Abstract
Contemporary therapies for erectile dysfunction are generally targeted towards older men and universally engage pharmacological and/or device related treatment options. Penile revascularization, using microvascular arterial bypass surgical techniques, is a non-pharmacological, non-device-related, and reconstructive surgical strategy for men with erectile dysfunction that was first described by Dr Vaclav Michal in 1973. Contemporary penile revascularization attempts to ‘cure' pure arteriogenic erectile dysfunction in young men with arterial occlusive pathology in the distal internal pudendal, common penile or proximal cavernosal artery secondary to focal endothelial injury from blunt pelvic, perineal or penile trauma. A microvascular anastomosis is fashioned between the donor inferior epigastric and recipient dorsal penile artery. Increased perfusion pressure is theoretically communicated to the cavernosal artery via perforating branches from the dorsal artery. This article will review the history, indications and pathophysiology of blunt trauma-induced focal arterial occlusive disease in young men with erectile dysfunction, current surgical techniques utilized and results of surgery. Contemporary use of penile revascularization is a logical and wanted therapeutic option to attempt to reverse erectile dysfunction in young men who have sustained blunt pelvic, perineal or penile trauma.
Keywords: erectile dysfunction, microvascular arterial bypass surgery, penile revascularization, traumatic arterial occlusive disease, vascular reconstructive surgery
Introduction
Penile revascularization was initially described by Dr Vaclav Michal in 1973.1 Since that time, other vascular surgeons have reported variations to Michal's techniques. All share the common surgical goal of restoring arterial blood flow and perfusion pressure to the corpora cavernosa in men with bothersome arteriogenic erectile dysfunction secondary to reduced cavernosal artery perfusion pressure. From a practical perspective, penile revascularization variations include donor inferior epigastric artery to: (i) recipient corpus cavernosum (original Michal surgery); (ii) recipient dorsal vein; and/or (iii) recipient dorsal penile artery.1,2,3,4,5 The diversity of procedures and heterogeneity of the patient population in the literature makes comparisons difficult. Across several penile revascularization studies, the microvascular arterial anastomosis between the donor inferior epigastric artery to the recipient dorsal artery has improved erectile function in selected populations of younger men, especially those who sustained blunt perineal, penile or pelvic trauma and sustained arterial occlusions in the distal internal pudendal artery, common penile artery and/or proximal cavernosal artery.
Our current understanding of erectile physiology and its relationship to biopsychosocial concerns, especially hormonal, neurological and vascular/endothelial function, has caused a paradigm shift in the treatment of erectile dysfunction. Until the turn of the century, especially in the 1960s and 1970s, erectile dysfunction was considered to be primarily psychological in origin. Erectile dysfunction is now universally considered to be a multidisciplinary6,7 biopsychosocial health-care concern, managed by the psychologist, sex therapist, pelvic floor physical therapist, psychiatrist, urologist, endocrinologist, neurologist and others as indicated.
There are new data, especially in the aging male with erectile dysfunction, that organic pathophysiologies frequently underlie the erectile impairment. In particular, in men with erectile dysfunction, there are hemodynamic data supporting frequent arterial and veno-occlusive vascular pathophysiologies and their significant association with vascular risk factors, systemic endothelial dysfunction and other vascular diseases.8,9
Young men with erectile dysfunction following blunt trauma
What about the male, 40 years and younger, with erectile dysfunction? While the biopsychosocial paradigm also applies to the younger male, the fact that a younger male can have an underlying vascular pathophysiology in the absence of classic risk factors for endothelial dysfunction10,11 such as hypertension, cigarette smoking, hyperlipidemia and diabetes, is not well appreciated nor understood by the majority of health-care providers.
What this overlooks is the reality that focal endothelial dysfunction leading to focal atherosclerosis has been well reported to occur in young men secondary to blunt trauma.12,13 The response to blunt trauma injury hypothesis of focal atherosclerosis has been well studied over the past three decades. It posits that the initiating event of the cascade of plaque formation begins with an injury to the endothelium with subsequent release of inflammatory cytokines, stimulation of smooth muscle proliferation and infiltration of macrophages. The endothelial injury can be a result of systemic disorders such as hyperlipidemia or hypertension, but can also be secondary to focal damage from blunt mechanical trauma against a bony structure. Blunt injury to arteries resulting in focal atherosclerosis has been well documented over a range of different anatomic locations such as the radial artery of construction workers14 or the brachial artery from a crutch injury.15 The same can be found in young men with a history of overt perineal trauma or even subclinical trauma.16 The distal internal pudendal artery, common penile artery and proximal cavernosal artery are particularly susceptible to injury given the fixed anatomic relationship to the ischio-pubic ramus as it passes through Alcock's Canal.
