Abstract
The skeleton is the most common metastatic organ in patients with prostate cancer (PCa). Non-invasive biomarkers that can facilitate the detection and monitoring of bone metastases are highly desirable. We designed this study to assess the expression patterns of serum miR-141 in patients with bone-metastatic PCa. Serum samples were collected to measure the miR-141 level in 56 patients, including six with benign prostatic hyperplasia (BPH), 20 with localized PCa and 30 with bone-metastatic PCa (10 with hormone-naive PCa, 10 with hormone-sensitive PCa and 10 with hormone-refractory PCa). A bone scan was performed for each patient with PCa to assess the number of bone lesions. The quantification of serum miR-141 levels was assayed by specific TaqMan qRT-PCR. The results showed that serum miR-141 levels were elevated in patients with bone metastasis (P<0.001). There was no statistically significant difference in the serum miR-141 levels between patients with BPH and patients with localized PCa. Using Kendall's bivariate correlation test, both the Gleason score and the number of bone-metastatic lesions were found to correlate with serum miR-141 levels (P=0.012 and P<0.001, respectively). The serum miR-141 level was found to be positively correlated with alkaline phosphatase (ALP) level in patients with skeletal metastasis, using Pearson's bivariate correlation test. No relationship was found between the serum miR-141 level and the serum prostate-specific antigen (PSA) level. We concluded that serum miR-141 levels are elevated in patients with bone-metastatic PCa and that patients with higher levels of serum miR-141 developed more bone lesions. Furthermore, serum miR-141 levels are correlated with serum ALP levels but not serum PSA levels.
Keywords: alkaline phosphatase (ALP), biological markers, bones, metastasis, microRNAs, miR-141, prostate-specific antigen (PSA), prostatic neoplasms, serum
Introduction
Prostate cancer (PCa) is the most frequently diagnosed malignancy and the second leading cause of cancer-related deaths in Western countries.1,2 In China, the incidence of PCa is markedly increasing each year,3,4 and most cases (70%) present in late stages and are not candidates for radical treatment. The skeleton is the most common site for PCa metastases, and no curative therapy exists once bone metastasis has occurred. However, the lack of sensitive and specific biomarkers for bone metastasis makes it difficult to detect and predict the status of this cancer.
MicroRNAs are a series of small non-coding RNAs with 18–24 nucleotides. They can regulate functional gene expression, and abundant evidence has revealed their aberrant expression in various types of human cancers.5,6,7 In addition, reliable methods for extracting miRNAs from bodily fluids, such as serum, have been well developed and well validated.8,9 Therefore, serum miRNAs could represent novel biomarkers for PCa.10,11 A study of plasma samples from PCa patients reported that plasma miR-141 levels can be used to screen for metastatic PCa with high sensitivity.8 However, the expression patterns of circulating miR-141 in patients with PCa have not been well established.
Based on these considerations, we hypothesized that serum miR-141 levels could be a novel biomarker for PCa and designed this study to evaluate the levels of serum miR-141 in patients with bone-metastatic PCa.
Materials and methods
Study subjects
From February 2008 to February 2009, five groups of patients, including six with benign prostatic hyperplasia (BPH), 20 with localized PCa (LPC), 10 with hormone-naive PCa (HNPC) and bone metastasis, 10 with hormone-sensitive PCa (HSPC) and bone metastasis and 10 with hormone-refractory PCa (HRPC) and bone metastasis, were prospectively enrolled in this study. After the appropriate informed consent and Institutional Review Board approval for each patient were obtained, 5 ml serum samples were collected from each patient at the first visit. All patients had a normal blood pressure and blood glucose level and showed no acute inflammation.
