluated sections to classify the lesions, and discrepancies in interpretation were discussed and resolved. Using criteria described previously 18, we classified proliferative epithelial lesions as adenocarcinoma, prostate intraepithelial neoplasia (PIN), dysplasia and hyperplasia (Figure 1). Adenocarcinoma was reserved for lesions that were clearly invasive.
Figure 1.
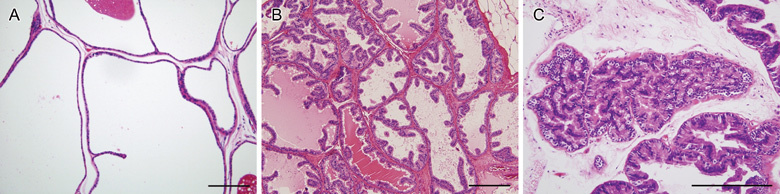
(A): Histological appearance of normal ventral prostate. A single layer of flattened and cuboidal epithelial cells lines the tubules with secretion in the lumen. Epithelial tubules, surrounded by a thin layer of smooth muscle cells, are distributed within the loosely organized stroma. Hematoxylin and eosin (H&E) stained (× 100). Hyperplasia with dysplasia of the dorsolateral prostate (B): columnar cells with increased growth of papillary epithelial cells are shown. Relatively decreased stroma was observed within the papillary projections, compared with normal prostate. H&E stained (× 100). Prostatic intraepithelial neoplasia of ventral prostate (C): prominent papillary projection with an increased complexity of the gland is shown. Nuclei were elongated and demonstrated hyperchromatic change. H&E stained (× 200). Bars = 100 μm.
Determination of serum and intraprostatic zinc concentration
Zinc concentration in serum and prostate, which was taken at the 20th week of treatment, was measured by the process described below. Blood (3 mL) sampled from inferior vena cava was centrifuged at 1 420 × g, at room temperature for 10 min, and serum was collected. Prostate tissues from each rat were excised, rinsed in phosphate-buffered saline and homogenized in 9 mL of autoclaved distilled water. The homogenates were centrifuged at 1 420 × g, at 4°C for 10 min. Serum and supernatants were incubated with 30°C of 24 μmol L−1 zinquin (AMRAD Corporation, Kew, Victoria, Australia) for 30 min at 37°C. Samples were then read on a Wallac 1420 Multilabel Counter (EG&G Wallac, Turku, Finland) at excitation and emission wavelengths of 370 nm and 460 nm, respectively.
All data were collated in SPSS version 12.0 (SPSS Inc., Chicago, IL, USA) and evaluated by the Kruskal–Wallis test, the Mann-Whitney U-test, the χ2-test and the unpaired t-test. P < 0.05 were accepted as significant.
Results
Serum and intraprostatic zinc concentration
All animals survived throughout the 20 weeks of experiment. The serum zinc level was similar without statistical difference (P = 0.082, by the Kruskal-Wallis test), and the average serum zinc concentration was 0.65 ± 0.09 μg mL−1. However, the intraprostatic zinc level showed a different aspect. In groups treated with zinc, intraprostatic zinc concentrations were significantly higher in the dorsolateral lobe than in the ventral lobe at average concentrations of 51.9 ± 12.8 and 10.8 ± 2.9 μg g−1, respectively (P = 0.001, by unpaired t-test). In addition to the difference in zinc concentration within the prostate itself, statistical differences in zinc concentration between treatment groups were found in both prostate lobes (P = 0.01 and 0.001, respectively, by the Kruskal-Wallis test). The intraprostatic zinc concentration in the dorsolateral lobe of group 1, in which tumors were induced in the absence of dietary zinc, was the lowest among treatment groups with an average of 36.3 ± 12.8 μg g−1. However, after oral zinc administration with tumor induction, the zinc concentration in the dorsolateral lobe of group 3 was significantly increased to 59.3 ± 17.8 μg g−1 (P = 0.004, by the Mann-Whitney U-test, Figure 2A), compared with that of control group 4 (54.5 ± 14.6 μg g−1, P = 0.75). The intraprostatic zinc concentration in the dorsolateral lobe of group 2, in which oral zinc was administrated in the absence of tumor induction, was 57.7 ± 6.0 μg g−1. This value is slightly higher than that of group 4; however, this difference was not determined to be significant (P = 0.88). A similar pattern was observed in the ventral lobe. Intraprostatic zinc concentrations were also lowest in group 1 (4.8 ± 1.7 μg g−1), compared with levels of 14.4 ± 4.8 μg g−1 in group 2, 12.1 ± 2.7 μg g−1 in group 3 and 14.1 ± 2.4 μg g−1 in group 4. In addition, after oral zinc administration with tumor induction, the zinc concentration of group 3 was significantly increased (P = 0.001, Figure 2B), compared with that of control group 4 (P = 0.07). The intraprostatic zinc concentration was highest in group 2, but the difference compared with group 4 was not found to be significant (P = 0.89).
