Abstract
Spirochaeta caldaria Pohlschroeder et al. 1995 is an obligately anaerobic, spiral-shaped bacterium that is motile via periplasmic flagella. The type strain, H1T, was isolated in 1990 from cyanobacterial mat samples collected at a freshwater hot spring in Oregon, USA, and is of interest because it enhances the degradation of cellulose when grown in co-culture with Clostridium thermocellum. Here we provide a taxonomic re-evaluation for S. caldaria based on phylogenetic analyses of 16S rRNA sequences and whole genomes, and propose the reclassification of S. caldaria and two other Spirochaeta species as members of the emended genus Treponema. Whereas genera such as Borrelia and Sphaerochaeta possess well-distinguished genomic features related to their divergent lifestyles, the physiological and functional genomic characteristics of Spirochaeta and Treponema appear to be intermixed and are of little taxonomic value. The 3,239,340 bp long genome of strain H1T with its 2,869 protein-coding and 59 RNA genes is a part of the Genomic Encyclopedia of Bacteria and Archaea project.
Keywords: obligately anaerobic, thermophilic, spiral-shaped, motile, periplasmic flagella, Gram-negative, chemoorganotrophic, Spirochaetaceae, Spirochaeta, Treponema, GEBA
Introduction
Strain H1T (= DSM 7334 = ATCC 51460) is the type strain of the species Spirochaeta caldaria [1,2] in the genus Spirochaeta (which currently contains 19 validly named species [3,4]) and was first isolated from cyanobacterial mat samples collected at a freshwater hot spring in Oregon, USA [1]. The genus name was derived from the latinized Greek words 'speira' meaning 'a coil' and 'chaitê' meaning 'hair', yielding the Neo-Latin 'Spirochaeta', 'coiled hair' [3]. The species epithet is derived from the Latin adjective 'caldaria', 'pertaining to warm water' (intended to mean inhabiting warm water) [3]. References to S. caldaria in PubMed are rather sparse. In 1996 Paster et al. reported S. caldaria as the closest relative to a spirochaete clone from the hindguts of an African higher termite, Nasutermites lujae [5], an observation that was underlined three years later when Lilburn et al. identified S. caldaria as a close relative of the majority of the ‘spirochaetes’ in the gut of the termite Reticulitermes flavipes [6]. In the same year (1999) Ohkuma et al. confirmed this observation for symbiotic ‘spirochaetes’ in the gut of diverse termites [7]. Here we present a summary classification and a set of features for S. caldaria strain H1T, together with the description of the complete genome sequencing and annotation.
Features of the organism
A representative genomic 16S rRNA sequence of S. caldaria H1T was compared using NCBI BLAST [8,9] under default settings (e.g., considering only the high-scoring segment pairs (HSPs) from the best 250 hits) with the most recent release of the Greengenes database [10] and the relative frequencies of taxa and keywords (reduced to their stem [11]) were determined, weighted by BLAST scores. The most frequently occurring genera were Spirochaeta (79.9%) and Treponema (20.1%) (17 hits in total). Regarding the two hits to sequences from members of the species, the average identity within HSPs was 99.4%, whereas the average coverage by HSPs was 98.4%. Regarding the five hits to sequences from other members of the genus, the average identity within HSPs was 94.3%, whereas the average coverage by HSPs was 96.3%. Among all other species, the one yielding the highest score was “Spirochaeta taiwanensis” AY35103, which corresponded to an identity of 95.2% and an HSP coverage of 94.4%. (Note that the Greengenes database uses the INSDC (= EMBL/NCBI/DDBJ) annotation, which is not an authoritative source for nomenclature or classification.) The highest-scoring environmental sequence was FJ462015 ('Microbial ecology industrial digester mesophilic anaerobic reactor fed effluent chemical industry clone 71a'), which showed an identity of 97.9% and an HSP coverage of 98.1%. The most frequently occurring keywords within the labels of all environmental samples which yielded hits were 'termit' (26.5%), 'hindgut' (17.8%), 'gut' (8.6%), 'homogen' (5.5%) and 'flagel' (2.1%) (233 hits in total), which is in line with previous observations about close relatives in termite guts [5-7]. Environmental samples which yielded hits of a higher score than the highest scoring species were not found.
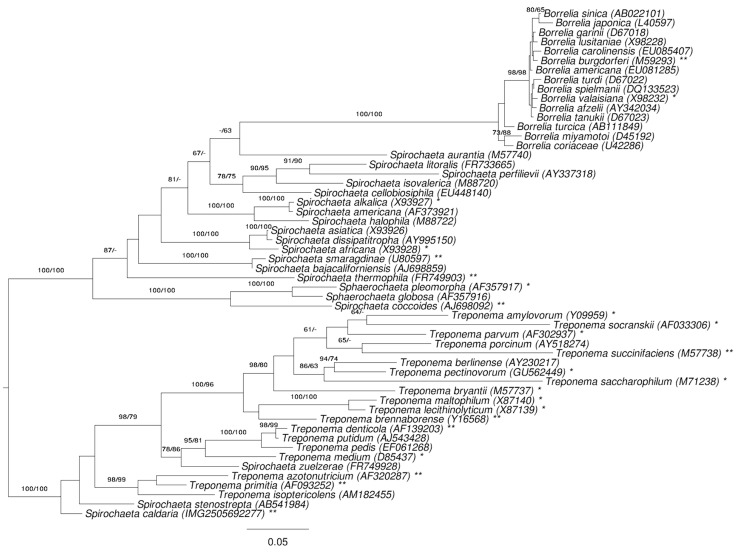
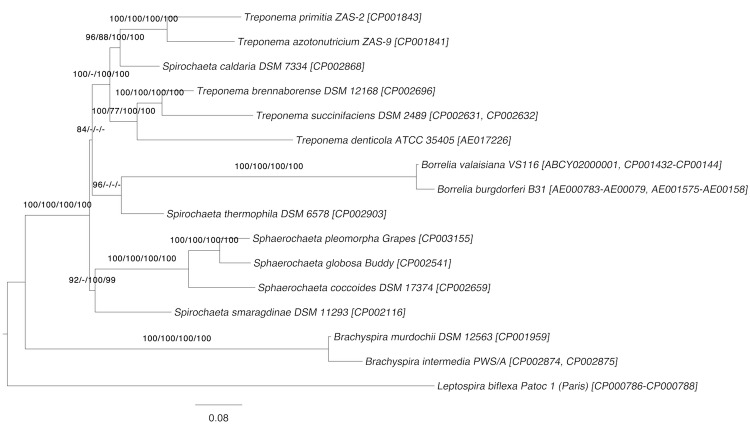
Figure 1 shows the phylogenetic neighborhood of S. caldaria in a 16S rRNA based tree. The sequences of the three 16S rRNA gene copies in the genome differ from each other by up to three nucleotides, and differ by up to four nucleotides from the previously published 16S rRNA sequence EU580141.
Figure 1.
Phylogenetic tree highlighting the position of S. caldaria relative to the type strains of the other species within the family Spirochaetaceae. The tree was inferred from 1,362 aligned characters [12,13] of the 16S rRNA gene sequence under the maximum likelihood (ML) criterion [14]. Rooting was done initially using the midpoint method [15] and then checked for its agreement with the current classification (Table 1). The branches are scaled in terms of the expected number of substitutions per site. Numbers adjacent to the branches are support values from 1,000 ML bootstrap replicates [16] (left) and from 1,000 maximum-parsimony bootstrap replicates [17] (right) if larger than 60%. Lineages with type strain genome sequencing projects registered in GOLD [18] are labeled with one asterisk, those also listed as 'Complete and Published' with two asterisks [19-23] (for S. thermophila, T. azotonutricium and T. primitia see CP002903, CP001841 and CP001883). Note: Spirochaeta coccoides was effectively renamed to Sphaerochaeta coccoides in [19] (see Validation List 147 [24].)
