Abstract
This study aims to evaluate the potential value of patient characteristics in predicting overall survival (OS) in patients with metastatic castration-resistant prostate cancer (mCRPC) treated with docetaxel-based thermotherapy. A total of 115 patients with mCRPC undergoing a docetaxel q3w regimen were enrolled in this study. A survival analysis was performed using the Kaplan–Meier method. Cox proportional hazards models were used to evaluate the prognostic value of all covariates for OS. OS was also analysed after stratifying patients according to the results of multivariate analysis. The median OS for the entire cohort was 17.0 months. The multivariate analysis showed that the prostate-specific antigen doubling time (PSADT), baseline haemoglobin (Hb) concentration, alkaline phosphatase (ALP) concentration, cycles of chemotherapy and time to castration resistance were independent prognostic factors of OS. According to the presence of PSADT <46.3 days and baseline ALP ≥110 IU l−1, all patients were divided into three risk groups: low-risk group (no risk factors), intermediate-risk group (one risk factor) and high-risk group (two risk factors). Median OSs for patients in low-, intermediate- and high-risk groups were 28.0 months (95% CI: 23.8–32.2), 21.0 months (95% CI: 18.9–23.1) and 11.0 months (95% CI: 7.6–14.4), respectively (P<0.001). In conclusion, PSADT, baseline Hb concentration, ALP concentration, cycles of chemotherapy and time to castration resistance were independent prognostic factors of OS in Chinese patients with mCRPC treated with docetaxel. PSADT combined with the baseline ALP concentration could be a useful risk stratification parameter for evaluating survival outcomes.
Keywords: castration-resistant, docetaxel, metastatic, overall survival, prognostic factor, prostate cancer
Introduction
Prostate cancer is the most frequently occurring cancer in men from Western countries and represents the second leading cause of cancer-related death, next to lung cancer.1 In China, the incidence of prostate cancer has increased dramatically over the past two decades, most likely due to economic development and lifestyle changes.2 Moreover, most newly diagnosed Chinese prostate cancer patients already have metastatic disease because prostate cancer screening using prostate-specific antigen (PSA) and digital rectal examination is not a routine practice in China.2
Androgen deprivation therapy is the initial treatment for metastatic prostate cancer and leads to improvement in bone pain, regression of soft tissue metastases, and a reduction in serum levels of PSA.3 Unfortunately, in virtually all patients, the disease inevitably advances to the androgen-independent stage within a median of 18–24 months after castration.4 Two randomized phase III studies, SWOG 99-164 and TAX 327,5 demonstrated that docetaxel-based chemotherapy confers a significant survival benefit of 20%–24% in patients with metastatic castration-resistant prostate cancer (mCRPC) compared with a mitoxantrone regimen.6 Nevertheless, many patients failed to respond to docetaxel chemotherapy and experienced considerable toxicity. Currently, the identification of readily available prognostic factors would be an essential step in optimising the management of patients being treated with docetaxel.
Several prognostic factors in patients with mCRPC have been reported, including Eastern Cooperative Oncology Group performance status (ECOG PS), baseline serum PSA level, haemoglobin (Hb), alkaline phosphatase (ALP), lactate dehydrogenase (LDH), prostate-specific antigen doubling time (PSADT), time to castration resistance and circulating tumor cells.7,8,9,10,11,12,13,14 However, the magnitude of the benefit provided by each factor has varied among studies, and the value of these factors in predicting overall survival (OS) for Chinese patients is still unclear. In the present study, we retrospectively analysed the data from 115 mCRPC patients treated with docetaxel to explore the prognostic factors of survival outcomes in Chinese patients.
Materials and methods
Patients and variables
The present study is based on data from 115 consecutive Chinese patients with mCRPC who received docetaxel-based chemotherapy at a standard dose and schedule5 at our institution from November 2005 to September 2011. These patients were diagnosed between October 1997 and July 2010. All eligible patients had histologically confirmed adenocarcinoma of the prostate and confirmed cases of mCRPC, which was determined by three sequential rises in serum PSA level with castrate levels of serum testosterone (<50 ng dl−1). All patients presented with metastatic disease(s) upon imaging (computed tomography, magnetic resonance imaging or whole body radionuclide bone scan). The time to castration resistance was calculated from the time of diagnosis until the confirmation of CRPC. The present study was approved by the Institutional Review Board of our institution, and written informed consent was obtained from each patient before enrolment.
