Abstract
Pain following depot intramuscular (IM) injection of oil vehicle-based drugs has been little studied. This study aimed to determine prospectively the prevalence, determinants, severity and functional consequences of pain during the week after IM injection of 1 000 mg testosterone undecanoate (TU) in a 4-mL castor oil vehicle. Androgen-deficient men receiving regular T replacement therapy at an academic andrology clinic were recruited to report pain scores using a coloured visual linear analogue scale at seven times over the first day and daily for a week after a deep IM gluteal injection. The time course and covariables influencing pain scores were analysed by mixed model analysis of variance (ANOVA). Following 168 injections in 125 men, pain was reported by 80% of men, peaking immediately after injection, reaching only moderate severity, lasting 1–2 days and returning to baseline by day 4. The pain required little analgesic use and produced minimal interference in daily activities. The time course of pain scores was reproducible in the 43 men who underwent two consecutive injections. Pain was more severe in men who had an earlier painful injection, but less severe in older and more obese men. There were negligible differences in post-injection pain experience between experienced nurses administering injections. Deep IM gluteal injection of depot TU in 4-mL castor oil is well tolerated and post-injection pain is influenced by earlier painful injection experience, as well as age and obesity.
Keywords: ageing, intramuscular injection, obesity, pain, testosterone
Introduction
Intramuscular (IM) injection is a route of administration used to deliver medication to either create a depot providing sustained drug delivery or to avoid gastrointestinal or hepatic first-pass drug metabolism 1. Although many IM-injected drugs are delivered in an aqueous vehicle, oil vehicle IM injections are confined to the delivery of sex steroids 2 or neuroleptic drugs 3, 4 as depot formulations. These involve the administration of pro-drug esters with the active drug released by side chain hydrolysis, a cleavage produced by ubiquitous nonspecific esterases.
Testosterone (T) has been used clinically in androgen replacement therapy for over six decades 5, administered by numerous routes, but a depot product with sustained duration of action is attractive, especially for younger men, for the longer intervals between treatments. For example, T pellets implanted at intervals of 6 months have a very high elective continuation rate relative to a cost-minimized choice of alternative T products 6. More recently, T undecanoate (TU) was developed as an IM injectable depot using an oil vehicle with a 12-week inter-injection interval that greatly exceeds older T ester products that typically required a 2-week inter-injection interval 7. However, owing to limited solubility, TU is marketed in a relatively large (4 mL) castor oil vehicle. Although TU has been administered for androgen replacement therapy for up to 10 years 8, 9, in healthy volunteers participating in male contraceptive studies [10, 11, 12, 13, 14], injection pain was recorded in few studies 11, 14, in which it was reported as infrequent and mild 11 or lasting 1–2 days 14, but quantitative details were lacking.
The complications of IM injections include local pain, bleeding, infection, intravascular delivery, tissue necrosis and scarring 15, 16; however, only pain is relatively common. Experimental animal studies show that oil-based injections produce local necrosis of muscle, leading to increases in circulating creatinine kinase levels [17, 18, 19]. Localized tissue damage at the injection site accounts for the pain, redness and sterile inflammation, mostly low grade and readily tolerated, following IM administration of oil vehicle injections 20. The irritation and inflammatory reactions arise from the intrinsic physicochemical properties of either the drugs, excipients 21 or vehicle 22, 23. Consequently, although post-injection pain is expected after any IM injection, the severity, duration and interference with regular activities may vary between individuals, injections and products, and has been subject to few systematic studies.
We previously studied pain following deep IM injection of a T ester in a 1-mL oil vehicle 24. Although the TU product information states that local pain occurs after injections, no systematic evaluation of post-injection pain with the unusually large injection volume (4 mL) is available. We therefore undertook a prospective observational study to determine the prevalence, predictors, severity and duration (time course) of gluteal pain and consequent impairment of activities of daily living for the week following deep IM injection of TU for androgen replacement therapy.
