Abstract
Maternal exposure to estrogenic xenobiotics or phthalates has been implicated in the distortion of early male reproductive development, referred to in humans as the testicular dysgenesis syndrome. It is not known, however, whether such early gestational and/or lactational exposure can influence the later adult-type Leydig cell phenotype. In this study, Sprague–Dawley rats were exposed to dibutyl phthalate (DBP; from gestational day (GD) 14.5 to postnatal day (PND) 6) or diethylstilbestrol (DES; from GD14.5 to GD16.5) during a short gestational/lactational window, and male offspring subsequently analysed for various postnatal testicular parameters. All offspring remained in good health throughout the study. Maternal xenobiotic treatment appeared to modify specific Leydig cell gene expression in male offspring, particularly during the dynamic phase of mid-puberty, with serum INSL3 concentrations showing that these compounds led to a faster attainment of peak values, and a modest acceleration of the pubertal trajectory. Part of this effect appeared to be due to a treatment-specific impact on Leydig cell proliferation during puberty for both xenobiotics. Taken together, these results support the notion that maternal exposure to certain xenobiotics can also influence the development of the adult-type Leydig cell population, possibly through an effect on the Leydig stem cell population.
Keywords: diethylstilbestrol (DES), INSL3, Leydig cells, dibutyl phthalate (DBP), puberty, testis
Introduction
Maternal exposure of male rats to endocrine disrupting xenobiotics such as dibutyl phthalate (DBP) or diethylstilbestrol (DES) is known to impact on the early development of the male reproductive system.1 In particular, several of the symptoms of what in humans has been termed the testicular dysgenesis syndrome (e.g., cryptorchidism and hypospadias) can be reproduced in some strains of rats by such early maternal exposure.1,2 The principal mechanism of action for these xenobiotics appears to be through disturbance of fetal testis development, involving most testicular cell types, including fetal Leydig cells and consequently, their production of fetal androgens, as well as the fetal hormone INSL3. The fetal population of Leydig cells is, however, quite discrete from those Leydig cells which develop in the testis postnatally, and which are presumed to differentiate de novo after birth from resident peritubular mesenchymal stem cells. Recently, Travison and colleagues3 suggested from data derived in the Massachussetts Male Aging Study that adult circulating testosterone concentrations might also be determined by early, possibly maternal, environmental factors, thereby adding reduced androgen production in the aging male to the possible list of symptoms linked to the testicular dysgenesis syndrome. This could imply either that endocrine disrupting xenobiotics might act directly on early Leydig stem cells or that by acting upon other cells in the testis, or on other components of the hypothalamo–pituitary–gonadal (HPG) axis, they could indirectly impact on adult-type Leydig cell differentiation and/or function. In support of this hypothesis, we recently demonstrated that regenerating adult-type Leydig cells in the testes of ethane dimethane sulfonate-treated rats indicated a disturbed differentiation trajectory following an early and short in vivo exposure to DBP and DES.4 Other studies have shown that similar early xenobiotic exposure can indeed disrupt the onset of puberty in female rats,5 hypothetically providing a rationale for understanding the association in immigrant girls between early xenobiotic exposures and precocious puberty.5
In the present study, pregnant Sprague–Dawley rats were given a moderate dose of either DBP or DES (here used as an estrogenic control) during a relatively short developmental window, such as to cause a minimal disruptive effect on the male reproductive system, though no other negative health outcomes. Both substances have been shown to impact on fetal Leydig cell function, reducing both INSL3 and androgen expression by the fetus.2,6,7 We have then assessed testis gene expression at three time points before, during and after puberty in male offspring. The findings indicate subtle but definite changes in the postnatal testicular phenotype.
Materials and methods
Ethics statement
All animal experimentation was approved by the University of Adelaide Animal Ethics Committee (approval No. S-019-2007) and adhered strictly to NIH guidelines.
Animals and treatments
Nine-week-old female Sprague–Dawley rats from Laboratory Animal Services (University of Adelaide, Adelaide, Australia) were mated overnight and checked the next morning for the presence of sperm in vaginal smears. The morning when sperm were observed in the vaginal smears was designated as gestation day 0.5 (GD0.5). The day when the pups were delivered was designated as postnatal day 1 (PND1). Pregnant rats and their offspring were maintained on soy-free standard AIN93G rodent diet (Specialty Feeds, Glen Forrest, Australia) throughout the experiment. Pregnant rats were divided into three groups, using randomization of body weights to ensure equal weight distribution among groups, and dosed as following (Figure 1): control group (n=8): orally gavaged with corn oil 1 ml kg−1 body weight every second day from GD14.5 to PND6 and subcutaneously injected with corn oil containing 0.5% ethanol on GD14.5 and GD16.5. DBP group (n=9): orally gavaged with DBP 500 mg kg−1 body weight in corn oil every second day from GD14.5 to PND6 and subcutaneously injected with corn oil containing 0.5% ethanol on GD14.5 and GD16.5. DES group (n=12): orally gavaged with corn oil 1 ml kg−1 body weight every second day from GD14.5 to PND6 and subcutaneously injected with DES 125 µg kg−1 body weight in corn oil containing 0.5% ethanol on GD14.5 and GD16.5 only. On PND2, excess pups were culled, retaining 10 pups (female/male ratio: ∼1∶1) per litter. On PND21, both male and female pups were weaned and caged with their littermates of the same gender. Progeny were culled by CO2 asphyxiation, as indicated, on PND10, 24 and 90 (Figure 1).
Figure 1.
