Abstract
Erectile dysfunction (ED) is a major complication of diabetes mellitus. Icariin has been shown to enhance erectile function through its bioactive form, icarisid II. This study investigates the effects of icarisid II on diabetic rats with ED and its potential mechanism via the assessment of advanced glycosylation end products (AGEs), autophagy, mTOR and the NO–cGMP pathway. Icarisid II was extracted from icariin by an enzymatic method. In the control and diabetic ED groups, rats were administered normal saline; in the icarisid II group, rats were administered icarisid II intragastrically. Erectile function was evaluated by measuring intracavernosal pressure/mean arterial pressure (ICP/MAP). AGE concentrations, nitric oxide synthase (NOS) activity and cGMP concentration were assessed by enzyme immunoassay. Cell proliferation was analysed using methyl thiazolyl tetrazolium assay and flow cytometry. Autophagosomes were observed by transmission electron microscopy, monodansylcadaverine staining and GFP-LC3 localisation. The expression of NOS isoforms and key proteins in autophagy were examined by western blot. Our results have shown that Icarisid II increased ICP/MAP values, the smooth muscle cell (SMC) growth curve, S phase and SMC/collagen fibril (SMC/CF) proportions and decreased Beclin 1 (P<0.05). Icarisid II significantly increased the proliferative index and p-p70S6K(Thr389) levels and decreased the numbers of autophagosomes and the levels of LC3-II (P<0.01). Icarisid II decreased AGE concentrations and increased cGMP concentration, NOS activity (P<0.05) and cNOS levels (P<0.01) in the diabetic ED group. Therefore, Icarisid II constitutes a promising compound for diabetic ED and might be involved in the upregulation of SMC proliferation and the NO–cGMP pathway and the downregulation of AGEs, autophagy and the mTOR pathway.
Keywords: advanced glycosylation end products (AGEs), autophagy, cell proliferation, diabetes mellitus (DM), erectile dysfunction (ED), icariin, icarisid II, mTOR, NO–cGMP, NOS activity
Introduction
Currently, some phosphodiesterase type 5 (PDE5) inhibitors have been widely used in the treatment of erectile dysfunction (ED).1,2,3 However, these drugs have many side effects, such as headaches and visual impairment, which show that it is impending for further research with highly selective PDE5 inhibitor and for the development of natural drugs.4 Investigations have suggested that the most metabolically active extract of Epimedium is icariin, which has been shown to exert inhibitory effects against PDE5.5 In addition to its erotogenic role, icariin has demonstrated testosterone-mimetic properties.6 Icariin has been shown to increase the intracavernous pressure in rats, which could be abolished nitric oxide synthase (NOS) and guanylate cyclase inhibitors.7 Furthermore, icariin has successfully ameliorated both castration-related and arteriogenic impairment of erectile function and has reduced penile neuron NOS concentration in a rodent model.8
However, the effect of icariin in inhibiting PDE5 is much weaker.9 Therefore, chemical modifications of the native structure of icariin have been extensively conducted to achieve high PDE5 inhibitory activity and multiple effects in the NO–cGMP pathway.10 In 2008, a modification of the native icariin compound with two hydroxyethyl moieties enhanced the inhibition of PDE5 80-fold.9 Icarisid II has also been successfully isolated and assessed its PDE5 inhibitory effect.11,12 This study investigates the effect of icarisid II on diabetic rats with ED and its potential mechanism via assessment of advanced glycosylation end products (AGEs), autophagy, mTOR and the NO–cGMP pathway.
Materials and methods
Animals and treatment
All experimental protocols were approved by the Institutional Animal Care and Use Committee at Peking University (Beijing, China). Male Wistar rats (Grade A, certificate no. scxk11-00-0006) were obtained from the Animal Breeding Center at the Peking University Health Science Center. Thirty rats with normal function with intracavernosal pressure/mean arterial pressure (ICP/MAP) values greater than 0.6 were chosen and classified as the control group. Sixty diabetic rats with erectile dysfunction and ICP/MAP values less than 0.45 were chosen from streptozotocin-induced diabetic male rats and were randomly divided into the diabetic ED group (n=30) and the icarisid II group (n=30). The rats were administered normal saline in the control and diabetic ED groups. In the icarisid II group, the rats were administered 10 mg/kg icarisid II intragastrically every day for 8 weeks. Body weights and glucometer tail-vein blood glucose levels (Bayer HealthCare, Tarrytown, NY, USA) were measured biweekly. Corpus cavernosum tissue specimens and blood samples were obtained from every rat in each group and were stored at −20 °C after 8 weeks.
ICP and MAP measurements
To exclude the impact of individual differences, we evaluated erection function using ICP/MAP. Before and after the eight-week treatment, surgery was performed under 2% isoflurane anaesthesia at 37 °C. The right corpora cavernosa and carotid arteries were isolated and cannulated with 23-G butterfly needles primed with 250 U/ml heparin-saline solution and were connected to a pressure transducer (Utah Medical Products, Midvale, UT, USA) for ICP and MAP measurements. The right cavernous nerve near the major pelvic ganglion was identified for electric stimulation (20 Hz, 5 V and 60 s).
Masson trichrome staining of cavernous tissue
To exclude the impact of individual differences, we evaluated the cavernous tissue via smooth muscle cell/collagen fibril (SMC/CF) staining. The cavernous tissue specimens from each group were fixed in 2% paraformaldehyde and embedded in paraffin. Sections (3 µm in thickness) were then stained with a Masson trichrome staining kit (Sigma-Aldrich, St Louis, MO, USA). The corpus cavernosum SMCs were red, and CFs were blue.
Glucose, AGEs, NOS activity and cGMP assay
Blood specimens were checked for blood glucose levels using the Roche Diagnostics Cobas Integra 400 Plus assay system (Roche Diagnostics, Indianapolis, IN, USA). Corpus cavernosum tissue specimens from each group were performed for AGEs (Cell Biolabs, Inc. San Diego, CA, USA), NOS activity and cGMP (Cayman Chemicals, Ann Arbor, MI, USA) with the corresponding ELISA kits according to the manufacturer's instructions.