Bicycle riding17,18,19 in particular is an activity that places the distal internal pudendal artery, common penile artery and proximal cavernosal artery at risk. Data from numerous studies have shown a strong association between bicycle riding, proximal cavernosal artery occlusion and erectile dysfunction. Marceau et al.19 revealed a relative risk of 1.72 for development of moderate to severe erectile dysfunction in men who rode >3 h a week. Munarriz et al.20 evaluated cavernosal blood velocity with a pharmacological erection sitting on a traditional narrow bicycle seat with nose extension versus a wide, noseless seat versus lying supine. There were no differences in cavernosal artery peak systolic velocities lying supine and sitting on a wide noseless seat. However, while sitting on a standard narrow seat with nose extension, cavernosal artery peak systolic velocities approached zero, reflecting the ease with which the cavernosal artery was significantly compressed by perineal pressure and the close association of the proximal cavernosal artery to the bony structure, the ischio-pubic ramus.20 A prospective study of bicycling police officers using a standard narrow seat with nose extension was conducted. Baseline erectile function and penile sensitivity were obtained. The officers were subsequently provided a narrow noseless bicycle seat for 6 months. At the conclusion of the study, there were significant improvements in erectile function and significant decreases in penile numbness.21
The Erectile Dysfunction Guideline Update from 2005 defined the arteriogenic index patient as an otherwise healthy man 55 years old or younger with recently acquired erectile dysfunction due to focal arterial occlusive disease. This definition excludes the vast majority of published data on a topic that often includes veno-occlusive dysfunction among the series.22 We herein review the evaluation and treatment options for a young man with suspected arteriogenic erectile dysfunction.
Patient selection—young man with suspected arteriogenic erectile dysfunction
As is the case in many aspects of surgery, patient selection is the key to optimizing clinical outcomes. As the Guideline Panel stated, the ideal patient for microvascular arterial bypass penile revascularization surgery, is the young man with a history of focal endothelial dysfunction and an absence of systemic endothelial dysfunction.
At San Diego Sexual Medicine, all men undergo a complete biopsychosocial evaluation. They undergo psychological evaluation with a sex therapist. Hormone blood tests are done to exclude testosterone and/or dihydrotestosterone deficiency, thyroid disease, hyper- or hypo-estrogenemia or prolactinoma. A physical examination is performed including qualitative sensory testing measuring vibration, hot and cold perception to assess the integrity of the sensory dorsal nerve branches of the pudendal nerve that are at risk from blunt pelvic and perineal trauma. A duplex Doppler ultrasound during pharmacological erection using maximal smooth muscle relaxation is performed. Right or left cavernosal artery peak systolic velocities less than 30 cm s−1 are considered abnormal. If veno-occlusive dysfunction is identified, end diastolic velocity values greater than 5 cm s−1 rule out the patient as a candidate for microvascular arterial bypass penile revascularization surgery. Should arteriogenic erectile dysfunction be suspected, dynamic infusion cavernosometry is performed to measure the right and left cavernosal artery systolic occlusion pressure and compare the values to the simultaneously recorded brachial artery systolic occlusion pressure. A pressure gradient more than 30 mmHg between the cavernosal and brachial artery systolic occlusion pressures is considered abnormal. The final assessment is the selective internal pudendal arteriogram during pharmacological erection. This provides anatomic information as to: (i) the site of the obstructing lesions in the distal internal pudendal artery, common penile artery and proximal cavernosal artery; (ii) the integrity of the right and left donor inferior epigastric arteries; (iii) the integrity of the recipient right and left cavernosal arteries; and (iv) the presence of arterial communications from the recipient dorsal arteries to the cavernosal arteries.
Conservative therapies
Patients must have intact trapping mechanisms to be considered appropriate for microvascular arterial bypass penile revascularization; therefore, many also respond well to oral phosphodiesterase-type 5 inhibitors and intracavernosal injections or intraurethral prostaglandin E1 suppositories.23,24,25 Other therapies for erectile dysfunction include a vacuum erection device or even penile prosthesis. It is our experience, however, that many of these young men with arteriogenic erectile dysfunction are not enthusiastic regarding a lifetime of medically or prosthetic assisted erections and prefer an opportunity to regain physiological penile erectile function whenever possible.