We retrospectively collected the variables, including patient characteristics at diagnosis (age, serum PSA level, Gleason score and metastatic site(s)) and characteristics at the start of chemotherapy (age, serum PSA level, PSADT, complete blood count, biochemistry profile, ECOG PS, metastatic site(s), radical prostatectomy, cycles of chemotherapy and time to castration resistance). All laboratory analyses were performed in the same laboratory.
Treatment
All patients were treated on a 21-day cycle, with 75 mg m−2 of docetaxel (Taxotere, Aventis, Essex, UK) administered intravenously on the first day and 5 mg of prednisone administered twice daily throughout the cycle. On days 1–3, dexamethasone was administered at a dosage of 10 mg twice daily to prevent potential acute hypersensitivity reactions or residual body fluid. The average number of chemotherapy cycles was 6 (range: 1–16).
Definition and calculation of PSADT
By definition, PSADT was defined as the time required for the PSA level to double. PSADT was calculated from the slope of the linear regression calculated from the natural logarithm values of all serial PSA (on the ordinate) versus time of measurement (on the abscissa), according to the following formula: PSADT=(ln2×T)/[ln(PSA2)−ln(PSA1)], where ln is the natural log, T is the time (in days) between PSA measurements, PSA2 is the PSA defining CRPC and PSA1 is the next most recent PSA level.15
The end point of the present study
The end point of the present study was the effect of patient characteristics on OS, calculated as the interval between the first day of docetaxel administration and the date of death or the last follow-up visit for censored (living) patients.
Statistical analysis
The number of chemotherapy cycles was classified according to the mean value, baseline gamma-glutamyl transpeptidase (GGT) and LDH concentrations were classified according to the upper limit of normal range and baseline Hb concentration was classified according to the cut-off point of 110 g l−1. All other characteristics were divided into dichotomous variables according to the median value. A survival analysis was performed using the Kaplan–Meier method, and the differences in OS rates were assessed by the log-rank test. The relationship between a given explanatory covariate and the survival data was evaluated using a univariate Cox regression analysis. Only variables which were statistically significant in the univariate analysis (P<0.05) were included in the multivariate model. A multivariate Cox proportional hazards regression analysis was used to analyse the results while controlling for important prognostic covariates that could potentially confound the analyses. OS was also analysed after stratifying patients by PSADT and baseline ALP concentration according to the results of the multivariate Cox regression analysis. For all statistical tests, a two-sided P value of less than 0.05 was considered statistically significant. All statistical analyses were performed using SPSS software version 16.0 (SPSS Inc., Chicago, IL, USA).
Results
Patient characteristics
Follow-up continued until 30 April 2012. During the follow-up period (median: 45.0 months; range: 2.0–74.0 months), 87 of 115 patients (75.6%) died, and all deaths were attributed to prostate cancer or prostate cancer–related complications. The median OS for the entire cohort was 17.0 months (95% CI: 13.8–20.2; range: 2.0–46.0 months). The Gleason scores were not available for 46 patients who came from rural areas and were diagnosed at local hospitals so long ago that the pathological specimens could not be obtained. The demographics and disease characteristics for all patients were summarized in Table 1.
Table 1. Patient characteristics at diagnosis and at the initiation of chemotherapy in 115 mCRPC patients treated with docetaxal.