Materials and methods
Participants
Participants with established androgen deficiency due to hypothalamic, pituitary or testis disorders (excluding 'andropause') and receiving regular T treatment were recruited from the Andrology Department, Concord Hospital, a teaching hospital of the University of Sydney Medical School. No incentives to participate were provided for men who continued to receive maintenance T replacement therapy at minimal out-of-pocket cost under the national health scheme. Participants were enrolled when they required re-treatment, consequent on return of their own distinctive symptoms of androgen deficiency 25. Further details of the participant population have been reported elsewhere 6, 25. The trial was approved by the Human Ethics Review Committee, Sydney South West Area Health Service (Concord zone), and all participants provided a written informed consent before entry.
Study procedures and assessments
Injections were administered to the recumbent patients by three experienced nurses within the department. The deep IM injection was administered slowly, over 3–5 min, using a 38-mm disposable 21-G needle into the upper, outer quadrant of the buttock.
Participants were surveyed prospectively to identify any pain around the gluteal region immediately before and then at fixed time intervals after TU injection (immediately and at 4 and 8 h after, at bedtime that evening, the following morning and then daily for 8 days). Using a standardized questionnaire, they were asked about previous experience with post-injection pain, and the presence, severity, use of analgesics and consequences such as interference with daily living (discomfort, disruption or cancellation of any domestic, occupational or recreational activities) of any post-injection pain. To quantify the pain, a coloured visual linear analogue pain scale was used (http://ergonomics.about.com/od/ergonomicbasics/ss/painscale.htm) that gives scores ranging from no (= 0) to extreme (= 10) distress, with a graded colour background ranging from green (low score), yellow (midscale score) to red (high score). The scale is standardized by referring to familiar benchmarks from daily life as single word descriptors for pain scores of 2 (annoying), 4 (uncomfortable), 6 (dreadful), 8 (horrible) and 10 (agonizing). Participants took home a coloured copy of the visual analogue scale for reference to scale and descriptors. Responses before and immediately after injection were completed before leaving the department, and post-clinic responses were obtained through telephone on the following day (for responses at 4 and 8 h, at bedtime, following morning and after 24 h) and 1 week later (for daily responses).
Data analysis
Data were analysed by mixed model and repeated measures analysis of variance (ANOVA) for longitudinal data, including covariates using NCSS 2007 software (NCSS, Kaysville, UT, USA). For the mixed model analysis, a restricted maximal likelihood approach using an autoregressive variance–covariance matrix structure allowing for different variance between time points was used. Categorical data were analyzed by Fishers exact test and associated odds ratios (OR) with 95% confidence intervals (CI) estimated using StatXact 5 software (Cytel, Boston, MA, USA). Total pain score was defined as the sum of all pain scores at the 13 study time points and any pain was defined as a pain score of > 0. Body surface area (BSA) was calculated using the Gehan–George formula 26. Results are reported as mean and SEM unless otherwise stated.
Results
The pain score outcomes and its determinants were analyzed from 168 injections administered to 125 men (Table 1), including 43 men who completed two consecutive injection treatments during the study period. Each injection required 13 pain scores, and all but two of 2 184 potential pain scores were completed (> 99.9%). All men had at least one previous TU injection before entry to the study. The data were analyzed with the injection (rather than the person) as the primary unit of analysis. The findings were unchanged if the analysis was restricted to the 125 men rather than their 168 injections as the unit of analysis other than slightly reducing the power of the analysis.
Table 1. Characteristics of patients.
| Patients' characteristics | Mean ± SEM | Quartiles | Range |
|---|---|---|---|
| Age (years) | 49 ± 1 | 39, 48, 61 | 18–77 |
| Height (cm) | 176 ± 1 | 172, 178, 182 | 154–194 |
| Weight (kg) | 88 ± 2 | 78, 86, 99 | 47–134 |
| BMI (kg m−2) | 28.2 ± 0.4 | 24.6, 27.9, 31.1 | 19.3–41.2 |
| BSA (m2) | 2.08 ± 0.03 | 1.94, 2.11, 2.22 | 1.43–2.69 |
| Earlier TU injections (number) | 5.9 ± 0.2 | 4, 6, 8 | 1–12 |
Abbreviations: BMI, body mass index; BSA, body surface area; TU, testosterone undecanote.