Morphology and physiology
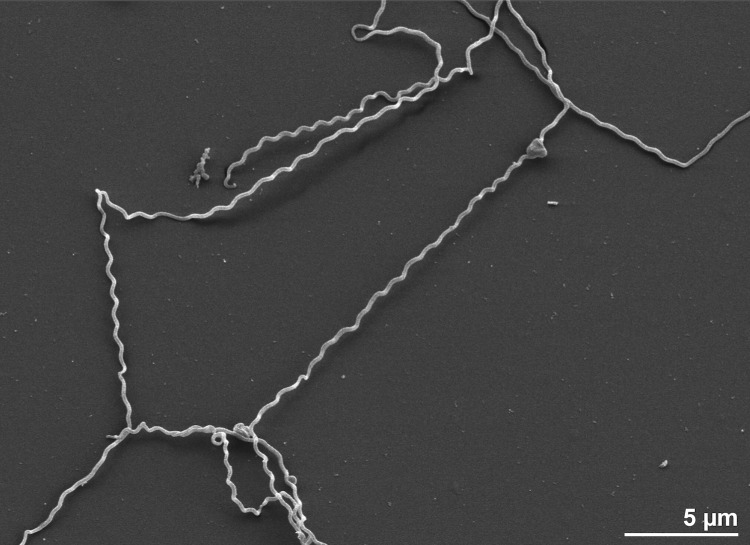
Cells of S. caldaria were helical, 0.2 to 0.3 µm in diameter and 15 to 25 µm in length (Figure 2); spherical bodies were seen in stationary-phase cultures (not visible in Figure 2). The cells are motile by two periplasmic flagella in a 1:2:1 arrangement [1]. S. caldaria is a Gram-negative, strictly anaerobic, thermophile (Table 1) with an optimal growth temperature between 48°C and 52°C, and no growth observed above 60°C or below 25°C [1]. The pH range for growth is 5.8-8.5, with an optimum at pH 7.2-7.5 [1]. S. caldaria tolerates a NaCl concentration of up to 0.25% (wt/vol), but no growth was observed in the presence of 0.4% (wt/vol) NaCl or higher concentrations [1]. On agar plates strain H1T forms white, fluffy, cotton-ball like colonies.
Figure 2.
Scanning electron micrograph of S. caldaria strain H1T
Table 1. Classification and general features of S. caldaria H1T according to the MIGS recommendations [25] and the NamesforLife database [4].
| MIGS ID | Property | Term | Evidence code |
|---|---|---|---|
| Current classification | Domain Bacteria | TAS [26] | |
| Phylum Spirochaetae | TAS [27,28] | ||
| Class Spirochaetes | TAS [29,30] | ||
| Order Spirochaetales | TAS [31,32] | ||
| Family Spirochaetaceae | TAS [30,31,33] | ||
| Genus Spirochaeta | TAS [31,34-36] | ||
| Species Spirochaeta caldaria | TAS [1,2] | ||
| Type strain H1 | TAS [1,2] | ||
| Gram stain | negative | TAS [2] | |
| Cell shape | spiral shaped | TAS [2] | |
| Motility | motile | TAS [2] | |
| Sporulation | none | TAS [2] | |
| Temperature range | thermophile | TAS [2] | |
| Optimum temperature | 48-52°C | TAS [2] | |
| Salinity | <0.4% | TAS [2] | |
| MIGS-22 | Oxygen requirement | obligately anaerobic | TAS [2] |
| Carbon source | carbohydrates | TAS [2] | |
| Energy metabolism | chemoorganotroph | TAS [2] | |
| MIGS-6 | Habitat | fresh water, hot spring | TAS [2] |
| MIGS-15 | Biotic relationship | free living | TAS [2] |
| MIGS-14 | Pathogenicity | none | TAS [2] |
| Biosafety level | 1 | TAS [37] | |
| Isolation | hot spring | TAS [2] | |
| MIGS-4 | Geographic location | Hunter's Hot Spring, Oregon | TAS [2] |
| MIGS-5 | Sample collection time | August 1990 | NAS |
| MIGS-4.1 | Latitude | 42.222 | NAS |
| MIGS-4.2 | Longitude | -120.368 | NAS |
| MIGS-4.3 | Depth | not reported | |
| MIGS-4.4 | Altitude | not reported |
Evidence codes - TAS: Traceable Author Statement (i.e., a direct report exists in the literature); NAS: Non-traceable Author Statement (i.e., not directly observed for the living, isolated sample, but based on a generally accepted property for the species, or anecdotal evidence). Evidence codes are from of the Gene Ontology project [75].
S. caldaria utilizes pentoses, hexoses and disaccharides as carbon and energy sources. Amino acids cannot be fermented. Glucose is fermented to H2, CO2, acetate and lactate as the main fermentation products, ethanol is not produced [1]. H1T is able to ferment L-arabinose, D-galactose, D-glucose, D-mannose, D-fructose, D-xylose, cellobiose, cellotriose, cellotetraose, lactose, maltose, sucrose and starch. D-ribose, mannitol, cellulose, xylan, glycerol, peptone, casein hydrolysate, and sodium acetate are not utilized [1]. Exogenous fatty acids, reported to be required by Treponema species for cellular lipid synthesis and growth [38], are not required. A supplement with vitamins is, however, required [1]. S. caldaria grows in the presence of rifampicin (100 µg/ml of medium), but growth is inhibited by penicillin G, neomycin, chloramphenicol or tetracycline (10 µg/ml of medium each) [1].
Genome sequencing and annotation
Genome project history
This organism was selected for sequencing on the basis of its phylogenetic position [39], and is part of the Genomic Encyclopedia of Bacteria and Archaea project [40]. The genome project is deposited in the Genomes On Line Database [18] and the complete genome sequence is deposited in GenBank. Sequencing, finishing and annotation were performed by the DOE Joint Genome Institute (JGI) using state of the art sequencing technology [41]. A summary of the project information is shown in Table 2.
Table 2. Genome sequencing project information.
| MIGS ID | Property | Term |
|---|---|---|
| MIGS-31 | Finishing quality | Finished |
| MIGS-28 | Libraries used | Three genomic libraries: one 454 pyrosequence standard library, one 454 PE library (12 kb insert size), one Illumina library |
| MIGS-29 | Sequencing platforms | Illumina GAii, 454 GS FLX Titanium |
| MIGS-31.2 | Sequencing coverage | 283.3 × Illumina; 26.8 × pyrosequence |
| MIGS-30 | Assemblers | Newbler version 2.3, Velvet 0.7.63, phrap version SPS - 4.24 |
| MIGS-32 | Gene calling method | Prodigal |
| INSDC ID | CP002868 | |
| GenBank Date of Release | August 12, 2011 | |
| GOLD ID | Gc01874 | |
| NCBI project ID | 46527 | |
| Database: IMG-GEBA | 2505679006 | |
| MIGS-13 | Source material identifier | DSM 7334 |
| Project relevance | Tree of Life, GEBA |
Growth conditions and DNA isolation
S. caldaria strain H1T, DSM 7334, was grown anaerobically in DSMZ medium 635 (Spirochaeta caldaria medium) [42] at 50°C. DNA was isolated from 0.5-1 g of cell paste using MasterPure Gram-positive DNA purification kit (Epicentre MGP04100) following the standard protocol as recommended by the manufacturer with modification st/DL for cell lysis as described in Wu et al. 2009 [40]. DNA is available through the DNA Bank Network [43].