Cell harvests, cell growth curve assay and cell cycle analysis
The primary SMCs were isolated from every rat of each group and were cultured in Dulbecco's modified Eagle's medium supplemented with 10% foetal calf serum, 100 U ml−1 penicillin and 100 mg ml−1 streptomycin (Invitrogen, Carlsbad, CA, USA). These primary rat SMCs were used in the corresponding experiments.
Cell proliferation capacity was investigated via a cell growth curve drawn using the MTT method, and the cell cycle was analysed using a flow cytometer (FACS; Becton Dickinson, Franklin Lakes, NJ, USA). The optical density (o.d.) values were measured at 570 nm and 630 nm with a microplate reader (Bio-Rad, Hercules, CA, USA). The growth curve was constructed according to o.d. values. Cell cycle S phase (DNA synthesis period) analysis and proliferative index (PI) were evaluated, with PI=(S+G2M)/(G0G1+S+G2M). For each sample, 2×105 cells were measured.
Autophagosome observations using transmission electron microscopy (TEM), monodansylcadaverine (MDC) staining or GFP-LC3 localisation
The SMCs were post-fixed in osmium tetroxide (OsO4) and embedded in Epon. Sections were then stained with uranyl acetate/lead citrate (Sigma-Aldrich) and viewed with a JEM1230 transmission electron microscope (JEOL, Tokyo, Japan). The SMCs on cover slips were stained with MDC (Sigma-Aldrich) and were observed with an SP5 confocal system (Leica, Solms, German) with excitation and emission filters with wavelengths of 380 nm and 525 nm, respectively.
The SMCs were transfected with GFP-LC3 plasmid using LipofectamineTM2000 reagent (Invitrogen) and were observed for LC3 distribution using the SP5 confocal system. Fifty non-overlapping SMCs in each specimen were randomly selected for autophagosome distribution analysis via TEM, MDC and GFP-LC3 assays.
Protein isolation and Western blot analysis
Cell lysates containing 100 µg protein were electrophoresed using sodium dodecyl sulphate–polyacrylamide gel electrophoresis and were then transferred to a polyvinylidene fluoride membrane (Millipore Corp., Bedford, MA, USA). Detection of target proteins on the membranes was performed with an electrochemiluminescence kit (Amersham Life Sciences Inc., Arlington Heights, IL, USA) using primary antibodies for nNOS, iNOS, eNOS, LC3-I/II, Beclin 1, p70S6K, p-p70S6K(Thr389) and β-actin (1∶1000, all antibodies were from Santa Cruz Biotech, Santa Cruz, CA, USA). After the hybridisation of the secondary antibodies, the resulting images were analysed using a BioRad GS-670 densitometer (BioRad) and UTHSCSA Image Tool for Windows (3.0) (University of Texas Medical School at San Antonio, San Antonion, TX, USA) to determine the integrated density value of each protein band.
Statistical analysis
All experiments were repeated three times, and the similar results were obtained. Data were expressed as mean±s.d. and were analysed using one-way ANOVA in the SPSS13.0 software package (SPSS Inc., Chicago, IL, USA). P<0.05 was considered statistically significant.
Results
Effects of icarisid II on ICP/MAP and SMCs/CF measurement
Compared to the diabetic ED group, the values for the ICP/MAP and SMC/CF measurements were higher in the icarisid II (P<0.05)and control groups (P<0.01). The values were lower in the icarisid II group than in the control group (P<0.05) (Figures 1 and 2).
Figure 1.
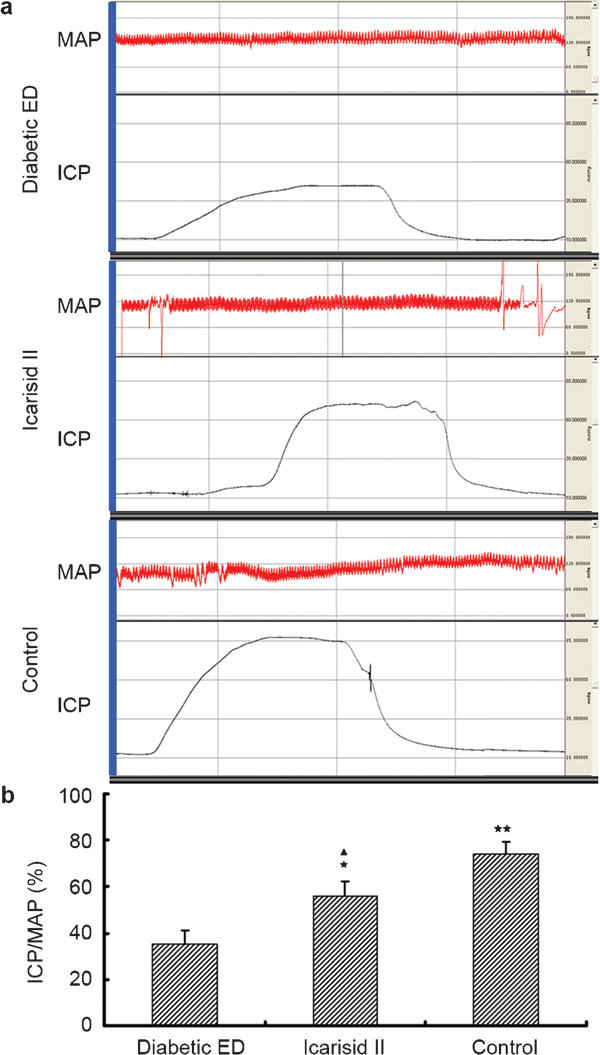
The ratio of intracavernosal pressure and mean arterial pressure (ICP/MAP) was investigated to evaluate erectile function in each group. (a) A Biopac physiograph displays the ICP (red curve) and MAP (black curve) values of representative rats in each group. (b) ICP/MAP levels were analysed with acqKnowledge software (BioPac Systems, Santa Barbara, CA,USA) and ANOVA. ★★P<0.01, ★P<0.05 vs. diabetic ED group. ▴P<0.05 vs. control group.