The pain score before injection was 0 in nearly all (96%) men. Following injection, any pain (defined as a pain score > 0) was reported after 80% of injections (Figure 1), with the peak post-injection pain score occurring most often at the first (immediate) post-injection time-point (in 98/168, 58%). The peak pain score ranged up to 7 (mean 2.0, median 2, mode 1) and exceeded a score of 4 after 40 injections (24%) and 6 or above after 4 injections (2.4%). The total and maximal pain scores were highly correlated (r = 0.80, P < 0.001). The mean pain score as well as the proportion reporting any pain at different time points returned to pre-injection levels by day 4 after injection.
Figure 1.
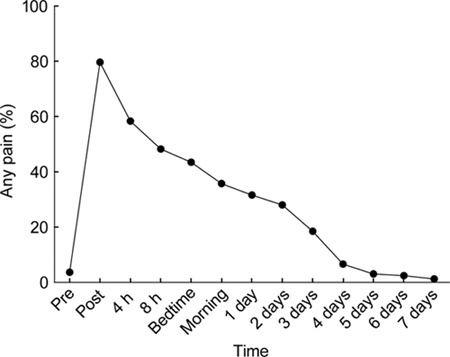
Any pain (defined as a pain score > 0) before and at various time intervals after 168 intramuscular (IM) injections of 1 000 mg testosterone undecanote (TU) in 4-mL castor oil vehicle into the upper outer quadrant of the buttock region.
Post-injection pain after any previous TU injection was common (140/168, 83%), and the time course of pain scores was significantly different (P < 0.001) according to earlier post-injection pain experience, which was a strong determinant of pain after the study injection (Figure 2). Analgesic use was associated with higher pain scores across the time course of the study (P = 0.004) and with higher total (14 ± 4 vs. 8 ± 1, P = 0.015) and maximal (3.4 ± 0.5 vs. 2.4 ± 0.1, P = 0.032) pain scores. Previous pain experience was a nonsignificant predictor of analgesic use after the study injection (17/140 [12%] vs. 1/28 [4%]; OR 3.7, exact 95% CI 0.5–162). The effect of analgesic use was not independent of previous pain experience, and when both variables were included in models, the influence of analgesic use was rendered nonsignificant (data not shown).
Figure 2.
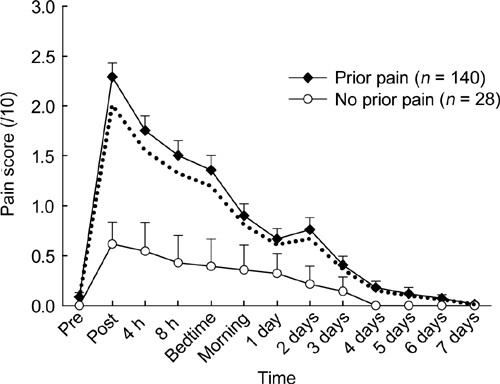
Pain scores are plotted as mean and SEM before and at various time intervals after intramuscular (IM) injection of 1 000 mg testosterone undecanote (TU) in 4-mL castor oil vehicle into the upper outer quadrant of the buttock region. The two plots correspond to men with or without a history of pain with earlier TU injections. Dotted line represents overall mean pain score for the whole study.
The time course of post-injection pain was reproducible, as there was no significant difference between time course of pain scores between first and second injection within any individual man (P = 0.90) in the 43 men who had two consecutive injections during the study period. Furthermore, the time course of pain scores for either first or second injection in the men having two injections during the study was indistinguishable (P > 0.9 for either first or second injection, data not shown) from men having only a single injection. There was a significant difference in time course of pain scores between nurses giving the injection (P = 0.01), but there were no differences in total or maximal pain score between nurses (P > 0.4).
Higher age (P < 0.001, Figure 3) and greater body mass index (BMI) (P = 0.003, Figure 4), as well as body weight and BSA (both P < 0.003, data not shown) were all significant covariates associated with consistently lower pain scores across the full-time course of the study. Using less powerful linear correlation with only a single value per person (vs. time course of 13 values), age was inversely correlated with total (r = – 0.33, P < 0.001) and maximal (r = – 0.39, P < 0.001) pain scores, but height, weight, BMI and BSA were not correlated with either of them (P > 0.4). Neither height nor number of previous injections influenced time course of pain scores, nor did they correlate with either total or maximal pain score (all P > 0.2).