Figure 2.
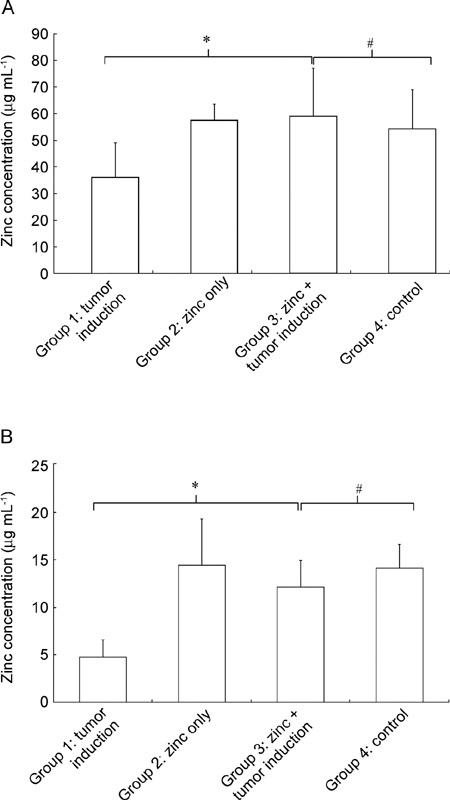
Zinc concentration in dorsolateral (A) and ventral (B) lobes of the prostate in each treatment group. *P < 0.05, comparison between groups 1 and 3; #P > 0.05, comparison between groups 3 and 4.
Development of PIN in each group
On histological examination, prostate adenocarcinoma was not found in any group. The prevalence of PIN in group 1, in which tumors were induced, was 33.3% in both dorsolateral and ventral lobes. However, after oral zinc administration with tumor induction in group 3, the incidence of PIN was increased to 53.5% and 46.7% for each prostate lobe, respectively (Table 1). Although PIN did not develop in any of the group 4 rats, zinc administration did induce PIN in both prostate lobes in group 2 (46.7% and 40%, respectively). This difference in PIN prevalence was found to be statistically significant for both lobes (P = 0.01 and 0.03, respectively, by the χ2-test).
Table 1. Summary of histological examination.
| Treatment group | Dorsolateral prostate (%) |
Ventral prostate (%) |
||||
|---|---|---|---|---|---|---|
| Hyperplasia | Dysplasia | PIN | Hyperplasia | Dysplasia | PIN | |
| G1: tumor induction | 13 (86.7) | 11 (73.3) | 5 (33.3) | 12 (80) | 10 (66.7) | 5 (33.3) |
| G2: zinc administration only | 14 (93.3) | 10 (66.7) | 7 (46.7) | 14 (93.3) | 9 (60) | 6 (40) |
| G3: zinc administration + tumor induction | 15 (100) | 13 (86.7) | 8 (53.3) | 15 (100) | 12 (80) | 7 (46.7) |
| G4: control | 7 (46.7) | 4 (26.7) | 0 | 6 (40) | 3 (20) | 0 |
Abbreviation: G, group; PIN, prostate intraepithelial neoplasia.