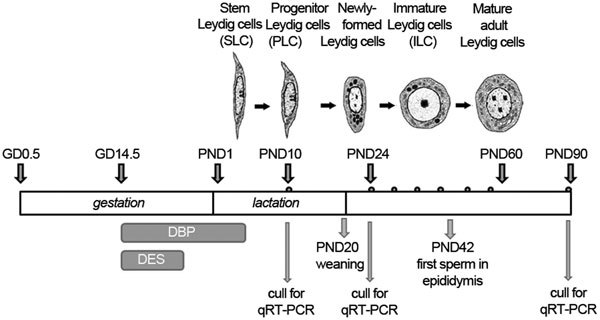
Diagram to illustrate the treatment and sampling regimen applied (small dots above the bar indicate approximate blood sampling times). The morphological changes during pubertal Leydig cell differentiation (after16,17,18) are illustrated at their corresponding times in the upper section of the figure. GD, gestational day; PND, postnatal day; DBP, dibutyl phthalate; DES, diethylstilbestrol.
Left and right testes were collected for RNA preparation and RT-PCR analysis, following snap-freezing in liquid nitrogen, and for Bouin-fixation, paraffin-embedding and cell counting, respectively. Only testes and blood from healthy animals, without gross testicular dysgenesis, were included. Trunk blood was collected from the vena cava at killing on days PND10, PND24 and PND 90. Peripheral blood was additionally collected by tail vein bleeding on PND30, PND35, PND40, PND45 and PND55. All samples were collected between 9:00 a.m. and 11:00 a.m.
Measurement of anogenital distance (AGD)
The AGD was measured from the base of the genitals to the centre of the anal opening for all pups before culling on PND2 and PND10. Pups were digitally photographed from the ventral aspect, including a precise 1-mm scale in each picture as reference point. All images were imported to the same computer and monitor for all measurements. These were repeated twice several days apart by the same observer, who was blinded to the treatment identities. The AGD for each animal was the average of the two values obtained from the repeat measurements.
Vaginal opening in female offspring
Female offspring were checked daily from PND29 onwards for the onset of sexual maturity as determined by the separation of the vaginal membrane (i.e., vaginal opening).
RNA analysis
RNA was extracted from total testis tissue using the TRIzol reagent (Life Technologies, Melbourne, Australia) and quality checked by electrophoresis. After treatment with RNAse-free DNAse using the TURBO DNA-free kit according to the manufacturer's instructions (Life Technologies), 3 µg total RNA per sample was reverse transcribed using Superscript II (Life Technologies) reverse transcriptase primed by oligo again according to the manufacturer's instructions.
Quantitative RT-PCR (qRT-PCR) was carried out as previously described8 using a Rotor-Gene 3000 (Corbett Research/Qiagen, Mortlake, Australia) real-time PCR cycler, and the ExTaq Sybr-Green pre-mix (Takara, Shiga, Japan), with oligonucleotide primers and PCR conditions as listed in Table 1. Primer pairs were designed using Primer 3 software to span at least one intron. For the luteinizing hormone receptor gene (Lhcgr), primers were chosen specifically to identify only full-length transcripts, since it is known that a number of shorter non-functional Lhcgr transcripts are generated during Leydig cell differentiation in the rat.9 Transcript levels were normalized against the ribosomal protein transcript S27a.10 For each PCR reaction, melt curves were calculated of final products which were subsequently checked by individual agarose gel electrophoresis for correct size, followed by sequencing. Results of qRT-PCR were processed using Q-gene96 software,11 and normalized gene expression (NE) calculated according to the equation NE=(Eref)CTref/(Etarget)CTtarget, where Etarget and Eref, and CTtarget and CTref are the PCR amplification efficiencies and the threshold cycles (where the fluorescence curve intersects the threshold) of the PCR amplification for the test transcript and S27a transcript, respectively.
Table 1. Oligonucleotide primers and conditions used for qRT-PCRs.
| Common name | Gene | Forward (5′-3′) | Reverse (5′-3′) | Size (bp) | Annealing temperature (°C) |
|---|---|---|---|---|---|
| Insl3 | Insl3 | ctgctactgctgctcctggctcta | gtggggacacagacccaaaa | 372 | 67 |
| 11β-HSD1 | Hsd11b1 | ggtcaacgtgtccatcactctc | catgatcctccttcctggattc | 214 | 62 |
| P450scc | Cyp11a1 | gtggcactcgtggggacag | accccaatgggcctctggta | 430 | 65 |
| 17β-HSD3 | Hsd17b3 | tggaaaagctacaggtcatctcag | ggttgaaacagaataaggggtcag | 455 | 60 |
| LH receptor | Lhcgr | ctcatcgccctgtcttcctact | tgcaaaagtctgcaaaggagag | 481 | 62 |
| 17α-hydroxylase | Cyp17a1 | gcagaggtttgacttggatgtg | gaggtatggatcggggatgtta | 180 | 64 |
| StAR | Star | ggaccttgaaaggctctgga | tacagcgtacgcttacgaag | 592 | 60 |
| S27a | Rps27a | ccaggataaggaaggaattcctcctg | ccagcaccacattcatcagaagg | 297 | 64 |
Leydig cell counting
Leydig cell numbers were estimated from testis sections of PND24 and PND90 rats using the fractionator and optical disector method.12,13 This method has been shown to be a precise approach to estimate the total number of various testicular cell types within the whole testis.13 The procedure was carried out as previously described.14 Each testis was cut into 10–15 slices of even thickness. These were laid out according to their position in the testis. Three slices were randomly selected and each exhaustively sectioned into 30-µm sections. The number of 30-µm sections from each testis slice was counted. Summation of the number of sections from all selected testis slices is number of total sections (f2). Again, using a randomly selected number, one section was mounted, subjected to Periodic-Acid-Schiff staining with Mayer's haematoxylin counterstaining, and cells were counted. Microscope images were captured on Spot analysis software (Diagnostic Instruments Inc., Sterling Heights, MI, USA) using a ×100 oil-free objective (numerical aperture 0.95, for PND24 testes) or ×40 oil-free objective (numerical aperture 0.65, for PND90 testes) on an Olympus BX-51 microscope attached with a Spot RT colour camera and OptiScan motorized stage system. The OptiScan motorized stage was programmed to move across the testis section in steps of 400 µm (for PND24 testis) or 1 mm (for PND90 testis) in the x-axis, then 400 µm (for PND24 testis) or 1 mm (for PND90 testis) in the y-axis, randomly and systematically to traverse the entire testis section and ensure that each cell has an equal chance of being counted. A 10×10 cm acetate counting frame was placed over the microscope image on the computer screen. The section was imaged through the z-axis, initially incorporating a guard zone of 2 µm. To avoid artefacts of the cutting surface, no Leydig cells were counted within the guard zone. The movement in the z-axis was measured by the attached microcator. Leydig cells fulfilling the counting frame rule15 were counted as they came into focus when the section was sectioned optically. Leydig cells were recognized by their oval to spherical nucleus with a thin rim of heterochromatin and prominent nucleolus.16,17,18 Finally, the entire testis cross-section was captured with ×1.25 objective for determination of the surface area of each testis section. The total number of Leydig cells within the whole testis was estimated by the formula19: N ^=Q×1/f1×1/f2×1/f3, where N ^ is the estimated total number, Q is the number of Leydig cells counted in the disector, f1 is the fraction of tissue (i.e., number of randomly selected slices/number of total slices), f2 is the fraction of sections (i.e., total number of randomly selected sections/number of total sections) and f3 is the sampling fraction (i.e., Σ volume of dissectors/Σ volume of the entire testis section).
