Abstract
C-type natriuretic peptide (CNP) is a 22-amino acid peptide and act as a local paracrine or autocrine regulator. There is growing evidence that CNP is involved in male reproductive processes. To investigate the role of CNP during spermatogenesis, we measured the mRNA expression of CNP and its specific membrane-bound natriuretic peptide receptor-B (NPR-B) using real-time RT-PCR in the testes of normal rats on different postnatal days. After that spermatogenesis dysfunction model induced by ornidazole was established with the aim to study the correlation of CNP with spermatogenic dysfunction. Then, Sertoli cells from 18- to 22-day-old healthy male rats were cultured in the presence of different CNP concentrations (1×10−6, 1×10−7 and 1×10−8 mol l−1), and the mRNA expression levels of androgen-binding protein, inhibin B and transferrin were examined at 0 min, 30 min, 1 h, 2 h, 4 h, 8 h, 12 h, 24 h and 48 h. During the postnatal development of rat testes, the highest mRNA expression levels of CNP and NPR-B were found at postnatal D0, and the levels then declined gradually, with a second CNP peak at postnatal D35. In the ornidazole-induced infertile rat testes, CNP gene expression was lower than in the uninduced rats (P<0.05), while NPR-B gene expression was greater (P<0.05). In cultured Sertoli cells, supplementation with CNP stimulated the gene expression of androgen-binding protein/inhibin B/transferrin, particularly at 12 h, and 1×10−7 mol l−1 CNP had the highest upregulation effect. The gene expression levels of CNP/NPR-B in rat testes at different postnatal stages and in infertile rat testes indicated that CNP may participate in the physiology and/or pathology related to spermatogenesis. Moreover, CNP regulated endocrine function in Sertoli cells. Taken together, these results showed that CNP is closely tied to spermatogenesis.
Keywords: C-type natriuretic peptide, natriuretic peptide receptor-B, Sertoli cell culture, spermatogenesis
Introduction
Natriuretic peptides belong to a family of small proteins that play a major role in the modulation of natriuresis, diuresis and vasodilatation. The natriuretic peptide family in mammals consists of three structurally related peptides: atrial natriuretic peptide (ANP), brain natriuretic peptide (BNP) and C-type natriuretic peptide (CNP). All three members contain the conserved sequence CFGXXXDRIXXXXGLGC, where X is any amino acid. The flanking cysteines form a 17-amino-acid disulphide-linked ring that is required for biological activity.1 Both ANP and BNP bind to natriuretic peptide receptor A (NPR-A) and are expressed in the heart and other organs. In contrast to ANP and BNP, which are mainly cardiovascular hormones, CNP is mainly produced by extracardiac tissues to act as a local paracrine or autocrine regulator.2 Its biological effects are mediated by intracellular cyclic GMP (cGMP) accumulation via specific membrane-bound natriuretic peptide receptor B (NPR-B) activation.3
There is growing evidence that CNP is involved in various reproductive processes.4 CNP−/− mice display dwarfism, and NPR-B−/− mice display dwarfism, female sterility and decreased adiposity.5, 6 In rats, uterine CNP levels are modulated by the oestrous cycle, with the highest expression during pro-oestrus.7 For instance, an intraperitoneal infusion of oestradiol increased uterine CNP in a dose-dependent fashion in ovariectomized mice.8 CNP concentrations in seminal plasma and seminal vesicle fluid were, respectively, 2000– and 100 000-fold greater than those found in the porcine brain. Seminal plasma (porcine) elevates cGMP in Balb/3T3 (235-fold), NIH/3T3 and Rat-2 cells, and may function during fertilisation.9 Nielsen et al.10 examined the tissue-specific expression of proCNP and CNP in extracts from 32 different porcine tissues, and the highest peptide concentrations were found in extracts from male reproductive tissues (such as the epididymis, seminal vesicles and prostate); CNP mRNA in the seminal vesicles and epididymis was 125-fold higher than in the other tissues examined.
Therefore, we hypothesize that CNP may play a key role in spermatogenesis. To further investigate the effect of CNP on spermatogenesis, the gene expression of CNP and NPR-B was examined by real-time quantitative RT-PCR in rat testes at different postnatal stages and in the testes of ornidazole-induced infertile rats; finally, CNP was added to Sertoli cell cultures, and its effects were observed on the endocrine function of Sertoli cells.