Discussion
Zinc is an essential trace element and a structural component of many proteins, including intracellular signaling enzymes and transcription factors 19. Zinc has an important role in nucleic acid metabolism, cell replication and tissue growth 20, and zinc deficiency is associated with a wide range of pathological conditions, including impaired immunity, retarded growth, brain development disorders and delayed wound healing 19, 21. Although zinc has been shown to be required for normal growth, many reports have suggested that zinc is also involved in cancer development. It may directly affect tumor cells by regulating gene expression profiles and/or cell viability, both of which are mediated in part by tumor-induced changes in zinc transporter expression. The expression level of zinc transporters in human tumors correlates with their malignancy, suggesting that alteration of intracellular zinc homeostasis can contribute to the severity of cancer 22, 23, 24. Conversely, zinc may indirectly influence tumor cells by affecting processes within the cancer microenvironment; the functions and/or activity levels of immune cells that attack tumor cells have been shown to be influenced by intracellular zinc concentrations within those cells 25, 26.
However, the relationship between tumor development and zinc levels seems to be complicated. Indeed, zinc concentrations are reduced in patients having carcinomas of the liver, gallbladder and digestive tract 27, 28, 29, whereas zinc concentrations are shown to be elevated in malignant tissues of the breast 30. In prostate malignancy, a decrease in zinc accumulation is one of the most consistent and persistent characteristics. Zinc concentrations diminish early in the course of prostate cancer, preceding the initial histological changes, and continue to decline during the ultimate progression toward hormone-independent growth 31, 32. These findings were matched with the results of our study, which also show the lowest intraprostatic zinc concentrations in the tumor induction group (group 1).
Although the association of zinc homeostasis with prostate malignancy has been defined, it is still unclear whether prostate malignancy causes the disruption of zinc homeostasis or vice versa 33. Although intraprostatic zinc concentration decreases in the early phases of prostate cancer tumorigenesis, this finding alone does not fulfill a causal relationship between chemoprevention and zinc iron supplement. It is well established that zinc is essential for cell survival, but it is also increasingly clear that zinc is toxic to cells when it accumulates to a certain level. As shown in our study, high dietary zinc, in addition to normal zinc uptake, promoted premalignant changes in the tumor induction model. Though the mechanism of zinc toxicity in not clearly defined, several studies have proposed possible solutions. Dubi et al. 34 observed that extracellular zinc ion triggers a metabotropic calcium ion rise that was apparent in the presence of citrate through a zinc-sensing receptor pathway. In the presence of this calcium response, extracellular zinc ion enhanced the growth and survival of the hormone-resistant prostate cancer cell line PC-3 significantly, as zinc enhances the activity of telomerase, an enzyme thought to be responsible for unlimited proliferation of tumor cells and the activity of which is increased in prostate cancer 35. In addition, zinc intake is directly associated with circulating levels of insulin-like growth factor-I, which are related to prostate carcinogenesis 36. Furthermore, it has been suggested that a loss of senescence potential in prostate cells, which was correlated with zinc concentration, may have a role in tumorigenesis. Supporting this point, Wong et al. 37 demonstrated an increasing percentage of senescent cells when treated with a high zinc concentration in the normal prostate cell line PNT2, a phenomenon that was not observed in the prostate cancer cell line LNCaP. This may explain the function of high zinc concentrations in the normal prostate in performing a regulatory role in maintaining senescence in healthy prostate tissue.
These results, in addition to our own findings, imply the ineffectiveness of dietary zinc supplement or zinc-based therapy. Furthermore, our findings show a potential harm in high-dose dietary zinc early in prostate malignant change. These findings may also suggest that a decrease in zinc concentration is a result of the tumorigenic process of prostate cancer rather than an inciting factor. Recent clinical trials also concur with this view, demonstrating a high-dose zinc intake of over 100 mg per day to be detrimental to the prevention of prostate cancer 12. Similarly, Gallus et al. 38 presented a multicenter study comparing 1 294 prostate cancer patients against a control group of 1 451, in which the relative risk of prostate cancer increased in accordance with increased zinc intake. In their study, the relative risk ratio of the high-dose zinc group was 1.56.
We also recognize the fact that there are some methodological weaknesses in this study. With the animal model induced by MNU, cyproterone acetate and testosterone, the incidence of prostate carcinomas was previously reported to approach 75% by approximately 52 weeks 39, and the incidence of PIN approached 40% by 20 weeks 18. We focused on the development of PIN, as it was possible to identify the chemopreventive effects or carcinogenic effects of zinc administration in an early period of malignant change, considering the multistep process and longer duration of tumorigenesis in prostate malignancy. In addition, this focus enabled us to avoid the difficulties induced by prolonged animal experimentation, mainly in the maintenance of animal survival and quality control. The limitation is that not all PIN lesions progress to prostate cancer, and PIN is not always a precursory lesion in prostate cancer. As such, it is possible that, with longer monitoring, the actual occurrence of prostate cancer may disagree with our results, which is why a longer experimental design is still needed in this field.