Immunoassays
Blood was collected into heparinized syringes and kept on ice for 30 min prior to preparing plasma, which was then stored at −20 °C until analysis. Total testosterone was measured using a well validated in-house time-resolved fluorescent immunoassay following cold ethanol extraction.20 INSL3 in blood was measured using a rodent-specific time-resolved fluorescent immunoassay, exactly as previously described.21 Luteinising hormone (LH) was estimated using a commercial rat LH ELISA kit (USCN Life, Wuhan, China), according to the manufacturer's protocol. The range of detection for this assay was 0.78 mIU ml−1–50 mIU ml−1. The assay was specific for natural or recombinant rat LH and had no reported cross-reactivity with other related proteins.
Statistical analysis
Comparisons between gene expression in control, DBP and DES groups were made using ANOVA, followed by post hoc two-tailed t-tests with the help of the Graphpad Prism version 5 package (Graphpad Software, La Jolla, CA).
Results
General effect of treatments
Administration of DBP to pregnant and lactating rats from GD14.5 to PND6 had no effect on maternal body weight or on the weights of progeny born, measured at PND 21 (data not shown). All animals appeared healthy throughout the experiment. All pregnant rats in the control and DBP groups delivered exclusively live pups, though there was some subsequent perinatal mortality as well as testicular dysgenesis (Table 2) for the DBP-treated offspring, as has been previously reported.2,22 Only those maternally DBP-treated animals which showed no evidence of gross testicular dysgenesis were included in subsequent analyses. Offspring gender ratio was not affected. Administration of DES to rats at high doses has earlier been associated with abortion of fetuses.23 For this reason, DES was given to pregnant rats only on GD14.5 and GD16.5, and not at all during lactation. Unlike the control and DBP-treated animals, the DES-treated rats showed a small (9% P<0.05) reduction in body weight at GD20.5 (data not shown). Two out of the initial 12 rats in this group had still-births, whereby no live pups were born (Table 2). Excluding these two rats, DES treatment led to a marginally smaller litter size (10.8±1.5; mean±s.e.m.), compared to control (13.6±1.0) or DBP (12.0±0.8) groups (not statistically significant). Once born, all pups grew well and healthily, with no differences in body weight between groups at PND10 (data not shown). At PND21, both male and female DES pups showed a 6%–7% increased body weight compared to control and DBP groups (P<0.05; data not shown), which, however, did not persist into adulthood. There was no evidence for any testicular dysgenesis among the male offspring maternally treated with DES (Table 2).
Table 2. Offspring phenotype following treatments.
| Control | DBP | DES | |
|---|---|---|---|
| No. of rats with live pups on PND1 | 8/8 | 9/9 | 10/12 |
| No. of rats with stillbirth | 0/8 | 0/9 | 2/12 |
| No. pups/littera | 13.63±0.98 | 12.00±0.82 | 9.00±1.75b |
| Total no. pups born/treatment group | 109 | 108 | 108 |
| No. pups found dead on PND2 | 1 | 21 | 24 |
| Pup survival rate (%) | 99.08 | 80.56 | 77.78 |
| Male offspring on PND24 with unilateral abdominal cryptorchidism | 0/8 (0%) | 2/9 (22%) | 1/9 (11%) |
| Male offspring with unilateral anorchia on PND24c | 0/8 (0%) | 3/9 (33%) | 0/9 (0%) |
| Male offspring with unilateral testicular dysgenesis on PND90d | 1/16 (6%) | 4/18 (22%) | 1/11 (9%) |
| Total testicular dysgenesise | 1/32 (3%) | 9/36 (25%) | 2/29 (7%) |
Abbreviations: DBP, dibutyl phthalate; DES, diethylstilbestrol.
Mean±s.e.m.
Including the two litters with still births (different from control and DBP groups; P<0.05). If the litters with still births are excluded, then the litter size becomes 10.80±1.52 (not statistically different).
Only one testis could be located on dissection.
One testis was considerably smaller than the other by at least 35%.
Sum of the three preceding categories.
AGD
There were no between-group differences in AGD for both male (control: 2.16±0.50 mm; mean±s.e.m.) and female (control 1.16±0.42) pups at PND2 (data not shown). However, at PND10, DBP-treated male pups showed a reduced AGD compared to controls (Figure 2), intermediate in size between male and female distances. DES treatment also reduced male AGD at PND10. Female AGD showed no effects of maternal treatment.