| Characteristics | Median |
|---|---|
| Characteristics at diagnosis | |
| Age (year) | 65 (49–81) |
| PSA (ng ml−1) | 100 (2.9–3034.4) |
| Gleason score (No. of patients, %) | |
| ≤6 | 5 (4.3) |
| 7 | 21 (18.3) |
| 8 | 15 (13.0) |
| 9 | 22 (19.1) |
| 10 | 6 (5.2) |
| Not available | 46 (40.0) |
| Metastatic site(s) (No. of patients, %) | |
| M0 | 24 (20.9) |
| M1a | 21 (18.3) |
| M1b | 68 (59.1) |
| M1c | 2 (1.7) |
| Characteristics at the start of chemotherapy | |
| Age (year) | 68 (51–82) |
| PSA (ng ml−1) | 90.5 (0.1–4066.4) |
| PSADT (day) | 46.3 (5.6–661.1) |
| WBC (×109 l−1) | 6.4 (3.2–14.7) |
| PTL (×109 l−1) | 165 (72–518) |
| Hb (g l−1) | 121 (68–150) |
| Albumin (g l−1) | 43.1 (31.2–50.7) |
| ALP (IU l−1) | 110 (33–1083) |
| GGT (IU l−1) | 33 (11–217) |
| LDH (IU l−1) | 234 (124–1693) |
| ECOG PS (No. of patients, %) | |
| 0 | 40 (34.8) |
| 1 | 65 (56.5) |
| 2 | 10 (8.7) |
| Metastatic site(s) (No. of patients, %) | |
| Lymph node | 39 (33.9) |
| Bone | 115 (100.0) |
| Viscera | 16 (13.9) |
| Lung | 9 (7.8) |
| Bladder | 4 (3.5) |
| Liver | 2 (1.7) |
| Brain | 1 (0.9) |
| RP (No. of patients, %) | |
| Yes | 12 (10.4) |
| No | 103 (89.6) |
| Cycles of chemotherapy | 5 (1–16) |
| Time to castration resistance (month) | 23 (2–114) |
Abbreviations: ALP, alkaline phosphatase; ECOG PS, Eastern Cooperative Oncology Group performance status; GGT, gamma-glutamyl transpeptidase; Hb, haemoglobin; LDH, lactate dehydrogenase; mCRPC, metastatic castration-resistant prostate cancer; PLT, platelet; PSA, prostate-specific antigen; PSADT, prostate-specific antigen doubling time; RP, radical prostatectomy; WBC, white blood cell.
Univariate and multivariate Cox regression analysis for OS
Kaplan–Meier and log-rank tests were used to assess OS and compare differences in survival time distributions among patient characteristics with respect to OS (Table 2).
Table 2. Summary of the association between patient characteristics and overall survival (OS) for all patients.
| Characteristics | No. of patients | No. of deaths | Median of OS (month) | Log-rank test P |
|---|---|---|---|---|
| All patients | 115 | 87 | 17.0 | — |
| Characteristics at diagnosis | ||||
| Age (year) | 0.726 | |||
| <65 | 51 | 35 | 18.0 | |
| ≥65 | 64 | 52 | 15.0 | |
| PSA (ng ml−1) | 0.790 | |||
| <100 | 40 | 29 | 20.0 | |
| ≥100 | 75 | 58 | 16.0 | |
| Metastases | 0.063 | |||
| Yes | 91 | 70 | 14.0 | |
| No | 24 | 17 | 18.0 | |
| Characteristics at the start of chemotherapy | ||||
| Age (year) | 0.548 | |||
| <68 | 55 | 37 | 15.0 | |
| ≥68 | 60 | 50 | 18.0 | |
| PSA (ng ml−1) | 0.016 | |||
| <90.5 | 57 | 36 | 19.0 | |
| ≥90.5 | 58 | 51 | 14.0 | |
| PSADT (day) | 0.001 | |||
| <46.3 | 57 | 48 | 14.0 | |
| ≥46.3 | 58 | 39 | 23.0 | |
| WBC (×109 l−1) | 0.552 | |||
| <6.4 | 56 | 46 | 19.0 | |
| ≥6.4 | 59 | 41 | 17.0 | |
| PTL (×109 l−1) | 0.824 | |||
| <165 | 57 | 48 | 16.0 | |
| ≥165 | 58 | 39 | 20.0 | |
| Hb (g l−1) | 0.002 | |||
| <110 | 28 | 24 | 10.0 | |
| ≥110 | 87 | 63 | 19.0 | |
| Albumin (g l−1) | 0.252 | |||
| <43.1 | 57 | 47 | 15.0 | |
| ≥43.1 | 58 | 40 | 18.0 | |
| ALP (IU l−1) | <0.001 | |||
| <110 | 57 | 39 | 26.0 | |
| ≥110 | 58 | 48 | 15.0 | |
| GGT (IU l−1) | <0.001 | |||
| <54 | 89 | 63 | 20.0 | |
| ≥54 | 26 | 24 | 11.0 | |
| LDH (IU l−1) | 0.027 | |||
| <250 | 71 | 44 | 21.0 | |
| ≥250 | 46 | 43 | 14.0 | |
| ECOG PS | 0.002 | |||
| 0 | 40 | 21 | 24.0 | |
| 1–2 | 75 | 66 | 15.0 | |
| Metastatic site(s) | 0.680 | |||
| Bone only | 99 | 73 | 17.0 | |
| Bone and viscera | 16 | 14 | 13.0 | |
| RP | 0.060 | |||
| Yes | 12 | 7 | 25.0 | |
| No | 103 | 80 | 16.0 | |
| Cycles of chemotherapy | <0.001 | |||
| <6 | 59 | 55 | 12.0 | |
| ≥6 | 56 | 32 | 24.0 | |
| Time to castration resistance (month) | 0.006 | |||
| <23 | 57 | 48 | 14.0 | |
| ≥23 | 58 | 39 | 21.0 | |
Abbreviations: ALP, alkaline phosphatase; ECOG PS, Eastern Cooperative Oncology Group performance status; GGT, gamma-glutamyl transpeptidase; Hb, haemoglobin; LDH, lactate dehydrogenase; PLT, platelet; PSA, prostate-specific antigen; PSADT, prostate-specific antigen doubling time; RP, radical prostatectomy; WBC, white blood cell.