Figure 2.
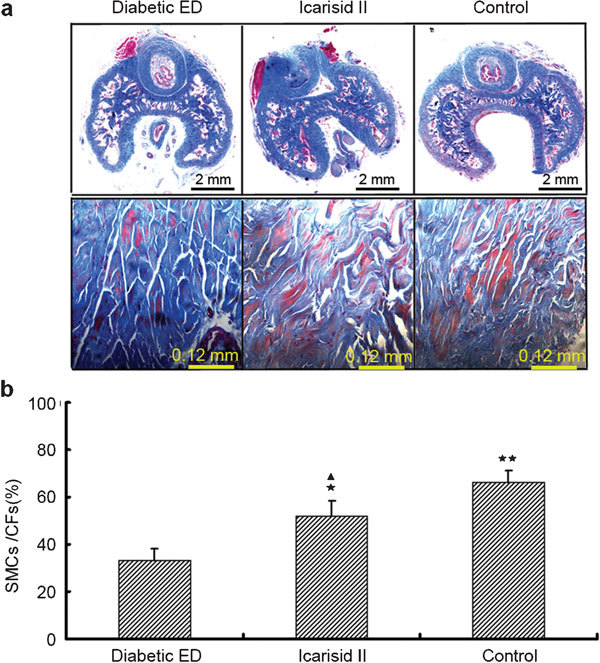
Morphological analysis of the ratio of smooth muscle cells/collagen fibrils (SMCs/CFs) in the corpus spongiosum from a representative of each group. (a) Masson's trichrome staining shows SMCs (red areas) and CFs (blue areas) of representative rats in each group. (b) The morphometric analysis of the area fraction (SMCs/CFs) was calculated using Leica QWin Pro V2.6 image analysis and processing software (Leica DMIRB, Leica, Wetzlar, Germany). Data are presented as the area fraction means from the 20 visual fields randomly selected from every specimen in each group. The differences were analysed by ANOVA. ★★P<0.01, ★P<0.05 vs. diabetic ED group. ▴P<0.05 vs. control group.
Effects of icarisid II on cell multiplication using cell growth curve and cycle analysis
Compared to the diabetic ED group, the SMCs grew faster and the o.d. values were greater in the icarisid II and control groups, especially at days 5, 6 and 7 (P<0.05) (Figure 3a). Compared to the diabetic ED group, the percentages of cells in S phase (DNA synthesis period) were higher in the icarisid II (P<0.05) and control groups (P<0.01), and the PIs were significantly higher in the icarisid II and control groups (P<0.01). However, there was no statistical difference between the icarisid II group and the control group (Figure 3).
Figure 3.
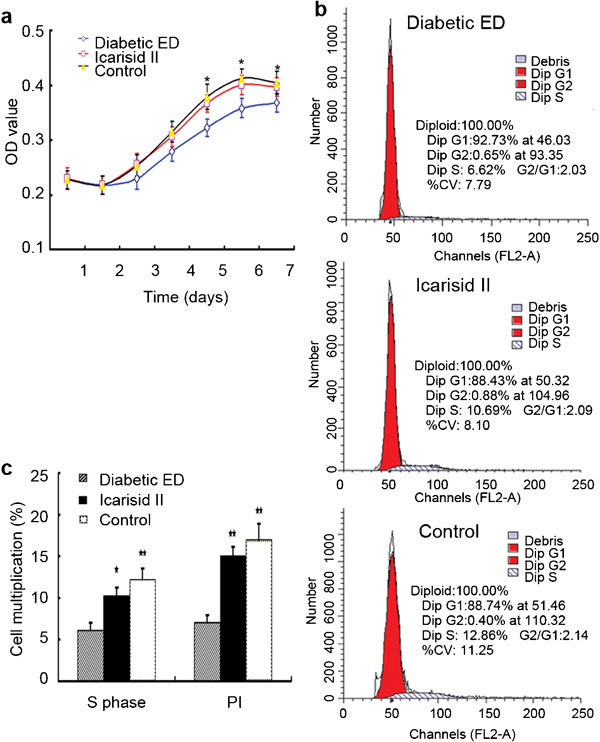
The cell growth curve and cell cycle analysis in the smooth muscle cells (SMCs) from each group. (a) The cell growth curve was assayed from the optical density (o.d.) values using the MTT method. (b) The cell cycle was analysed by flow cytometry. (c) The differences were then analysed by ANOVA. ★★P<0.01 and ★P<0.05 vs. the diabetic ED group. PI, proliferative index.
Effects of icarisid II on autophagosomes using TEM, MDC and GFP-LC3 localisation assays
Compared to the diabetic ED group, autophagosome quantities were significantly lower in the icarisid II and control groups (P<0.01). However, autophagosome quantities in the icarisid II group were still greater than those in the control group (P<0.05) (Figure 4).
Figure 4.
