Glutamate is extensively involved in metabolic and oncogenic pathways. Univariate and multivariate analyses showed that serum glutamate levels directly correlated with Gleason score (≤6 vs. ≥8) and primary prostate cancer (PCa) aggressiveness. Compared with Caucasian Americans, serum glutamate levels were higher in African Americans with metastatic castrate-resistant PCa than in the patients with primary tumors. Glutamate deprivation or blockade with pharmacological inhibitors of glutamate release or metabotropic glutamate receptor 1 (GRM1) decreased growth, migration and invasion, and induced apoptosis in both androgen-stimulated and androgen-independent PCa cells. Immunohistochemical staining showed GRM1 overexpression in primary or metastatic PCa tissues. These data highlight the potential of glutamate as a biomarker of aggressiveness in primary PCa and target its metabolic and signaling activities for therapeutic interventions.
Malignant cells exhibit altered metabolic profiles and bioenergetic needs which reflect the shift from more efficient metabolism of glucose and mitochondrial adenosine triphosphate (ATP) production to aerobic glycolysis, a wasteful glucose consumption leading to increased lactate production and other bioenergetic demands despite accessibility to oxygen.1 Since metabolic pathways and key metabolites are tissue-specific and different during the multistep process of neoplastic progression, the search for a universal metabolic phenotype or metabolite in cancer is neither fruitful nor rational. Our knowledge of metabolic changes during various stages of prostate carcinogenesis is very limited. Not surprisingly, our options for using metabolites as biomarkers of malignant transformation and progression or therapeutic targets are limited. The prostate gland in the normal and neoplastic state has a distinct and unique reservoir of metabolites, most prominently in terms of production of prostate-specific antigen, polyamines and citrate.2
In our search for serum metabolic biomarker(s), we were interested in those small molecules or metabolites which are specifically used as bioenergetic substrates or metabolic precursors of major biosynthetic or lipogenic pathways. Using this approach, glutamate appeared to have unique characteristics. Glutamate, as an intermediate metabolite, is actively involved in the tricarboxylic acid cycle and lipogenesis pathways that are biologically relevant in prostate carcinogenesis and castrate-resistant progression. Glutamate is considered a cell signaling molecule in addition to its extensive role in bioenergetics, biosynthetic pathways and maintenance of other amino acids and nucleotide pools. One important characteristic of glutamate is that its level in the systemic circulation is kept within a stable range3,4 and its abnormal circulatory level reflects pathological conditions or disturbances in metabolic homeostasis as has been shown in our study.5
To examine clinical significance of glutamate in PCa, serum glutamate levels were examined in healthy adult males (n=60) and patients with primary PCa (n=197) or metastatic castrate-resistant PCa (n=109). Statistical analyses showed a significant positive correlation between serum glutamate and Gleason score (≤7 vs. ≥8) both in African Americans and Caucasian Americans. There was a significant association between race and serum glutamate levels, even when adjusting for clinical status of the research subjects. Overall, the serum glutamate levels were generally higher in African Americans than Caucasian Americans. Glutamate levels for both the African Americans and Caucasian Americans followed the same general trend within aggressiveness subgroups of primary PCa, but diverged greatly when going from the high aggressiveness subgroup (Gleason sum ≥8, or prostate-specific antigen >20 ng ml−1, or Gleason sum=7, and stage cT3–cT4) to the metastatic castrate-resistant PCa.5
The biological relevance of glutamate in PCa cells was established by testing the effect of glutamate deprivation or pharmacological antagonists in PCa cells. Glutamate deprivation or blockade with metabotropic glutamate receptor 1 (GRM1)-antagonists (i.e., Riluzole, BAY 36-7620) decreased PCa cell growth, migration and invasion, and led to apoptotic-cell death as demonstrated by dose-dependent increases in cleaved caspases-3, -7 and -9, poly (ADP-ribose) polymerase (PARP) and phospho-H2AXSer139 levels.5
Glutamate can enter mammalian cells by glutamate transporters (GT), ionotropic glutamate receptors (iGluRs) or metabotropic glutamate receptors (mGluRs). The expression of various members of glutamate uptake/receptors system in PCa is not known. We chose to study GRM1 expression in benign and malignant prostate tissues for the following reasons: (i) previous reports have indicated an oncogenic function and transforming ability for GRM1 in breast cancer and melanoma;6,7,8 and (ii) our in vitro studies have proven GRM1 antagonists ability to induce apoptosis and significantly decreased PCa cells growth, migration and invasion. GRM1 expression was positive in basal cells of normal or benign prostatic hyperplasia (BPH), but weak or no expression in luminal acinar epithelial cells (Figure 1). Moderate to intense cytoplasmic staining was also noted in high-grade prostate intraepithelial neoplasia (HGPIN) and in tumors with Gleason scores 6 and 7. Intense cytoplasmic staining was noted in malignant acinar cells of Gleason score ≥8 tumors, and PCa cells invading into extraprostatic adipose and muscular tissues, infiltrating around nerves or metastasized to bone (Figure 1). Overall, our descriptive immunohistochemical analysis was suggestive of a progressive enhancement of GRM1 overexpression from normal or hyperplastic glands (n=10), to HGPIN (n=10), to primary (n=10), locally advanced (n=5) and then metastatic PCa (n=6). In addition, GRM1 expression in androgen-stimulated LNCaP or primary African American (i.e., E006AA) PCa cell lines was less than androgen-independent PC-3, DU145 and androgen-independent bone metastatic PCa cell lines including VCaP and MDA-PCa 2b (of African American origin).5
Figure 1.
