Abstract
The higher frequency of varicocele in men with infertility has drawn attention and resulted in increased research at the molecular level towards treatments. The aim of this study was to investigate the role of tumor necrosis factor (TNF)-related apoptosis-inducing ligand (TRAIL) and its receptors in varicocele-induced testicular dysfunction in an experimental rat model. The rats were divided into three groups: control, sham and varicocele. Varicoceles in rats were induced by partial ligation of the left renal vein and left testes. The rats were analyzed 13 weeks after surgery. The degree of DNA fragmentation within cells in the testis was determined using terminal deoxynucleotidyl transferase deoxyuridine triphosphate nick end labeling (TUNEL) assay. Tubule degeneration was evaluated using the Johnsen score. The expression of TRAIL and its receptors was detected by immunohistochemical and Western blotting techniques. The apoptotic index, Johnsen score and the expression of TRAIL and TRAIL receptors were examined. The data are presented as the mean±s.d. and were analyzed using computer software. The Kruskal–Wallis and Dunn's multiple comparison tests were used in the statistical analyses. The germ cell apoptotic index was increased in rats with varicoceles when compared with the sham and control groups (P=0.0031). The Johnsen score was significantly decreased in the varicocele group when compared with the sham and control groups (P<0.0001). Immunohistochemical and Western blotting analyses showed that after varicocele induction, the expression of TRAIL-R1 and TRAIL-R4 in germ cells was increased and the expression of TRAIL-R2 was decreased. There are no significant differences among the groups in terms of TRAIL and TRAIL-R3 receptor expression. The results of this study indicate that TRAIL and its receptors may have a potential role in the pathogenesis of varicocele-induced testicular dysfunction.
Keywords: apoptosis, infertility, testis, TNF-related apoptosis inducing ligand (TRAIL), varicocele
Introduction
A varicocele is an abnormal enlargement of the pampiniform plexus of the spermatic cord. This abnormality is the most common cause of male infertility and occurs in approximately 21%–81% of infertile men.1 The association of a varicocele with infertility has been recognized for centuries.2 Varicoceles are ordinarily accompanied by a decreased testicular volume, an impaired sperm quality and a decline in Leydig cell function. However, the exact mechanism that causes this abnormality remains to be determined.3 Several theories have been suggested to explain the mechanism by which varicoceles impair male fertility. These theories include increased apoptosis, increased DNA damage in sperm, oxidative stress, tissue hypoxia, degenerative changes in the seminiferous tubule and immunological infertility caused by the increased apoptosis of germ cells.4,5,6,7,8,9 However, the presence of a varicocele does not necessarily cause infertility or worsen semen viability. Therefore, further studies are still necessary to understand the relationship between varicoceles and male infertility.
Recent investigations have reported that nuclear factor-kappa B (NF-κB) activation involves varicocele-induced and postchemotherapy testicular dysfunction.10 NF-κB is a key regulator of inflammatory gene expression, and its activation can be stimulated by oxidative stress.11 Although there are several mediators that may increase the levels of NF-κB in different pathologies, evidence suggests that the death ligand, tumor necrosis factor (TNF)-α-related apoptosis-inducing ligand (TRAIL), a recently discovered member of the TNF family, may be another factor that stimulates NF-κB, thus implicating this ligand in varicocele-mediated pathogenesis.12 TRAIL, a type II transmembrane protein with a molecular weight of 40 kDa, is known to stimulate the apoptotic pathway in transformed cells and the NF-κB signal pathway in the presence of autoimmune and inflammatory responses.13 Currently, five TRAIL receptors belonging to the TNF-α receptor superfamily have been identified. Two of them, DR4/TRAIL-R1 and DR5/TRAIL-R2, contain a cytoplasmic death domain that transmits apoptotic signals in response to TRAIL.14 Two other cellular TRAIL receptors, DcR1/TRAIL-R3, a glycosylphosphatidylinositol-linked protein without an intracellular domain, and DcR2/TRAIL-R4, containing a truncated death domain, have been identified. TRAIL-R4 binds TRAIL without activation of the apoptotic machinery and seems to antagonize the death domain containing TRAIL receptors.15 Finally, osteoprotegerin, a regulator of osteoclastogenesis, was reported to be a soluble receptor for TRAIL.16,17,18 Immunohistochemical studies indicate that TRAIL, TRAIL-R2 and TRAIL-R4 were detected in Leydig cells, whereas the ligands and all receptors were localized in germ cells.19,20 The aim of this study is to investigate the possible role of TRAIL pathway activation in the pathogenesis of infertility in an experimental rat model.
Materials and methods
A total of 18 adult Wistar male rats were housed at 24 °C under controlled conditions with free access to standard rat chow and water. The experimental protocol was approved by the Animal Care and Usage Committee of Akdeniz University and was in accordance with the Declaration of Helsinki and International Association for the Study of Pain guidelines.