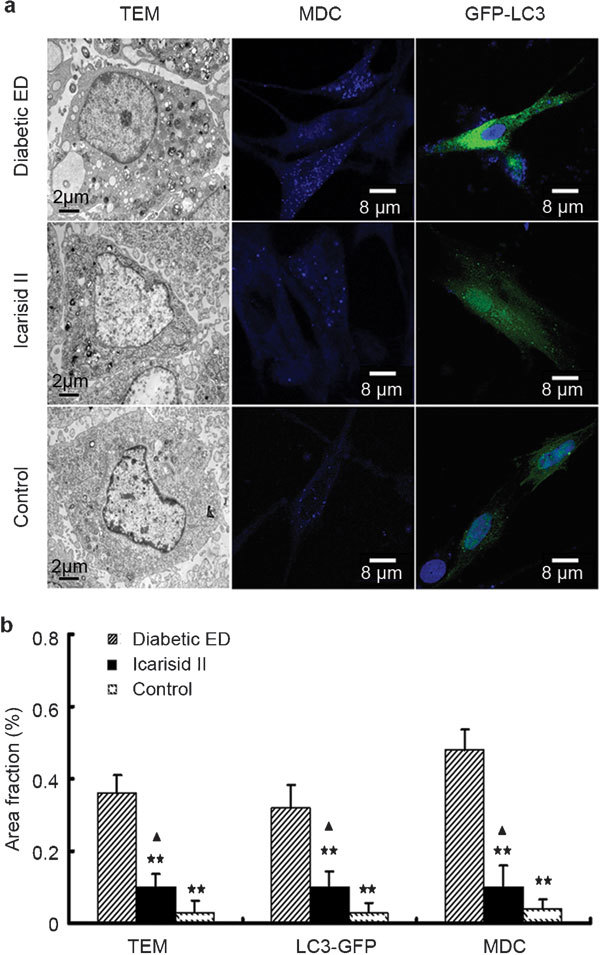
Morphological analysis of autophagy in the smooth muscle cells (SMCs) from each group. (a) Punctate autophagosomes were observed using transmission electron microscopy monodansylcadaverine staining and GFP-LC3 transfection localisation methods. (b) The area fractions (autophagosomes/cytoplasm) were calculated using Leica QWin Pro V2.6 image analysis and processing software. Data were presented as the means of the area fractions (autophagosomes/cytoplasm) in the 50 cells randomly selected from every specimen in each group. The differences were then analysed by ANOVA. ★★P<0.01 vs. the diabetic erectile dysfunction (ED) group. ▴P<0.05 vs. the control group.
Effects of icarisid II on the expression levels of key proteins in the mTOR pathway
Compared to the diabetic ED group, LC3-II and Beclin 1 expression levels were significantly lower in the icarisid II group (P<0.01 and P<0.05, respectively), and they were significantly lower in the control group (P<0.01). Compared to the diabetic ED group, p-p70S6K(Thr389) expression levels were significantly higher in the icarisid II and control groups (P<0.01). However, LC3-II and Beclin 1 expression levels were still higher in the icarisid II group than those in the control group (P<0.05 and P<0.01, respectively), and the p-p70S6K(Thr389) expression level was still lower in the icarisid II group than that in the control group (P<0.01). However, the p70S6K expression levels were not significantly different (P>0.05) (Figure 5).
Figure 5.
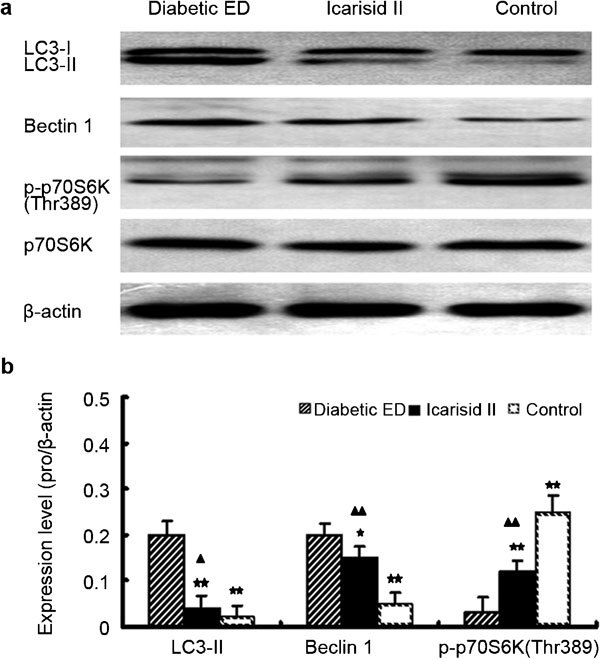
Expression of mTOR signalling pathway proteins in the corpus cavernosum tissue from each group. (a) Western blot of LC3-I/II, Beclin 1, p70S6K, p-p70S6K(Thr389) and β-actin. (b) The relative expression levels were calculated by normalising to β-actin. The differences were then analysed by ANOVA. ★★P<0.01 and ★P<0.05 vs. the diabetic erectile dysfunction (ED) group. ▴▴P<0.01 and ▴P<0.05 vs. the control group.
Effects of icarisid II on glucose level, AGE concentrations, NOS activity and cGMP concentration
Blood glucose levels were lower in the control group than those in the diabetic ED and icarisid II groups (P<0.01). Concentrations of AGEs were higher in the diabetic ED group than those in the icarisid II and control groups (P<0.05 and P<0.01, respectively), and AGE concentrations were higher in the icarisid II group than those in the control group (P<0.05). NOS activity levels and cGMP concentrations were lower in the diabetic ED group than those in the icarisid II and control group (P<0.05 and P<0.01, respectively), but they were still lower in the icarisid II group compared to the control group (P<0.05) (Table 1).
Table 1. Comparisons of glucose levels, AGE concentrations, NOS activity levels and cGMP concentrations among groups (x±s.d.).
| Group | Icarisid | Control | Diabetic ED |
|---|---|---|---|
| Blood sugar (mmol l−1) | 23.71±3.92 | 6.87±0.83★★ | 29.06±4.75 |
| AGEs (pg ml−1) | 54.28±2.76★,▴ | 48.83±2.19★★ | 62.35±4.63 |
| NOS activity (U mg−1) | 0.36±0.04★,▴ | 0.49±0.06★★ | 0.21±0.04 |
| cGMP content (pmol mg−1 min−1) | 0.42±0.04★,▴ | 0.53±0.06★★ | 0.27±0.04 |
P<0.01 and bP<0.05 vs. the diabetic ED group.
P<0.05 vs. the control group.