Genome sequencing and assembly
The genome was sequenced using a combination of Illumina and 454 sequencing platforms. All general aspects of library construction and sequencing can be found at the JGI website [44]. Pyrosequencing reads were assembled using the Newbler assembler (Roche). The initial Newbler assembly, consisting of 60 contigs in one scaffold, was converted into a phrap [45] assembly by making fake reads from the consensus, to collect the read pairs in the 454 paired end library. Illumina GAii sequencing data (899.9 Mb) was assembled with Velvet [46] and the consensus sequences were shredded into 2.0 kb overlapped fake reads and assembled together with the 454 data. The 454 draft assembly was based on 121.6 Mb 454 draft data and all of the 454 paired end data. Newbler parameters are -consed -a 50 -l 350 -g -m -ml 20. The Phred/Phrap/Consed software package [45] was used for sequence assembly and quality assessment in the subsequent finishing process. After the shotgun stage, reads were assembled with parallel phrap (High Performance Software, LLC). Possible mis-assemblies were corrected with gapResolution [44], Dupfinisher [46], or sequencing clones bridging PCR fragments with subcloning. Gaps between contigs were closed by editing in Consed, by PCR and by Bubble PCR primer walks (J.-F. Chang, unpublished). A total of 519 additional reactions and 5 shatter libraries were necessary to close gaps and to raise the quality of the finished sequence. Illumina reads were also used to correct potential base errors and increase consensus quality using a software Polisher developed at JGI [47]. The error rate of the completed genome sequence is less than 1 in 100,000. Together, the combination of the Illumina and 454 sequencing platforms provided 310.1 × coverage of the genome. The final assembly contained 285,090 pyrosequence and 24,996,639 Illumina reads.
Genome annotation
Genes were identified using Prodigal [48] as part of the DOE-JGI genome annotation pipeline [24], followed by a round of manual curation using the JGI GenePRIMP pipeline [49]. The predicted CDSs were translated and used to search the National Center for Biotechnology Information (NCBI) nonredundant database, UniProt, TIGR-Fam, Pfam, PRIAM, KEGG, COG, and InterPro databases. Additional gene prediction analysis and functional annotation was performed within the Integrated Microbial Genomes - Expert Review (IMG-ER) platform [50].
Genome properties
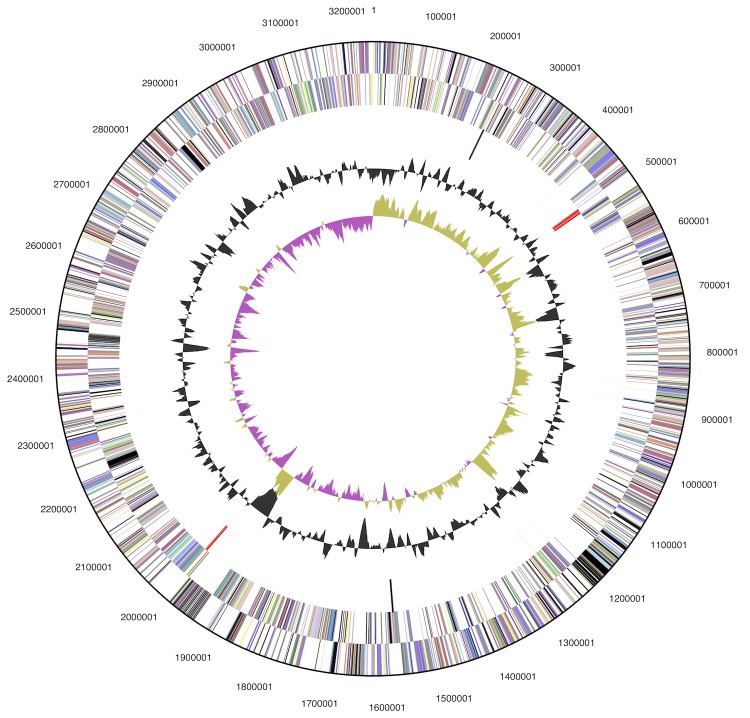
The genome consists of a 3,239,340 bp long chromosome with a G+C content of 45.6% (Table 3 and Figure 3). Of the 2,928 genes predicted, 2,869 were protein-coding genes, and 59 RNAs; 80 pseudogenes were also identified. The majority of the protein-coding genes (71.0%) were assigned a putative function while the remaining ones were annotated as hypothetical proteins. The distribution of genes into COGs functional categories is presented in Table 4.
Table 3. Genome Statistics.
| Attribute | Value | % of Total |
|---|---|---|
| Genome size (bp) | 3,239,340 | 100.00% |
| DNA coding region (bp) | 2,965,950 | 91.56% |
| DNA G+C content (bp) | 1,476,358 | 45.58% |
| Number of replicons | 1 | |
| Extrachromosomal elements | 0 | |
| Total genes | 2,928 | 100.00% |
| RNA genes | 59 | 2.02% |
| rRNA operons | 3 | |
| Protein-coding genes | 2,869 | 97.98% |
| Pseudo genes | 80 | 2.73% |
| Genes with function prediction | 2,078 | 70.97% |
| Genes in paralog clusters | 1,319 | 45.05% |
| Genes assigned to COGs | 2,270 | 77.53% |
| Genes assigned Pfam domains | 2,260 | 77.19% |
| Genes with signal peptides | 527 | 18.00% |
| Genes with transmembrane helices | 762 | 26.02% |
| CRISPR repeats | 0 |
Figure 3.
Graphical map of the chromosome. From outside to the center: Genes on forward strand (color by COG categories), Genes on reverse strand (color by COG categories), RNA genes (tRNAs green, rRNAs red, other RNAs black), GC content (black), GC skew (purple/olive).
Table 4. Number of genes associated with the general COG functional categories.
| Code | Value | %age | Description |
|---|---|---|---|
| J | 158 | 6.3 | Translation, ribosomal structure and biogenesis |
| A | 0 | 0.0 | RNA processing and modification |
| K | 156 | 6.2 | Transcription |
| L | 125 | 5.0 | Replication, recombination and repair |
| B | 2 | 0.1 | Chromatin structure and dynamics |
| D | 33 | 1.3 | Cell cycle control, cell division, chromosome partitioning |
| Y | 0 | 0.0 | Nuclear structure |
| V | 40 | 1.6 | Defense mechanisms |
| T | 228 | 9.1 | Signal transduction mechanisms |
| M | 142 | 5.7 | Cell wall/membrane/envelope biogenesis |
| N | 86 | 3.4 | Cell motility |
| Z | 0 | 0.0 | Cytoskeleton |
| W | 0 | 0.0 | Extracellular structures |
| U | 50 | 2.0 | Intracellular trafficking, secretion, and vesicular transport |
| O | 91 | 3.6 | Posttranslational modification, protein turnover, chaperones |
| C | 134 | 5.3 | Energy production and conversion |
| G | 296 | 11.8 | Carbohydrate transport and metabolism |
| E | 188 | 7.5 | Amino acid transport and metabolism |
| F | 67 | 2.7 | Nucleotide transport and metabolism |
| H | 77 | 3.1 | Coenzyme transport and metabolism |
| I | 60 | 2.4 | Lipid transport and metabolism |
| P | 77 | 3.1 | Inorganic ion transport and metabolism |
| Q | 26 | 1.1 | Secondary metabolites biosynthesis, transport and catabolism |
| R | 293 | 11.7 | General function prediction only |
| S | 182 | 7.3 | Function unknown |
| - | 658 | 22.5 | Not in COGs |
Insights from the genome sequence, and taxonomic conclusions for S. caldaria
Comparative genomics
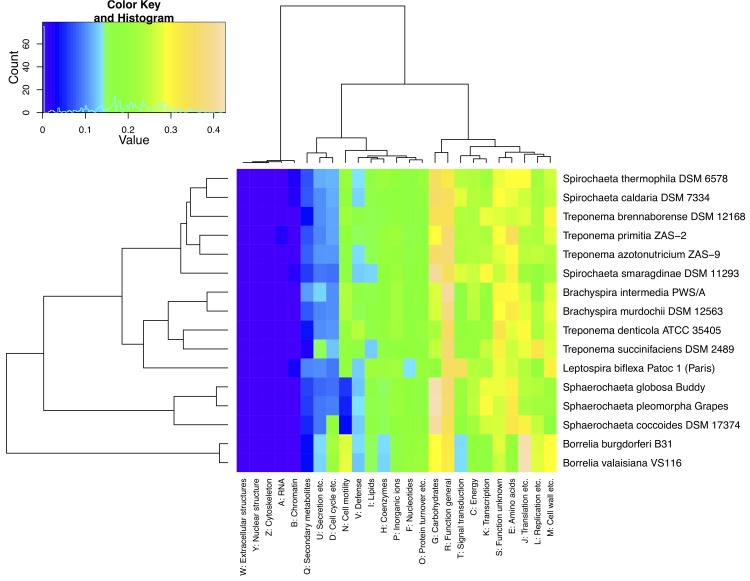
To assess the composition of the completed Spirochaetes type-strain genomes, we extracted the COG IDs from their IMG annotations [50] and determined the absolute and relative numbers of genes present in each COG category [51]. Heatmaps were generated using the opm package [52] for the statistical environment R [53] from the arcsine-square root transformed (see, e.g., p. 386 in [54] for the rationale of this transformation) COG proportions (Fig. 4) and from the log-transformed absolute numbers (data not shown). The results indicate that the relative COG category content mainly reflects changes in life style, with the intracellular parasites (Borrelia spp.) and the coccoid forms (Sphaerochaeta spp.) forming clusters of their own.