Figure 2.
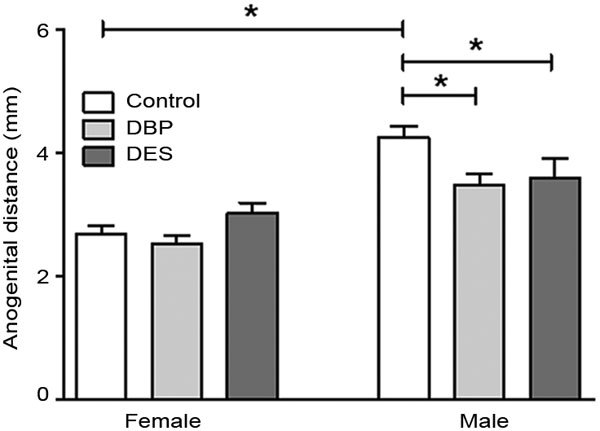
Anogenital distance measured at PND10 (mean±s.e.m, n=11–16) for male and female pups maternally treated with DBP or DES. Horizontal bars and asterisks indicate statistical differences (P<0.05). DBP, dibutyl phthalate; DES, diethylstilbestrol. PND, postnatal day.
Vaginal opening
The day of vaginal opening was also continually monitored for the female pups from PND29 as an indicator of female puberty. All treatment groups showed no effect compared to controls (day 34.5±2.2; mean±s.e.m.; data not shown), reinforcing the lack of effect of the treatment paradigm on more general health parameters.
Effect of maternal DBP and DES treatments on testis parameters
Although relative testis size appeared to be modulated by the specific maternal treatments (Figure 3b), with DBP-treated male offspring having relatively smaller testes at PND10 and PND24, though larger testes at PND90, this was less evident when assessing absolute testis weights (Figure 3a). Then only the DES-treated animals at PND24 (i.e., at a very dynamic phase of puberty with accelerated testis growth) showed evidence for increased testis weight.
Figure 3.

Absolute (a) and relative (b) testis weights at PND10, PND24 and PND90 for control, maternally DBP-treated and maternally DES-treated rats, as indicated (mean±s.e.m.; n=8–16). Circulating testosterone (c) and LH (d) concentrations for the same treatment groups as above (mean±s.e.m.; n=6). (e) T/LH ratio for individual rats as a measure of Leydig cell capacity. (f) Absolute LC numbers per testis stereologically counted using the optical disector method at PND24 and PND90 for control, maternally DBP-treated, and maternally DES-treated rats (mean±s.e.m.; n=3–5). (g) The quotient of circulating INSL3 concentration relative to estimated LC number per testis within individual animals (mean±s.e.m.; n=3–5), as an indicator of individual Leydig cell capacity. Unless otherwise indicated, asterisks show statistical differences (P<0.05). LC, Leydig cell; LH, luteinizing hormone; PND, postnatal day; T, testosterone.
Stereological analysis of the testes showed that total Leydig cell numbers per testis were increased at PND24 only in the DES treatment group (Figure 3f). By PND90, there were no notable differences between both treatment groups and controls. Immunological analysis of total testosterone and LH in peripheral blood showed no differences for any treatment at any time point (Figure 3c and 3d), although a trend towards higher values for LH was evident in the DES-treated group at PND10 (Figure 3d). The ratio of T to LH within individuals has been interpreted as a measure of total Leydig cell functional capacity.24,25 Although there was a major increase in this parameter (mean±s.e.m. for all groups combined; P<0.001) between PND24 (0.037±0.031; n=18) and PND90 (0.443±0.482; n=18), as the Leydig cells differentiate and the HPG axis attains its final mature status (Figure 3e), there were no differences between treatment groups at all time points. Figure 3g indicates the ratio of circulating INSL3 (see below) to Leydig cell numbers per testis for those individual rats where this was measured. As such, it therefore offers an indication of individual Leydig cell functional capacity. Importantly, what this figure shows is that in spite of increased Leydig cell numbers at PND24, treatment does not appear to have altered their individual capacity to generate INSL3. This is different at PND90, where in the control group, Leydig cells appear to have reduced their individual capacity to make INSL3, compared to PND24, though not in the two treated groups.
The peptide hormone INSL3 is also considered a measure of Leydig cell functional capacity.26 Analysis of circulating INSL3 (Figure 4) showed that up to PND40 (i.e., during puberty), values for INSL3 tended to be higher in the DBP- and DES-treated groups compared to controls, though subsequent to this time, particularly the DES-treated group indicated lower circulating INSL3 compared to the control group. Besides the increased total INSL3 production (area under the curve) evident for the DBP treated group, there is also a clear shift in the median for each treatment group (Figure 4), with this parameter being at PND42 for the controls, PND38 for the DBP-treated group and PND37 for the DES-treated group. The median was calculated as the value bisecting to 50% the area under the curve, and then rounded to the nearest whole day.
Figure 4.
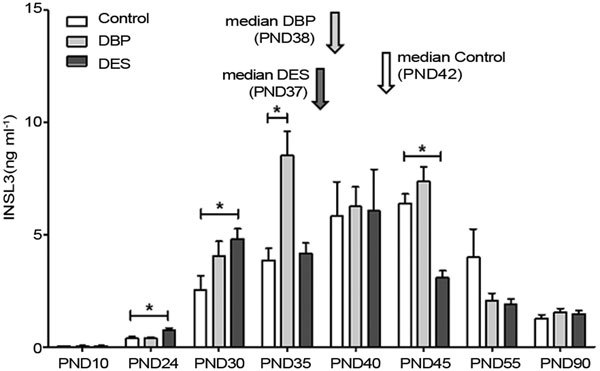
Circulating serum INSL3 concentration in control, maternally DBP-treated and maternally DES-treated rats at different ages, as indicated (mean±s.e.m.; n=6). Calculated medians are indicated by vertical arrows. Asterisks indicate statistical differences (P<0.05). DBP, dibutylphthalate; DES, diethylstilbestrol; PND, postnatal day.