Effects of icarisid II on the expression of NOS isoforms
Compared to the diabetic ED group, nNOS and eNOS expression levels were higher in the icarisid II and control groups (P<0.01), and iNOS expression levels were lower in the icarisid II and control groups (P<0.01). However, the eNOS expression level was still lower in the icarisid II group compared with the control group (P<0.01) (Figure 6).
Figure 6.
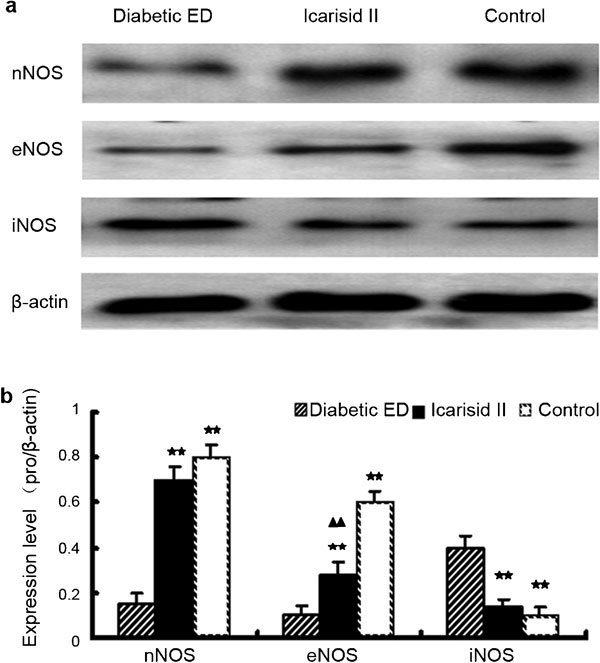
Expression of nitric oxide synthase (NOS) isoforms in the corpus cavernosum tissue from each group. (a) Western blot of nNOS, eNOS and iNOS expression. (b) The relative expression levels were calculated by normalising to β-actin. The differences were then analysed by ANOVA. ★★P<0.01 vs. the diabetic erectile dysfunction (ED) group. ▴▴P<0.01 vs. the control group.
Discussion
Icarisid II might enhance the proliferation of SMCs and attenuate excessive autophagy in diabetic ED rats by regulating the mTOR signalling pathway
It is well known that SMCs/CFs are significantly reduced in the erectile tissue of ED patients.13,15 The renin-angiotensin system plays an important role in causing SMC fibrosis, and angiotensin II type I receptor blockers and angiotensin-sconverting enzyme inhibitors can ameliorate SMC fibrosis and extend the life span of SMCs in animal models.16,19 Tankyrase 1 also has a similar effect.14,15 This study determined that a decrease in erectile function coincided with the downregulation of SMC proliferation and SMC/CF proportions in diabetic ED rats and that icarisid II could ameliorate these effects.
The autophagy phenomenon was first recorded with the observation of autophagosomes by TEM in 1962, which is considered the diagnostic gold standard of autophagy detection.20 MDC staining, GFP-LC3 fluorescent localisation and key protein expression levels by Western blot are normally used for autophagy study.21 LC3 is located on the membrane surfaces of preautophagic vacuoles and autophagic vacuoles. LC3-II reflects autophagy activity to some extent and is a common membrane marker for autophagic vacuoles.22 This study found that excessive autophagy occurred in diabetic ED rats and was attenuated by icarisid II, which was confirmed by LC3-II expression by western blot and with autophagosome characterisation via TEM, MDC staining and GFP-LC3 localisation.
The signal transduction molecules in autophagy are complex and the mTOR pathway is widely studied in autophagy.23 TOR kinase is a negative control element in autophagy and might play an important role in the regulation of cell growth.24,25 Beclin 1 is a regulatory protein in autophagy.26 Beclin 1 is involved in autophagosome formation and forms a complex with class III PI3K.27,28 Beclin 1 participates in the formation of autophagosomes and plays an important role in cell growth by regulating autophagy activity;29 studies have shown that autophagy activity is lower in Beclin 1 knockout mice30 and that Beclin 1 is critical for autophagy activity.31 P70S6K regulates 5′-TOP mRNA translation and biosynthesis, which plays a critical role in the growth of the cytoskeleton through the phosphorylation of the S6 protein.32,33 This study determined that Beclin 1 levels were significantly increased in diabetic ED rats and that icarisid II decreased these levels. This study also found that the proliferation of SMCs and p-p70S6K(Thr389) levels were significantly decreased in diabetic ED rats and that icarisid II treatment resulted in increases in both.
This study found that the decreases in erectile function, SMC proliferation and SMC/CF proportions coincided with autophagy upregulation and mTOR pathway downregulation in diabetic ED rats.
Icarisid II might ameliorate erectile function in diabetic ED rats via a downregulation in AGE concentrations and an upregulation in the NO–cGMP pathway
Diabetes mellitus, the third serious chronic disease in humans, may induce diabetic ED.34 Corpus cavernosum SMC fibrosis and cavernous nerve damage occurs in nearly 70% of diabetic patients.35,36 In such patients, treatment with existing drugs is almost always ineffectual because drugs such as PDE5 inhibitors require the presence of intact and undamaged penile nerves and SMCs. Therefore, there is a great need for a new compound that has the capacity to help regenerate damaged nerves and SMCs.