Fig. 4.
Heatmap showing the distribution of transformed relative COG category counts. The rows represent the genomes, the columns the COG categories. Both rows and columns were rearranged according to their overall (dis-)similarities as represented by the dendrograms on the left and upper side, respectively; for technical details see the opm manual [52].
Expectedly, the Sphaerochaeta genomes are impoverished with regard to category N (“Cell motility”). The genomes of the flagellated forms, however, also differ regarding their proportion of genes in this category. Hence, we calculated the correlation between this proportion and the average number of flagella reported for each species in the literature [55] (Fig. 5, left side). The correlation was high (0.917) and significant (p < 10-07). The number of flagella obviously has a historical component, with flagella lacking in one clade (Sphaerochaeta) and the number of flagella being particularly high in other clades (Borrelia, Brachyspira). To rule out a pseudocorrelation caused by common ancestry (see chapter in [56] for the background), we thus converted the data to phylogeny-independent contrasts using the CONTRASTS program available in the PHYLIP package [57] and the ML tree inferred from the 16S rRNAs from the genome sequences as the underlying phylogeny. The correlation between the contrasts was almost as high (0.818) and significant (p < 10-05) (Fig. 5, right side). Thus, Spirochaetes appear to rely on increasing their number of motility genes for increasing their number of flagella.
Fig. 5.
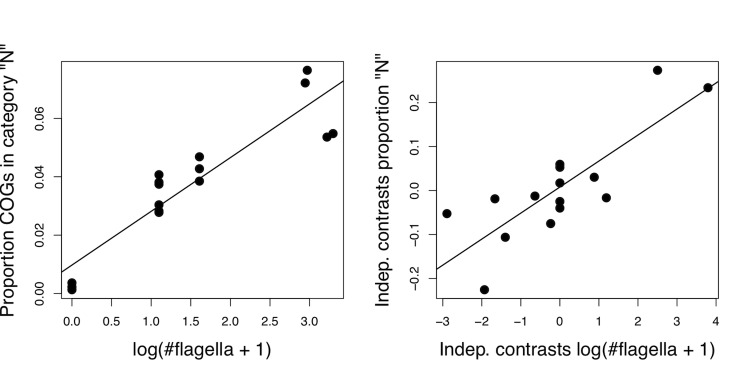
Scatter plots showing the relationship between the number of flagella and the proportion of genes in the COG category N (“Cell motility”). The left picture is based on the uncorrected data, whereas the right graph plots the phylogeny-independent contrast calculated from the numbers used in the left graph. The lines represent the corresponding linear models. For the magnitudes and significances of the correlations, see the main text.
Other relations to the life-style include the lower proportions of many COG categories in the reduced genomes of the Borrelia species, apparently as an adaptation to their lifestyle (the higher proportion of genes in category J is simply due to the absolute number of genes in this category being held constant during genome reduction; data not shown), which is considered by some as parasitic, and symbiotic by others. The coccoid forms have an increased proportion of genes in category G, related to carbohydrate transport and metabolism, but this seems not be directly linked to the loss of the typical spirochaete shape, as the Spirochaeta smaragdinae genome shows a similarly high proportion of G genes (Fig. 4) and in absolute terms has more genes in this category than S. coccoides (data not shown). The coccoid forms have fewer genes in the cell-wall related category M, but this also holds for S. smaragdinae.
Further, there seem to be more genes in the defense-related category V in the genomes of the host-associated but non-intracellular genera Brachyspira and Treponema, but there are exceptions to this rule, such as T. azotonutricium (Fig. 4). In contrast to the other genera, neither Spirochaeta nor Treponema appear as homogeneous genera the COG content of their genomes, even if one considers that S. caldaria might be better placed in Treponema (see below).
Taxonomic interpretation for S. caldaria and neighboring species in the family Spirochaetaceae according to 16S rRNA data
Based on physiological characteristics, the G+C content and the comparison of 16S rRNA sequences, strain H1T was classified into the genus Spirochaeta [1]. S. caldaria H1T is free living, saccharolytic, obligate anaerobe and possess the ultrastructural features typical of spirochetes. S. caldaria differs from all other Spirochaeta species, with respect to its thermophilic growth temperature, with the exception of Spirochaeta thermophila, which has a temperature optimum between 66 and 68°C [1]. In contrast to the mesophilic Spirochaeta species, S. caldaria does not produce ethanol as an end-product of D-glucose fermentation [1].
Based on a 16S rRNA sequence comparison, S. caldaria as well as Spirochaeta zuelzerae and Spirochaeta stenostrepta are more closely related to species of Treponema (Fig. 1). To rule out the possibility that the discrepancies between 16S rRNA data and taxonomic classification were not caused by either a mix-up or contamination of cultures, we cross-compared the 16S rRNA sequences deposited in INSDC for S. caldaria (EU580141 and M71240 in addition to the herein published whole genome sequence), S. stenostrepta (AB541984, FR733664, and M88724) and S. zuelzerae (FR749928, FR749929 and M88725), respectively. Besides poor sequence quality towards the ends of some sequence deposits, differences between accessions annotated as originating from the same species were not apparent.
The 16S rRNA data and the taxonomic classification of Spirochaetaceae are in significant conflict with each other. This problem has already been addressed in detail in one of the previous reports of the GEBA series [19]. The analysis shown in [19] used the classification as phylogenetic constraint, paired-site tests [56] to assess the significance of the differences between the resulting trees, and the ParaFit tests to determine the leaves of the trees that cause these differences [58]. One of the consequences of the earlier study was the assignment of Spirochaeta coccoides to Sphaerochaeta (compare Fig. 1 with Fig. 5 below). We here focus on our current target species, S. caldaria, and comparably problematic taxa.
Phylogenomic analyses
According to the results from 16S rRNA analysis (Fig. 1) a comparative analysis the genome sequences of Spirochaeta africana (GenBank CP003282) and Treponema primitia (GenBank CP001843) were performed. The genomes of the sequenced Spirochaeta species and T. primitia differ significantly in their size. Compared to the genome of T. primitia (4.1 Mb, 3,579 protein-coding genes) the genomes of S. caldaria (3.2 Mb, 2,928 protein coding genes), and S. africana (3.3 Mb, 3,874 protein-coding genes) are smaller in size.