Materials and methods
Materials
The male Sprague–Dawley rats came from the Laboratory Animal Centre of Tongji Medical College (Wuhan, China). They were maintained under standard conditions (12 h light/12 h dark cycle; 35–60% relative humidity). Rat feed and tap water were available ad libitum.
Methods
Real-time RT-PCR
Total RNA was extracted from testes of rats at different postnatal ages (D0, D5, D10, D15, D21, D28, D35, D42 and D56). The day of birth was designated as postnatal D0. Two to four rats were sampled per stage. The experiment was repeated three times to estimate expression stability. Total RNA was extracted using Trizol reagent (Invitrogen, Breda, The Netherlands) according to the manufacturer's protocol. Briefly, approximately 50 mg of testes tissue was pipetted into 1 ml Trizol reagent. Total RNA was reverse transcribed into first-strand cDNA using a First Strand cDNA Synthesis Kit (Toyobo, Tokyo, Japan). Each RT reaction mixture contained 3 µg of total RNA, 1× RT buffer, 0.5 mmol L−1 of each dNTP, 1 µg of oligo d(T)20, 400 IU Moloney murine leukaemia virus reverse transcriptase, 40 IU RNasin and H2O to a final volume of 40 µl. The reaction mixture was incubated at 42 °C for 20 min and then at 99 °C for 5 min. Real-time RT-PCR was performed as previously described.11 The primer pairs used in RT-PCR are listed in Table 1. The amplified products were recovered from gels using a gel extraction kit. They were then used as templates for amplification of 103–108 copies to create a standard curve.
Table 1. Real-time RT-PCR primers.
| Gene | Forward primer | Reverse primer | Length (bp) |
|---|---|---|---|
| β-actin | 5′ TCC TCC CTG GAG AAG AGC TA 3′ | 5′ TCA GGA GGA GCA ATG ATC TTG 3′ | 302 |
| rCNP | 5′ CTG CTC GCG CTA CTC TCA CT 3′ | 5′ AAA GCA GCC TTT GGA CAA GC 3′ | 300 |
| rNPR | 5′ ACG ACC AGC TAA GGT TAC GCA 3′ | 5′ CAG GAG GTC CTT TTC GCT CTC 3′ | 282 |
| rABP | 5′ TCC GAT ACC ACC AAG CAC AAG 3′ | 5′ TCA GGA AAG CTG GGA ACA CTG 3′ | 260 |
| rINHB | 5′ CCA CTG GCT ACT ACG GGA ACT 3′ | 5′ CAC TCC TCC ACG ATC ATG TTG 3′ | 241 |
| rTRF | 5′ CCA AGC TCC AAA CCA TGT TGT 3′ | 5′ GCA GGC TTC TAG GAG TCG TGA 3′ | 283 |
Abbreviations: ABP, androgen-binding protein; CNP, C-type natriuretic peptide; INHB, inhibin B; NPR, natriuretic peptide receptor; TRF, transferrin.
Establishment of ornidazole-induced infertility in rats
Twenty adult male rats weighting 200–220 g were randomly divided into two groups. One group was treated with ornidazole (200 mg kg−1 d−1) dissolved in 0.5% carboxymethylcellulose solution, and the other group was only treated with 0.5% carboxymethylcellulose solution as control. All of the rats were treated by gastric gavage for 20 consecutive days. Treated rats were killed by cervical dislocation, and their testes were removed and decapsulated. Real-time RT-PCR was performed as described above.
Sertoli cell culture and treatment of Sertoli cells with CNP peptide
To assess the effect of CNP on Sertoli cells, CNP peptide (N8768; Sigma-Aldrich, St Louis, MO, USA) was added to Sertoli cell cultures. Rat Sertoli cells were isolated and cultured as previously described.12 Some Sertoli cell cultures were treated with different concentrations of CNP (10−6, 10−7 and 10−8 mol l−1), which were regarded as the experimental groups, and an untreated culture served as the control group. Each experiment was repeated three times. The cultured cells were cultured for 72 h before the CNP was added. Treated culture cells were harvested after 0 min, 30 min, 1 h, 2 h, 4 h, 8 h, 12 h, 24 h and 48 h. The mRNA levels of androgen-binding protein (ABP), inhibin B (INHB) and transferrin (TRF) in each sample were determined by real-time RT-PCR.