We have reported that icariin preserved erectile function in castrated rats with nNOS preservation.37 Further, icariin stimulated myocardial cell differentiation by the generation of reactive oxygen species, which increased p38MAPK levels.38 Chung et al.39 found that icariin increased MEK/ERK and PI3K/Akt/eNOS path-dependent protein levels in human umbilical vein endothelial cells. Icariin has also been shown to enhance eNOS expression and NO production in human endothelial cells and to decrease caspase-3 expression and cellular apoptosis in response to hydrogen peroxide.40 Icarisid II has been reported to be the main bioactive form of icariin in vivo.41 We innovatively isolated and purified icarisid II and found that icarisid II increases intracellular cGMP levels through the enhancement of nNOS expression and NOS activity in rat corpus cavernosum tissue in vitro.42
AGEs form as the result of the nonenzymatic glycosylation of proteins through a process by which a reducing sugar attaches to an amino group of an amino acid residue and then undergoes rearrangement to form a ketoamine-linked sugar.43,44 AGEs accumulate in diabetic tissue, and AGE concentrations are increased in the penile cavernosal smooth muscle tissues and the vascular beds of diabetic ED patients.45 AGEs interfere with ion channels, gap junctions and receptors so that calcium ion release and blood flow both decrease; as a result, the SMC relaxation mechanism is impaired.46 Seftel et al.47 reported that AGEs quenched the production of epoxide and NO in endotheliocytes and speculated on a pathophysiologic mechanism for AGE-mediated ED via the upregulation of iNOS and the downregulation of eNOS. However, Chen et al.48 reported that AGEs could attenuate the activity of cNOS (nNOS and eNOS) and could increase iNOS activity in rat cavernosum tissue, resulting in the impairment of penile erectile function. Ishibashi reported that vardenafil could block the AGE-induced upregulation of MCP-1 mRNA levels in HUVECs by suppressing AGE receptor expression levels and subsequent ROS generation via the elevation of cGMP levels.49
This study found that a decrease in erectile function coincided with an upregulation in AGE concentrations and a downregulation in the NO–cGMP pathway in diabetic ED rats and that icarisid II could ameliorate these effects. We also observed that icarisid II increased erectile function, SMC proliferation and SMC/CF proportions and decreased AGE concentrations in diabetic ED rats. These findings might be related to the upregulation of the NO–cGMP pathway and the downregulation of autophagy and the mTOR pathway. Further study is needed.
Author contributions
JZ carried out the design of the study, drafted the manuscript and participated in every parts of the experiment. AML participated in the design of the study, performed the statistical analysis, Protein isolation and Western blot analysis. BXL participated in the design of the study, Intracavernosal pressure (ICP) and mean arterial pressure (MAP) measurement. FH participated in cell harvest, cell growth curve and cell cycle analysis. FL participated in NOS activity. SZX participated in Masson trichrome staining of cavernous tissue. GQK participated in Masson trichrome staining of cavernous tissue. SJC participated in animals and treatment. CGM participated in cGMP assay. XL participated in Glucose and AGEs. SPS participated in Statistical Analysis. ZLJ participated in the design of the study, Autophagosome observation with transmission electron microscopy (TEM), Monodansylcadaverine (MDC) staining or GFP-LC3 localization. ZCX participated in the design of the study, conceived of the study, participated in its design and coordination, helped to draft the manuscript and have given final approval of the version to be published. All authors read and approved the final manuscript.
Acknowledgments
We are grateful to Tamotsu Yoshimori for providing the GFP-LC3 plasmid. This work was supported by the National Natural Science Foundation of China (No. 30670818 and NO.30772285).
The authors have no conflicting interests to disclose.
References
- Lin CS, Xin ZC, Lue TF. Phosphodiesterases as therapeutic targets. Urology. 2003;61:685–91. doi: 10.1016/s0090-4295(02)02439-1. [DOI] [PubMed] [Google Scholar]
- Sharma R. Novel phosphodiesterase-5 inhibitors: current indications and future directions. Indian J Med Sci. 2007;61:667–79. [PubMed] [Google Scholar]
- Corbin JD, Francis SH. Pharmacology of phosphodiesterase-5 inhibitors. Int J Clin Pract. 2002;56:453–9. [PubMed] [Google Scholar]
- Küthe A, Montorsi F, Andersson KE, Stief CG. Phosphodiesterase inhibitors for the treatment of erectile dysfunction. Curr Opin Investig Drugs. 2002;3:1489–95. [PubMed] [Google Scholar]
- Jiang Z, Hu B, Wang J, Tang Q, Tan Y, et al. Effect of icariin on cyclic GMP levels and on the mRNA expression of cGMP-binding cGMP-specific phosphodiesterase (PDE5) in penile cavernosum. J Huazhong Univ Sci Technolog Med Sci. 2006;26:460–2. doi: 10.1007/s11596-006-0421-y. [DOI] [PubMed] [Google Scholar]
- Zhang ZB, Yang QT. The testosterone mimetic properties of icariin. Asian J Androl. 2006;8:601–5. doi: 10.1111/j.1745-7262.2006.00197.x. [DOI] [PubMed] [Google Scholar]