Experimental rat model of varicocele
Twenty-week-old adult rats were used for the control group (n=6). The animals underwent a sham operation at 7 weeks and were studied 13 weeks later (n=6). Animals in the experimental varicocele group underwent partial ligation of the left renal vein at 7 weeks and were studied 13 weeks later. A total of six animals underwent the varicocele surgery, and all six of them developed varicoceles. The establishment of the control and experimental groups is described elsewhere.21 After identification of the left varicocele by observation of the dilatation of the internal spermatic veins, both testicles were harvested from the same incision. Sham-operated rats underwent a similar procedure: the left renal vein was freed by dissection but was not ligated. The animals were euthanized 13 weeks after renal vein ligation, and the left testicular tissues were removed for immunohistochemistry and Western blotting analysis.
Histological evaluation
The left testicular tissues of rats were fixed in Bouin's fixative for 4 h, dehydrated in ethanol, embedded in paraffin and stained with hematoxylin and eosin for histopathological evaluation. Tubule degeneration was evaluated in each group using Johnsen scores. This scoring system is principally based on the progressive degeneration of germinal epithelium and subsequent loss of the most mature cell types during testicular damage. Johnsen score was also used to categorize the level of spermatogenesis in the control testes and compare with the scores of the varicocele-induced testes. A grade from 1 to 10 was given to each tubule cross-section according to the following criteria: 10=complete spermatogenesis and perfect tubules; 9=many spermatozoa present and disorganized spermatogenesis; 8=only a few spermatozoa present; 7=no spermatozoa but many spermatids present; 6=only a few spermatids present; 5=no spermatozoa or spermatids but many spermatocytes present; 4=only a few spermatocytes present; 3=only spermatogonia present; 2=no germ cells but only Sertoli cells present; 1=no germ cells and no Sertoli cells present.22
Evaluation of DNA damage
The apoptotic index in germ cells was determined by the terminal deoxynucleotidyl transferase deoxyuridine triphosphate nick end labeling (TUNEL) assay. Paraffin sections of 5-µm thickness from the testes tissues were washed twice in phosphate-buffered solution (PBS) for 5 min. Following the incubation of slides with the permeabilization solution (0.1% Triton X-100 in 0.1% sodium citrate) for 8 min at 4 °C and washing twice with PBS for 5 min, the labeling reaction was performed using 50 µl of TUNEL reagent for each sample, except for the negative controls, in which the reagent was added without enzyme. The slides were then incubated for 1 h at 37 °C. After washing with PBS, the slides were incubated with a converter reagent for 30 min at 37 °C. Color development to localize the cells containing labeled DNA strand breaks was performed by incubating the slides with fast red substrate solution for 5 min. The TUNEL assays were conducted using a cell death detection kit (Roche, Mannheim, Germany). The apoptotic index was determined by a Zeiss Axioplan microscope (Thornwood, NY, USA). All seminiferous tubules from each group were observed, and the numbers of TUNEL-positive and TUNEL-negative seminiferous tubules were recorded. The seminiferous tubules with apoptotic cell signals were defined as positive seminiferous tubules. The positive seminiferous tubule rate was calculated by multiplying the ratio of positive tubules to the total number of tubules by 100%.
Immunohistochemical scoring of TRAIL and its receptors
The tissue sections were deparaffinized and blocked from endogenous peroxidase activity with methanol containing 3% H2O2 for 10 min and from nonspecific binding with Ultra V Block (Labvision, Freemont, CA, USA) for 7 min at room temperature. Rabbit polyclonal TRAIL (Ab 2435; Abcam, Cambridge, MA, USA), TRAIL-R1 (Ab 8414), TRAIL-R2 (Ab 8416), TRAIL-R3 (Ab 2087) and TRAIL-R4 (Ab 2019) primary antibodies were applied at dilutions of 1∶500, 1∶750, 1∶300, 1∶1000 and 1∶600, respectively and incubated overnight at 4 °C in a humidified chamber. The sections were washed in PBS and incubated with biotinylated horse anti-mouse IgG and anti-rabbit IgG (3 mg ml−1; Vector, Burlingame, CA, USA) at a dilution of 1∶500 for 45 min at room temperature. The antigen–antibody complex was detected using an avidin–biotin horseradish peroxidase complex with a Universal LSAB Kit (Dako, Glostrup, Denmark). Diaminobenzidine (3, 3′-diaminobenzidine tetrahydrochloride dihydrate; Sigma, St. Louis, MO, USA) was used as the chromogen, and the sections were mounted with Permount (Fisher Chemicals, Zurich, Switzerland) on