An estimate of the overall similarity among S. caldaria, S. africana and T. primitia was computed with the Genome-to-Genome Distance Calculator (GGDC) [59,60]. This system calculates the distances by comparing the genomes to obtain HSPs (high-scoring segment pairs) and inferring distances from the set of formulas (1, HSP length / total length; 2, identities / HSP length; 3, identities / total length). Table 5 shows the results of the pairwise comparison.
Table 5. Pairwise comparison of S. caldaria with S. africana and T. primitia using the GGDC-Genome-to-Genome Distance Calculator.
| HSP length / total length [%] |
identities / HSP length [%] |
identities / total length [%] |
||
|---|---|---|---|---|
| S. caldaria | S. africana | 1.62 | 84.50 | 1.37 |
| S. caldaria | T. primitia | 6.04 | 81.92 | 4.95 |
| T. primitia | S. africana | 1.34 | 83.99 | 1.12 |
The comparison of S. caldaria with T. primitia yielded the highest scores, 6.04% of the average of genome length are covered with HSPs. The identity within the HSPs was 81.92%, whereas the identity over the whole genome was 4.95%. Lower similarity scores were observed in the comparison of S. caldaria with S. africana in which only 1.62% of the average of both genome lengths are covered with HSPs. The identity within these HSPs was 84.5%, whereas the identity over the whole genome was only 1.37%.
As expected, those distances relating HSP coverage (formula 1) and number of identical base pairs within HSPs to total genome length (formula 3) are higher between S. caldaria and T. primitia than between S. caldaria and S. africana. That the distances relating the number of identical base pairs to total HSP length (formula 2) behave differently indicates that the genomic similarities between S. caldaria and S. africana are limited to more conserved sequences, a kind of saturation phenomenon [59].
Amino-acid sequences from 16 Spirochaetaceae and outgroups (other Spirochaetes families) completed type-strain genomes were retrieved from INSDC and used in a phylogenomic analysis of the group, as described previously [19,61]. One of the previous taxonomic consequences for the genus Spirochaeta was the assignment of S. coccoides to the genus Sphaerochaeta [19]. Here the gene-content phylogeny from the previously conducted analyses is depicted together with the bootstrap support values from all four applied approaches (Fig. 6).
Figure 6.
Phylogenetic tree inferred from completely sequenced genomes of the Spirochaeta type strains. The tree was inferred from 11,131 gene-content characters under the maximum likelihood (ML) criterion and rooted with Leptospira. The branches are scaled in terms of the expected number of substitutions per site. Numbers above the branches are bootstrapping support values (if larger than 60%) from (i) maximum-likelihood gene-content analysis; (ii) maximum-parsimony gene-content analysis; (iii) maximum-likelihood supermatrix analysis; (iv) maximum-parsimony supermatrix analysis. For further details see [19].
All phylogenomic methods support the sister-group relationship of S. caldaria and two Treponema species, T. azotonutricium and T. primitia (88-100%). These methods corroborate the results of the 16S rRNA analysis that Treponema is paraphyletic. It was previously concluded that taxonomic revisions were necessary [19]. Here we revisit the definitions of Spirochaeta and Treponema and formally propose a number of revisions and emendations to solve these problems.
Phenotypic data and taxonomic interpretation
Table 6 presents an overview of key morphological and physiological features of S. caldaria, S. zuelzerae and S. stenostrepta compared with the genus descriptions of Spirochaeta and Treponema.
Table 6. Typical features of reference taxa compared to the three Spirochaeta species placed within Treponema.
| Spirochaeta caldaria [1] | Spirochaeta zuelzerae [62] | Spirochaeta stenostrepta [63] | Genus Spirochaeta [31,34-36,55] | Genus Treponema [55,64] | |
|---|---|---|---|---|---|
| Cell shape | helical | helical | helical | helical; spherical bodies under unfavorable growth conditions | helical; spherical bodies under unfavorable growth conditions |
| Pathogenicity | non pathogenic | non pathogenic | non pathogenic | non pathogenic | some species are pathogenic |
| Biotic relationship | free living | free living | free living | free living | primarily host-associated |
| Size [µm] | 0.2-0.3 by 15-45 | 0.2-0.35 by 8-16 | 0.2-0.3 by 15-45 | 0.2-0.75 by 5-250 | 0.1-0.7 by 5-20 |
| Motility | motile | motile | motile | motile | motile |
| Flagellation | flagella 1-2-1 | flagella 1-2-1 | 2 periplasmic flagella | 2 periplasmic flagella (exception: S. plicatillis, which has many flagella) | one or more periplasmic flagella |
| Relationship to O2 | obligately anaerobe | obligately anaerobe | obligately anaerobe | obligately anaerobe or facultatively anaerobe | obligately anaerobe or microaerophilic |
| Utilizes | carbohydrates, no amino acids |
carbohydrates | carbohydrates | a variety of carbohydrates, no amino acids |
carbohydrates or amino acids |
| Fermentation products | acetate, lactate, CO2, H2 | acetate, lactate, CO2, H2 | acetate, ethanol, CO2, H2, (lactate) | acetate, ethanol, CO2, H2 | |
|
G+C content [mol%] |
46 | 56 | 60 | 51-65 [35] 44-65 [34] |
37-54 |
The genus descriptions of Spirochaeta and Treponema evolved during the decades, and became less restrictive and differentiating. This makes a correct diagnosis of the genera within the family Spirochaetaceae difficult. In 2010, Leschine and Paster listed characteristics for the differentiation of the genus Spirochaeta from other genera of spirochetes [65]. In contrast to the genus Treponema, members of the genus Spirochaeta are free-living and cannot use amino acids as energy source. S. caldaria, S. zuelzerae and S. stenostrepta have both characteristics (Table 6), but based on 16S rRNA comparison these three Spirochaeta spp. are more closely related to species of Treponema [65]. The utilization of amino acids is not a restrictive criterion as some Treponema species also lack the ability to use amino acids as an energy source (T. bryantii [66], T. parvum [67], T. pectinovorum [68] and T. porcinum [69]). As a consequence of the existence of free-living species of Spirochaeta, which are more closely related to species of Treponema, Leschine and Paster suggest that “free-living“ vs. “host-associated” may not be a reliable taxonomic criterion to differentiate species of Spirochaeta and Treponema [65].
Spirochaeta zuelzerae was originally described by Veldkamp in 1960 [62] as “Treponema zuelzerae”. Based on existing classification key at the time [70], Veldkamp placed his spirochete, on the basis of its cell length to the Spirochaetaceae and in its serological similarity to the genus Treponema, into the family “Treponemaceae”. Canale-Parola et al. 1968 criticized the classification based on cell length, as the size can vary depending on the growth phase of the culture [25]. Because of the similarity between Veldkamp’s spirochete and other species of Spirochaeta Canale-Parola et al. (1968) suggested that T. zuelzerae should be included in the genus Spirochaeta, as Spirochaeta zuelzerae. Thus the name S. zuelzerae was revived and validly published [71].
Apparently the phenotypic definitions of both genera are vague and non-differential. The range of the features expressed as continuous numbers (cell size, GC content) numerically overlap, and the ranges of the other, discrete features logically overlap. Even the biotic relationships are expressed merely as a tendency, with Treponema assumed to be “primarily host-associated”; a criterion that has been questioned earlier [65]. S. stenostrepta and S. zuelzerae do not fit the description of Treponema, and only with regard to a single character, the GC content, which can hardly outweigh the phylogenetic evidence presented in Fig. 1, Fig. 6 and [19]. As far as this can be inferred from the distribution of relative COG counts (Fig. 4), genomic data make it unlikely that physiological characteristics can be found to differentiate between Spirochaeta and Treponema.