Figure 3.
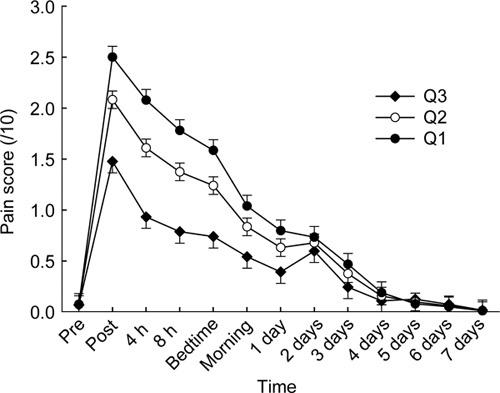
Pain scores are plotted as mean and SEM before and at various time intervals after intramuscular (IM) injection of 1 000 mg testosterone undecanote (TU) in 4-mL castor oil vehicle into the upper outer quadrant of the buttock region. The three plots correspond to the pain score time course evaluated at three quartiles for age (Q1, 39 years; Q2, 48 years, Q3, 61 years).
Figure 4.
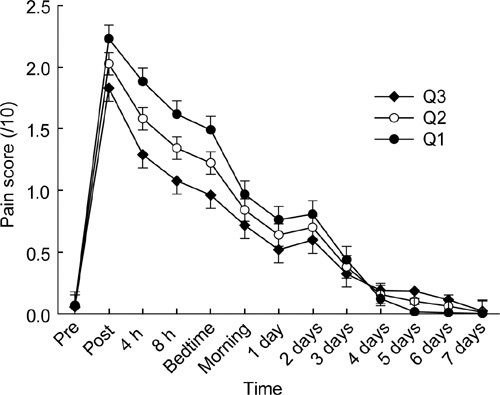
Pain scores are plotted as mean and SEM before and at various time intervals after intramuscular (IM) injection of 1 000 mg testosterone undecanote (TU) in 4-mL castor oil vehicle into the upper outer quadrant of the buttock region. The three plots correspond to the pain score time course evaluated at three quartiles for BMI (Q1, 24.6 kg m− 2; Q2, 27.9 kg m− 2; Q3, 31.1 kg m− 2).
Participants reported minimal interference in activities of daily living. No other significant or unexpected adverse effects were observed during the study. In particular, no episode of cough and/or syncopal feelings within minutes after IM oil vehicle injection, presumed to be due to venous oil microembolism 14, 24, was reported after the close scrutiny of 168 injections. The present study provides an upper confidence limit on the incidence of this idiosyncratic side effect of < 2% per injection, consistent with the previous estimate of 1.5% 24.
Discussion
The present findings confirm that post-injection pain after a deep IM injection of 4-mL TU in castor oil frequently causes pain of low to moderate severity for up to 3 days after injection 27. This post-injection pain requires only infrequent analgesic use and produces little disturbance to the daily living activities. The peak pain score occurred immediately after the injection to reach a mean pain score of < 2.5 on a 10-point scale. This corresponds most closely to a descriptor of 'annoying' and less severe than a descriptor of 'uncomfortable'. As this study included only men returning for a TU injection, the prevalence of pain could have been underestimated if men discontinuing TU treatment after a first painful injection were excluded; however, as this was rare, such underestimation is likely to have been minor. The higher prevalence of any pain reported in this study (80% vs. 29%) compared with our previous study of T enanthate administered in a 1-mL oil vehicle 24 may be because of either the larger injection volume or more systematic and intensive pain surveillance in this study. Yet such injection pain is underreported in longer and larger studies of TU administration, which record either no or infrequent injection pain 8, 9, 10, 11, 12, 13, 14.