The results of univariate and multivariate Cox regression analysis for OS in 115 patients were summarized in Table 3. The univariate analysis of baseline clinical characteristics identified a total of nine statistically significant predictors of OS: baseline serum PSA level, PSADT, baseline Hb concentration, ALP concentration, GGT concentration, LDH concentration, ECOG PS, cycles of chemotherapy and time to castration resistance. Subsequent multivariate analysis reserved PSADT (P=0.005; HR=0.407), baseline Hb concentration (P=0.028; HR=0.495), baseline ALP concentration (P=0.019; HR=1.934), cycles of chemotherapy (P<0.001; HR=0.379) and time to castration resistance (P=0.030; HR=0.548) as independent prognostic factors of OS. The median OS for patients with PSADT <46.3 days and ≥46.3 days were 14.0 months (95% CI: 10.5–17.5) and 23.0 months (95% CI: 19.9–26.1), respectively (P=0.001). OS curves were reported in Figure 1. Median OS for patients with ALP ≥110 IU l−1 and <110 IU l−1 were 15.0 months (95% CI: 12.1–17.9) and 26.0 months (95% CI: 21.0–30.9), respectively (P<0.001). OS curves were reported in Figure 2.
Table 3. Summary of the association between patient characteristics and overall survival: Cox univariate and multivariate regression analysis.
| Univariate analysis | Multivariate analysis | |||||
|---|---|---|---|---|---|---|
| Characteristics | P | HR | 95% CI | P | HR | 95% CI |
| Characteristics at diagnosis | ||||||
| Age | 0.728 | 1.081 | 0.696–1.678 | |||
| PSA | 0.791 | 1.069 | 0.652–1.754 | |||
| Metastases | 0.063 | 1.571 | 0.817–3.168 | |||
| Characteristics at the start of chemotherapy | ||||||
| Age | 0.549 | 1.139 | 0.744–1.744 | |||
| PSA | 0.019 | 1.672 | 1.088–2.571 | 0.973 | 1.024 | 0.569–1.842 |
| PSADT | 0.002 | 0.501 | 0.326–0.770 | 0.005 | 0.407 | 0.237–0.701 |
| WBC | 0.499 | 1.183 | 0.727–1.927 | |||
| PLT | 0.942 | 0.982 | 0.589–1.613 | |||
| Hb | 0.005 | 0.469 | 0.278–0.792 | 0.028 | 0.495 | 0.265–0.925 |
| Albumin | 0.256 | 0.715 | 0.400–1.276 | |||
| ALP | <0.001 | 2.157 | 1.400–3.321 | 0.019 | 1.934 | 1.112–3.363 |
| GGT | <0.001 | 2.792 | 1.603–4.865 | 0.087 | 1.709 | 0.925–3.157 |
| LDH | 0.032 | 1.691 | 1.046–2.736 | 0.405 | 1.268 | 0.725–2.219 |
| ECOG PS | 0.003 | 2.147 | 1.307–3.527 | 0.053 | 1.950 | 0.990–3.839 |
| Metastatic site(s) | 0.680 | 1.166 | 0.656–2.070 | |||
| RP | 0.060 | 0.433 | 0.174–1.080 | |||
| Cycles of chemotherapy | <0.001 | 0.335 | 0.214–0.535 | <0.001 | 0.379 | 0.225–0.639 |
| Time to castration resistance | 0.007 | 0.557 | 0.365–0.855 | 0.030 | 0.548 | 0.318–0.943 |
Abbreviations: ALP, alkaline phosphatase; CI, confidence interval; ECOG PS, Eastern Cooperative Oncology Group performance status; GGT, gamma-glutamyl transpeptidase; Hb, haemoglobin; HR, hazard ratio; LDH, lactate dehydrogenase; PLT, platelet; PSA, prostate-specific antigen; PSADT, prostate-specific antigen doubling time; RP, radical prostatectomy; WBC, white blood cell.