On the basis of the phylogenetic evidence presented above (Fig. 1, Fig. 6) and in [19], the reclassification of S. caldaria, S. stenostrepta and S. zuelzerae into the genus Treponema is proposed. This also makes emendation of the genus necessary, as the current description excludes a small number of features found in these three species. Our proposal is based on two principles, (i) that all taxa should be monophyletic (or, more precisely, no taxon should be demonstrably non-monophyletic) [39,42,72,73] and (ii) that as few taxonomic changes should be conducted as possible. The second principle rules out the alternative solution to merge both genera (which would then also make the inclusion of Sphaerochaeta and perhaps Borrelia necessary).
Emended description of the genus Treponema Schaudinn 1905 emend. Smibert 1974 (Approved Lists 1980)
The description of the genus Treponema is the one given by Norris et al. [74], with the following modification.
The GC content is between 37 and 60 mol%. The biotic relationship is either host associated or free living.
Description of Treponema caldaria (Pohlschroeder et al. 1994) Abt, Göker and Klenk, comb. nov.
Basonym: Spirochaeta caldaria Pohlschroeder et al. 1994.
The characteristics of the species are given in the species description by Pohlschroeder et al. 1994 [1].
The type strain is H1T (= DSM 7334 = ATCC 51460).
Description of Treponema stenostrepta (Zuelzer et al. 1912) Abt, Göker and Klenk, comb. nov.
Basonym: Spirochaeta stenostrepta (Zuelzer et al. 1912)
The characteristics of the species are given in the species description by Zuelzer et al. 1912 [63]. The type strain is Z1T (= DSM 2028 = ATCC 25083).
Description of Treponema zuelzerae (Canale-Parola 1980) Abt, Göker and Klenk, comb. nov.
Basonym: Spirochaeta zuelzerae (ex Veldkamp 1960) Canale-Parola 1980,
This species was originally described by Veldkamp 1960 as “Treponema zuelzerae” [62] but that name did not appear on the Approved Lists. The name was subsequently revived and validly published as Spirochaeta zuelzerae [25,71].
The characteristics of the species are given in the species description by Veldkamp 1960 [62]. The type strain is ATCC 19044 (= DSM 1903).
Acknowledgements
We would like to gratefully acknowledge the help of Maren Schröder for growing S. caldaria cultures and Evelyne-Marie Bramilla for DNA extraction and quality control (both at DSMZ). This work was performed under the auspices of the US Department of Energy Office of Science, Biological and Environmental Research Program, and by the University of California, Lawrence Berkeley National Laboratory under contract No. DE-AC02-05CH11231, Lawrence Livermore National Laboratory under Contract No. DE-AC52-07NA27344, and Los Alamos National Laboratory under contract No. DE-AC02-06NA25396, UT-Battelle and Oak Ridge National Laboratory under contract DE-AC05-00OR22725, as well as German Research Foundation (DFG) INST 599/1-2.
References
- 1.Pohlschroeder M, Leschine SB, Canale-Parola E. Spirochaeta caldaria sp. nov., a thermophilic bacterium that enhances cellulose degradation by Clostridium thermocellum. Arch Microbiol 1994; 161:17-24 10.1007/BF00248889 [DOI] [Google Scholar]
- 2.Validation List N. ° 55. Int J Syst Bacteriol 1995; 45:879-880 [Google Scholar]
- 3.Euzéby JP. List of bacterial names with standing in nomenclature: A folder available on the Internet. Int J Syst Bacteriol 1997; 47:590-592 10.1099/00207713-47-2-590 [DOI] [PubMed] [Google Scholar]
- 4.Garrity G. NamesforLife. BrowserTool takes expertise out of the database and puts it right in the browser. Microbiol Today 2010; 37:9 [Google Scholar]
- 5.Paster BJ, Dewhirst FE, Cooke SM, Fussing V, Poulsen LK, Breznak JA. Phylogeny of not-yet-cultures spirochaetes from termite guts. Appl Environ Microbiol 1996; 62:347-352 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Lilburn TG, Schmidt TM, Breznak JA. Phylogenetic diversity of termite gut spirochaetes. Environ Microbiol 1999; 1:331-345 10.1046/j.1462-2920.1999.00043.x [DOI] [PubMed] [Google Scholar]
- 7.Ohkuma M, Iida T, Kudo T. Phylogenetic relationships of spirochaetes in the gut of diverse termites. FEMS Microbiol Lett 1999; 181:123-129 10.1111/j.1574-6968.1999.tb08834.x [DOI] [PubMed] [Google Scholar]
- 8.Altschul SF, Gish W, Miller W, Myers EW, Lipman DJ. Basic local alignment search tool. J Mol Biol 1990; 215:403-410 [DOI] [PubMed] [Google Scholar]
- 9.Korf I, Yandell M, Bedell J. BLAST, O'Reilly, Sebastopol, 2003. [Google Scholar]
- 10.DeSantis TZ, Hugenholtz P, Larsen N, Rojas M, Brodie EL, Keller K, Huber T, Dalevi D, Hu P, Andersen GL. Greengenes, a Chimera-Checked 16S rRNA Gene Database and Workbench Compatible with ARB. Appl Environ Microbiol 2006; 72:5069-5072 10.1128/AEM.03006-05 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Porter MF. An algorithm for suffix stripping. Program: electronic library and information systems 1980; 14:130-137.