Another possible point of contention is zinc dosage. In this study, zinc was supplied as 227 mg L−1 through drinking water, as described by other researchers, mainly because of the simplicity in administration and calculation. To compensate for water consumption and body weight change for groups exposed to zinc during the course of the experiment, we supplied an average of 26.48 mg kg−1 additional zinc in the ordinary meal. This concentration is higher than that administered in other in vivo experiments, in which zinc was supplied at about 2.25–1.5 mg kg−1 40. Thus, further study with varying zinc concentrations and administration routes is necessary to clarify this variable.
In summary, intraprostatic zinc levels decreased after tumor induction in this in vivo study. After the administration of high doses of dietary zinc with tumor induction, the intraprostatic zinc concentration increased significantly compared with levels of the control group, and the prevalence of PIN was rather increased. These results suggest potential harm in high-dose dietary zinc early in prostate malignant change. However, to adequately understand the role of dietary zinc in the development of prostate cancer, longer studies to induce adenocarcinoma with various doses of dietary zinc should be conducted.
References
- Neuhouser ML, Kristal AR, Patterson RE, Goodman PJ, Thompson IM.Dietary supplement use in the Prostate Cancer Prevention Trial. Implications for prevention trials Nutr Cancer 20013912–8. [DOI] [PubMed] [Google Scholar]
- Giovannucci E. Medical history and etiology of prostate cancer. Epidemiol Rev. 2001;23:159–62. doi: 10.1093/oxfordjournals.epirev.a000783. [DOI] [PubMed] [Google Scholar]
- Jung KW, Yim SH, Kong HJ, Hwang SY, Won YJ, et al. Cancer survival in Korea 1993-2002: a population-based study. J Korean Med Sci. 2007;22:S5–10. doi: 10.3346/jkms.2007.22.S.S5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Costello LC, Franklin RB. The clinical relevance of the metabolism of prostate cancer; zinc and tumor suppression: connecting the dots. Molecular Cancer. 2006;17:1–13. doi: 10.1186/1476-4598-5-17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Feng P, Liang JY, Cuan ZX, Franklin RB. Zinc induces mitochondria apoptogenesis in prostate cells. Mol Urol. 2006;4:31–5. [PubMed] [Google Scholar]
- Liang JY, Liu YY, Zou J, Franklin FB, Costello LC. Inhibitory effect of zinc on human prostatic carcinoma cell growth. Prostate. 1999;40:200–7. doi: 10.1002/(sici)1097-0045(19990801)40:3<200::aid-pros8>3.0.co;2-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kristal AR, Stanford JL, Cohen JH, Wicklund K, Patterson RE. Vitamin and mineral supplement use is associated with reduced risk of prostate cancer. Cancer Epidemiol Biomark Prev. 1999;8:887–92. [PubMed] [Google Scholar]
- Key TJ, Silcocks PB, Davey GK, Appleby PN, Bishop DT. A case-control study of diet and prostate cancer. Br J Cancer. 1997;76:678–87. doi: 10.1038/bjc.1997.445. [DOI] [PMC free article] [PubMed] [Google Scholar]
- West DW, Slattery ML, Robison LM, French TK, Mahoney AW. Adult dietary intake and prostate cancer risk in Utah: a case-control study with special emphasis on aggressive tumors. Cancer Causes Control. 1991;2:85–94. doi: 10.1007/BF00053126. [DOI] [PubMed] [Google Scholar]