Baseline data information
Clinical and biochemical features, such as age, height, weight, serum prostate-specific antigen (PSA), serum testosterone, serum alkaline phosphatase (ALP), Gleason score of PCa, size of prostate, status of lymph node metastasis and number of bone lesions, were recorded in detail. A bone scan was performed for all patients with PCa to access the number of bone lesions. HRPC was defined by the following criteria: disease progression during androgen-ablation therapy despite secondary hormone therapy, at least 4 weeks withdrawal of flutamide (or 6 weeks in the case of bicalutamide) before enrolment, increasing serum levels of PSA on three consecutive measurements obtained at least 1 week apart, and a serum testosterone value of less than 50 ng dl−1. After the collection of serum samples, the 20 localized PCa patients were treated by radical prostatectomy; the 10 HNPC patients were administered goserelin 3.6 mg subcutaneously monthly and bicalutamide 50 mg orally once a day; the 10 HSPC patients continued their former hormonal therapy of goserelin 3.6 mg subcutaneously monthly and bicalutamide 50 mg orally once a day; and the 10 HRPC patients were treated with docetaxel 75 mg m−2 intravenously on day 2 plus prednisone 5 mg orally twice daily on days 1–21, with 21 days in each cycle.12
MicroRNA isolation and real-time RT-PCR assay
Total RNA was isolated from each serum sample using Trizol reagent (Invitrogen, Carlsbad, CA, USA) as previously described.13 The expression levels of mature miR-141 were assessed by qRT-PCR using specific TaqMan Assays (Applied Biosystems, Carlsbad, CA, USA). Amplifications were run on the Eppendorf Mastercycler ep Real-Time PCR System (Eppendorf, Hamburg, Germany). The PCR results were analysed using the Mastercycler ep Realplex Program and reported as relative quantities with respect to a calibrator sample using the 2−ΔCt method. U6 snRNA was used as a normalizer.
Data analyses
All of the analyses were performed using the statistical software package SPSS 15.0 (SPSS Inc, Chicago, IL, USA). The levels of miR-141 in each group were defined as the mean±s.d. from at least two separate experiments performed in triplicate. A one-way analysis of variance test was used to compare the means of the miR-141 levels among the five groups. Independent samples t-tests were performed to compare the differences among the groups. Pearson's or Kendall's correlation coefficients were compared among clinical features and serum miR-141 levels. A two-tailed P<0.05 was considered statistically significant.
Results
The median age of the 56 patients was 68.5 (54–81) years old. The clinical features of the patients, the Ct values of serum miR-141, the Ct values of U6, and the 2−ΔCt exchanged values of miR-141 are listed in Table 1. No significant differences were detected among the five groups (group 1, BPH patients; group 2, localized PCa patients; group 3, HNPC patients; group 4, HSPC patients; and group 5, HRPC patients) in patient age, height or weight (P>0.05). Higher serum PSA levels were observed in groups 3 and 5 (P<0.001), and castration levels of serum testosterone were discovered in groups 4 and 5 under androgen-deprivation therapy (P<0.001). Patients with BPH had larger prostate sizes (P=0.029).
Table 1. Clinical characteristics of the 56 patients in five groups. Data is expressed as mean±s.d.
| Characteristics | BPH (n=6) | LPC (n=20) | HNPC (n=10) | HSPC (n=10) | HRPC (n=10) | P value* |
|---|---|---|---|---|---|---|
| Age (year) | 66.8±3.5 | 68.3±1.9 | 70.1±2.5 | 69.7±1.5 | 68.6±1.7 | 0.136 |
| Height (cm) | 170.0±4.8 | 171.0±3.7 | 168.7±6.5 | 168.9±5.3 | 172.3±6.9 | 0.469 |
| Weight (kg) | 68.7±10.1 | 72.8±6.9 | 66.4±8.8 | 68.4±7.3 | 75.9±12.4 | 0.125 |
| Serum PSA level (ng ml−1) | 6.99±5.42 | 9.27±5.43 | 245.90±202.19 | 12.79±11.68 | 136.32±128.51 | <0.001 |
| Serum testosterone level (ng dl−1) | 4.48±1.45 | 4.63±1.27 | 3.97±1.13 | 0.16±0.11 | 0.20±0.17 | <0.001 |
| Serum alkaline phosphatase (U l−1) | 66.12±42.79 | 79.45±39.50 | 441.20±302.33 | 237.60±165.25 | 300.40±179.63 | <0.001 |
| Tumour Gleason score | ||||||
| ≤7 | — | 16 | 4 | 5 | 2 | — |
| >7 | — | 4 | 6 | 5 | 8 | — |
| Size of prostate (ml) | 55.8±12.8 | 34.5±13.2 | 42.5±15.6 | 41.1±11.1 | 43.7±11.9 | 0.029 |
| number of bone lesions | ||||||
| ≤2 | — | 0 | 4 | 5 | 3 | — |
| 3–5 | — | 0 | 4 | 2 | 4 | — |
| ≥6 | — | 0 | 2 | 3 | 3 | — |
| Lymph node metastasis | ||||||
| Yes | — | 2 | 6 | 7 | 6 | — |
| No | — | 18 | 4 | 3 | 4 | — |
| Ct value of miR-141 | 34.51±1.65 | 33.77±1.87 | 30.27±0.99 | 31.19±1.41 | 30.02±1.07 | <0.001 |
| Ct value of U6 | 22.48±1.26 | 22.73±1.32 | 22.36±1.08 | 22.51±1.17 | 21.94±0.96 | 0.636 |
| Level of miR-141 (2−ΔCt) (×10−4) | 4.79±3.05 | 10.19±3.00 | 50.53±10.12 | 38.59±13.34 | 46.39±9.93 | <0.001 |
Abbreviations: ANOVA, analysis of variance; BPH, benign prostatic hyperplasia; LPC: localized prostate cancer; HNPC, hormone-naive prostate cancer; HRPC, hormone-refractory prostate cancer; HSPC, hormone-sensitive prostate cancer; PSA, prostate-specific antigen.