Within that overall moderate pain experience, patient features influence the severity and time course of post-injection pain scores. The most prominent was earlier painful experience from similar injection(s), but modifying influences also included age and obesity such that higher age and weight were associated with consistently lower pain scores. The influence of earlier pain experience, but not the number of previous TU injections, is consistent with either that certain men are more prone to experience post-injection pain or that an earlier painful experience(s) with injection may sensitize or continue to influence subsequent post-injection pain experience. Similar observations supporting the latter mechanism are reported in well-controlled studies, mostly in children, showing that psychological techniques, such as distraction 28 and/or topical local anaesthetic 29 or vapour coolant 30, significantly reduce IM injection pain and may prevent entrenching of long-term needle phobia 31, 32. The role played by expectation, with or without involving semivoluntary muscular tensing before subsequent injections, in this study remains speculative. As this study provides observational data, it cannot distinguish definitively between these possibilities, whereas a truly experimental design involving creating injection pain is unlikely to be feasible. The lack of significant differences between experienced nurses in giving injections in this study may not necessarily be extrapolated to less experienced injectors such as inexperienced medical staff or those administering few injections or non-medically trained family members.
The moderating influences of higher age and body weight, but not height, on post-injection pain could have either a neurogenic (central or peripheral nerve) or local mechanism. Central and/or peripheral neurogenic differences in pain thresholds with ageing 33 and in obese individuals are described, although both increased 34, 35 and decreased 36, 37, 38 sensitivity to various pain stimuli, although not to injections, are reported in obesity. Even when obesity and altered pain thresholds co-exist, no direct relationship has been shown between them, as for example, the increased pain sensitivity of obese individuals is not altered by surgically induced weight loss 39, nor is there any relationship between obesity and reduced sensory thresholds in Prader-Willi syndrome 40. The relevance of these changes to post-injection pain remains speculative.
Although effects of obesity on pain perception are difficult to fully discount, a more plausible explanation is that greater body weight is associated with a thicker subdermal fat layer over the buttocks, modifying local tissue effects of the injection. An important variable is the subdermal fat pad thickness, which may exceed the length of standard injection needles so that an IM injection may not deposit the injectate into gluteal muscles 41, 42. On the other hand, the local inflammatory reaction to pressure and/or necrosis around the injection site does not appear much different between the skeletal muscle and fat 19. Although the volume and site of injection significantly influence pharmacokinetics of injectable androgen ester in oil vehicle in men 43, limited nonhuman experimental data suggests that the pharmacokinetics of drug delivery into the bloodstream from injectable oil vehicle depots may not differ substantially between injections into subdermal fat or muscle 20. At extremes, such injury and/or inflammatory effects may also cause rare side effects such as sciatic nerve injury following gluteal IM injections 44.
In summary, we show that local pain is a common experience after IM injection of TU in a 4-mL castor oil vehicle into the gluteal muscle. However, in most men, pain is of short duration and low intensity. These features, together with the long interval between injections, contribute to the high patient acceptability and continuation rates compared with other shorter-acting forms of T replacement therapy. Previous underreporting 8, 9, 10, 12, 13, 14 or underestimation 11, 14 of TU-related injection pain may be an unrecognized limitation for the most effective clinical uses of TU, which assume a high level of therapeutic compliance for optimal, life-long androgen replacement therapy 45, or for a well-accepted TU-based combination of hormonal male contraceptive regimens 46.
Disclosure statement
The author's department has received partial funding from BayerSchering for other investigator-initiated studies of testosterone undecanoate, but not this study.
Acknowledgments
We are grateful to Dr Fiona Blyth for helpful comments on the paper and to the support from the Swiss National Research Foundation and the Lichtenstein–Stiftung, Basel, Switzerland (GS).
References
- Greenblatt DJ, Koch-Weser J. Intramuscular injection of drugs. N Engl J Med. 1976;295:542–6. doi: 10.1056/NEJM197609022951006. [DOI] [PubMed] [Google Scholar]
- Junkman K. Long-acting steroids in reproduction. Recent Prog Horm Res. 1957;13:380–419. [PubMed] [Google Scholar]
- Altamura AC, Sassella F, Santini A, Montresor C, Fumagalli S, et al. Intramuscular preparations of antipsychotics: uses and relevance in clinical practice. Drugs. 2003;63:493–512. doi: 10.2165/00003495-200363050-00004. [DOI] [PubMed] [Google Scholar]
- Bhanji NH, Chouinard G, Margolese HC. A review of compliance, depot intramuscular antipsychotics and the new long-acting injectable atypical antipsychotic risperidone in schizophrenia. Eur Neuropsychopharmacol. 2004;14:87–92. doi: 10.1016/S0924-977X(03)00109-3. [DOI] [PubMed] [Google Scholar]
- Handelsman DJ.Androgen Physiology, Pharmacology and AbuseIn: DeGroot LJ, Jameson JL, editors. Endocrinology6th edn. Philadelphia: Elsevier Saunders; 2010. in press).