- Andersson SO, Wolk A, Bergström R, Giovannucci E, Lindgren C, et al. Energy, nutrient intake and prostate cancer risk: a population-based case-control study in Sweden. Int J Cancer. 1996;68:716–22. doi: 10.1002/(SICI)1097-0215(19961211)68:6<716::AID-IJC4>3.0.CO;2-6. [DOI] [PubMed] [Google Scholar]
- Kolonel LN, Yoshizawa CN, Hankin JH. Diet and prostatic cancer: a case-control study in Hawaii. Am J Epidemiol. 1988;127:999–1012. doi: 10.1093/oxfordjournals.aje.a114903. [DOI] [PubMed] [Google Scholar]
- Leitzmann MF, Stampfer MJ, Wu K, Colditz GA, Willett WC, et al. Zinc supplement use and risk of prostate cancer. J Natl Cancer Inst. 2003;95:1004–7. doi: 10.1093/jnci/95.13.1004. [DOI] [PubMed] [Google Scholar]
- American Physiological Society; World Medical Association General Assembly. Guiding Principles for Research Involving Animals and Human Beings Am J Physiol Cell Physiol 20022823. [DOI] [PubMed] [Google Scholar]
- Boileau TW, Liao Z, Kim S, Lemeshow S, Erdman JW, Jr, et al. Prostate carcinogenesis in N-methyl-N-nitrosourea (NMU)-testosterone-treated rats fed tomato powder, lycopene, or energy-restricted diets. J Natl Cancer Inst. 2003;95:1578–86. doi: 10.1093/jnci/djg081. [DOI] [PubMed] [Google Scholar]
- Sidhu P, Garg ML, Dhawan DK. Protective role of zinc in nickel induced hepatotoxicity in rats. Chem Biol Interact. 2003;20:199–209. doi: 10.1016/j.cbi.2004.09.012. [DOI] [PubMed] [Google Scholar]
- González-Reimers E, Durán-Castellón MC, Martín-Olivera R, López-Lirola A, Santolaria-Fernández F, et al. Effect of zinc supplementation on ethanol-mediated bone alterations. Food Chem Toxicol. 2005;43:1497–505. doi: 10.1016/j.fct.2005.04.003. [DOI] [PubMed] [Google Scholar]
- Unsal C, Celik JB, Toy H, Esen H, Otelcioglu S. Protective role of zinc pretreatment in hepatotoxicity induced by halothane. Eur J Anaesthesiol. 2008;25:810–5. doi: 10.1017/S0265021508004523. [DOI] [PubMed] [Google Scholar]
- Arunkumar A, Vijayababu MR, Venkataraman P, Senthilkumar K, Arunakaran J. Chemoprevention of rat prostate carcinogenesis by diallyl disulfide, an organosulfur compound of garlic. Biol Pharm Bull. 2006;29:375–9. doi: 10.1248/bpb.29.375. [DOI] [PubMed] [Google Scholar]
- Prasad AS. Zinc: an overview. Nutrition. 1995;11:93–9. [PubMed] [Google Scholar]
- Lee S, Simpson M, Nimmo M, Xu Z. Low zinc intake suppressed N-methyl-N-nitrosourea-induced mammary tumorigenesis in Sprague-Dawley rats. Carcinogenesis. 2004;25:1879–85. doi: 10.1093/carcin/bgh214. [DOI] [PubMed] [Google Scholar]
- Kim YJ, Kim MY, Kim HO, Lee MD, Park YM. Acrodermatitis enteropathica-like eruption associated with combined nutritional deficiency. J Korean Med Sci. 2005;20:908–11. doi: 10.3346/jkms.2005.20.5.908. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eide DJ. The SLC39 family of metal ion transporters. Pflugers Arch. 2004;447:796–800. doi: 10.1007/s00424-003-1074-3. [DOI] [PubMed] [Google Scholar]
- Zhao H, Eide D. The yeast ZRT1 gene encodes the zinc transporter protein of a high-affinity uptake system induced by zinc limitation. Proc Natl Acad Sci USA. 1996;93:2454–8. doi: 10.1073/pnas.93.6.2454. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eide D, Broderius M, Fett J, Guerinot ML. A novel iron-regulated metal transporter from plants identified by functional expression in yeast. Proc Natl Acad Sci USA. 1996;93:5624–8. doi: 10.1073/pnas.93.11.5624. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fraker PJ, King LE, Laakko T, Vollmer TL. The dynamic link between the integrity of the immune system and zinc status. J Nutr. 2000;130:1399–406. doi: 10.1093/jn/130.5.1399S. [DOI] [PubMed] [Google Scholar]