—: not applicable.
*One-way ANOVA test is used.
Patients with bone metastasis expressed higher levels of serum miR-141 than those without bone metastasis
Elevated levels of serum miR-141 were detected in the HNPC, HSPC and HRPC patients with bone metastasis; and patients with BPH and LPC had relatively lower levels of serum miR-141 (one-way analysis of variance test, P<0.001) illustrated in Figure 1. The levels of serum miR-141 in HSPC patients were comparable but not significantly lower than those in patients with HNPC (P=0.552) and HRPC (P=0.543), respectively, by independent samples t-test.
Figure 1.
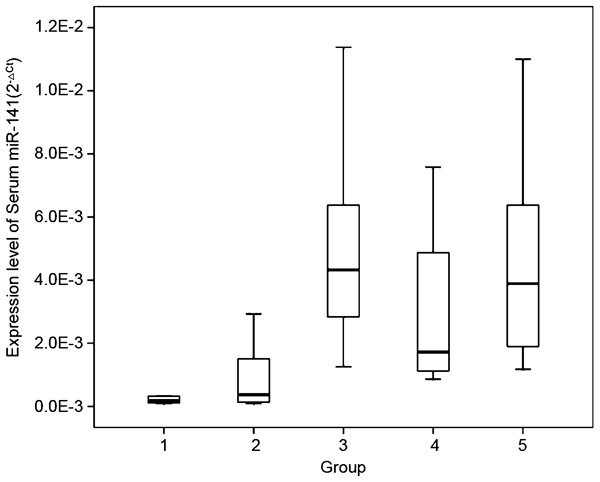
Box plot of different serum miR-141 expression levels in different groups of patients (group 1, BPH patients, n=6; group 2, LPC patients, n=20; group 3, HNPC patients, n=10; group 4, HSPC patients, n=10; group 5, HRPC patients, n=10). By one-way ANOVA test and independent samples t-test, serum miR-141 expressed higher in the patients with bone metastasis (HNPC, HSPC and HRPC patients) than those in BPH and LPC patients (P<0.001). Serum miR-141 levels were comparable in HNPC and HRPC patients, a bit higher than those in HSPC, but not statistically significant (independent samples t-test, P=0.552 and P=0.543, respectively). ANOVA, analysis of variance; BPH, benign prostatic hyperplasia; LPC, localized prostate cancer; HNPC, hormone-naive prostate cancer; HRPC, hormone-refractory prostate cancer; HSPC, hormone-sensitive prostate cancer.
Levels of serum miR-141 were correlated with serum ALP levels, but not PSA levels, in PCa patients with bone metastasis
Serum miR-141 levels were found to be positively correlated with ALP levels in HNPC (P=0.002), HSPC (P=0.001) and HRPC (P=0.003) patients, using Pearson's bivariate correlation test; patients with a higher ALP level had a higher miR-141 level in each of the three groups. There was no such correlation in the LPC group (P=0.638), as illustrated in Figure 2. No statistical relationship between miR-141 level and PSA level was detected in any of the five groups.
Figure 2.