Microvascular arterial bypass penile revascularization surgery
The aim of surgery is to bypass the obstructed distal internal pudendal/common penile/proximal cavernosal arteries and deliver increased systolic perfusion pressure and blood flow from the donor inferior epigastric artery to the recipient dorsal penile artery. Ideally, the increased dorsal artery systolic perfusion pressure and blood flow will be communicated to the cavernosal artery via perforating branches between the dorsal penile artery and the cavernosal artery.26,27,28
Technique
The patient is positioned supine on the operating room table with his arms tucked. Great care is taken to ensure proper padding and positioning of the blood pressure cuff to minimize the risk of any iatrogenic injury to the ulnar nerve at the elbow. Ulnar nerve injury results in numbness to the fifth finger and half of the fourth finger. Sequential compression devices are placed around each calf to minimize postoperative venous thromboembolic disease. Preoperative antibiotics are given within one hour of skin incision (cefazolin, or vancomycin if allergic). The arteriogram films are reviewed prior to the skin incision to confirm the integrity of the most suitable donor inferior epigastric artery as well as the most suitable recipient dorsal penile artery. The surgery can be divided into three parts, dorsal artery dissection, inferior epigastric harvesting and microvascular anastomosis.
Dorsal artery dissection
A 14-French Foley catheter is placed after the patient has been prepped and draped. Loupe magnification (×2.5) and a headlight are utilized to aid visualization during the first two portions of the case. A curvilinear 3 cm penoscrotal incision is fashioned opposite to the planned abdominal incision for the donor inferior epigastric artery harvest (Figure 1a). Using a finger-sweep technique, Dartos and Bucks fascia are entered to bluntly expose the corpus spongiosum and both tunica albuginea. The penis is then everted through this incision and fixed in place with six yellow blunt hooks using a Scott or Lone-Star ring retractor to expose the dorsal neurovascular bundle. This approach allows the surgeon to also preserve the critical fundiform ligament, and maintain erect penile length postoperatively. Should the patient develop a partial erection at any time during the case, intracavernosal alpha-adrenergic agonist, 250 µg phenylephrine, may be provided.
Figure 1.
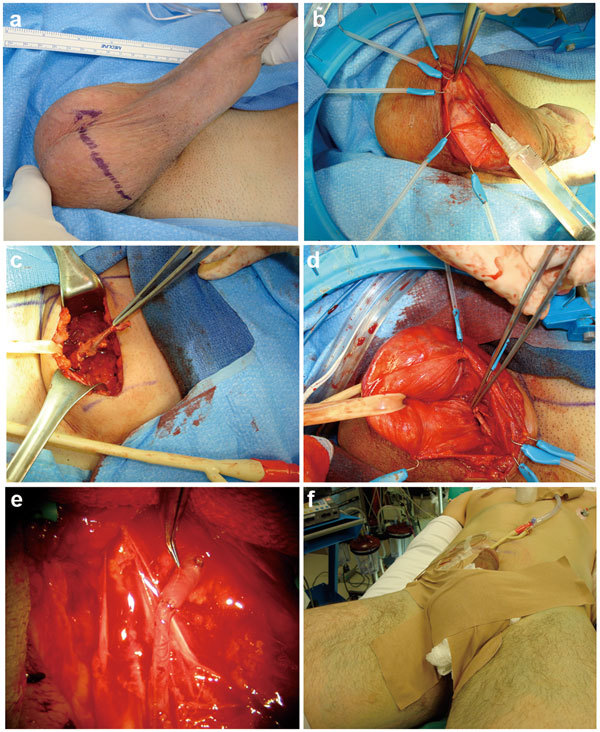
Microvascular arterial bypass penile revascularization surgery. (a) Oblique, inguinal scrotal incision on side of scrotum opposite of donor inferior epigastric harvest. (b) Preservation of fundiform ligament with Scott retractor hooks provides excellent exposure of dorsal neurovascular bundle. Careful initial dissection of recipient dorsal penile artery is fashioned. (c) Transverse abdominal incision is fashioned to harvest the donor inferior epigastric artery. The donor inferior epigastric artery has been transected around the level of the umbilicus and is ready for transfer to the base of the penis. (d) The donor inferior epigastric artery has been transferred through the internal ring, through the inguinal canal and out the external ring underneath the fundiform ligament to lie at the base of the penis at the dorsal neurovascular bundle. (e) The microvascular arterial anastomosis between the donor inferior epigastric artery and the recipient dorsal penile artery using interrupted 10-0 nylon sutures has been completed. (f) The postoperative compressive dressing over the scrotum is in place. The On-Q catheters are in place for pain control—they are placed above and below the rectus muscle and stay in place for 3 days postoperation. The Foley catheter is in place and is removed the following morning. The hands are tucked in at the sides to prevent ulnar nerve injury.