glass slides and then evaluated under a light microscope. For controls, sections were incubated with non-immune IgG3 (MAB007; R&D Systems, Minneapolis, MN, USA) and rabbit serum (X0902; Dako) at the same concentrations as the primary antibodies. Pictures were taken while viewing with an Axiophot microscope (Zeiss, Oberkochen, Germany). Two independent histologists who were blinded to the names of the antibodies used for the staining performed the immunohistochemical scoring. Briefly, both the intensity and the marker distribution (percentage of positively stained epithelial cells) were used to obtain the immune staining scores. The intensity was scored as follows: 0, negative; 1, weak; 2, moderate; and 3, strong staining. The marker distribution was also scored as 0, less than 10% 1, between 10% and 40% 2, between 40% and 70% and 3, more than 70% of the epithelial cells were stained on the specimen. Adding the scores of both the intensity and the marker distribution for a given animal provided the final immune staining score.23,24
Western blotting
After homogenization of the left testis, the supernatants were collected, and the protein concentrations were determined. One hundred micrograms of each protein sample was loaded into each lane. The samples were subjected to SDS-polyacrylamide gel electrophoresis (SDS-PAGE) under standard conditions and transferred onto PVDF membranes (BioRad Comp., Philadelphia, PA, USA). The SDS gel used was a Bis-Tris Gel (Philadelphia, PA, USA). To evaluate the expression of TRAIL and its receptors, the membranes were blocked for 1 h with 5% non-fat dry milk and were incubated with TRAIL-L (dilution: 1∶50), TRAIL-R1 (dilution: 1∶500), TRAIL-R2 (dilution: 1∶500), TRAIL-R3 (dilution: 1∶200) and TRAIL-R4 (dilution: 1∶200) primary antibodies at 4 °C overnight. After washing, the membranes were incubated with anti-rabbit IgG horseradish peroxidase conjugate diluted to 1∶5000 for 1 h at room temperature. Immunolabeling was visualized using the chemiluminescence-based SuperSignal CL HRP Substrate System and the membranes were subsequently exposed to Hyperfilm. Beta-actin, as an internal standard, was also examined to confirm equal loading of the proteins. The band intensities from the Hyperfilm were evaluated using Alpha Digidoc software (Alpha Innotech, Corp., San Leandro, CA, USA) as a measure of protein expression levels.
Results
A total of 18 rats in three groups were evaluated after 13 weeks in an experimental rat varicocele model. All categorical variables for the groups are shown in Table 1. The mean left testicular vein diameter reached 1.03±0.08 mm after 13 weeks and this is significantly different compared with the control and sham-operated rats (0.13±0.05 min and 0.15±0.05 min, respectively; P=0.0017). There was no difference between the groups in body weight. The ratio of left testis to body weight in varicocele-induced rats was also significantly smaller compared with the controls, indicating testicular tissue loss (P=0.0157). When the testicular sections were examined using light microscopy, we observed similar tubule counts in the testes of all of the groups. While normal spermatogenesis and testicular histology were observed in the control and sham-operated groups, abnormal spermatogenic cell types in damaged seminiferous tubules were observed in varicocele-induced rats. Johnsen scores, indicating tubule degeneration, showed a significant decrease in varicocele-induced testes and confirmed the testicular damage (Table 1). The DNA fragmentation index values, as evaluated with the TUNEL method and using the ratio of apoptotic germ cells to non-apoptotic cells, were found to be significantly higher in the varicocele group (Table 1 and Figure 1).
Table 1. The results of body weight, left testicle weight, vein diameters, Johnsen score, spermatic tubule counts and apoptotic indices of the groups.
| Control (n=6) | Sham (n=6) | Varicocele (n=6) | P | |
|---|---|---|---|---|
| Body weight (g) | 379.80±27.06 | 362.50±51.55 | 338.20±31.72 | 0.2404 |
| Left testicle weight (g) | 2.02±0.10 | 1.91±0.12 | 1.44±0.18 | <0.0001* |
| Vein diameter (mm) | 0.13±0.05 | 0.15±0.05 | 1.03±0.08 | 0.0017* |
| Johnsen score | 9.20±1.01 | 9.20±1.09 | 6.92±2.36 | <0.0001* |
| Spermatic tubule count | 401±33 | 413±16 | 403±31 | 0.4233 |
| Apoptotic index (%) | 12.9±2.04 | 12.3±1.9 | 26.4±2.5 | 0.0031* |
*P<0.05 compared with control group by Kruskal–Wallis test. The data are presented as the mean±s.d.
Figure 1.
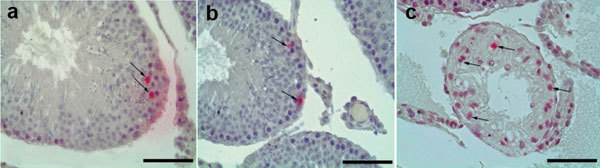
The results of TUNEL in all groups. Red points and black arrows show apoptotic cells. (a) Control gruop; (b) Sham group; (c) varicocele group. Scale bars=50 µm. TUNEL, transferase deoxyuridine triphosphate nick end labeling.