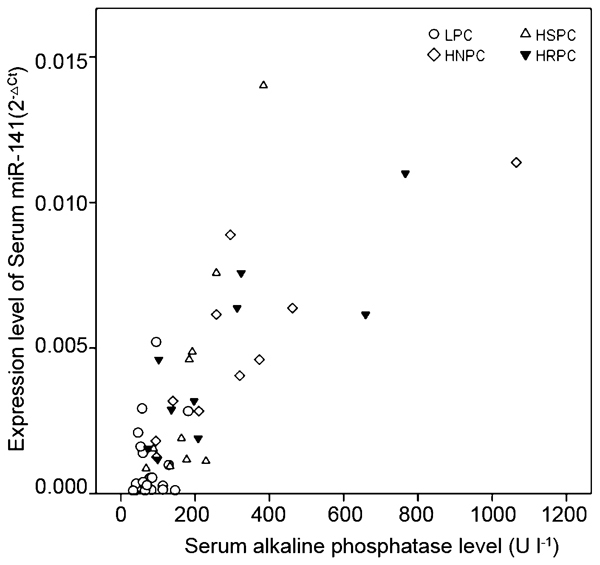
Scatter diagram and Pearson's bivariate correlation test between serum miR-141 levels and serum ALP levels. Serum miR-141 was positively parallel to ALP in HNPC, HSPC and HRPC patients (P=0.002, P=0.001 and P=0.003, respectively). There was no significant correlation between serum miR-141 and ALP in LPC (P=0.638). ALP, alkaline phosphatase; LPC, localized prostate cancer; HNPC, hormone-naive prostate cancer; HRPC, hormone-refractory prostate cancer; HSPC, hormone-sensitive prostate cancer.
A higher serum miR-141 level was correlated with more bone metastatic lesions and a higher Gleason score
Using Kendall's bivariate correlation test, serum miR-141 levels were found to correlate with the number of bone lesions in patients with bone metastasis (P<0.001), and this correlation remained when the tests were performed separately in each of the three groups (HNPC, HSPC, and HRPC; P=0.028, P=0.014, and P=0.028, respectively), as illustrated in Figure 3. Serum miR-141 levels were also found to be related to the Gleason scores of patients with PCa, and patients with higher Gleason scores had higher serum miR-141 levels (P=0.012). No relationships were detected between miRNA-141 level and the patient's age, height, weight, size of prostate or testosterone level (P>0.05 for all comparisons).
Figure 3.
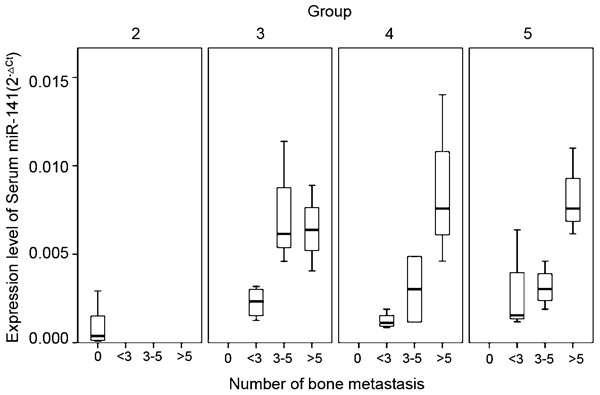
Box plot of different serum miR-141 expression levels for patients with different number of bone lesions (0, <3, 3–5 and >5) in different subgroups of patients with prostate cancer (group 2, LPC patients, n=20; group 3, HNPC patients, n=10; group 4, HSPC patients, n=10; group 5, HRPC patients, n=10). By Kendall's bivariate correlation test, serum miR-141 levels were positively correlate with the number of bone lesions in patients with bone metastasis (P<0.001), and this correlation remained when tests were done separately in three different subgroups (HNPC, HSPC and HRPC) (P=0.028, P=0.014 and P=0.028, respectively). LPC, localized prostate cancer; HNPC, hormone-naive prostate cancer; HRPC, hormone-refractory prostate cancer; HSPC, hormone-sensitive prostate cancer.
Discussion
In the current study, preliminary results revealed the expression levels of miR-141 in patients with PCa and indicated that miR-141 may be a biomarker for bone metastasis. First, patients with bone-metastatic PCa had higher serum miR-141 levels than those with localized PCa or BPH; second, patients with more bone lesions had higher serum miR-141 levels; and third, serum miR-141 levels were correlated with serum ALP levels and tumour Gleason scores.