The neurovascular bundle is exposed and dissection is carried out proximally underneath the fundiform ligament (Figure 1b). During this portion of the case, great care is taken to minimize any trauma to the neurovascular bundle. Detailed dissection of the dorsal penile artery will be performed later under the microscope. Liberal use of papaverine hydrochloride irrigation is utilized to minimize vasospasm and potential endothelial injury. Blunt dissection is then performed toward the external ring to allow for subsequent transfer of the inferior epigastric artery to the base of the penis. The penis is returned to its natural position and temporary skin staples are used to approximate the scrotal incision.
Inferior epigastric artery harvest
The skin is marked following Langer's line three-fourths distance from the pubis to the umbilicus. A 5-cm semilunar incision is made and carried down to the anterior rectus fascia. The fascia is cleared superiorly and inferiorly preserving the perforating vessels and nerves. A longitudinal paramedian incision is then made sharply in the fascia. The edge of the fascia is grasped with Kocher clamps and the belly of the rectus muscle is bluntly mobilized medially. If necessary, tendinous rectus muscle intersections are dissected with electrocautery. As the muscle belly is rotated medially the inferior epigastric vessels, one artery and two veins, are identified on the under surface of the muscle in the preperitoneal plane. Papaverine hydrochloride solution is utilized again to topically bathe the vessel and minimize vasospasm. The vessels are encircled with a vessel loop and the inferior epigastric bundle is dissected using blunt dissection cranially to the level of the umbilicus and caudally to near its origin off the femoral artery. Bipolar cautery with a microvascular tip is carefully used to cauterize arterial branches from the inferior epigastric bundle to the rectus muscles and pelvic sidewall. The cranial aspect is then triply clipped with ligaclips and divided. The inferior epigastric bundle is widely pulsatile and is approximately 16 cm in length (Figure 1c).
The temporary scrotal staples are removed and accounted for. The previously dissected path through the external ring, through the inguinal canal and out the internal ring, is palpated from above and below. This ensures that there are no fascial bands that could kink or obstruct the donor inferior epigastric artery as it is transferred from the abdominal field to the base of the penis. A long uterine packing forcep is passed from the scrotal incision, underneath the fundiform ligament, through the external ring, through the inguinal canal and out the internal ring. This forcep is then visualized in the abdominal field. The ligaclip on the end of the epigastric bundle is carefully grasped with the long uterine packing forcep and under direct vision is delivered down to the penile field by passing through the internal ring, through the inguinal canal and out the external ring, and underneath the fundiform ligament to lie at the base of the penis. This step is of critical importance to prevent twisting or kinking of the vessel that could in any compromise blood flow (Figure 1d).
Once the vessel is successfully transferred, then the abdominal field is inspected to ensure hemostasis. An On-Q Pain Pump may be used to provide continuous local anesthesia to the abdominal field for a period of 3 days postoperation. Two soaker catheters are used: one placed below the fascia to directly bathe the muscle belly; the other placed above the fascia. This will deliver 0.25% Marcaine at 2 ml per hour directly to the surgical bed. We have found that the use of the On-Q pump has dramatically reduced the need for postoperative narcotics.
The fascia is closed with two running 0 Vicryl sutures, the deep dermis approximated with 3-0 Vicryl and the subcuticular layer closed with a running 4-0 Monocryl suture. Dermabond is placed over the abdominal incision and on the skin exit locations of the two soaker catheters.
Microvascular anastomosis
The penis is once again everted through the scrotal incision and fixed retraction is obtained with the Scott ring retractor. Again, great care is taken to not cause any injury to the neurovascular bundle through retraction. The donor inferior epigastric vessel is brought next to the previously selected dorsal artery to ensure that the anastomosis can be developed without tension. The cross-table operating microscope is then brought into the field. For intraluminal irrigation, a dilute mix of heparin and papaverine is used to minimize clot formation as well as vasospasm. The previously used full strength papaverine solution is removed from the field at this point as the low pH could damage the arterial endothelium.