Immunohistochemical staining of control (Figure 2a–2e) and sham (Figure 2f–2j) seminiferous tubules with complete spermatogenesis revealed a pronounced expression of TRAIL and its four receptors in the germ cells (Table 2). Analysis for the subcellular localization of TRAIL-R1 showed a weak immunostaining localized in primary spermatocytes (Figure 2a and 2f). Spermatogonia, primary spermatocytes, and interstitial cells were highly immunoreactive for TRAIL-R2 (Figure 2b and 2g). Low TRAIL-R3 expression was present in the cytoplasmic extrusions of spermatozoa at stage VIII (Figure 2c and 2h). Blood vessel walls localized in the interstitial area were immunopositive for TRAIL-R3 protein (Figure 2c and 2h). The protein expression of TRAIL-R4 was localized in cytoplasmic extrusions of the spermatozoa in the control and sham groups (Figure 2d and 2i). TRAIL was present in the cytoplasmic extrusions of elongated sperm and the cytoplasm of interstitial cells (Figure 2e and 2j). In the varicocele group, TRAIL-R1 protein expression was considerably increased (P=0.0029) (Figure 2k). TRAIL-R1 expression was present in spermatogonia and primary spermatocytes in the varicocele-induced testes (Figure 2k). However, the expression of TRAIL-R2 protein in the varicocele group decreased significantly in spermatogonia, primary spermatocytes and interstitial cells (P=0.0235) (Figure 2l). Moreover, in the varicocele group, expressions of TRAIL-R3 (P=0.7188), TRAIL-R4 (P=0.0110) and TRAIL (P=0.8447) proteins were similar to those of the control and sham groups (Figure 2m–2o, respectively).
Figure 2.
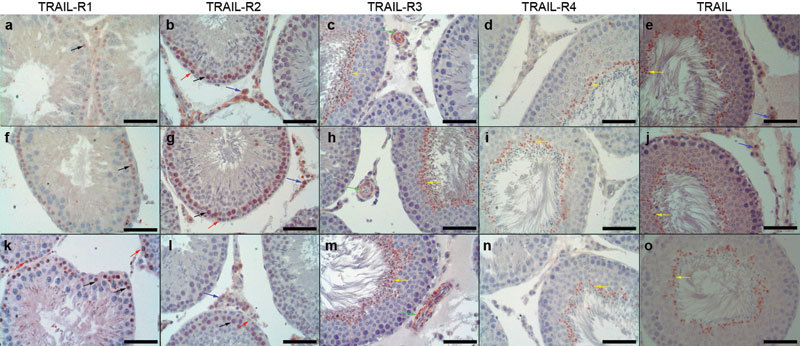
Immuno-labeling of TRAIL and its receptors in all groups (black arrow: primary spermatocyte; red arrow: spermatogonia; blue arrow: interstitial cell; yellow arrow: the cytoplasmic extrusion of sperm; green arrow: wall of the vessel). (a-e) Control gruop; (f-j) Sham group; (k-o) varicocele group. Scale bars=50 µm. TRAIL, tumor necrosis factor-related apoptosis-inducing ligand.
Table 2. Immune staining scores (intensity scores+marker distribution scores) of TRAIL and its receptors.
| Control (n=6) | Sham (n=6) | Varicocele (n=6) | P | |
|---|---|---|---|---|
| TRAIL-R1 | 2.50±0.83 (2–4) | 2.66±0.81 (2–4) | 5.00±0.63 (4–6) | 0.0029* |
| TRAIL-R2 | 4.16±0.40 (4–5) | 4.50±0.54 (4–5) | 3.33±0.81 (2–4) | 0.0235* |
| TRAIL-R3 | 3.66±0.81 (2–4) | 3.66±0.51 (3–4) | 4.00±0.89 (3–5) | 0.7188 |
| TRAIL-R4 | 2.33±0.51 (2–3) | 2.16±0.75 (1–3) | 3.50±0.54 (3–4) | 0.0110* |
| TRAIL | 1.83±0.75 (1–3) | 1.66±0.51 (1–2) | 1.83±0.40 (1–2) | 0.8447 |
Abbreviation: TRAIL, tumor necrosis factor-related apoptosis-inducing ligand.
The first number is the total of the intensity scores. The second number is the total of marker distribution scores. Intensity scores (0, negative; 1, weak; 2, moderate; 3, strong staining); marker distribution scores (0, less than 10% 1, between 10% and 40% 2, between 40% and 70% 3, more than 70% of the epithelial cells stained on the specimen). *P<0.05 compared with control group by Dunn's multipl comparison test. The data are presented as the mean±s.d.
Western blotting data confirmed the results of the immunohistochemical analysis. While the protein expression of TRAIL-R1 (P=0.0062) and R4 (P=0.0028) receptors increased, the receptor protein expressions of TRAIL-R2 (P=0.0062) decreased in the varicocele group compared with the control and sham-operated rats. Similar to the immunohistochemical data, there were no differences in TRAIL (P=0.9599) and TRAIL-R3 (P=0.8948) receptor protein expression of varicocele-induced rats compared with other groups (Table 3 and Figure 3).