- Handelsman DJ, Mackey MA, Howe C, Turner L, Conway AJ. Analysis of testosterone implants for androgen replacement therapy. Clin Endocrinol (Oxf) 1997;47:311–6. doi: 10.1046/j.1365-2265.1997.2521050.x. [DOI] [PubMed] [Google Scholar]
- Jockenhovel F, Minnemann T, Schubert M, Freude S, Hubler D, et al. Comparison of long-acting testosterone undecanoate formulation versus testosterone enanthate on sexual function and mood in hypogonadal. men. Eur J Endocrinol. 2009;160:815–9. doi: 10.1530/EJE-08-0830. [DOI] [PubMed] [Google Scholar]
- Zitzmann M, Nieschlag E. Androgen receptor gene CAG repeat length and body mass index modulate the safety of long-term intramuscular testosterone undecanoate therapy in hypogonadal men. J Clin Endocrinol Metab. 2007. 92:3844–53. doi: 10.1210/jc.2007-0620. [DOI] [PubMed] [Google Scholar]
- Jacobeit JW, Gooren LJ, Schulte HM. Safety aspects of 36 months of administration of long-acting intramuscular testosterone undecanoate for treatment of female-to-male transgender individuals. Eur J Endocrinol. 2009;161:795–8. doi: 10.1530/EJE-09-0412. [DOI] [PubMed] [Google Scholar]
- Kamischke A, Venherm S, Ploger D, von Eckardstein S, Nieschlag E. Intramuscular testosterone undecanoate and norethisterone enanthate in a clinical trial for male contraception. J Clin Endocrinol Metab. 2001;86:303–9. doi: 10.1210/jcem.86.1.7057. [DOI] [PubMed] [Google Scholar]
- Meriggiola MC, Cerpolini S, Bremner WJ, Mbizvo MT, Vogelsong KM, et al. Acceptability of an injectable male contraceptive regimen of norethisterone enanthate and testosterone undecanoate for men. Hum Reprod. 2006;21:2033–40. doi: 10.1093/humrep/del094. [DOI] [PubMed] [Google Scholar]
- Meriggiola MC, Armillotta F, Costantino A, Altieri P, Saad F, et al. Effects of testosterone undecanoate administered alone or in combination with letrozole or dutasteride in female to male transsexuals. J Sex Med. 2008;5:2442–53. doi: 10.1111/j.1743-6109.2008.00909.x. [DOI] [PubMed] [Google Scholar]
- Mommers E, Kersemaekers WM, Elliesen J, Kepers M, Apter D, et al. Male hormonal contraception: a double-blind, placebo-controlled study. J Clin Endocrinol Metab. 2008;93:2572–80. doi: 10.1210/jc.2008-0265. [DOI] [PubMed] [Google Scholar]
- Gu Y, Liang X, Wu W, Liu M, Song S, et al. Multicenter contraceptive efficacy trial of injectable testosterone undecanoate in Chinese men. J Clin Endocrinol Metab. 2009;94:1910–5. doi: 10.1210/jc.2008-1846. [DOI] [PubMed] [Google Scholar]
- Greenblatt DJ, Allen MD. Intramuscular injection-site complications. J Am Med Assoc. 1978;240:542–4. [PubMed] [Google Scholar]
- Hay J. Complications at site of injection of depot neuroleptics. BMJ. 1995;311:421. doi: 10.1136/bmj.311.7002.421. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Steiness E, Rasmussen F, Svendsen O, Nielsen P. A comparative study of serum creatine phosphokinase (CPK) activity in rabbits, pigs and humans after intramuscular injection of local damaging drugs. Acta Pharmacol Toxicol (Copenh) 1978;42:357–64. doi: 10.1111/j.1600-0773.1978.tb02217.x. [DOI] [PubMed] [Google Scholar]