Microvascular dissection of the selected dorsal artery is then performed using standard microvascular technique. Great care is taken to not injure the dorsal nerves as well as to preserve the perforating branches through the tunica into the corpus cavernosum as these perforating arterial communications are responsible for the therapeutic effect of the procedure. Once the dorsal artery is sufficiently mobilized, based on the relative length of the transferred donor inferior epigastric artery in the base of the penis, the distal aspect is occluded with a gold-plated, low-pressure microvascular clamp. The proximal aspect of the recipient dorsal artery is clipped and then divided. An end-to-end anastomosis is made rather than an end-to-side because there is dramatically less turbulence across an end-to-end anastomosis. The adventitia of the dorsal artery is dissected free only so far as to prevent its incorporation in the anastomosis, as this would risk postoperative arterial anastomosis occlusion. Removal of the adventitia is performed via a sleeve, circumcision-like dissection technique. The anastomosis involves the inferior epigastric artery connected to the distal, not proximal, dorsal penile artery. There are usually numerous connecting artery branches passing through the tunical albuginea from the distal dorsal artery to the cavernosal artery, which can be seen on preoperative selective internal pudendal arteriography. It is presumed that the increased perfusion pressure passes to the cavernosal artery through these communicating arterial branches.
The transferred donor inferior epigastric artery is then clamped with two gold-plated, low-pressure microvascular clamps. The end of the donor inferior epigastric artery is newly transected and its adventitia carefully is removed at its distal edge. Overdissection of either the donor inferior epigastric artery or the recipient dorsal artery is avoided to preserve the small vasovasorum and the health of each artery in the anastomosis.
Prior to beginning the anastomosis, a white Merocel sponge background is brought into the field to isolate the vessels from the surrounding structures and aid in visualization. The anastomosis is performed using 10-0 nylon interrupted sutures. The 10-0 nylon sutures are passed from the outside of the lumen through the arterial wall to the inside of the lumen on the side of the donor inferior epigastric artery. The suture is then passed into the lumen of the recipient dorsal artery and is passed through the arterial wall out the lumen of the recipient dorsal penile artery. Four quadrant stitches are placed first and a tail is left on each stitch to aid in placement of subsequent stitches. The dilute heparin-papaverine irrigation solution is injected intraluminally to distend the vessels and reduce the risk of back-walling. Once the quadrant stitches are in place, 12–14 more interrupted stitches are placed to complete the anastomosis. Should there be a size discrepancy between the donor inferior epigastric and the recipient dorsal penile arteries, positioning the four quadrant stitches first prevents a dog-ear from forming.
When the anastomosis is completed, the distal gold-plated, low-pressure microvascular clamp on the recipient dorsal artery is released first and the anastomosis is inspected. Retrograde filling of the donor inferior epigastric artery should be observed.
The two gold-plated, low-pressure microvascular clamps on the donor inferior epigastric clamps are then removed. Brisk pulsation should be observed. If any additional stitches are needed to reinforce the anastomosis, they are placed. Some bleeding is sometimes seen emanating from the needle holes. These can be safely observed and on occasion a small amount of hemostatic agent can be gently wrapped around the anastomosis to promote hemostasis (Figure 1e).
At this point, a complete sponge and needle count is performed and the penis is released from fixed retraction. It is gently replaced in its orthotopic and normal location and the penoscrotal incision is closed. The dartos layer is approximated with 3-0 Vicryl sutures in a running fashion and the skin is closed with a subcuticular 4-0 Monocryl. Dermabond is applied to the scrotal and abdominal incisions. Benzoin is placed in the perineum and lower abdomen. The scrotal incision is covered with fluffs and two 4-inch Elastoplast segments, approximately 18 cm, are passed from the thigh to the opposite side of the abdomen to develop a compressive dressing that stays in place over night. The Foley catheter is fixed to the abdomen with a Statlock, left to straight drainage overnight and is removed the following morning. A large scrotal support is placed over the compressive dressing (Figure 1f).
Complications
A traumatic disruption of the anastomosis is possible within the first few weeks following surgery. Patients are given strict instructions to not engage in any sort of sexual activity including masturbation for 6 weeks postoperatively. No effort is made to discourage normal nocturnal erections. Other possible complications include decreased penile sensation secondary to injury of the dorsal nerves, penile pain and loss of penile length, although we feel that this risk has been diminished by the technique of preserving the fundiform ligament. We do not see glans hyperemia as a complication with anastomosis of the donor inferior epigastric artery to the dorsal artery. In more than 30 years of performing this surgery, we have seen only two cases of mild pulmonary embolism and three cases of temporary ulnar neuropathy.
Surgical results
Use of nightly low-dose phosphodiesterase-type 5 inhibitors for a 6-month postoperative period may help these young men with erectile dysfunction increase their self-confidence and ultimately improve recovery. To optimize outcomes, it is important to continue to engage the patient's psychological recovery throughout the process. It is not uncommon for these men to be anxious about the patency of their anastomosis and their resulting function.