Since the focus of this study was to assess any impact of maternal treatment on adult-type Leydig cell function, analysis of Leydig cell-specific gene expression was also undertaken. First of all expression profiles for all seven transcripts being analysed was carried out on rat testis samples collected throughout puberty (Figure 5) using quantitative real-time RT-PCR (qRT-PCR). This indicated that for each gene, there was a different trajectory of expression, reflecting different patterns of expression within the testis. For all gene transcripts, PND24 appears to represent a particularly dynamic phase of Leydig cell gene expression, corresponding in controls to the transition from the immature to mature Leydig cell phenotype. In contrast, PND90 represents a stable adult phase, whereby any overexpression during puberty and the establishment of the HPG axis appears now to be subdued. Using these selected time points, the expression of these genes was then measured by qRT-PCR, specifically comparing the effects of maternal DBP and DES treatments versus vehicle controls (Figure 6). For the Star, Hsd11b1 and Hsd17b3 gene products, there was an effect of DBP treatment at PND10, and a trend also at this time for the side-chain cleavage enzyme gene, Cyp11a1 (Figure 6). DES treatment at this time point showed an effect only for the Star gene transcript. At PND24, an effect was only evident for the full-length LH receptor gene transcript (Lhcgr; Figure 6e), and only after DES treatment, though trends were also observed for this treatment for Insl3 and Cyp11a1 gene transcripts. A trend was also evident upon DBP treatment for Insl3 gene transcripts at PND24. There were no effects of either treatment for any transcript at PND90, except for a trend to higher values in the DES group for Insl3 (P=0.06).
Figure 5.
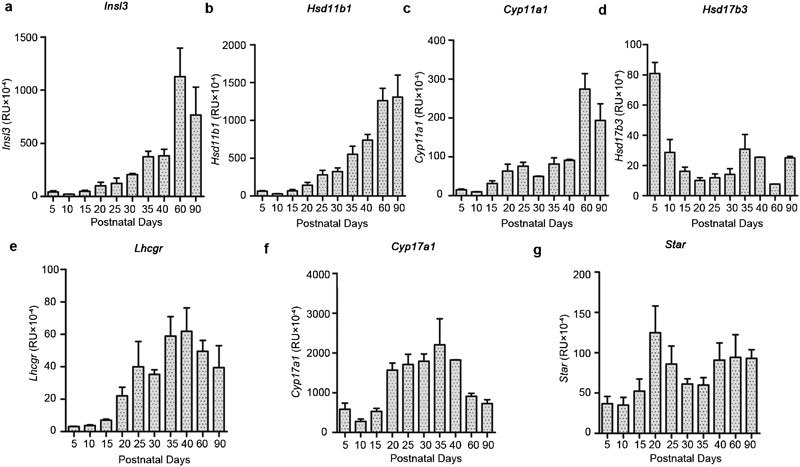
Transcript profiles measured for specific genes by qRT-PCR in RNA extracted from intact testes of untreated Sprague–Dawley rats culled at the days indicated (mean±s.e.m.; n=4). All transcripts are expressed normalized against the housekeeping control transcript for ribosomal protein S27a.
Figure 6.
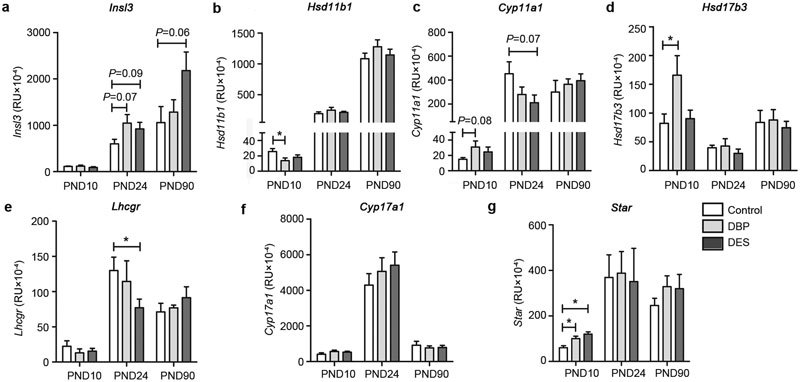
Relative gene transcript amount in RNA from testes of control, maternally DBP-treated and maternally DES-treated rats at PND10, PND24 and PND90 (mean±s.e.m.; n=6). All transcripts are expressed normalized against the housekeeping control transcript for ribosomal protein S27a. Unless otherwise indicated, horizontal bars and asterisks (*) indicate statistical differences (P<0.05). DBP, dibutylphthalate; DES, diethylstilbestrol; PND, postnatal day.
Discussion
In this pilot study, and as in earlier studies,27,28,29 a dosing regimen for both DES and DBP was chosen, which had as the primary objective sufficient xenobiotic exposure to induce minimal though statistically significant disruption of the classic end point of a reduced AGD in male pups, though without obvious effects on general health parameters. Thus, any change observed in specific parameters is not attributable to a general health deficit, but should be due to a specific disruption of those features. For this reason also, the few maternally DBP-treated offspring exhibiting gross testicular dysgenesis or atrophy were also excluded, so as not to invoke effects which might be the consequences of gross anatomical aberration. These criteria were met and strongly supported by the generally low within- and between-group variance in most parameters.