- Ning H, Xin ZC, Lin G, Banie L, Lue TF, et al. Effects of icariin on phosphodiesterase-5 activity in vitro and cyclic guanosine monophosphate level in cavernous smooth muscle cells. Urology. 2006;68:1350–4. doi: 10.1016/j.urology.2006.09.031. [DOI] [PubMed] [Google Scholar]
- Tian L, Xin ZC, Liu WJ, Yang YM, Liu G, et al. Effects of icariin on the erectile function and expression of nitrogen oxide synthase isoforms in corpus cavernosum of arteriogenic erectile dysfunction rat model. Zhonghua Yi Xue Za Zhi. 2004;84:954–7. [PubMed] [Google Scholar]
- Xin ZC, Kim EK, Lin CS, Liu WJ, Tian L, et al. Effects of icariin on cGMP-specific PDE5 and cAMP-specific PDE4 activities. Asian J Androl. 2003;5:15–8. [PubMed] [Google Scholar]
- Xin H, Li WR, Zhou F, Li GY, et al. Effects of icariin on improving erectile function in streptozotocin-induced diabetic rats. J Sex Med. 2011;8:2761–72. doi: 10.1111/j.1743-6109.2011.02421.x. [DOI] [PubMed] [Google Scholar]
- Dell'Agli M, Galli GV, Dal Cero E, Belluti F, Matera R, et al. Potent inhibition of human phosphodiesterase-5 by icariin derivatives. J Nat Prod. 2008;71:1513–7. doi: 10.1021/np800049y. [DOI] [PubMed] [Google Scholar]
- Li WK, Zhang RY, Xiao PG. Flavonoids from Epimedium wanshanense. Phytochemistry. 1996;43:527–30. doi: 10.1016/0031-9422(96)00187-2. [DOI] [PubMed] [Google Scholar]
- Wang H, Liu Y, Huai Q, Cai J, Zoraghi R, et al. Multiple conformations of phosphodiesterase-5: implications for enzyme function and drug development. J Biol Chem. 2006;281:21469–79. doi: 10.1074/jbc.M512527200. [DOI] [PubMed] [Google Scholar]
- Zhang J, Wu XJ, Yuan YM, Liu BX, Cui WS, et al. Relationship of Tankyrase 1 and autophagy on erectile dysfunction in aging rats. Chin J Androl. 2010;24:7–11. [Google Scholar]
- Zhang J, Wu XJ, Zhuo DX, Liu T, Li WR, et al. Effect of tankyrase 1 on autophagy in the corpus cavernosum smooth muscle cells from ageing rats with erectile dysfunction and its potential mechanism. Asian J Androl. 2010;12:744–52. doi: 10.1038/aja.2010.44. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhu YC, Zhu YZ, Gohlke P, Stauss HM, Unger T. Effects of angiotensin-converting enzyme inhibition and angiotensin II AT1 receptor antagonism on cardiac parameters in left ventricular hypertrophy. Am J Cardiol. 1997;80:110A–7A. doi: 10.1016/s0002-9149(97)00465-7. [DOI] [PubMed] [Google Scholar]
- Kuno A, Yamada T, Masuda K, Ogawa K, Sogawa M, et al. Angiotensin-converting enzyme inhibitor attenuates pancreatic inflammation and fibrosis in male Wistar Bonn/Kobori rats. Gastroenterology. 2003;124:1010–9. doi: 10.1053/gast.2003.50147. [DOI] [PubMed] [Google Scholar]
- Yamada T, Kuno A, Ogawa K, Tang M, Masuda K, et al. Combination therapy with an angiotensin-converting enzyme inhibitor and an angiotensin II receptor blocker synergistically suppresses chronic pancreatitis in rats. J Pharmacol Exp Ther. 2005;313:36–45. doi: 10.1124/jpet.104.077883. [DOI] [PubMed] [Google Scholar]
- Miyazaki R, Ichiki T, Hashimoto T, Inanaga K, Imayama I, et al. SIRT1, a longevity gene, downregulates angiotensin II type 1 receptor expression in vascular smooth muscle cells. Arterioscler Thromb Vasc Biol. 2008;28:1263–9. doi: 10.1161/ATVBAHA.108.166991. [DOI] [PubMed] [Google Scholar]
- Mizushima N. Methods for monitoring autophagy. Int J Biochem Cell Biol. 2004;36:2491–502. doi: 10.1016/j.biocel.2004.02.005. [DOI] [PubMed] [Google Scholar]
- Liu Y, Schiff M, Czymmek K, Tallóczy Z, Levine B, et al. Autophagy regulates programmed cell death during the plant innate immune response. Cell. 2005;121:567–77. doi: 10.1016/j.cell.2005.03.007. [DOI] [PubMed] [Google Scholar]
- Kabeya Y, Mizushima N, Ueno T, Yamamoto A, Kirisako T, et al. LC3, a mammalian homologue of yeast Apg8p, is localized in autophagosome membranes after processing. EMBO J. 2000;19:5720–8. doi: 10.1093/emboj/19.21.5720. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rouschop KM, Wouters BG. Regulation of autophagy through multiple independent hypoxic signaling pathways. Curr Mol Med. 2009;9:417–24. doi: 10.2174/156652409788167131. [DOI] [PubMed] [Google Scholar]
- Schmelzle T, Hall MN. TOR, a central controller of cell growth. Cell. 2000;103:253–62. doi: 10.1016/s0092-8674(00)00117-3. [DOI] [PubMed] [Google Scholar]
- The Science News Staff Breakthrough of the year. Areas to watch in 2005. Science. 2004;306:2014. doi: 10.1126/science.306.5704.2014. [DOI] [PubMed] [Google Scholar]
- Maiuri MC, Criollo A, Kroemer G. Crosstalk between apoptosis and autophagy within the Beclin 1 interactome. EMBO J. 2010;29:515–6. doi: 10.1038/emboj.2009.377. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Meijer AJ, Codogno P. Regulation and role of autophagy in mammalian cells. Int J Biochem Cell Biol. 2004;36:2445–62. doi: 10.1016/j.biocel.2004.02.002. [DOI] [PubMed] [Google Scholar]
- Itakura E, Mizushima N. Atg14 and UVRAG: mutually exclusive subunits of mammalian Beclin 1–PI3K complexes. Autophagy. 2009;5:534–6. doi: 10.4161/auto.5.4.8062. [DOI] [PubMed] [Google Scholar]
- Aita VM, Liang XH, Murty VV, Pincus DL, Yu W, et al. Cloning and genomic organization of beclin 1, a candidate tumor suppressor gene on chromosome 17q21. Genomics. 1999;59:59–65. doi: 10.1006/geno.1999.5851. [DOI] [PubMed] [Google Scholar]