Circulating miRNAs, including miR-141, have recently been indicated to be practical and promising biomarkers for the non-invasive diagnosis of various tumour entities, including PCa.14 Mitchell et al.8 were the first to describe the overexpression of circulating miR-141 in advanced PCa, and Bryant et al.15 discovered an association of miR-141 with metastatic PCa. In addition, Yaman Agaoglu et al.16 found that the levels of miR-141 were higher in patients with metastatic PCa than in patients with localized disease. A more recent prospective study further revealed that circulating miR-141 is present at elevated levels in patients with metastatic PCa and that the miR-141 levels of these patients also correlated with their Gleason scores or lymph-node status.11 Furthermore, serum miR-141 was found to be elevated in metastatic castration-resistant PCa.17 Our results further verified that serum miR-141 is overexpressed in PCa patients with bone metastasis, whereas no significant difference was noted in the serum miR-141 levels of BPH and LPC patients. The existing data suggested that miR-141 was released into the blood and would be detectable in the serum only in patients with advanced disease, indicating that miR-141 plays a role in tumour metastasis, not tumour formation. A serum PSA level of less than 20 ng ml−1 was a precise predictor of a lack of metastases. Nevertheless, a PSA level of higher than 20 ng ml−1 was not sensitive enough to indicate metastatic disease.18 In the current study, miR-141 is hypothesized to promote the ability of prostate tumour cells to disseminate to bone and other organs, and miR-141 levels could be a supplementary or alternative marker for metastasis in those patients whose PSA levels are higher than 20 ng ml−1.
Additionally, we revealed that serum miR-141 levels were correlated with the number of bone lesions and the serum ALP level. Bone metastases in PCa patients have mostly been characterized as osteogenic lesions.19,20 Serum ALP, which is considered a bone formation marker and is usually present at a higher level during osteoblastic activity, including the presence of osteogenic metastases, has been used as a biomarker for bone metastasis in various types of malignant tumours.21,22,23,24 However, because ALP is produced not only by osteoblasts but also by various other organs, including the liver, it is not a specific biomarker for bone metastasis in PCa patients.25 Other bone markers, such as serum osteocalcin and serum type I procollagen C-propeptide, are also indicators of osteoblast activity; nevertheless, none of these other markers are specific biomarkers for bone metastasis.26,27 In the current study, higher miR-141 levels indicated a higher number of bone lesions, which suggests that we may be able to use serum miR-141 as a specific marker for bone metastasis.
Despite these favourable results, several important issues must be addressed. The expression of miR-141 was measured as a relative quantity in the 56 patients in this study, so it might not be easy to translate or validate these data in other groups of patients. Additionally, we found it difficult to assign a specific ‘cutoff' to validate these results. Given the relatively small sample size of the current study, patients with metastatic PCa but no bone metastases were not included in our cohort. A larger subset of patients who have metastases—both with and without bone lesions—will be needed to verify the current data and to further demonstrate that serum miR-141 is a specific marker only for bone metastasis. The relationships between serum miR-141 and treatment outcomes (such as response to hormonal therapy and overall survival) were not examined in the present study. To obtain these data, serial levels of serum samples should be taken throughout the whole period of treatment and follow-up. In the meantime, the association between serum miR-141 and the treatment efficacy of denosumab and zoladronic acid is also of great interest. Furthermore, the mechanisms underlying our findings are largely unknown. Further investigation into the relationship between miR-141 and the genes associated with bone metastasis, such as RANKL (receptor activator of nuclear factor-kB ligand, which is expressed on the surface of osteoblasts and stromal cells),28,29,30 is being carried out at our institution, and the expected results may help uncover the mechanism of bone metastasis.
Conclusions
Serum miR-141 levels were elevated in PCa patients with bone metastasis, and patients with higher levels of serum miR-141 harboured more bone-metastatic lesions. The levels of serum miR-141 were also correlated with serum ALP levels but not PSA levels in PCa patients with bone metastasis. Therefore, miR-141 is a potential biomarker for the diagnosis and evaluation of bone metastasis in PCa patients.
Author contributions
DWY conceived of the study, supervised the experiments, analysed the data and contributed to the manuscript preparation. HLZ and XJQ made equal contributions to this article. HLZ participated in the design of the study, qRT-PCR, review of related literatures and the manuscript draft. XJQ contributed in the collection and analyses of the final data and in the preparation of the manuscript. DLC carried out the qRT-PCR analyses. YZ participated in the design of the study and performed the statistical analysis. BD performed the statistical analysis. XDY and SLZ conceived of the study, participated in its design and coordination and helped to draft the manuscript. All authors read and approved the final manuscript.
The authors declare that they have no competing financial interests.