We counsel patients that not all will experience fully restored natural erections and currently, we do not scientifically know what combination of preoperative factors determine the best clinical outcome. In our experience, critical factors including quality, luminal diameter and length of the donor artery, quality and luminal diameter of the recipient artery, quality and luminal diameter of the communications between the recipient artery and donor artery (Figure 2), and age less than 50 years are clinically relevant.
Figure 2.
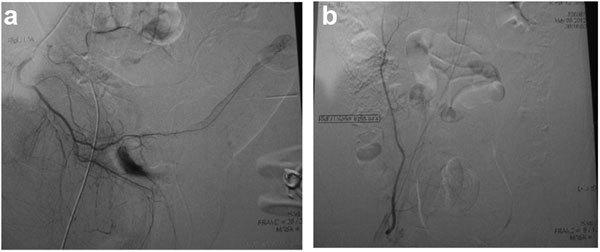
Surgical results. (a) Selective internal pudendal arteriogram showing trauma-related (bicycle accident) arterial occlusive pathology at the proximal cavernosal artery in a 21-year male with erectile dysfunction. (b) Selective infusion of contrast in the donor inferior epigastric artery reveals excellent length, excellent luminal diameter and freedom from arterial occlusive disease.
In our experience, approximately two-thirds of patients will realize significant improvement in postoperative erectile function. Individual results after this penile revascularization can be striking with one patient reporting that he could ‘build a house on his erection'. In one series, the total International Index of Erectile Function score increased from a mean of 35.5 to 56.2 and their Erectile Function domain increased from 14.8 to 23.8 after surgery. A total of 73% of patients scored 21 or greater in the Erectile Function domain. Importantly, 87% would recommend the surgery to someone else and 89% reported subjective improvement in their sexual function after surgery.
Over the years, we have accumulated numerous young patients, approximately 60% of all operated, who have had successful postoperative results, who have been followed for years, who regularly communicate with this office, including approximately 20% that have now 10 years outcome data since the surgery. The vast majority of these patients have graciously counseled new patients who may have preoperative questions or concerns. On the other hand, there have been approximately 10%–15% of patients who have been lost to follow-up and not ever examined or evaluated after 6 weeks postoperation. It is not known the clinical outcome of such patients. In general, patients who undergo this surgery want to move on with their lives. We have performed a second penile revascularization procedure in approximately 10 (0.67%) patients of 1500. The success rate of the second procedure is the same 60% as the first procedure. Outright, no improvement of the procedure occurs in approximately 25%—the explanation perhaps involves biopsychosocial considerations. We have now performed 15 postoperative selective inferior epigastric arteriograms that have excitedly documented the postoperative viability of the microvascular surgery (Figure 3). In patients who fail surgery, they persist on pharmacological management of their erectile dysfunction. Since such patients have excellent veno-occlusive function, pharmacological management is usually successful, although not what the patient wished/hoped for postoperatively. We have had no mortalities and one case of postoperative deep vein thrombosis and pulmonary embolism. We have had one case of deep wound infection, which responded to standard incision and drainage procedures. Penile shortening occurs in approximately 5%—from injury/tightening/scarring of the fundiform ligament.
Figure 3.
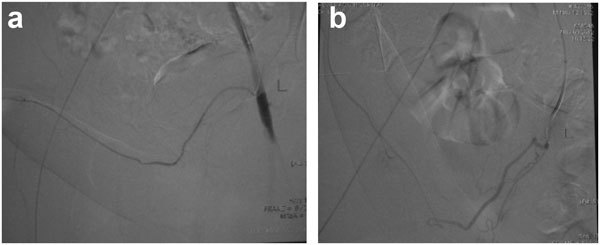
Postoperative selective inferior epigastric arteriogram revealing intact microvascular arterial anastomosis. (a) Early film. (b) delayed film.
Conclusions
Dr Goldstein has performed penile revascularization since 1981 after having the privilege and honor of being trained and mentored by Dr Vaclav Michal at that time. The procedure has evolved in all aspects including surgical indication, patient selection and surgical technique. Currently, at San Diego Sexual Medicine, we perform 30–40 penile revascularization procedures annually in the above-described fashion.