- 12.Lee C, Grasso C, Sharlow MF. Multiple sequence alignment using partial order graphs. Bioinformatics 2002; 18:452-464 10.1093/bioinformatics/18.3.452 [DOI] [PubMed] [Google Scholar]
- 13.Castresana J. Selection of conserved blocks from multiple alignments for their use in phylogenetic analysis. Mol Biol Evol 2000; 17:540-552 10.1093/oxfordjournals.molbev.a026334 [DOI] [PubMed] [Google Scholar]
- 14.Stamatakis A, Hoover P, Rougemont J. A rapid bootstrap algorithm for the RAxML web-servers. Syst Biol 2008; 57:758-771 10.1080/10635150802429642 [DOI] [PubMed] [Google Scholar]
- 15.Hess PN, De Moraes Russo CA. An empirical test of the midpoint rooting method. Biol J Linn Soc Lond 2007; 92:669-674 10.1111/j.1095-8312.2007.00864.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Pattengale ND, Alipour M, Bininda-Emonds ORP, Moret BME, Stamatakis A. How Many Bootstrap Replicates Are Necessary? Lect Notes Comput Sci 2009; 5541:184-200 10.1007/978-3-642-02008-7_13 [DOI] [Google Scholar]
- 17.Swofford DL. PAUP*: Phylogenetic Analysis Using Parsimony (*and Other Methods), Version 4.0 b10. Sinauer Associates, Sunderland, 2002. [Google Scholar]
- 18.Pagani I, Liolios K, Jansson J, Chen IM, Smirnova T, Nosrat B, Markowitz VM, Kyrpides NC. The Genomes OnLine Database (GOLD) v.4: status of genomic and metagenomic projects and their associated metadata. Nucleic Acids Res 2012; 40:D571-D579 10.1093/nar/gkr1100 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Abt B, Han C, Scheuner C, Lu M, Lapidus A, Nolan M, Lucas S, Hammon N, Deshpande S, Cheng JF, et al. Complete genome sequence of the termite hindgut bacterium Spirochaeta coccoides type strain (SPN1T), reclassification in the genus Sphaerochaeta as Sphaerochaeta coccoides comb. nov. and emendations of the family Spirochaetaceae and the genus Sphaerochaeta. Stand Genomic Sci 2012; 6:194-209 10.4056/sigs.2796069 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Fraser CM, Casjens S, Huang WM, Sutton GG, Clayton RA, Lathigra R, White O, Ketchum KA, Dodson R, Hickey EK, et al. Genomic sequence of a Lyme disease spirochaete, Borrelia burgdorferi. Nature 1997; 390:580-586 10.1038/37551 [DOI] [PubMed] [Google Scholar]
- 21.Mavromatis K, Yasawong M, Chertkov O, Lapidus A, Lucas S, Nolan M, Glavina Del Rio T, Tice H, Cheng JF, Pitluck S, et al. Complete genome sequence of Spirochaeta smaragdinae type strain (SEBR4228T). Stand Genomic Sci 2010; 3:136-144 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Han C, Gronow S, Teshima H, Lapidus A, Nolan M, Lucas S, Hammon N, Deshpande S, Cheng JF, Zeytun A, et al. Complete genome sequence of Treponema succinifaciens type strain (6091T). Stand Genomic Sci 2011; 4:361-370 10.4056/sigs.1984594 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Seshadri R, Myers GS, Tettelin H, Eisen JA, Heidelberg JF, Dodson RJ, Davidsen TM, DeBoy RT, Fouts DE, Haft DH, et al. Comparison of the genome of the oral pathogen Treponema denticola with other spirochete genomes. Proc Natl Acad Sci USA 2004; 101:5646-5651 10.1073/pnas.0307639101 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Mavromatis K, Ivanova NN, Chen IM, Szeto E, Markowitz VM, Kyrpides NC. The DOE-JGI Standard operating procedure for the annotations of microbial genomes. Stand Genomic Sci 2009; 1:63-67 10.4056/sigs.632 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Canale-Parola E, Udris Z, Mandel M. The classification of free-living spirochetes. Arch Mikrobiol 1968; 63:385-397 10.1007/BF00412124 [DOI] [PubMed] [Google Scholar]
- 26.Woese CR, Kandler O, Wheelis ML. Towards a natural system of organisms. Proposal for the domains Archaea and Bacteria. Proc Natl Acad Sci USA 1990; 87:4576-4579 10.1073/pnas.87.12.4576 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Garrity G, Holt JG. Phylum B17 Spirochaetes phy. nov. Garrity and Holt. In: Garrity GM, Boone DR, Castenholz RW (eds), Bergey's Manual of Systematic Bacteriology, Second Edition, Volume 1, Springer, New York, 2001, p. 138. [Google Scholar]
- 28.Judicial Commission of the International Committee on Systematics of Prokaryotes The nomenclatural types of the orders Acholeplasmatales, Halanaerobiales, Halobacteriales, Methanobacteriales, Methanococcales, Methanomicrobiales, Planctomycetales, Prochlorales, Sulfolobales, Thermococcales, Thermoproteales and Verrucomicrobiales are the genera Acholeplasma, Halanaerobium, Halobacterium, Methanobacterium, Methanococcus, Methanomicrobium, Planctomyces, Prochloron, Sulfolobus, Thermococcus, Thermoproteus and Verrucomicrobium, respectively. Opinion 79. Int J Syst Evol Microbiol 2005; 55:517-518 10.1099/ijs.0.63548-0 [DOI] [PubMed] [Google Scholar]
- 29.Ludwig W, Euzeby J, Whitman WG. Draft taxonomic outline of the Bacteroidetes, Planctomycetes, Chlamydiae, Spirochaetes, Fibrobacteres, Fusobacteria, Acidobacteria, Verrucomicrobia, Dictyoglomi, and Gemmatimonadetes http://www.bergeys.org/outlines/Bergeys_Vol_4_Outline.pdf Taxonomic Outline 2008.
- 30.Abt B, Han C, Scheuner C, Lu M, Lapidus A, Nolan M, Lucas S, Hammon N, Deshpande S, Cheng JF, et al. Complete genome sequence of the termite hindgut bacterium Spirochaeta coccoides type strain (SPN1), reclassification in the genus Sphaerochaeta as Sphaerochaeta coccoides comb. nov. and emendations of the family Spirochaetaceae and the genus Sphaerochaeta. Stand Genomic Sci 2012; 6:194-209 10.4056/sigs.2796069 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Skerman VBD, McGowan V, Sneath PHA. Approved Lists of Bacterial Names. Int J Syst Bacteriol 1980; 30:225-420 10.1099/00207713-30-1-225 [DOI] [PubMed] [Google Scholar]
- 32.Buchanan RE. Studies in the nomenclature and classification of bacteria. II. The primary subdivisions of the Schizomycetes. J Bacteriol 1917; 2:155-164 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Swellengrebel NH. Sur la cytologie comparée des spirochètes et des spirilles. [Paris]. Ann Inst Pasteur (Paris) 1907; 21:562-586 [Google Scholar]
- 34.Pikuta EV, Hoover RB, Bej AK, Marsic D, Whitman WB, Krader P. Spirochaeta dissipatitropha sp. nov., an alkaliphilic, obligately anaerobic bacterium, and emended description of the genus Spirochaeta Ehrenberg 1835. Int J Syst Evol Microbiol 2009; 59:1798-1804 [DOI] [PubMed] [Google Scholar]
- 35.Canale-Parola E. Genus I. Spirochaeta Ehrenberg 1835, 313. In: Buchanan RE, Gibbons NE (eds), Bergey's Manual of Determinative Bacteriology, Eighth Edition, The Williams and Wilkins Co., Baltimore, 1974, p. 168-171. [Google Scholar]
- 36.Ehrenberg CG. Dritter Beitrag zur Erkenntniss grosser Organisation in der Richtung des kleinsten Raumes. Abhandlungen der Preussischen Akademie der Wissenschaften (Berlin), 1835, p. 143-336. [Google Scholar]
- 37.BAuA. 2010, Classification of bacteria and archaea in risk groups. http://www.baua.de TRBA 466, p. 206.