Figure 1.
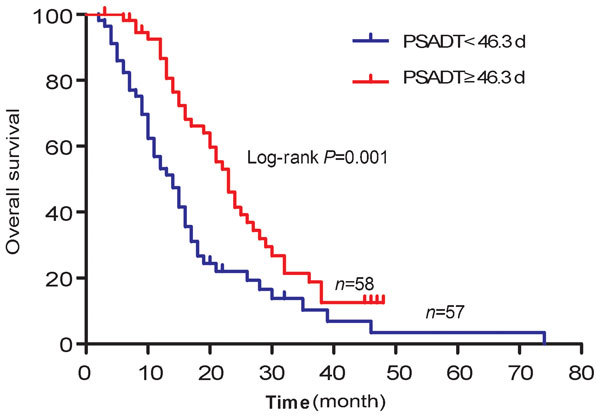
Overall survival curves for 115 mCRPC patients treated with docetaxel, divided into PSADT <46.3 days and PSADT ≥46.3 days groups. mCRPC, metastatic castration-resistant prostate cancer; PSADT, prostate-specific antigen doubling time.
Figure 2.
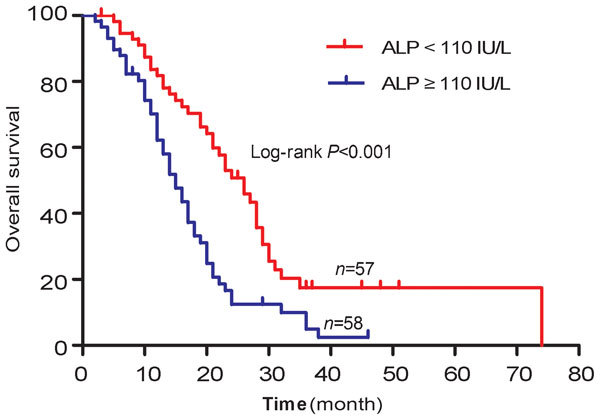
Overall survival curves for 115 mCRPC patients treated with docetaxel, divided into ALP <110 IU l−1 and ALP ≥110 IU l−1 groups. ALP, alkaline phosphatase; mCRPC, metastatic castration-resistant prostate cancer.
Risk stratification according to PSADT and baseline ALP concentration
According to the presence of PSADT <46.3 days and baseline ALP ≥110 IU l−1, which were two of most statistically significant risk factors in the multivariate analysis, we divided the entire cohort into the following three risk groups: low-risk group (no risk factors), intermediate-risk group (one risk factor, either PSADT <46.3 days or ALP ≥110 IU l−1) and high-risk group (two risk factors, both PSADT <46.3 days and ALP ≥110 IU l−1). The median OSs for patients in low-, intermediate- and high-risk groups were 28.0 months (95% CI: 23.8–32.2), 21.0 months (95% CI: 18.9–23.1) and 11.0 months (95% CI: 7.6–14.4), respectively. The hazard ratios of intermediate- and high-risk to low-risk were 1.927 (95% CI: 1.093–3.398) and 10.143 (95% CI: 3.982–25.836), respectively. The OS curves according to risk stratification were distinctly tiered and statistically significant (P<0.001), as shown in Figure 3.
Figure 3.