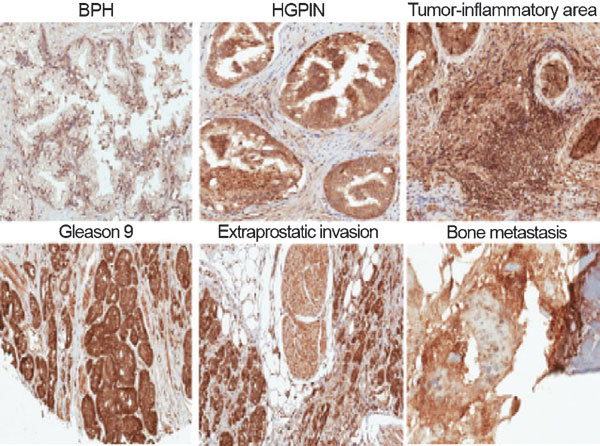
Immunohistochemical staining of GRM1 in normal and malignant prostate tissues. BPH shows nuclear staining in basal cells and absence of staining in normal luminal acinar cells. Moderate to intense cytoplasmic staining is noted in HGPIN. Intense nuclear and cytoplasmic staining is noted in tissue infiltrating macrophages, stromal cells and PCa cells within the tumor-inflammatory area. Intense cytoplasmic staining was observed in malignant luminal acinar cells of a Gleason 9 (5+4) tumor, and in tumor cells invading into extraprostatic adipose and muscle tissues. Intense anti-GRM1 staining was noted in scattered metastatic PCa cells within fragmented pieces of bone. Original magnification: ×200. BPH, benign prostate hyperplasia; GRM1, metabotropic glutamate receptor 1; HGPIN, high-grade prostate-intraepithelial neoplasia; PCa, prostate cancer.
Large-scale immunohistochemical studies are required to cover a range of clinical conditions (e.g., BPH, normal glands, primary or metastatic PCa from untreated patients, castrate-resistant primary or metastatic tumors, etc.) and racial groups (Caucasian Americans and African Americans). Such studies will provide us enough power to perform comprehensive statistical analyses to compare in a quantitative manner the GRM1 staining intensity associated with demographic, predictive or prognostic clinicohistopathological variables of interest. The above report on biological and clinical relevance of glutamate and its receptor GRM1 has provided proof of concept for circulatory metabolites that could serve not just as intermediate or endpoint metabolites, but also as prognostic or diagnostic biomarkers for clinical discrimination of aggressive tumors from indolent ones and as potential therapeutic targets for PCa. Our data support the potential of Riluzole (Rilutek; Sanofi-Aventis), a US Food and Drug Administration-approved GRM1-antagonist and a well-tolerated oral medicine for possible therapeutic interventions in PCa.
References
- Koppenal W, Bounds PL, Dang CV. Otto Warburg's contributions to current concepts of cancer metabolism. Nat Rev. 2011;11:325–37. doi: 10.1038/nrc3038. [DOI] [PubMed] [Google Scholar]
- Trock BJ. Application of metabolomics to prostate cancer. Urol Oncol. 2011;29:572–81. doi: 10.1016/j.urolonc.2011.08.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stegink LD, Filer LJ, Jr, Baker GL. Plasma amino acid concentrations in normal adults fed meals with added monosodium L-glutamate and aspartame. J Nutr. 1983;113:1851–60. doi: 10.1093/jn/113.9.1851. [DOI] [PubMed] [Google Scholar]
- Stegink LD, Filer LJ, Jr, Baker GL. Plasma and erythrocyte amino acid levels in normal adult subjects fed a high protein meal with and without added monosodium glutamate. J Nutr. 1982;112:1953–60. doi: 10.1093/jn/112.10.1953. [DOI] [PubMed] [Google Scholar]
- Koochekpour S, Majumdar S, Azabdaftari G, Attwood K, Scioneaux R, et al. Serum Glutamate levels correlate with Gleason score and glutamate blockade decreases proliferation, migration, and invasion and induces apoptosis in prostate cancer cells. Clin Cancer Res. 2012;18:5888–901. doi: 10.1158/1078-0432.CCR-12-1308. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pollock PM, Cohen-Solal K, Sood R, Namkoong J, Martino JJ, et al. Melanoma mouse model implicates metabotropic glutamate signaling in melanocytic neoplasia. Nat Genet. 2003;34:108–12. doi: 10.1038/ng1148. [DOI] [PubMed] [Google Scholar]
- Namkoong J, Shin SS, Lee HJ, Marín YE, Wall BA, et al. Metabotropic glutamate receptor 1 and glutamate signaling in human melanoma. Cancer Res. 2007;67:2298–305. doi: 10.1158/0008-5472.CAN-06-3665. [DOI] [PubMed] [Google Scholar]
- Speyer CL, Smith JS, Banda M, Devries JA, Mekani T, et al. Metabotropic glutamate receptor-1: a potential therapeutic target for the treatment of breast cancer. Breast Cancer Res Treat. 2012;132:565–73. doi: 10.1007/s10549-011-1624-x. [DOI] [PMC free article] [PubMed] [Google Scholar]