Table 3. Western blotting analyses (intensity measurements of the protein (µg) expressions) of the groups.
| Control (n=6) | Sham (n=6) | Varicocele (n=6) | P | |
|---|---|---|---|---|
| TRAIL-R1 | 0.32±0.08 (0.21–0.43) | 0.33±0.13 (0.19–0.56) | 0.71±0.26 (0.48–1.22) | 0.0062* |
| TRAIL-R2 | 2.10±0.53 (1.14–2.63) | 2.22±0.31 (1.92–2.83) | 0.84±0.74 (0.006–1.86) | 0.0062* |
| TRAIL-R3 | 1.09±0.52 (0.35–1.81) | 1.11±0.40 (0.72–1.80) | 1.02±0.49 (0.54–1.88) | 0.8948 |
| TRAIL-R4 | 0.26±0.13 (0.15–0.48) | 0.36±0.14 (0.13–0.56) | 1.06±0.23 (0.78–1.33) | 0.0028* |
| TRAIL | 1.36±0.49 (0.63–2.07) | 1.38±0.39 (0.92–2.08) | 1.47±0.48 (1.07–2.40) | 0.9599 |
Abbreviation: TRAIL, tumor necrosis factor-related apoptosis-inducing ligand.
*P<0.05 compared with control group by Kruskal–Wallis method. The data are presented as the mean±s.d.
Figure 3.
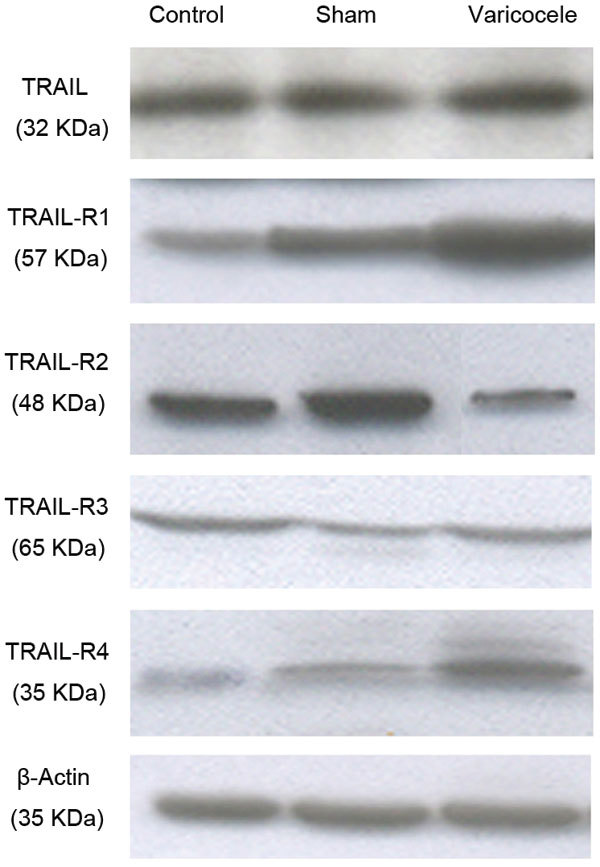
Bands corresponding with TRAIL and its receptors protein expression were measured. TRAIL (P=0.9599); TRAIL-R1 (P=0.0062); TRAIL-R2 (P=0.0062); TRAIL-R3 (P=0.8948); TRAIL-R4 (P=0.0028). TRAIL, tumor necrosis factor-related apoptosis-inducing ligand.
Discussion
Varicocele has incidence rates of 4.4%–22.6% in the general population, 21%–41% in men with primary infertility and 75%–81% in men with secondary infertility.2 To date, several factors have been proposed to account for varicocele-induced testicular injuries and it appears that the pathophysiology of varicocele is complex and multifaceted. The higher frequency of varicocele appearances in men with infertility has drawn attention and resulted in increased research at the molecular level towards treatments.3,9 The present study was performed to investigate the role of TRAIL and its receptors in the pathogenesis of varicocele-induced testicular dysfunction. We chose a well-established, experimental varicocele rat model that mimics the varicocele in humans.21 In our study, the varicocele model was created successfully and showed significantly increased spermatic vein diameters and decreased Johnsen scores 13 weeks after the varicocele was induced.