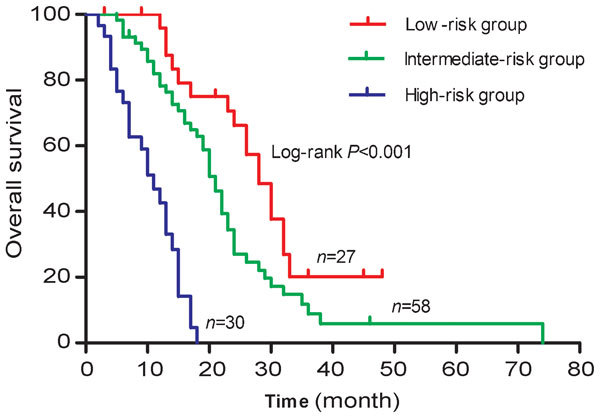
Overall survival curves for 115 mCRPC patients treated with docetaxel according to the risk groups. According to the presence of PSADT <46.3 days and/or ALP ≥110 IU l−1, each patient was divided into low-, intermediate- and high-risk groups. ALP, alkaline phosphatase; mCRPC, metastatic castration-resistant prostate cancer; PSADT, prostate-specific antigen doubling time.
Discussion
This retrospective observation study demonstrates the significant value of several prechemotherapeutic variables, including PSADT, baseline Hb concentration, baseline ALP concentration, cycles of chemotherapy and time to castration resistance, as independent prognostic factors of OS in patients with mCRPC treated with docetaxel-based chemotherapy.
The PSADT, which assumes that tumour growth is logarithmic, is a simple tool used to determine the growth rate of the serum PSA level. There are several reports available regarding the prognostic impact of PSADT for prostate cancer patients.10,16,17 D'Amico et al.16 suggested that the PSADT should be confirmed as a useful surrogate marker for survival and as a tool to identify patients at risk of progression after radical prostatectomy or radiotherapy. Teeter et al.17 reported that in an older, racially diverse cohort, a short PSADT (less than 9 months) on recurrence after radical prostatectomy was associated with worse all-cause mortality. Recently, an analysis of a TAX327 cohort indicated that baseline PSADT was an independent predictor of survival, demonstrating that patients with a short PASDT (less than 55 days) had a lower chance of survival compared with patients who had a more indolent PSADT.10 As mentioned above, our study also demonstrated that a rapid increase in the serum PSA level, namely, a short PSADT, is independently associated with a negative survival outcome. The median OSs for patients with PSADT <46.3 days and those with PSADT ≥46.3 days were 14.0 months and 23.0 months, respectively (P=0.001). Consequently, the PSADT was a valuable tool capable of selecting high-risk patients before the onset of chemotherapy. It could be used to assess prognosis after relapse and to guide therapeutic strategy in patients being treated with docetaxel.
According to the inclusion criteria, all 115 patients developed bone metastases. Bone metastases from prostate cancer are characterized by both excessive bone formation and resorption due to the increased number and activity of osteoblasts and osteoclasts.18 Total serum ALP, a routinely and successively measured marker that is inexpensive and readily available, could partly reflect osteoblastic activity. Although total serum ALP is a relatively nonspecific biomarker and can be elevated by either bone or liver metastasis, patients with bone metastasis and an elevated baseline ALP are likely to have bone as the dominant source of ALP.8 Additionally, patients with mCRPC rarely have liver metastasis that could cause ALP elevations (only 2 of 115 patients had liver metastasis in this study). A study of 643 patients indicated that the serum levels of total ALP and bone-specific alkaline phosphatase were correlated highly and that total ALP primarily reflected bone-specific alkaline phosphatase in men with mCRPC and bone metastasis.18 In large multivariate analyses of patients with metastatic androgen-independent prostate cancer, elevated levels of serum total ALP were independently associated with shorter OS.19,20 Our current results also indicted that patients with baseline serum total ALP ≥110 IU l−1 had shorter OS than patients with baseline serum total ALP <110 IU l−1 (15.0 months vs. 26.0 months, P<0.001). Baseline ALP ≥110 IU l−1 predicted a nearly two-fold increased risk of death (HR=1.934).