Statistical analysis
The expression level of target genes was determined by dividing the target gene expression level by β-actin expression level. The data were presented as mean±SD. Gene expression of CNP and NPR-B in rat testes at different postnatal stages and in the testes of ornidazole-induced infertile rats was evaluated by one-way ANOVA; gene expression of ABP, INHB and TRF following stimulation by CNP was analysed using general linear models—repeated measures ANOVA. Statistical analyses were performed using SPSS software version 11.5 (SPSS Inc., Chicago, IL, USA). Statistical significance was assumed at P<0.05.
Results
Specific amplification and standard curve
Melting curve analysis demonstrated that each of the PCR products amplified a single predominant product with a distinct melting temperature, as shown in Figure 1a. The predicted length of each product was confirmed by agarose gel electrophoresis, as shown in Figure 1b.
Figure 1.
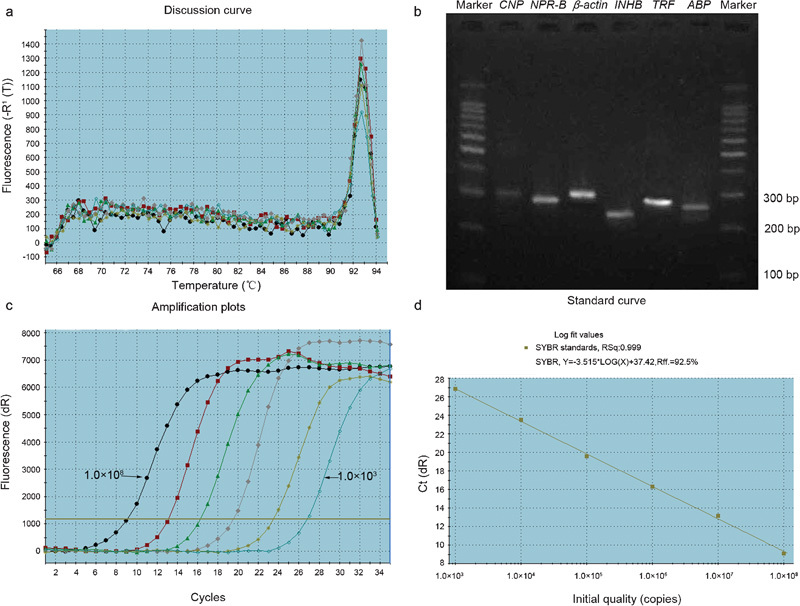
Specific amplification by real-time RT-PCR. (a) A melting curve analysis of a CNP amplification reaction showed a sharp, high peak of the melting temperature (92.8 °C), indicating the presence of a specific product that melts at this temperature; the differentially colored curve corresponds to the same colored curve in (c). (b) Agarose gel electrophoresis analysis demonstrates that this peak corresponds to a single band. (c) A plot of fluorescence from 103 to 108 copies of CNP. (d) A linear standard curve from 103 to 108 copies of CNP. ABP, androgen-binding protein; CNP, C-type natriuretic peptide; INHB, inhibin B; NPR B, natriuretic peptide receptor-B; TRF, transferrin.
The standard curves of CNP, NPR-B and β-actin exhibited a linear relationship from 103 to 108 copies, with a correlation coefficient >0.99, as shown in Figure 1c and d.
Gene expression of CNP and NPR-B in postnatal rat testes
The gene expression of CNP peaked at D0 and then declined gradually, reaching its lowest level on postnatal D21. Subsequently, its expression increased again at postnatal D28, reaching a second minor peak at postnatal D35, and declined until D42 (Figure 2a). The gene expression of NPR-B also peaked at the time point just after birth (D0) and then declined, reaching its lowest level at postnatal D42 (Figure 2b), which was similar to the expression pattern of CNP, except on postnatal D35.
Figure 2.
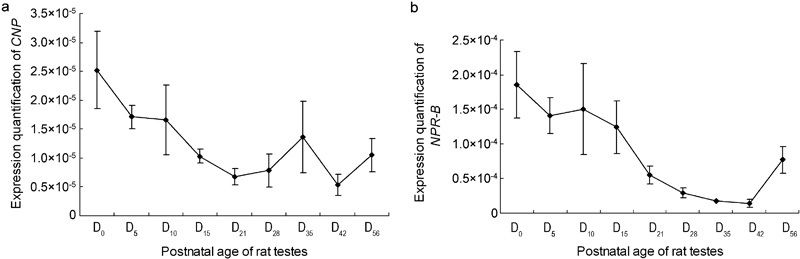
Gene expression of CNP/NPR-B in the testes of rats at postnatal stages. The results are expressed as mean±s.e.m. from three different experiments. (a) Gene expression of CNP in the testes of rats at postnatal stages. (b) Gene expression of NPR-B in the testes of rats at postnatal stages. CNP, C-type natriuretic peptide; NPR-B, natriuretic peptide receptor B.