This study has focused on the long-term effects of maternal exposure to DBP and DES in male rats and in particular on the phenotype of adult-type Leydig cells in the intact testes. It does not aim therefore to reassess the known acute effects of maternal exposure leading to gross testicular dysgenesis (DBP), or to perinatal death (DES) that have been previously reported.2,22,23 The observations relating to this pathology are essentially in agreement with those described by other authors.2,22,30 As measures of Leydig cell phenotype, we have assessed specific Leydig cell numbers and gene expression shortly after cessation of treatment at PND10, at a single dynamic time point during their pubertal differentiation (PND24), and after the establishment of adult endocrine stability at PND90. Additionally, we have assessed the secretion into the circulation of the Leydig cell peptide hormone INSL3, which we have shown is expressed constitutively, and accurately reflects the numbers and/or differentiation status of Leydig cells in humans and other species.26 Total circulating testosterone was also measured, as was circulating LH. Both parameters were assessed only for single time points per day (from 9:00 a.m. to 11:00 a.m.), and therefore being pulsatile hormones, such measures are of statistical value only for the sample population, where the T/LH ratio should reflect the average capacity of Leydig cells for steroidogenesis.24,25 Although on PND24 at the beginning of puberty, there appeared to be a trend for both DES and DBP groups to have lower individual T/LH ratios compared to controls (data not shown), this did not reach statistical significance (P=0.2), and was definitely not maintained at the later time point (PND90).
Although it is normal practice to assess testicular growth during puberty, as for most other organs, as relative to whole body weight, for the testis this may be spurious. Postnatal growth of the testis is largely due to specific proliferation of Leydig and Sertoli cells, as well as of the germinal epithelium, as a consequence of the activation of the hormones of the HPG axis during puberty, and thus, is not necessarily governed by the same factors as for the general postnatal growth of other organs and tissues. Thus, the absolute testis weight for a cohort of identically aged rats appears to be a better assessment of the impact of maternal exposure to xenobiotics, compared to the relative testis weight. Importantly, these results indicate an effect on testis weight only of gestational DES treatment, and only at PND24, the most dynamic phase of testicular growth. This observation is also in full agreement with the estimate of total Leydig cell numbers per testis, as well as with the secretion profiles for the peptide hormone INSL3. Leydig cell numbers were close to those previously reported by Zirkin and Ewing31 and Hardy et al.17 Close inspection of the testicular histology (data not shown) indicated that the increase in Leydig cell numbers did not appear to be associated with any kind of focal hyperplasia as has been reported for the fetal testis under DBP treatment.32 Nor was there any evidence for focal atrophy in any of the testes examined, which, however, did exclude any of the grossly dysgenic testes resulting from maternal DBP treatment. Taken together, all of these data strongly suggest that particularly maternal estrogen exposure serves to accelerate postnatal Leydig cell differentiation and proliferation, resulting in an advance in the median value of INSL3 secretion from PND42 (control) to PND37 (DES treatment). The values for circulating LH at PND10 and PND24 would support this argument, suggesting an impact of gestational xenoestrogen exposure also on the development of the HPG axis. In contrast, maternal DBP treatment has little if any effect on either testis growth or Leydig cell numbers, although the circulating INSL3 profiles do suggest modest acceleration in Leydig cell differentiation, leading to earlier and higher circulating levels of INSL3, for example, at PND35, and which is reflected in the trends to increased Insl3 mRNA at PND24. The lack of xenobiotic impact on adult testosterone level is not surprising given its high within-animal fluctuation, and has been reported previously.29
Looking at specific testicular gene expression, it is also evident that maternal DBP treatment is having a similar positive impact on those gene transcripts involved in the first stages of the establishment of steroidogenesis, namely, Cyp11a1, Hsd17b3 and Star, though only at PND10. Interestingly, transcripts for the full-length LH receptor (Lhcgr) as well as for the side-chain cleavage enzyme (Cyp11a1), which are both relatively early-intermediate markers of Leydig cell differentiation, are both reduced for the DES-treated group at PND24. Note that Insl3 is a relatively late marker of Leydig cell differentiation. Taken together, these observations again support the notion that early estrogenic exposure is causing a temporal disruption of normal Leydig cell proliferation and differentiation, with early progenitor Leydig cells proliferating longer in an immature state (PND10), but then differentiating faster (PND24) to complete puberty somewhat earlier (median circulating INSL3 at PND37). The DBP effects are probably comparable to these, or intermediate between DES and control groups, though less intense, leading to few differences in the measured parameters compared to controls. These results are similar to those recently reported for maternal bisphenol A exposure, which appears to have comparable estrogenic effects on adult-type Leydig cell differentiation.33 There is also a good correlation between the findings reported here and our recent study on the influence of DBP and DES upon adult-type Leydig cell regeneration following ethane dimethane sulfonate ablation,4 where also the kinetics of Leydig cell differentiation was modulated by these xenobiotics.
This study has also highlighted the value of measuring circulating INSL3 during puberty, by emphasizing what has earlier been referred to as the ‘overshoot' effect.21 As Leydig cells differentiate during puberty, concomitant with the large and initially not well regulated pulses of LH from the pituitary, they initially give rise to an overproduction of INSL3 as a measure of Leydig cell differentiation status. Unlike testosterone, whose enzymatic production is acutely regulated by feedback from the pituitary, INSL3 is constitutively expressed.26 Circulating testosterone, therefore, does not reveal such an overshoot effect, but once a maximum is attained, the testosterone level is held more or less constant by acute feedback regulation from the HPG axis. Following the overshoot at around day 40, circulating INSL3 production, and presumably Leydig cell activity, relaxes to yield stable and considerably lower adult levels by about days 60–90, when Leydig cell differentiation status has become regularized at a lower level as a consequence of chronic HPG control. This is reflected also in the reduced measure of INSL3 per individual Leydig cell. Interestingly, although this cannot be statistically supported, the results suggest that at PND90, both DBP and DES treatments lead to a modest reduction in LH and in total Leydig cell numbers per testis, which then appear to maintain their pubertal level of INSL3 production per individual cell.