- Prasad AS. Clinical and biochemical manifestation zinc deficiency in human subjects. J Pharmacol. 1985;16:344–52. [PubMed] [Google Scholar]
- Hu S, Zhang M, Lv Z, Bi J, Dong Y, et al. Expression of zinc-fingers and homeoboxes 2 in hepatocellular carcinogenesis: a tissue microarray and clinicopathological analysis. Neoplasma. 2007;54:207–11. [PubMed] [Google Scholar]
- Gupta SK, Singh SP, Shukla VK. Copper, zinc, and Cu/Zn ratio in carcinoma of the gallbladder. J Surg Oncol. 2005;91:204–8. doi: 10.1002/jso.20306. [DOI] [PubMed] [Google Scholar]
- Rudolf E, Klvacová L, John S, Cervinka M. Zinc alters cytoskeletal integrity and migration in colon cancer cells. Acta Medica. 2008;51:51–7. [PubMed] [Google Scholar]
- Taylor KM, Vichova P, Jordan N, Hiscox S, Hendley R, et al. ZIP7-mediated intracellular zinc transport contributes to aberrant growth factor signaling in antihormone-resistant breast cancer cells. Endocrinology. 2008;149:4912–20. doi: 10.1210/en.2008-0351. [DOI] [PubMed] [Google Scholar]
- Franklin RB, Feng P, Milon B, Desouki MM, Singh KK, et al. hZIP1zinc uptake transporter down regulation and zinc depletion in prostate cancer. Mol Cancer. 2005;4:32–7. doi: 10.1186/1476-4598-4-32. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zaichick V, Sviridova TV, Zaichick SV. Zinc in the human prostate gland: normal, hyperplastica nd cancerous. Int Urol Nephrol. 1997;29:565–74. doi: 10.1007/BF02552202. [DOI] [PubMed] [Google Scholar]
- Iguchi K, Otsuka T, Usui S, Ishii K, Onishi T, et al. Zinc and metallothionein levels and expression of zinc transporters in androgen-independent subline of LNCaP cells. J Androl. 2004;25:154–61. doi: 10.1002/j.1939-4640.2004.tb02771.x. [DOI] [PubMed] [Google Scholar]
- Dubi N, Gheber L, Fishman D, Sekler I, Hershfinkel M. Extracellular zinc and zinc-citrate, acting through a putative zinc-sensing receptor, regulate growth and survival of prostate cancer cells. Carcinogenesis. 2008;29:1692–700. doi: 10.1093/carcin/bgn027. [DOI] [PubMed] [Google Scholar]
- Sommerfeld HJ, Meeker AK, Piatyszek MA, Bova GS, Shay JW, et al. Telomerase activity: a prevalent marker of malignant human prostate tissue. Cancer Res. 1996;56:218–22. [PubMed] [Google Scholar]
- Holmes MD, Pollak MN, Willett WC, Hankinson SE. Dietary correlates of plasma insulin-like growth factor I and insulin-like growth factor binding protein 3 concentrations. Cancer Epidemiol Biomarkers Prev. 2002;11:852–61. [PubMed] [Google Scholar]
- Wong PF, Abubakar S. LNCaP prostate cancer cells are insensitive to zinc-induced senescence. J Trace Elem Med Biol. 2008;22:242–7. doi: 10.1016/j.jtemb.2008.03.008. [DOI] [PubMed] [Google Scholar]
- Gallus S, Foschi R, Negri E, Talamini R, Franceschi S, et al. Dietary zinc and prostate cancer risk: a case-control study from Italy. Eur Urol. 2007;52:1052–6. doi: 10.1016/j.eururo.2007.01.094. [DOI] [PubMed] [Google Scholar]
- Boileau TW, Liao Z, Kim S, Lemeshow S, Erdman JW, Jr, et al. Prostate carcinogenesis inN-methyl-N-nitrosourea (NMU)-testosterone-treated rats fed tomato powder, lycopene, or energy-restricted diets. J Natl Cancer Inst. 2003;95:1578–86. doi: 10.1093/jnci/djg081. [DOI] [PubMed] [Google Scholar]
- Feng P, Li TL, Guan ZX, Franklin RB, Costello LC. Effect of zinc on prostatic tumorigenicity in nude mice. Ann N Y Acad Sci. 2003;1010:316–20. doi: 10.1196/annals.1299.056. [DOI] [PubMed] [Google Scholar]