Our analysis revealed that the correlation coefficient between CNP and NPR-B was 0.807, which showed that the gene expression of CNP and NPR-B during postnatal stages had a strong positive relationship.
Gene expression of CNP and NPR-B in rat testes subjected to ornidazole-induced infertility
The mRNA level of CNP in the testes of ornidazole-induced infertile rats was lower than that in the testes of uninduced rats (P=0.0401). However, the mRNA level of NPR-B was higher than that in the uninduced rat testes (P=0.0067), as shown in Table 2.
Table 2. Gene expression of CNP and NPR-B in the untreated group and ornidazole group (x̄±s.d., one-way ANOVA).
| CNP/β-actin (×10−6) | NPR-B/β-actin (×10−5) | |
|---|---|---|
| Untreated group (n=10) | 6.29±4.90 | 1.87±1.07 |
| Ornidazole group (n=10) | 2.75±1.19 | 3.16±1.06 |
| P value | 0.0401 | 0.0067 |
Abbreviations: CNP, C-type natriuretic peptide; NPR-B, natriuretic peptide receptor B.
CNP can stimulate the gene expression of ABP, INHB and TRF in cultured Sertoli cells
The gene expression levels of ABP, INHB and TRF were investigated by real-time RT-PCR quantification in cultured Sertoli cells after treatment with CNP. The amount of ABP mRNA significantly higher (P<0.05) than the control at the 8 h time point after 10−8 mol l−1 CNP was added (P<0.05, compared with the control group) and was significantly higher than the control (P<0.05) at 8 h, 12 h and 24 h after 10−7 mol l−1 CNP was added; when 10−6 mol l−1 CNP was present in the culture medium, the level of ABP mRNA was higher than the control (P<0.05) at 30 min, 1 h, 2 h, 4 h, 8 h and 12 h. Among all treatments, the ABP mRNA expression was most robust at the 12-h time point following 10−7 mol l−1 CNP treatment (Figure 3a). The amount of INHB mRNAs was higher than the control (P<0.05) at 8, 12, 24 and 48 h when 10−7 mol l−1 CNP was added and peaked at 12 h; there were no differences when either 10−8 or 10−6 mol l−1 CNP was added to the cell cultures (P>0.05, compared with the control group; Figure 3b). The level of TRF mRNA was higher than the control (P<0.05) at 8 and 12 h when 10−7 mol l−1 CNP was added and peaked at 12 h; when 10−6 mol l−1 CNP was added, the level of TRF mRNA was higher than the control (P<0.05) at 30 min, 1 h, 2 h, 4 h, 8 h, 12 h and 24 h (P<0.05, compared with the control group; Figure 3c). In summary, the gene expression levels of ABP, INHB and TRF in the CNP-treated Sertoli cells increased (P<0.05, compared with control group) and peaked after 12 h of treatment. Among the different concentrations examined, 10−7 mol l−1 CNP had the strongest effect on the expression of these three genes. These results imply that CNP could stimulate the endocrine function of cultured Sertoli cells.
Figure 3.