Apoptosis is one of the main pathophysiologic mechanisms of testicular dysfunction as a result of varicoceles and therefore, molecules that play a role in apoptotic processes have been studied.25 Similar to previous reports, the TUNEL results of the present study supported those findings by showing an increase in cells that show DNA damage in varicocele-induced testes. The relationship between the apoptotic pathway and TRAIL is also a promising area of scientific interest for researchers investigating malignant or inflammatory processes.23,24,26,30
Recently, it was reported that TRAIL and its receptors are expressed in rat testis during normal development, and that the TRAIL protein is present in different germ cell types, whereas its receptors were predominantly detected in postmeiotic germ cells.19 There are no reports in the literature concerning TRAIL and TRAIL receptor expression in the testicular sections of rats with varicoceles evaluated by immunohistochemical staining and Western blotting. There are similar reports, but they do not use testicular rat tissue. Grataroli et al.20 reported the immunolocalization of TRAIL , its receptors and their identification by analyzing proteins and mRNA in human testes. They found that postmeiotic germ cells immuno-expressed both TRAIL and its four receptors, while Leydig cells expressed TRAIL, TRAIL-R2 and TRAIL-R4. Their results were globally consistent with those observed previously in the adult rat testis.19 In contrast with the rat testis, TRAIL-R1 was also immunodetected in myoid and Sertoli cells in the human testis.20 Our immunohistochemical data were similar to those in the Grataroli et al. study.19 We found that, TRAIL-R1 was specifically present in meiotic spermatocytes only in the control testis. However, after varicocele induction, expression of TRAIL-R1 was observed in both spermatogonia and meiotic spermatocytes. Moreover, the other death receptor, TRAIL-R2, was present in spermatogonia, meiotic spermatocytes and interstitial cells. The expression pattern of TRAIL-R2 did not change with varicocele induction but the expression level was significantly lower in all of these cell types. We also obtained a single specific band at the predicted molecular weight for both TRAIL proteins in the Western blotting, indicating that our antibodies could specifically recognize either TRAIL-R1 or TRAIL-R2. Although the spermatogonia expression of TRAIL-R2 in our study differs from the current literature, the presence of TRAIL-R1 and TRAIL-R2 in meiotic spermatocytes is consistent with the findings of Grataroli et al.19 Moreover, in accordance with our TUNEL results, it seems that TRAIL death receptor signaling could be activated in meiotic spermatocytes. The two decoy receptors, TRAIL-R3 and TRAIL-R4, were shown to be expressed by elongated spermatozoa as reported by Grataroli et al.19 They also found that both of these decoy receptors were present in round and elongated spermatids.19 In our study, we demonstrated that TRAIL-R4 expression (P=0.0028) increased in Western blotting analyses. Thus, it is possible that the changes after varicocele induction might be related to the sperm quality rather than anti-apoptotic functions of this molecule.
Indeed, under denaturing and reducing conditions on SDS-PAGE, TRAIL-R4 protein from rat testis migrated as two protein bands. This result suggests the presence of extensive post-translational modification, or unusual structural motifs (or both) in the testicular TRAIL-R4 protein. However, the unusual migration of this receptor on SDS-polyacrylamide gel electrophoresis gels as different forms of TRAIL-R4 has also been previously reported in 293 T cells.28 Previous studies indicate that overexpression of TRAIL-R4 activates the transcription factor NF-κB and protects cells against TRAIL-induced DNA damage, suggesting that TRAIL-R4, unlike TRAIL-R3, has a functional, intracellular signaling domain.14,29 Thus, TRAIL-R4 might protect cells from TRAIL-induced DNA damage by either acting as a decoy receptor or transducing an anti-apoptotic signal. Currently, the intracellular signaling pathways responsible for TRAIL receptor-mediated NF-κB activation are unclear, and the mechanisms responsible for TRAIL receptor-induced apoptosis are controversial. In addition to TRAIL-R4, it has been shown that TRAIL-R1 and TRAIL-R2 can also activate NF-κB.15,30 As shown recently, overexpression of TRAIL-R4 did not protect cells from DNA damage induced by TRAIL-R1. Activation of NF-κB alone is in sufficient to block the DNA damage induced by TRAIL receptors, and an alternative mechanism other than NF-κB activation may account for the protective role of TRAIL-R4 on TRAIL-induced DNA damage.30 Our present study suggests that despite the high levels of TRAIL-R4 in varicocele-bearing testicles of rats, the increase in death receptor TRAIL-R1 levels might be associated with the initiation of TRAIL pathways in varicocele-induced testicular dysfunction.
In conclusion, this study evaluated the role of TRAIL and its receptors in testis with varicocele, and examined whether this family of proteins contributes to the mechanism of increased DNA damage at the molecular level. We demonstrated that TRAIL and its receptors may play a role in apoptosis signaling and serve as an induction mechanism on cell tissues associated with the experimental varicocele rat model.
Author contributions
ITK, CCO and OC helped supervise the fieldwork, designed the study's analysis plan and drafted the manuscript. OC and OK designed the study, directed its implementation, including quality assurance and control and reviewed the manuscript. OK, MT and CCO helped conducting the literature review and prepared the ‘Materials and Methods' section of the manuscript. OC conducted the fieldwork and reviewed the manuscript. All authors read and approved the final manuscript.
Acknowledgments
This study was supported by the research foundation of Akdeniz University (No. 2009.04.0103.001).
All authors declare that there are no competing financial interests.