To our knowledge, no study to date has been performed to clarify whether PSADT, combined with baseline ALP concentration, contributes to independent prognostic information for OS in mCRPC patients. Our study demonstrated that patients with a short PSADT and a high baseline ALP concentration were at the highest risk of decreased OS, patients with a long PSADT and a low baseline ALP concentration were at the lowest risk of death, and patients with one of the two risk factors fell into the intermediate-risk category. This suggests that mCRPC patients can be risk-stratified according to PSADT and baseline ALP concentration. Therefore, when estimating the prognosis for mCRPC patients being treated with docetaxel, both PSADT and ALP concentration should be calculated in order to provide more accurate prognostic information. Furthermore, we suggest that the combination of a short PSADT and a high baseline ALP concentration could be used as a guide for the early initiation of chemotherapy for patients with mCRPC.
Previous studies have demonstrated that the baseline PSA level, which is generally considered to be related to the tumour burden, could be a prognostic factor of OS for mCRPC patients.9 In our study, an elevated baseline PSA level was shown to be associated with shorter OS in univariate analysis. However, the multivariate analysis failed to confirm baseline PSA level as an independent prognostic factor of OS (P=0.407). This might be a result of the relatively small sample size and the possible selection bias, as patients with ECOG PS greater than score 2 were excluded in this study.
In the present study, we identified prognostic factors using only prechemotherapeutic variables, which are easily measured by standard assays in most institutions. This facilitates the oncologist's ability to predict the survival outcomes for mCRPC patients before the initiation of chemotherapy. However, potential flaws in the current study include its retrospective nature and the relatively small sample size. Future prospective studies should be performed to validate our results in a larger population of patients.
Conclusions
In conclusion, this retrospective analysis demonstrated that PSADT, baseline Hb concentration, baseline ALP concentration, cycles of chemotherapy and time to castration resistance could provide independent prognostic information on OS in Chinese patients with mCRPC treated with docetaxel. These variables can be used to optimize the management of mCRPC. In addition, PSADT combined with baseline ALP concentration could be a useful risk stratification parameter for evaluating survival outcomes.
Author contributions
YYQ and BD designed the study, collected, analysed and interpreted the clinical data, wrote and revised the manuscript. YYK reviewed pathological slides and revised the manuscript. DWY supervised the project and revised the manuscript. XDY, SLZ, HLZ, CGM and WYY collected partial patients' clinical data and followed up with patients. All the authors vouched for the respective data and analysis, approved the final version and agreed to publish the manuscript.
Acknowledgments
This study was supported in part by grants from National Natural Science Foundation of China (Nos. 30801149 and 30973009).
The authors declare that they have no financial or commercial interests related to this study.
References
- Ferlay J, Autier P, Boniol M, Heanue M, Colombet M, et al. Estimates of the cancer incidence and mortality in Europe in 2006. Ann Oncol. 2007;18:581–92. doi: 10.1093/annonc/mdl498. [DOI] [PubMed] [Google Scholar]
- Dai B, Ye DW, Kong YY, Shen YJ, Wang BH. Individualized prostate biopsy strategy for Chinese patients with different prostate-specific antigen levels. Asian J Androl. 2008;10:325–31. doi: 10.1111/j.1745-7262.2008.00345.x. [DOI] [PubMed] [Google Scholar]
- Crawford ED, Eisenberger MA, McLeod DG, Spaulding JT, Benson R, et al. A controlled trial of leuprolide with and without flutamide in prostatic carcinoma. N Engl J Med. 1989;321:419–24. doi: 10.1056/NEJM198908173210702. [DOI] [PubMed] [Google Scholar]
- Petrylak DP, Tangen CM, Hussain MH, Lara PN, Jones JA, et al. Docetaxel and estramustine compared with mitoxantrone and prednisone for advanced refractory prostate cancer. N Engl J Med. 2004;351:1513–20. doi: 10.1056/NEJMoa041318. [DOI] [PubMed] [Google Scholar]