Barriers and obstacles to its more widespread adoption by sexual medicine physicians include: the need for specialized preoperative testing such as duplex Doppler ultrasound, dynamic cavernosometry and selective internal pudendal arteriography, the length and difficulty of the surgical procedure, the need for specialized microvascular training, the usual limited reimbursement by medical insurance, and the label that the surgery remains as ‘experimental' despite the fact that, at least one surgeon, has already performed over 1500 such cases since 1981. Not withstanding, penile revascularization or microvascular arterial bypass surgery remains a true for-cause treatment for erectile dysfunction. More work needs to be accomplished to continue to elucidate exactly which young men with erectile dysfunction will benefit most from penile revascularization. Now that the Erectile Dysfunction Guideline Update Panel has defined the index patient, attempts can be made to establish uniformity in patient selection and surgical technique, so robust multi-institutional clinical outcome data can one day be obtained.
The authors declare no competing financial interests.
References
- Michal V, Kramár R, Pospíchal J, Hejhal L.[Direct arterial anastomosis on corpora cavernosa penis in the therapy of erective impotence.] Rozhl Chir 197352587–90.Czech. [PubMed] [Google Scholar]
- Michal V, Kramár R, Pospíchal J. Femoro-pudendal by-pass, internal iliac thromboendarterectomy and direct arterial anastomosis to the cavernous body in the treatment of erectile impotence. Bull Soc Int Chir. 1974;33:343–50. [PubMed] [Google Scholar]
- Michal V. Arterial disease as a cause of impotence. Clin Endocrinol Metab. 1982;11:725–48. doi: 10.1016/s0300-595x(82)80010-8. [DOI] [PubMed] [Google Scholar]
- Kayıgil O, Okulu E, Aldemir M, Onen E. Penile revascularization in vasculogenic erectile dysfunction (ED): long-term follow-up. BJU Int. 2011;109:109–15. doi: 10.1111/j.1464-410X.2011.10293.x. [DOI] [PubMed] [Google Scholar]
- Sarramon JP, Malavaud B, Braud F, Bertrand N, Vaessen C, et al. Evaluation of male sexual function by the International Index of Erectile Function after deep dorsal vein arterialization of the penis. J Urol. 2001;166:576–80. [PubMed] [Google Scholar]
- Carvalho J, Nobre P. Biopsychosocial determinants of men's sexual desire: testing an integrative model. J Sex Med. 2011;8:754–63. doi: 10.1111/j.1743-6109.2010.02156.x. [DOI] [PubMed] [Google Scholar]
- Rosenbaum TY. How well is the multidisciplinary model working. J Sex Med. 2011;8:2957–8. doi: 10.1111/j.1743-6109.2011.02527.x. [DOI] [PubMed] [Google Scholar]
- García-Malpartida K, Mármol R, Jover A, Gómez-Martínez MJ, Solá-Izquierdo E, et al. Relationship between erectile dysfunction and silent myocardial ischemia in type 2 diabetic patients with no known macrovascular complications. J Sex Med. 2011;8:2606–16. doi: 10.1111/j.1743-6109.2011.02365.x. [DOI] [PubMed] [Google Scholar]
- El-Sakka AI, Morsy AM, Fagih BI. Severity of erectile dysfunction could predict left ventricular diastolic dysfunction in patients without overt cardiac complaint. J Sex Med. 2011;8:2590–7. doi: 10.1111/j.1743-6109.2011.02350.x. [DOI] [PubMed] [Google Scholar]
- Chung SD, Chen YK, Kang JH, Keller JJ, Huang CC, et al. Population-based estimates of medical comorbidities in erectile dysfunction in a Taiwanese population. J Sex Med. 2011;8:3316–24. doi: 10.1111/j.1743-6109.2011.02496.x. [DOI] [PubMed] [Google Scholar]
- Rastrelli G, Corona G, Monami M, Melani C, Balzi D, et al. Poor response to alprostadil ICI test is associated with arteriogenic erectile dysfunction and higher risk of major adverse cardiovascular events. J Sex Med. 2011;8:3433–45. doi: 10.1111/j.1743-6109.2011.02474.x. [DOI] [PubMed] [Google Scholar]