References
- Ghanem MA, Safan MA, Ghanem AA, Dohle GR. The role of varicocele sclerotherapy in men with severe oligo-astheno-teratozoospermia. Asian J Androl. 2011;13:867–71. doi: 10.1038/aja.2011.48. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Will MA, Swain J, Fode M, Sonksen J, Christman GM, et al. The great debate: varicocele treatment and impact on fertility. Fertil Steril. 2011;95:841–52. doi: 10.1016/j.fertnstert.2011.01.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lopushnyan NA, Walsh TJ. Surgical techniques for the management of male infertility. Asian J Androl. 2012;14:94–102. doi: 10.1038/aja.2011.62. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cheng D, Zheng XM, Li SW, Yang ZW, Hu LQ. Effects of epidermal growth factor on sperm content and motility of rats with surgically induced varicoceles. Asian J Androl. 2006;8:713–7. doi: 10.1111/j.1745-7262.2006.00202.x. [DOI] [PubMed] [Google Scholar]
- Akkoyunlu G, Erdogru T, Seval Y, Ustunel I, Koksal T, et al. Immunolocalization of glial cell-derived neurotrophic factor (GDNF) and its receptor GFR-alpha1 in varicocele-induced rat testis. Acta Histochem. 2007;109:130–7. doi: 10.1016/j.acthis.2006.11.003. [DOI] [PubMed] [Google Scholar]
- Koksal IT, Tefekli A, Usta M, Erol H, Abbasoglu S, et al. The role of reactive oxygen species in testicular dysfunction associated with varicocele. BJU Int. 2000;86:549–52. doi: 10.1046/j.1464-410x.2000.00755.x. [DOI] [PubMed] [Google Scholar]
- Zini A, Al-Hathal N. Antioxidant therapy in male infertility: fact or fiction. Asian J Androl. 2011;13:374–81. doi: 10.1038/aja.2010.182. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Turker Koksal I, Erdogru T, Gulkesen H, Sezer C, Usta M, et al. The potential role of inducible nitric oxide synthase (iNOS) activity in the testicular dysfunction associated with varicocele: an experimental study. Int Urol Nephrol. 2004;36:67–72. doi: 10.1023/b:urol.0000032687.58462.4f. [DOI] [PubMed] [Google Scholar]
- Tekcan M, Koksal IT, Tasatargil A, Kutlu O, Gungor E, et al. Potential role of poly(ADP-ribose) polymerase activation in the pathogenesis of experimental left varicocele. J Androl. 2012;33:122–32. doi: 10.2164/jandrol.110.012203. [DOI] [PubMed] [Google Scholar]
- Tugcu V, Gedikbasi A, Mutlu B, Guner E, Uhri M, et al. Increased testicular 8-hydroxy-2′-deoxyguanosine (8-OHdG) and inducible nitric oxide synthetase (iNOS) and nuclear factor kappaB (NF-kappaB) expressions in experimental rat varicocele. Arch Ital Urol Androl. 2010;82:148–53. [PubMed] [Google Scholar]
- Ilbey YO, Ozbek E, Simsek A, Cekmen M, Otunctemur A, et al. Chemoprotective effect of a nuclear factor-kappaB inhibitor, pyrrolidine dithiocarbamate, against cisplatin-induced testicular damage in rats. J Androl. 2009;30:505–14. doi: 10.2164/jandrol.108.006270. [DOI] [PubMed] [Google Scholar]
- Wiley SR, Schooley K, Smolak PJ, Din WS, Huang CP, et al. Identification and characterization of a new member of the TNF family that induces apoptosis. Immunity. 1995;3:673–82. doi: 10.1016/1074-7613(95)90057-8. [DOI] [PubMed] [Google Scholar]
- Nesterov A, Lu X, Johnson M, Miller GJ, Ivashchenko Y, et al. Elevated AKT activity protects the prostate cancer cell line LNCaP from TRAIL-induced apoptosis. J Biol Chem. 2001;276:10767–74. doi: 10.1074/jbc.M005196200. [DOI] [PubMed] [Google Scholar]
- Degli-Esposti MA, Dougall WC, Smolak PJ, Waugh JY, Smith CA, et al. The novel receptor TRAIL-R4 induces NF-kappaB and protects against TRAIL-mediated apoptosis, yet retains an incomplete death domain. Immunity. 1997;7:813–20. doi: 10.1016/s1074-7613(00)80399-4. [DOI] [PubMed] [Google Scholar]
- Schneider P, Thome M, Burns K, Bodmer JL, Hofmann K, et al. TRAIL receptors 1 (DR4) and 2 (DR5) signal FADD-dependent apoptosis and activate NF-kappaB. Immunity. 1997;7:831–6. doi: 10.1016/s1074-7613(00)80401-x. [DOI] [PubMed] [Google Scholar]