- Yue Z, Horton A, Bravin M, DeJager PL, Selimi F, et al. A novel protein complex linking the delta 2 glutamate receptor and autophagy: implications for neurodegeneration in lurcher mice. Neuron. 2002;35:921–33. doi: 10.1016/s0896-6273(02)00861-9. [DOI] [PubMed] [Google Scholar]
- Pattingre S, Tassa A, Qu X, Garuti R, Liang XH, et al. Bcl-2 antiapoptotic proteins inhibit Beclin 1-dependent autophagy. Cell. 2005;122:927–39. doi: 10.1016/j.cell.2005.07.002. [DOI] [PubMed] [Google Scholar]
- Lekmine F, Uddin S, Sassano A, Parmar S, Brachmann SM, et al. Activation of the p70 S6 kinase and phosphorylation of the 4E-BP1 repressor of mRNA translation by type I interferons. J Biol Chem. 2003;278:27772–80. doi: 10.1074/jbc.M301364200. [DOI] [PubMed] [Google Scholar]
- Stolovich M, Tang H, Hornstein E, Levy G, Cohen R, et al. Transduction of growth or mitogenic signals into translational activation of TOP mRNAs is fully reliant on the phosphatidylinositol 3-kinase-mediated pathway but requires neither S6K1 nor rpS6 phosphorylation. Mol Cell Biol. 2002;22:8101–13. doi: 10.1128/MCB.22.23.8101-8113.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- UK Prospective Diabetes Study (UKPDS) Group Intensive blood-glucose control with sulphonylureas or insulin compared with conventional treatment and risk of comp lications in patients with type 2 diabetes (UKPDS33) Lancet. 1998;352:837–53. [PubMed] [Google Scholar]
- Lue TF. Neurogenic erectile dysfunction. Clin Auton Res. 2001;11:285–94. doi: 10.1007/BF02332973. [DOI] [PubMed] [Google Scholar]
- Vileikyte L, Leventhal H, Gonzalez JS, Peyrot M, Rubin RR, et al. Diabetic peripheral neuropathy and depressive symptoms: the association revisited. Diabetes Care. 2005;28:2378–83. doi: 10.2337/diacare.28.10.2378. [DOI] [PubMed] [Google Scholar]
- Liu WJ, Xin ZC, Xin H, Yuan YM, Tian L, et al. Effects of icariin on erectile function and expression of nitric oxide synthase isoforms in castrated rats. Asian J Androl. 2005;7:381–8. doi: 10.1111/j.1745-7262.2005.00066.x. [DOI] [PubMed] [Google Scholar]
- Ding L, Liang XG, Hu Y, Zhu DY, Lou YJ, et al. Involvement of p38MAPK and reactive oxygen species in icariin-induced cardiomyocyte differentiation of murine embryonic stem cells in vitro. Stem Cells Dev. 2008;17:751–60. doi: 10.1089/scd.2007.0206. [DOI] [PubMed] [Google Scholar]
- Chung BH, Kim JD, Kim CK, Kim JH, Won MH, et al. icariin stimulates angiogenesis by activating the MEK/ERK- and PI3K/Akt/eNOS-dependent signal pathways in human endothelial cells. Biochem Biophys Res Commun. 2008;376:404–8. doi: 10.1016/j.bbrc.2008.09.001. [DOI] [PubMed] [Google Scholar]
- Wang YK, Huang ZQ. Protective effects of icariin on human umbilical vein endothelial cell injury induced by H2O2in vitro. Pharmacol Res. 2005;52:174–82. doi: 10.1016/j.phrs.2005.02.023. [DOI] [PubMed] [Google Scholar]
- Liu J, Lou YJ. Determination of icariin and metabolites in rat serum by capillary zone electrophoresis: rat pharmacokinetic studies after administration of icariin. J Pharm Biomed Anal. 2004;36:365–70. doi: 10.1016/j.jpba.2004.06.021. [DOI] [PubMed] [Google Scholar]
- Zhang J, Wang YB, Ma CG, Liu T, Li WR, et al. Icarisid II, a PDE5 inhibitor from Epimedium Wanshanense, increases cellular cGMP by enhancing NOS in diabetic ED rats corpus cavernosum tissue Andrologiae-pub ahead of print 6 July 2011; doi: 10.1111/j.1439-0272.2010.01144.x. [DOI] [PubMed]
- Ulrich P, Cerami A. Protein glycation, diabetes, and aging. Recent Prog Horm Res. 2001;56:1–21. doi: 10.1210/rp.56.1.1. [DOI] [PubMed] [Google Scholar]
- Jiaan DB, Seftel AD, Fogarty J, Hampel N, Cruz W, et al. Age-related increase in an advanced glycation end product in penile tissue. World J Urol. 1995;13:369–75. doi: 10.1007/BF00191219. [DOI] [PubMed] [Google Scholar]
- Usta MF, Bivalacqua TJ, Yang DY, Ramanitharan A, Sell DR, et al. The protective effect of aminoguanidine on erectile function in streptozotocin diabetic rats. J Urol. 2003;170 4Pt1:1437–42. doi: 10.1097/01.ju.0000077557.45582.f3. [DOI] [PubMed] [Google Scholar]
- Melman A, Christ GJ. Integrative erectile biology. The effects of age and disease on gap junctions and ion channels and their potential value to the treatment of erectile dysfunction. Urol Clin North Am. 2001;28:217–31. doi: 10.1016/s0094-0143(05)70133-6. [DOI] [PubMed] [Google Scholar]
- Seftel AD, Vaziri ND, Ni Z, Razmjouei K, Fogarty J, et al. Advanced glycation end products in human penis: elevation in diabetic tissue, site of deposition, and possible effect through iNOS or eNOS. Urology. 1997;50:1016–26. doi: 10.1016/S0090-4295(97)00512-8. [DOI] [PubMed] [Google Scholar]
- Chen D, Dai YT, Chen YS ZY. Effect of advanced glycation end products on the content of nitric oxide and the activity of nitric oxide synthase in rat cavernosum. J Nanjing Univ. 2007;43:157–63. [Google Scholar]
- Ishibashi Y, Matsui T, Takeuchi M, Yamagishi S. Vardenafil, an inhibitor of phosphodiesterase-5, blocks advanced glycation end product (AGE)-induced up-regulation of monocyte chemoattractant protein-1 mRNA levels in endothelial cells by suppressing AGE receptor (RAGE) expression via elevation of cGMP. Clin Exp Med. 2011;11:131–52. doi: 10.1007/s10238-010-0109-2. [DOI] [PubMed] [Google Scholar]