- Ross R. The pathogenesis of atherosclerosis: a perspective for the 1990s. Nature. 1993;362:801–9. doi: 10.1038/362801a0. [DOI] [PubMed] [Google Scholar]
- Banai S, Shou M, Correa R, Jaklitsch MT, Douek PC, et al. Rabbit ear model of injury-induced arterial smooth muscle cell proliferation. Kinetics, reproducibility, and implications. Circ Res. 1991;69:748–56. doi: 10.1161/01.res.69.3.748. [DOI] [PubMed] [Google Scholar]
- Ninković M, Wechselberger G, Schwabegger A, Anderl H. The instep free flap to resurface palmar defects of the hand. Plast Reconstr Surg. 1996;97:1489–93. doi: 10.1097/00006534-199606000-00030. [DOI] [PubMed] [Google Scholar]
- Moon IS, Hwang JK, Kim JI. Recurrent upper extremity embolism due to a crutch-induced arterial injury: a different cause of upper extremity embolism. Ann Vasc Surg. 2010;24:554.e7–e12. doi: 10.1016/j.avsg.2009.11.005. [DOI] [PubMed] [Google Scholar]
- Lehmann K, Schöpke W, Hauri D. Subclinical trauma to perineum: a possible etiology of erectile dysfunction in young men. Eur Urol. 1995;27:306–10. doi: 10.1159/000475186. [DOI] [PubMed] [Google Scholar]
- Bressel E, Nash D, Dolny D. Association between attributes of a cyclist and bicycle seat pressure. J Sex Med. 2010;7:3424–33. doi: 10.1111/j.1743-6109.2010.01905.x. [DOI] [PubMed] [Google Scholar]
- Sommer F, Goldstein I, Korda JB. Bicycle riding and erectile dysfunction: a review. J Sex Med. 2010;7:2346–58. doi: 10.1111/j.1743-6109.2009.01664.x. [DOI] [PubMed] [Google Scholar]
- Marceau L, Kleinman K, Goldstein I, McKinlay J. Does bicycling contribute to the risk of erectile dysfunction? Results from the Massachusetts Male Aging Study (MMAS) Int J Impot Res. 2001;13:298–302. doi: 10.1038/sj.ijir.3900733. [DOI] [PubMed] [Google Scholar]
- Munarriz R, Huang V, Uberoi J, Maitland S, Payton T, et al. Only the nose knows: penile hemodynamic study of the perineum–saddle interface in men with erectile dysfunction utilizing bicycle saddles and seats with and without nose extensions. J Sex Med. 2005;2:612–9. doi: 10.1111/j.1743-6109.2005.00089.x. [DOI] [PubMed] [Google Scholar]
- Schrader SM, Breitenstein MJ, Lowe BD. Cutting off the nose to save the penis. J Sex Med. 2008;5:1932–40. doi: 10.1111/j.1743-6109.2008.00867.x. [DOI] [PubMed] [Google Scholar]
- Manning M, Jünemann KP, Scheepe JR, Braun P, Krautschick A, et al. Long-term followup and selection criteria for penile revascularization in erectile failure. J Urol. 1998;160:1680–4. [PubMed] [Google Scholar]
- Corona G, Mondaini N, Ungar A, Razzoli E, Rossi A, et al. Phosphodiesterase type 5 (PDE5) inhibitors in erectile dysfunction: the proper drug for the proper patient. J Sex Med. 2011;8:3418–32. doi: 10.1111/j.1743-6109.2011.02473.x. [DOI] [PubMed] [Google Scholar]
- Debruyne FM, Gittelman M, Sperling H, Börner M, Beneke M. Time to onset of action of vardenafil: a retrospective analysis of the pivotal trials for the orodispersible and film-coated tablet formulations. J Sex Med. 2011;8:2912–23. doi: 10.1111/j.1743-6109.2011.02462.x. [DOI] [PubMed] [Google Scholar]
- Hsiao W, Bennett N, Guhring P, Narus J, Mulhall JP. Satisfaction profiles in men using intracavernosal injection therapy. J Sex Med. 2011;8:512–7. doi: 10.1111/j.1743-6109.2010.02093.x. [DOI] [PubMed] [Google Scholar]
- Hellstrom WJ, Montague DK, Moncada I, Carson C, Minhas S, et al. Implants, mechanical devices, and vascular surgery for erectile dysfunction. J Sex Med. 2010;7 (1 Pt 2:501–23. doi: 10.1111/j.1743-6109.2009.01626.x. [DOI] [PubMed] [Google Scholar]
- Raynor MC, Davis R, Hellstrom WJ. Robot-assisted vessel harvesting for penile revascularization. J Sex Med. 2010;7 (1 Pt 1:293–7. doi: 10.1111/j.1743-6109.2009.01462.x. [DOI] [PubMed] [Google Scholar]
- Goldstein I, Bastuba M, Lurie A, Lubisich J. Penile revascularization. J Sex Med. 2008;5:2018–21. doi: 10.1111/j.1743-6109.2008.00957.x. [DOI] [PubMed] [Google Scholar]