- Pan G, O'Rourke K, Chinnaiyan AM, Gentz R, Ebner R, et al. The receptor for the cytotoxic ligand TRAIL. Science. 1997;276:111–3. doi: 10.1126/science.276.5309.111. [DOI] [PubMed] [Google Scholar]
- Pan G, Ni J, Wei YF, Yu G, Gentz R, et al. An antagonist decoy receptor and a death domain-containing receptor for TRAIL. Science. 1997;277:815–8. doi: 10.1126/science.277.5327.815. [DOI] [PubMed] [Google Scholar]
- MacFarlane M, Ahmad M, Srinivasula SM, Fernandes-Alnemri T, Cohen GM, et al. Identification and molecular cloning of two novel receptors for the cytotoxic ligand TRAIL. J Biol Chem. 1997;272:25417–20. doi: 10.1074/jbc.272.41.25417. [DOI] [PubMed] [Google Scholar]
- Grataroli R, Vindrieux D, Gougeon A, Benahmed M. Expression of tumor necrosis factor-alpha-related apoptosis-inducing ligand and its receptors in rat testis during development. Biol Reprod. 2002;66:1707–15. doi: 10.1095/biolreprod66.6.1707. [DOI] [PubMed] [Google Scholar]
- Grataroli R, Vindrieux D, Selva J, Felsenheld C, Ruffion A, et al. Characterization of tumour necrosis factor-alpha-related apoptosis-inducing ligand and its receptors in the adult human testis. Mol Hum Reprod. 2004;10:123–8. doi: 10.1093/molehr/gah016. [DOI] [PubMed] [Google Scholar]
- Turner TT. The study of varicocele through the use of animal models. Hum Reprod Update. 2001;7:78–84. doi: 10.1093/humupd/7.1.78. [DOI] [PubMed] [Google Scholar]
- Mizuno K, Hayashi Y, Kojima Y, Kurokawa S, Sasaki S, et al. Influence for testicular development and histological peculiarity in the testes of flutamide-induced cryptorchid rat model. Int J Urol. 2007;14:67–72. doi: 10.1111/j.1442-2042.2006.01654.x. [DOI] [PubMed] [Google Scholar]
- Koksal IT, Sanlioglu AD, Kutlu O, Sanlioglu S. Effects of androgen ablation therapy in TRAIL death ligand and its receptors expression in advanced prostate cancer. Urol Int. 2010;84:445–51. doi: 10.1159/000304510. [DOI] [PubMed] [Google Scholar]
- Kutlu O, Akkaya E, Koksal IT, Bassorgun IC, Ciftcioglu MA, et al. Importance of TNF-related apoptosis-inducing ligand in pathogenesis of interstitial cystitis. Int Urol Nephrol. 2010;42:393–9. doi: 10.1007/s11255-009-9632-z. [DOI] [PubMed] [Google Scholar]
- Lee JD, Lee TH, Cheng WH, Jeng SY. Involved intrinsic apoptotic pathway of testicular tissues in varicocele-induced rats. World J Urol. 2009;27:527–32. doi: 10.1007/s00345-008-0367-8. [DOI] [PubMed] [Google Scholar]
- Koksal IT, Sanlioglu AD, Karacay B, Griffith TS, Sanlioglu S. Tumor necrosis factor-related apoptosis inducing ligand-R4 decoy receptor expression is correlated with high Gleason scores, prostate-specific antigen recurrence, and decreased survival in patients with prostate carcinoma. Urol Oncol. 2008;26:158–65. doi: 10.1016/j.urolonc.2007.01.022. [DOI] [PubMed] [Google Scholar]
- Sanlioglu AD, Karacay B, Koksal IT, Griffith TS, Sanlioglu S. DcR2 (TRAIL-R4) siRNA and adenovirus delivery of TRAIL (Ad5hTRAIL) break down in vitro tumorigenic potential of prostate carcinoma cells. Cancer Gene Ther. 2007;14:976–84. doi: 10.1038/sj.cgt.7701087. [DOI] [PubMed] [Google Scholar]
- Schneider P, Bodmer JL, Thome M, Hofmann K, Holler N, et al. Characterization of two receptors for TRAIL. FEBS Lett. 1997;416:329–34. doi: 10.1016/s0014-5793(97)01231-3. [DOI] [PubMed] [Google Scholar]
- Sanlioglu AD, Koksal IT, Karacay B, Baykara M, Luleci G, et al. Adenovirus-mediated IKKbetaKA expression sensitizes prostate carcinoma cells to TRAIL-induced apoptosis. Cancer Gene Ther. 2006;13:21–31. doi: 10.1038/sj.cgt.7700877. [DOI] [PubMed] [Google Scholar]
- Hu WH, Johnson H, Shu HB. Tumor necrosis factor-related apoptosis-inducing ligand receptors signal NF-kappaB and JNK activation and apoptosis through distinct pathways. J Biol Chem. 1999;274:30603–10. doi: 10.1074/jbc.274.43.30603. [DOI] [PubMed] [Google Scholar]