References
- Siegel R, Naishadham D, Jemal A. Cancer statistics, 2012. CA: Cancer J Clin. 2012;62:10–29. doi: 10.3322/caac.20138. [DOI] [PubMed] [Google Scholar]
- Quinn M, Babb P. Patterns and trends in prostate cancer incidence, survival, prevalence and mortality. Part I: international comparisons. BJU Int. 2002;90:162–73. doi: 10.1046/j.1464-410x.2002.2822.x. [DOI] [PubMed] [Google Scholar]
- Peyromaure EM, Mao K, Sun Y, Xia S, Jiang N, et al. A comparative study of prostate cancer detection and management in China and in France. Can J Urol. 2009;16:4472–7. [PubMed] [Google Scholar]
- Peyromaure M, Debre B, Mao K, Zhang G, Wang Y, et al. Management of prostate cancer in China: a multicenter report of 6 institutions. J Urol. 2005;174:1794–7. doi: 10.1097/01.ju.0000176817.46279.93. [DOI] [PubMed] [Google Scholar]
- Slack FJ, Weidhaas JB. MicroRNA in cancer prognosis. New Engl J Med. 2008;359:2720–2. doi: 10.1056/NEJMe0808667. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Taylor DD, Gercel-Taylor C. MicroRNA signatures of tumor-derived exosomes as diagnostic biomarkers of ovarian cancer. Gynecol Oncol. 2008;110:13–21. doi: 10.1016/j.ygyno.2008.04.033. [DOI] [PubMed] [Google Scholar]
- Farazi TA, Spitzer JI, Morozov P, Tuschl T. miRNAs in human cancer. J Pathol. 2011;223:102–15. doi: 10.1002/path.2806. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mitchell PS, Parkin RK, Kroh EM, Fritz BR, Wyman SK, et al. Circulating microRNAs as stable blood-based markers for cancer detection. Proc Natl Acad Sci USA. 2008;105:10513–8. doi: 10.1073/pnas.0804549105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gilad S, Meiri E, Yogev Y, Benjamin S, Lebanony D, et al. Serum microRNAs are promising novel biomarkers. PloS One. 2008;3:e3148. doi: 10.1371/journal.pone.0003148. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lu Z, Liu M, Stribinskis V, Klinge CM, Ramos KS, et al. MicroRNA-21 promotes cell transformation by targeting the programmed cell death 4 gene. Oncogene. 2008;27:4373–9. doi: 10.1038/onc.2008.72. [DOI] [PubMed] [Google Scholar]
- Brase JC, Johannes M, Schlomm T, Falth M, Haese A, et al. Circulating miRNAs are correlated with tumor progression in prostate cancer. Int J Cancer. 2011;128:608–16. doi: 10.1002/ijc.25376. [DOI] [PubMed] [Google Scholar]
- Tannock IF, de Wit R, Berry WR, Horti J, Pluzanska A, et al. Docetaxel plus prednisone or mitoxantrone plus prednisone for advanced prostate cancer. New Engl J Med. 2004;351:1502–12. doi: 10.1056/NEJMoa040720. [DOI] [PubMed] [Google Scholar]
- Zhong XY, Holzgreve W, Huang DJ. Isolation of cell-free RNA from maternal plasma. Methods Mol Biol. 2008;444:269–73. doi: 10.1007/978-1-59745-066-9_21. [DOI] [PubMed] [Google Scholar]
- Cheng H, Zhang L, Cogdell DE, Zheng H, Schetter AJ, et al. Circulating plasma MiR-141 is a novel biomarker for metastatic colon cancer and predicts poor prognosis. PLoS One. 2011;6:e17745. doi: 10.1371/journal.pone.0017745. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bryant RJ, Pawlowski T, Catto JW, Marsden G, Vessella RL, et al. Changes in circulating microRNA levels associated with prostate cancer. Br J Cancer. 2012;106:768–74. doi: 10.1038/bjc.2011.595. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yaman Agaoglu F, Kovancilar M, Dizdar Y, Darendeliler E, Holdenrieder S, et al. Investigation of miR-21, miR-141, and miR-221 in blood circulation of patients with prostate cancer. Tumour Biol. 2011;32:583–8. doi: 10.1007/s13277-011-0154-9. [DOI] [PubMed] [Google Scholar]