- Svendsen O, Rasmussen F, Nielsen P, Steiness E. The loss of creatine phosphokinase (CK) from intramuscular injection sites in rabbits. A predictive tool for local toxicity. Acta Pharmacol Toxicol (Copenh) 1979;44:324–8. doi: 10.1111/j.1600-0773.1979.tb02338.x. [DOI] [PubMed] [Google Scholar]
- Svendsen O, Blom L. Intramuscular injection and muscle damage: effects of concentration, volume, injection speed and vehicle. Arch Toxicol. 1984;Suppl 7:472–5. doi: 10.1007/978-3-642-69132-4_96. [DOI] [PubMed] [Google Scholar]
- Svendsen O, Blom L, Aaes-Jorgensen T, Larsen JJ. Local toxicity of different drugs after intramuscular or intralipomatous injection in pigs: serum concentrations after three different formulations of cis(Z)-clopenthixol. Acta Pharmacol Toxicol (Copenh) 1985;57:78–87. doi: 10.1111/j.1600-0773.1985.tb00014.x. [DOI] [PubMed] [Google Scholar]
- Akers MJ. Excipient-drug interactions in parenteral formulations. J Pharm Sci. 2002;91:2283–300. doi: 10.1002/jps.10154. [DOI] [PubMed] [Google Scholar]
- Balogh K. The histologic appearance of corticosteroid injection sites. Arch Pathol Lab Med. 1986;110:1168–72. [PubMed] [Google Scholar]
- Symmers W. Simulation of cancer by oil granuloma of therapeutic origin. Br Med J. 1955;2:1536–9. doi: 10.1136/bmj.2.4955.1536. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mackey MA, Conway AJ, Handelsman DJ. Tolerability of intramuscular injections of testosterone ester in an oil vehicle. Hum Reprod. 1995;10:862–5. doi: 10.1093/oxfordjournals.humrep.a136051. [DOI] [PubMed] [Google Scholar]
- Kelleher S, Conway AJ, Handelsman DJ. Blood testosterone threshold for androgen deficiency symptoms. J Clin Endocrinol Metab. 2004;89:3813–7. doi: 10.1210/jc.2004-0143. [DOI] [PubMed] [Google Scholar]
- Bailey BJ, Briars GL. Estimating the surface area of the human body. Stat Med. 1996;15:1325–32. doi: 10.1002/(SICI)1097-0258(19960715)15:13<1325::AID-SIM233>3.0.CO;2-K. [DOI] [PubMed] [Google Scholar]
- Saad F, Kamischke A, Yassin A, Zitzmann M, Schubert M, et al. More than eight years' hands-on experience with the novel long-acting parenteral testosterone undecanoate. Asian J Androl. 2007;9:291–7. doi: 10.1111/j.1745-7262.2007.00275.x. [DOI] [PubMed] [Google Scholar]
- Fowler-Kerry S, Lander JR. Management of injection pain in children. Pain. 1987;30:169–75. doi: 10.1016/0304-3959(87)91072-4. [DOI] [PubMed] [Google Scholar]
- Halperin BA, Halperin SA, McGrath P, Smith B, Houston T. Use of lidocaine-prilocaine patch to decrease intramuscular injection pain does not adversely affect the antibody response to diphtheria-tetanus-acellular pertussis-inactivated poliovirus-Haemophilus influenzae type b conjugate and hepatitis B vaccines in infants from birth to six months of age. Pediatr Infect Dis J. 2002;21:399–405. doi: 10.1097/00006454-200205000-00010. [DOI] [PubMed] [Google Scholar]
- Mawhorter S, Daugherty L, Ford A, Hughes R, Metzger D, et al. Topical vapocoolant quickly and effectively reduces vaccine-associated pain: results of a randomized, single-blinded, placebo-controlled study. J Travel Med. 2004;11:267–72. doi: 10.2310/7060.2004.19101. [DOI] [PubMed] [Google Scholar]