- 38.Livermore BP, Russell CJ. Lipids of the Spirochaetales: Comparison of the lipids of several members of the genera Spirochaeta, Treponema, and Leptospira. J Bacteriol 1974; 120:1268-1273 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Klenk HP, Göker M. En route to a genome-based classification of Archaea and Bacteria? Syst Appl Microbiol 2010; 33:175-182 10.1016/j.syapm.2010.03.003 [DOI] [PubMed] [Google Scholar]
- 40.Wu D, Hugenholtz P, Mavromatis K, Pukall R, Dalin E, Ivanova NN, Kunin V, Goodwin L, Wu M, Tindall BJ, et al. A phylogeny-driven genomic encyclopaedia of Bacteria and Archaea. Nature 2009; 462:1056-1060 10.1038/nature08656 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Mavromatis K, Land ML, Brettin TS, Quest DJ, Copeland A, Clum A, Goodwin L, Woyke T, Lapidus A, Klenk HP, et al. The fast changing landscape of sequencing technologies and their impact on microbial genome assemblies and annotation. PLoS ONE 2012; 7:e48837 10.1371/journal.pone.0048837 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Farris JS. The information content of the phylogenetic system. Syst Zool 1979; 28:483-519 10.2307/2412562 [DOI] [Google Scholar]
- 43.Gemeinholzer B, Dröge G, Zetzsche H, Haszprunar G, Klenk HP, Güntsch A, Berendsohn WG, Wägele JW. The DNA Bank Network: the start from a German initiative. Biopreserv Biobank 2011; 9:51-55 10.1089/bio.2010.0029 [DOI] [PubMed] [Google Scholar]
- 44.http://www.jgi.doe.gov/
- 45.The Phred/Phrap/Consed software package. http://www.phrap.com
- 46.Zerbino DR, Birney E. Velvet: algorithms for de novo short read assembly using de Bruijn graphs. Genome Res 2008; 18:821-829 10.1101/gr.074492.107 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Lapidus A, LaButti K, Foster B, Lowry S, Trong S, Goltsman E. POLISHER: An effective tool for using ultra short reads in microbial genome assembly and finishing. AGBT, Marco Island, FL, 2008. [Google Scholar]
- 48.Hyatt D, Chen GL, LoCascio PF, Land ML, Larimer FW, Hauser LJ. Prodigal: prokaryotic gene recognition and translation initiation site identification. BMC Bioinformatics 2010; 11:119 10.1186/1471-2105-11-119 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Pati A, Ivanova NN, Mikhailova N, Ovchinnikova G, Hooper SD, Lykidis A, Kyrpides NC. GenePRIMP: a gene prediction improvement pipeline for prokaryotic genomes. Nat Methods 2010; 7:455-457 10.1038/nmeth.1457 [DOI] [PubMed] [Google Scholar]
- 50.Markowitz VM, Ivanova NN, Chen IMA, Chu K, Kyrpides NC. IMG ER: a system for microbial genome annotation expert review and curation. Bioinformatics 2009; 25:2271-2278 10.1093/bioinformatics/btp393 [DOI] [PubMed] [Google Scholar]
- 51.Tatusov RL, Koonin EV, Lipman DJ. A genomic perspective on protein families. Science 1997; 278:631-637 10.1126/science.278.5338.631 [DOI] [PubMed] [Google Scholar]
- 52.Vaas LAI, Sikorski J, Michael V, Göker M, Klenk HP. Visualization and curve-parameter estimation strategies for efficient exploration of Phenotype Microarray kinetics. PLoS ONE 2012; 7:e34846 10.1371/journal.pone.0034846 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.R Development Core Team. R: A language and environment for statistical computing. R Foundation for Statistical Computing, Vienna, Austria, 2001. [Google Scholar]
- 54.Sokal RR, Rohlf JS. Biometry. The principles and practice of statistics in biological research. W.H. Freeman and Company, San Francisco 1969. [Google Scholar]
- 55.Paster BJ. Phylum XV. Spirochaetes Garrity and Holt 2001. In: Krieg NR, Staley JT, Brown DR, Hedlund BP, Paster BJ, Ward NL, Ludwig W, Whitman WB (eds), Bergey’s Manual of Systematic Bacteriology, Second Edition, Volume 4, Springer, new York, 2010, p. 471. [Google Scholar]
- 56.Felsenstein J. Inferring phylogenies. Sinauer Associates Inc., Sunderland, Massachusetts 2004. [Google Scholar]
- 57.Felsenstein J. Phylogenies and the comparative method. Am Nat 1985; 125:1-12 10.1086/284325 [DOI] [Google Scholar]
- 58.Stamatakis A, Auch AF, Meier-Kolthoff J, Göker M. AxPcoords & parallel AxParafit: statistical co-phylogenetic analyses on thousands of taxa. BMC Bioinformatics 2007; 8:405 10.1186/1471-2105-8-405 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Auch AF, Von Jan M, Klenk HP, Göker M. Digital DNA-DNA hybridization for microbial species delineation by means of genome-to-genome sequence comparison. Stand Genomic Sci 2010; 2:117-134 10.4056/sigs.531120 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Auch AF, Klenk HP, Göker M. Standard operating procedure for calculating genome-to-genome distances based on high-scoring segment pairs. Stand Genomic Sci 2010; 2:142-148 10.4056/sigs.541628 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Anderson I, Scheuner C, Göker M, Mavromatis K, Hooper SD, Porat I, Klenk HP, Ivanova N, Kyrpides N. Novel Insights into the diversity of catabolic metabolism from ten haloarchaeal genomes. PLoS ONE 2011; 6:e20237 10.1371/journal.pone.0020237 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Veldkamp H. Isolation and characteristics of Treponema zuelzerae nov. spec., an anaerobic, free-living spirochete. Antonie van Leeuwenhoek 1960; 26:103-125 10.1007/BF02538999 [DOI] [PubMed] [Google Scholar]
- 63.Zuelzer M. Über Spirochaeta plicatilis Ehrenberg und deren Verwandtschaftsbeziehungen. Arch Protistenkd 1912; 24:1-59 [Google Scholar]
- 64.Schaudinn F. Dtsch Med Wochenschr 1905; 31:1728 10.1055/s-0029-1188418 [DOI] [Google Scholar]
- 65.Leschine S, Paster BJ. Genus I. Spirochaeta Ehrenberg 1835. In: Krieg NR, Staley JT, Brown DR, Hedlund BP, Paster BJ, Ward NL, Ludwig W, Whitman WB (eds), Bergey’s Manual of Systematic Bacteriology, Second Edition, Volume 4, Springer, New York, 2010, p. 473. [Google Scholar]
- 66.Stanton TB, Canale-Parola E. Treponema bryantii sp. nov., a rumen spirochete that interacts with cellulolytic bacteria. Arch Microbiol 1980; 127:145-156 10.1007/BF00428018 [DOI] [PubMed] [Google Scholar]
- 67.Wyss C, Dewhirst FE, Gmür R, Thurnherr T. Yi Xue, Schüpbach, Guggenheim B, Paster BJ. Treponema parvum sp. nov., a small, glucoronic or galacturonic acid-dependent oral spirochaete from lesions of human periodontitis and acute necrotizing ulcerative gingivitis. Int J Syst Evol Microbiol 2001; 51:955-962 10.1099/00207713-51-3-955 [DOI] [PubMed] [Google Scholar]
- 68.Smibert RM, Burmeister JA. Treponema pectinovorum sp. nov. isolated from humans with periodontitis. Int J Syst Bacteriol 1983; 33:852-856 10.1099/00207713-33-4-852 [DOI] [Google Scholar]
- 69.Nordhoff M, Taras D, Macha M, Tedin K, Busse HJ, Wieler LH. Treponema berlinense sp. nov. and Treponema porcinum sp. nov., novel spirochaetes isolated from porcine faeces. Int J Syst Evol Microbiol 2005; 55:1675-1680 10.1099/ijs.0.63388-0 [DOI] [PubMed] [Google Scholar]
- 70.Breed RS, Murray EGD, Smith NR. Bergey's manual of determinative bacteriology, 7th ed. Baltimore: Williams an Wilkins Co. 1957. [Google Scholar]
- 71.Canale-Parola E. Revival of the names Spirochaeta litoralis, Spirochaeta zuelzerae, and Spirochaeta auranti. Int J Syst Bacteriol 1980; 30:594 10.1099/00207713-30-3-594 [DOI] [Google Scholar]
- 72.Hennig W. Phylogenetic systematics. Annu Rev Entomol 1965; 10:97-116 10.1146/annurev.en.10.010165.000525 [DOI] [Google Scholar]
- 73.Wiley EO, Lieberman BS. Phylogenetics. Theory and practice of phylogenetic systematics, Second Edition. Wiley-Blackwell, Hoboken 2011. [Google Scholar]
- 74.Norris SJ, Paster BJ, Smibert RM. Genus IV. Treponema Schaudinn 1905. In: Krieg NR, Staley JT, Brown DR, Hedlund BP, Paster BJ, Ward NL, Ludwig W, Whitman WB (eds), Bergey’s Manual of Systematic Bacteriology, Second Edition, Volume 4, Springer, New York, 2010, p. 501. [Google Scholar]
- 75.Ashburner M, Ball CA, Blake JA, Botstein D, Butler H, Cherry JM, Davis AP, Dolinski K, Dwight SS, Eppig JT, et al. Gene Ontology: tool for the unification of biology. Nat Genet 2000; 25:25-29 10.1038/75556 [DOI] [PMC free article] [PubMed] [Google Scholar]