- Selth LA, Townley S, Gillis JL, Ochnik AM, Murti K, et al. Discovery of circulating microRNAs associated with human prostate cancer using a mouse model of disease. Int J Cancer. 2012;131:652–61. doi: 10.1002/ijc.26405. [DOI] [PubMed] [Google Scholar]
- Stamey TA, Yang N, Hay AR, McNeal JE, Freiha FS, et al. Prostate-specific antigen as a serum marker for adenocarcinoma of the prostate. N Engl J Med. 1987;317:909–16. doi: 10.1056/NEJM198710083171501. [DOI] [PubMed] [Google Scholar]
- Norgaard M, Jensen AO, Jacobsen JB, Cetin K, Fryzek JP, et al. Skeletal related events, bone metastasis and survival of prostate cancer: a population based cohort study in Denmark (1999 to 2007) J Urol. 2010;184:162–7. doi: 10.1016/j.juro.2010.03.034. [DOI] [PubMed] [Google Scholar]
- Clarke NW, Hart CA, Brown MD. Molecular mechanisms of metastasis in prostate cancer. Asian J Androl. 2009;11:57–67. doi: 10.1038/aja.2008.29. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Akimoto S, Furuya Y, Akakura K, Ito H. Comparison of markers of bone formation and resorption in prostate cancer patients to predict bone metastasis. Endocr J. 1998;45:97–104. doi: 10.1507/endocrj.45.97. [DOI] [PubMed] [Google Scholar]
- Akimoto S, Inomiya H, Furuya Y, Akakura K, Ito H. Prognostic value of the serum levels of bone formation and bone resorption markers in prostate cancer patients with bone metastasis. Eur Urol. 1998;34:142–7. doi: 10.1159/000019700. [DOI] [PubMed] [Google Scholar]
- Akimoto S, Furuya Y, Akakura K, Shimazaki J, Ito H. Inability of bone turnover marker as a strong prognostic indicator in prostate cancer patients with bone metastasis: comparison with the extent of disease (EOD) grade. Prostate. 1999;38:28–34. doi: 10.1002/(sici)1097-0045(19990101)38:1<28::aid-pros3>3.0.co;2-m. [DOI] [PubMed] [Google Scholar]
- Tamada T, Sone T, Tomomitsu T, Jo Y, Tanaka H, et al. Biochemical markers for the detection of bone metastasis in patients with prostate cancer: diagnostic efficacy and the effect of hormonal therapy. J Bone Miner Metab. 2001;19:45–51. doi: 10.1007/s007740170059. [DOI] [PubMed] [Google Scholar]
- Sonpavde G, Pond GR, Berry WR, de Wit R, Armstrong AJ, et al. Serum alkaline phosphatase changes predict survival independent of PSA changes in men with castration-resistant prostate cancer and bone metastasis receiving chemotherapy. Urol Oncol. 2012;30:607–13. doi: 10.1016/j.urolonc.2010.07.002. [DOI] [PubMed] [Google Scholar]
- Garnero P, Buchs N, Zekri J, Rizzoli R, Coleman RE, et al. Markers of bone turnover for the management of patients with bone metastases from prostate cancer. Br J Cancer. 2000;82:858–64. doi: 10.1054/bjoc.1999.1012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Agrawal S, Patil KP, Dunsmuir WD. Molecular markers in prostate cancer. Part II: potential roles in management. Asian J Androl. 2009;11:22–7. doi: 10.1038/aja.2008.23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Whang PG, Schwarz EM, Gamradt SC, Dougall WC, Lieberman JR. The effects of RANK blockade and osteoclast depletion in a model of pure osteoblastic prostate cancer metastasis in bone. J Orthop Res. 2005;23:1475–83. doi: 10.1016/j.orthres.2005.05.004.1100230634. [DOI] [PubMed] [Google Scholar]
- Chen G, Sircar K, Aprikian A, Potti A, Goltzman D, et al. Expression of RANKL/RANK/OPG in primary and metastatic human prostate cancer as markers of disease stage and functional regulation. Cancer. 2006;107:289–98. doi: 10.1002/cncr.21978. [DOI] [PubMed] [Google Scholar]
- Saad F, Markus R, Goessl C. Targeting the receptor activator of nuclear factor-kappaB (RANK) ligand in prostate cancer bone metastases. BJU Int. 2008;101:1071–5. doi: 10.1111/j.1464-410X.2007.07364.x. [DOI] [PubMed] [Google Scholar]