- Tannock IF, de Wit R, Berry WR, Horti J, Pluzanska A, et al. Docetaxel and prednisone or mitoxantrone plus prednisone for advanced prostate cancer. N Engl J Med. 2004;351:1502–12. doi: 10.1056/NEJMoa040720. [DOI] [PubMed] [Google Scholar]
- Petrylak DP. The treatment of hormone-refractory prostate cancer: docetaxel and beyond. Rev Urol. 2006;8 Suppl 2:48–55. [PMC free article] [PubMed] [Google Scholar]
- He J, Zeng ZC, Yang P, Chen B, Jiang W, et al. Clinical features and prognostic factors for patients with bone metastases from prostate cancer. Asian J Androl. 2012;14:505–8. doi: 10.1038/aja.2012.24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sonpavde G, Pond GR, Berry WR, de Wit R, Armstrong AJ.Serum alkaline phosphatase changes predict survival independent of PSA changes in men with castration-resistant prostate cancer and bone metastasis receiving chemotherapy. Urol Oncol 2010. e-pub ahead of print 29 Sep 2010; doi:10.1016/j.urolonc.2010.07.002. [DOI] [PubMed]
- Saad F, Segal S, Eastham J.Prostate-specific antigen kinetics and outcomes in patients with bone metastases from castration-resistant prostate cancer treated with or without zoledronic acid. Eur Urol 2012. e-pub ahead of print 12 May 2012; doi:10.1016/j.eururo.2012.05.007. [DOI] [PubMed]
- Armstrong AJ, Garrett-Mayer ES, Yang YC, de Wit R, Tannock IF. A contemporary prognostic nomogram for men with hormone-refractory metastatic prostate cancer: a TAX327 study analysis. Clin Cancer Res. 2007;13:6396–403. doi: 10.1158/1078-0432.CCR-07-1036. [DOI] [PubMed] [Google Scholar]
- Bournakis E, Efstathiou E, Varkaris A, Wen S, Chrisofos M, et al. Time to castration resistance is an independent predictor of castration-resistant prostate cancer survival. Anticancer Res. 2011;31:1475–82. [PubMed] [Google Scholar]
- de Bono JS, Scher HI, Montgomery RB, Parker C, Miller MC, et al. Circulating tumor cells predict survival benefit from treatment in metastatic castration-resistant prostate cancer. Clin Cancer Res. 2008;14:6302–9. doi: 10.1158/1078-0432.CCR-08-0872. [DOI] [PubMed] [Google Scholar]
- Darshan MS, Loftus MS, Thadani-Mulero M, Levy BP, Escuin D, et al. Taxane-induced blockade to nuclear accumulation of the androgen receptor predicts clinical responses in metastatic prostate cancer. Cancer Res. 2011;71:6019–29. doi: 10.1158/0008-5472.CAN-11-1417. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Armstrong AJ, Eisenberger MA, Halabi S, Oudard S, Nanus DM, et al. Biomarkers in the management and treatment of men with metastatic castration-resistant prostate cancer. Eur Urol. 2012;61:549–59. doi: 10.1016/j.eururo.2011.11.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pound CR, Partin AW, Eisenberger MA, Chan DW, Pearson JD. Natural history of progression after PSA elevation following radical prostatectomy. JAMA. 1999;281:1591–7. doi: 10.1001/jama.281.17.1591. [DOI] [PubMed] [Google Scholar]
- D'Amico AV, Cote K, Loffredo M, Renshaw AA, Schultz D. Determinants of prostate cancer-specific survival after radiation therapy for patients with clinically localized prostate cancer. J Clin Onocol. 2002;20:4567–73. doi: 10.1200/JCO.2002.03.061. [DOI] [PubMed] [Google Scholar]
- Teeter AE, Presti JC, Jr, Aronson WJ, Terris MK, Kane CJ, et al. Does PSADT after radical prostatectomy correlate with overall survival?—a report from the SEARCH database group. Urology. 2011;77:149–53. doi: 10.1016/j.urology.2010.04.071. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cook RJ, Coleman R, Brown J, Lipton A, Major P, et al. Markers of bone metabolism and survival in men with hormone-refractory metastatic prostate cancer. Clin Cancer Res. 2006;12:3361–7. doi: 10.1158/1078-0432.CCR-06-0269. [DOI] [PubMed] [Google Scholar]
- Smaletz O, Scher HI, Small EJ, Verbel DA, McMillan A, et al. Nomogram for overall survival of patients with progressive metastatic prostate cancer after castration. J Clin Oncol. 2002;20:3972–82. doi: 10.1200/JCO.2002.11.021. [DOI] [PubMed] [Google Scholar]
- Halabi S, Small EJ, Kantoff PW, Kattan MW, Kaplan EB, et al. Prognostic model for predicting survival in men with hormone-refractory metastatic prostate cancer. J Clin Oncol. 2003;21:1232–7. doi: 10.1200/JCO.2003.06.100. [DOI] [PubMed] [Google Scholar]