The results of this study suggest that there may indeed be long-term though subtle consequences of maternal xenobiotic exposure for adult testis function, particularly during puberty and the establishment of the stable adult phenotype. Obviously, more detail is needed in order to explore precisely the mechanisms by which these long-term changes are achieved.
Author contributions
RI was principally responsible for the conception and supervision of the project. KH carried out most of the experimental work. HN provided expert assistance with the histology and stereology. RAI was largely responsible for supervising the analytical design and experimentation. All authors read and approved the final manuscript.
Acknowledgments
We gratefully acknowledge the help and support from Laboratory Animal Services, University of Adelaide. We also thank Professor Jeff Schwartz (Griffith University) for helpful advice, as well as to Ms Navdeep Mann, Ms Lee Ling Tan and Mr Damien Hunter for assistance with some of the animal experimentation, and the PCR analysis. We also wish to acknowledge the kind help and guidance of Dr Maree Gould and Dr Peter Hurst (University of Otago) in regard to the stereology. This work was funded in part through an Australian Research Council (http://www.arc.gov.au) project grant to RI (DP 0773315), and through scholarship support by the University of Adelaide to KH.
The authors declare no competing financial interests
References
- Sharpe RM, Skakkebaek NE. Testicular dysgenesis syndrome: mechanistic insights and potential new downstream effects. Fertil Steril. 2008;89 2 Suppl:e33–8. doi: 10.1016/j.fertnstert.2007.12.026. [DOI] [PubMed] [Google Scholar]
- Wilson VS, Howdeshell KL, Lambright CS, Furr J, Earl Gray L., Jr Differential expression of the phthalate syndrome in male Sprague–Dawley and Wistar rats after in utero DEHP exposure. Toxicol Lett. 2007;170:177–84. doi: 10.1016/j.toxlet.2007.03.004. [DOI] [PubMed] [Google Scholar]
- Travison TG, Araujo AB, O‘Donnell AB, Kupelian V, McKinlay JB. A population-level decline in serum testosterone levels in American men. J Clin Endocrinol Metab. 2007;92:196–202. doi: 10.1210/jc.2006-1375. [DOI] [PubMed] [Google Scholar]
- Heng K, Anand-Ivell R, Teerds K, Ivell R. The endocrine disruptors dibutyl phthalate (DBP) and diethylstilbestrol (DES) influence Leydig cell regeneration following ethane dimethane sulfonate (EDS) treatment of adult male rats. Int J Androl. 2012;35:353–63. doi: 10.1111/j.1365-2605.2011.01231.x. [DOI] [PubMed] [Google Scholar]
- Rasier G, Parent AS, Gérard A, Lebrethon MC, Bourguignon JP. Early maturation of gonadotropin-releasing hormone secretion and sexual precocity after exposure of infant female rats to estradiol or dichlorodiphenyltrichloroethane. Biol Reprod. 2007;77:734–42. doi: 10.1095/biolreprod.106.059303. [DOI] [PubMed] [Google Scholar]
- Emmen JM, McLuskey A, Adham IM, Engel W, Verhoef-Post M, et al. Involvement of insulin-like factor 3 (Insl3) in diethylstilbestrol-induced cryptorchidism. Endocrinology. 2000;141:846–9. doi: 10.1210/endo.141.2.7379. [DOI] [PubMed] [Google Scholar]
- McKinnell C, Sharpe RM, Mahood K, Hallmark N, Scott H, et al. Expression of Insulin-like factor 3 (Insl3) protein in the rat testis during fetal and postnatal development and in relation to cryptorchidism induced by in utero exposure to Di (n-butyl) phthalate. Endocrinology. 2005;146:4536–44. doi: 10.1210/en.2005-0676. [DOI] [PubMed] [Google Scholar]
- Anand-Ivell R, Relan V, Balvers M, Fritsch M, Bathgate RA, et al. Expression of the insulin-like peptide 3 (INSL3) hormone-receptor (LGR8) system in the testis. Biol Reprod. 2006;74:945–53. doi: 10.1095/biolreprod.105.048165. [DOI] [PubMed] [Google Scholar]
- Veldhuizen-Tsoerkan MB, Ivell R, Teerds K. Human chorionic gonadotrophin (hCG)-induced changes in luteinizing hormone/hCG receptor messenger ribonucleic acid transcript levels in the testis of adult, hypophysectomized, ethane dimethyl sulphonate treated rats. Mol Cell Endocrinol. 1994;105:37–44. doi: 10.1016/0303-7207(94)90033-7. [DOI] [PubMed] [Google Scholar]
- Lee PD, Sladek R, Greenwood CM, Hudson TJ. Control genes and variability: absence of ubiquitous reference transcripts in diverse mammalian expression studies. Genome Res. 2002;12:292–7. doi: 10.1101/gr.217802. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Muller PY, Janovjak H, Miserez AR, Dobbie Z. Processing of gene expression data generated by quantitative real-time RT-PCR. Biotechniques. 2002;32:1372–79. [PubMed] [Google Scholar]
- Gundersen HJ. Stereology of arbitrary particles. A review of unbiased number and size estimators and the presentation of some new ones, in memory of William R. Thompson. J Microsc. 1986;143:3–45. [PubMed] [Google Scholar]
- Wreford NG. Theory and practice of stereological techniques applied to the estimation of cell number and nuclear volume in the testis. Microsc Res Tech. 1995;32:423–36. doi: 10.1002/jemt.1070320505. [DOI] [PubMed] [Google Scholar]