- Nir Y, Paz A, Sabo E, Potasman I. Fear of injections in young adults: prevalence and associations. Am J Trop Med Hyg. 2003;68:341–4. [PubMed] [Google Scholar]
- Jacobson RM, Swan A, Adegbenro A, Ludington SL, Wollan PC, et al. Making vaccines more acceptable–methods to prevent and minimize pain and other common adverse events associated with vaccines. Vaccine. 2001;19:2418–27. doi: 10.1016/s0264-410x(00)00466-7. [DOI] [PubMed] [Google Scholar]
- Gagliese L. Pain and aging: the emergence of a new subfield of pain research. J Pain. 2009;10:343–53. doi: 10.1016/j.jpain.2008.10.013. [DOI] [PubMed] [Google Scholar]
- McKendall MJ, Haier RJ. Pain sensitivity and obesity. Psychiatry Res. 1983;8:119–25. doi: 10.1016/0165-1781(83)90099-9. [DOI] [PubMed] [Google Scholar]
- Pradalier A, Dry J, Willer JC, Boureau F. [Obesity and the nociceptive reflex (author's transl)] Pathol Biol (Paris) 1980;28:462–4. [PubMed] [Google Scholar]
- Zahorska-Markiewicz B, Zych P, Kucio C. Pain sensitivity in obesity. Acta Physiol Pol. 1988;39:183–7. [PubMed] [Google Scholar]
- Khimich S. Level of sensitivity of pain in patients with obesity. Acta Chir Hung. 1997;36:166–7. [PubMed] [Google Scholar]
- Miscio G, Guastamacchia G, Brunani A, Priano L, Baudo S, et al. Obesity and peripheral neuropathy risk: a dangerous liaison. J Peripher Nerv Syst. 2005;10:354–8. doi: 10.1111/j.1085-9489.2005.00047.x. [DOI] [PubMed] [Google Scholar]
- Buskila D, Neumann L, Malkin S, Levi I. Does nonarticlar tenderness change after bariatric surgery. Obes Surg. 2005;15:1243–6. doi: 10.1381/096089205774512609. [DOI] [PubMed] [Google Scholar]
- Priano L, Miscio G, Grugni G, Milano E, Baudo S, et al. On the origin of sensory impairment and altered pain perception in Prader-Willi syndrome: A neurophysiological study. Eur J Pain. 2009;13:829–35. doi: 10.1016/j.ejpain.2008.09.011. [DOI] [PubMed] [Google Scholar]
- Cockshott WP, Thompson GT, Howlett LJ, Seeley ET. Intramuscular or intralipomatous injections. N Engl J Med. 1982;307:356–8. doi: 10.1056/NEJM198208053070607. [DOI] [PubMed] [Google Scholar]
- Burbridge BE. Computed tomographic measurement of gluteal subcutaneous fat thickness in reference to failure of gluteal intramuscular injections. Can Assoc Radiol J. 2007;58:72–5. [PubMed] [Google Scholar]
- Minto C, Howe C, Wishart S, Conway AJ, Handelsman DJ. Pharmacokinetics and pharmacodynamics of nandrolone esters in oil vehicle: effects of ester, injection site and volume. J Pharmacol Exp Ther. 1997;281:93–102. [PubMed] [Google Scholar]
- Small SP. Preventing sciatic nerve injury from intramuscular injections: literature review. J Adv Nurs. 2004;47:287–96. doi: 10.1111/j.1365-2648.2004.03092.x. [DOI] [PubMed] [Google Scholar]
- Aminorroaya A, Kelleher S, Conway AJ, Ly LP, Handelsman DJ. Adequacy of androgen replacement influences bone density response to testosterone in androgen-deficient men. Eur J Endocrinol. 2005;152:881–6. doi: 10.1530/eje.1.01920. [DOI] [PubMed] [Google Scholar]
- Handelsman DJ.Male ContraceptionIn: DeGroot LJ, Jameson JL, editors. Endocrinology6th edn. Philadelphia: Elsevier Saunders; 2010. in press).