- Gould ML, Hurst PR, Nicholson HD. The effects of oestrogen receptors alpha and beta on testicular cell number and steroidogenesis in mice. Reproduction. 2007;134:271–9. doi: 10.1530/REP-07-0025. [DOI] [PubMed] [Google Scholar]
- Gundersen HJ. Notes on the estimation of the numerical density of arbitrary profiles: the edge effect. J Microsc. 1977;111:219–23. [Google Scholar]
- Ariyaratne HB, Mills N, Mason JI, Mendis-Handagama SM. Effects of thyroid hormone on Leydig cell regeneration in the adult rat following ethane dimethane sulphonate treatment. Biol Reprod. 2000;63:1115–23. doi: 10.1095/biolreprod63.4.1115. [DOI] [PubMed] [Google Scholar]
- Hardy MP, Zirkin BR, Ewing LL. Kinetic studies on the development of the adult population of Leydig cells in testes of the pubertal rat. Endocrinology. 1989;124:762–70. doi: 10.1210/endo-124-2-762. [DOI] [PubMed] [Google Scholar]
- Teerds KJ, de Rooij DG, Rommerts FF, van den Hurk R, Wensing CJ. Stimulation of the proliferation and differentiation of Leydig cell precursors after the destruction of existing Leydig cells with ethane dimethyl sulphonate (EDS) can take place in the absence of LH. J Androl. 1989;10:472–7. doi: 10.1002/j.1939-4640.1989.tb00143.x. [DOI] [PubMed] [Google Scholar]
- Howard V, Reed MG. New York; Springer; 1998. Unbiased Stereology: Three-dimensional Measurement in Microscopy. [Google Scholar]
- Anand-Ivell R, Ivell R, Driscoll DA, Manson J. INSL3 levels in amniotic fluid from human male fetuses. Hum Reprod. 2008;23:1180–6. doi: 10.1093/humrep/den038. [DOI] [PubMed] [Google Scholar]
- Anand-Ivell R, Heng K, Hafen B, Setchell B, Ivell R. Dynamics of INSL3 peptide expression in the rodent testis. Biol Reprod. 2009;81:480–7. doi: 10.1095/biolreprod.109.077552. [DOI] [PubMed] [Google Scholar]
- Foster PM. Disruption of reproductive development in male rat offspring following in utero exposure to phthalate esters. Int J Androl. 2006;29:140–7. doi: 10.1111/j.1365-2605.2005.00563.x. [DOI] [PubMed] [Google Scholar]
- Kawaguchi H, Miyoshi N, Miyamoto Y, Souda M, Umekita Y, et al. Effects of exposure period and dose of diethylstilbestrol on pregnancy in rats. J Vet Med Sci. 2009;71:1309–15. doi: 10.1292/jvms.001309. [DOI] [PubMed] [Google Scholar]
- Andersson AM, Jorgensen N, Frydelund-Larsen L, Rajpert-De Meyts E, Skakkebaek NE. Impaired Leydig cell function in infertile men: a study of 357 idiopathic infertile men and 318 proven fertile controls. J Clin Endocrinol Metab. 2006;89:3161–7. doi: 10.1210/jc.2003-031786. [DOI] [PubMed] [Google Scholar]
- de Kretser DM. Editorial: is spermatogenic damage associated with Leydig cell dysfunction. J Clin Endocrinol Metab. 2006;89:3158–60. doi: 10.1210/jc.2004-0741. [DOI] [PubMed] [Google Scholar]
- Ivell R, Anand-Ivell R. The biology of insulin-like factor 3 (INSL3) in human reproduction. Hum Reprod Update. 2009;15:463–76. doi: 10.1093/humupd/dmp011. [DOI] [PubMed] [Google Scholar]
- Mylchreest E, Sar M, Wallace DG, Foster PM. Fetal testosterone insufficiency and abnormal proliferation of Leydig cells and gonocytes in rats exposed to di(n-butyl) phthalate. Reprod Toxicol. 2002;16:19–28. doi: 10.1016/s0890-6238(01)00201-5. [DOI] [PubMed] [Google Scholar]
- Mylchreest E, Wallace DG, Cattley RC, Foster PM. Dose-dependent alterations in androgen-regulated male reproductive development in rats exposed to di(n-butyl) phthalate during late gestation. Toxicol Sci. 2000;55:143–51. doi: 10.1093/toxsci/55.1.143. [DOI] [PubMed] [Google Scholar]
- Howdeshell KL, Furr J, Lambright CR, Rider CV, Wilson VS, et al. Cumulative effects of dibutyl phthalate and diethylhexyl phthalate on male rat reproductive tract development: altered fetal steroid hormones and genes. Toxicol Sci. 2007;99:190–202. doi: 10.1093/toxsci/kfm069. [DOI] [PubMed] [Google Scholar]
- Fisher JS, Macpherson S, Marchetti N, Sharpe RM. Human ‘testicular dysgenesis syndrome': a possible model using in-utero exposure of the rat to dibutyl phthalate. Hum Reprod. 2003;18:1383–94. doi: 10.1093/humrep/deg273. [DOI] [PubMed] [Google Scholar]
- Zirkin BR, Ewing LL. Leydig cell differentiation during maturation of the rat testis: a stereological study of cell number and ultrastructure. Anat Rec. 1987;219:157–63. doi: 10.1002/ar.1092190208. [DOI] [PubMed] [Google Scholar]
- Mahood IK, Hallmark N, McKinnell C, Walker M, Fisher JS, et al. Abnormal Leydig cell aggregation in the fetal testis of rats exposed to di (n-butyl) phthalate and its possible role in testicular dysgenesis. Endocrinology. 2005;146:613–23. doi: 10.1210/en.2004-0671. [DOI] [PubMed] [Google Scholar]
- Nanjappa MK, Simon L, Akingbemi BT. The industrial chemical bisphenol A (BPA) interferes with proliferative activity and development of steroidogenic capacity in rat Leydig cells. Biol Reprod. 2012;86:135. doi: 10.1095/biolreprod.111.095349. [DOI] [PMC free article] [PubMed] [Google Scholar]