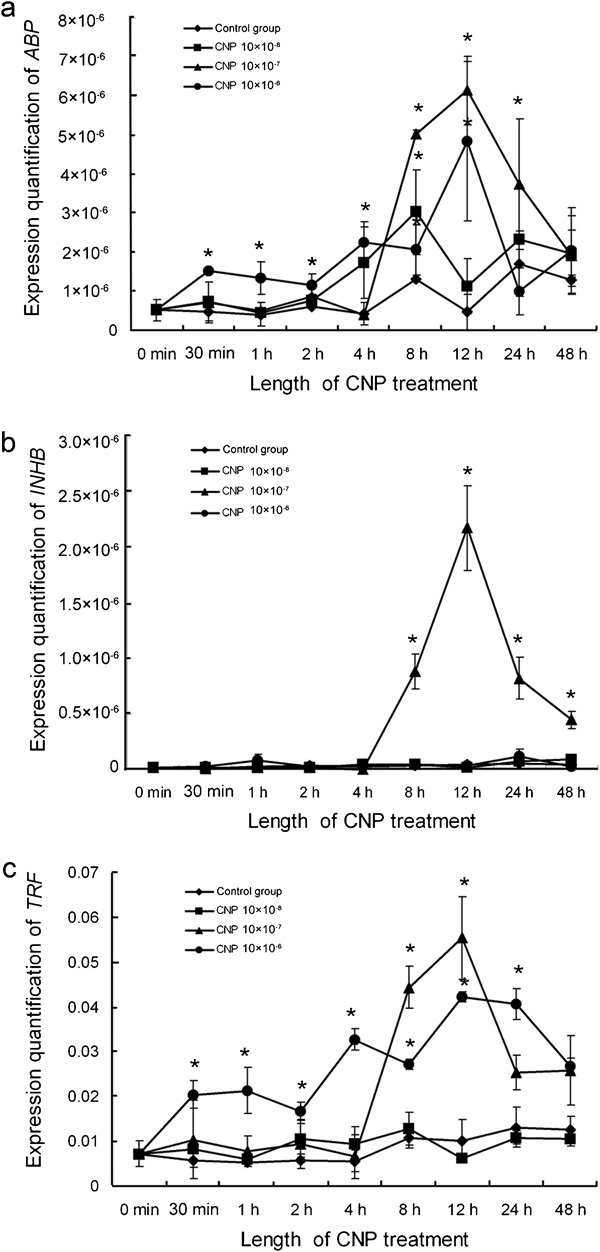
Gene expression of ABP, INHB and TRF in cultured Sertoli cells treated with CNP. (a) ABP gene expression in cultured Sertoli cells treated with CNP. (b) INHB gene expression in cultured Sertoli cells treated with CNP. (c) TRF gene expression in cultured Sertoli cells treated with CNP. The results are expressed as mean±s.e.m. from three different experiments. *P<0.05, compared with the control. ABP, androgen-binding protein; CNP, C-type natriuretic peptide; INHB, inhibin B; TRF, transferrin.
Discussion
Spermatogenesis is a complex differentiation process that creates functional sperm from an initially undifferentiated germ cell. The complete spermatogenesis cycle for rats requires 52–54 days. The process from birth to the first batch of mature spermatids released from the testes is called the first wave of spermatogenesis. In rats, there are only male germ cells (gonocytes) and Sertoli cells in the seminiferous tubules at postnatal D1. The first 3–5 days of postnatal life, when the gonocytes gradually proliferate and move towards the seminiferous tubule basal lamina, are crucial for the successful initiation of spermatogenesis.13 The first wave of meiotic and postmeiotic germ cell development in rats occurs after postnatal D15.14 Round, postmeiotic spermatid appear at postnatal D28 and transform into elongated spermatids at postnatal D40.15 With the release of the first mature spermatids from the testes around postnatal D44, the first wave of spermatogenesis is completed. CNP was originally isolated as a 22-amino acid peptide from the porcine brain16 and was later found in the testes. In this study, we showed that CNP and NPR-B exhibited similar gene expression patterns, except for postnatal D35, in the first wave of spermatogenesis. The CNP and NPR-B gene expression levels peaked at D0, which indicated that CNP and NPR-B have an intimate relationship with the onset of spermatogenesis. Among all of the different spermatogenic cells, the CNP mRNA level was greatest in the round sperm cells.17 In this study, the gene expression of CNP reached a second peak at postnatal D35, when the round sperm cells were abundant. The CNP mRNA level in mouse testes after birth exhibited a similar pattern to that in rat testes and was also found to increased at D20 when round sperm cells were abundant.17 These results suggest that CNP may be involved in spermiogenesis.
Mammalian spermatogenesis is a highly organized event under the tight control of both endocrine and paracrine factors. If this event is not properly organized, it will result in spermatogenic dysfunction. Spermatogenesis dysfunction models have been established with the aim to study the functions of those factors involved in spermatogenesis. Ornidazole is a nitroimidazole derivative after arilin and tinidazole. The antimicrobial activity of ornidazole is due to the reduction of a nitro group to a more reactive amine; this amine attacks microbial DNA, bringing about a loss of the helical structure of DNA, inducing DNA breakage, inhibiting further synthesis and ultimately causing the degradation of existing DNA. Ornidazole exerts a rapid and reversible antifertility effect in male rats.18 Ornidazole can reduce sperm motility, attenuate the function of spermatogenesis in testes and decrease sperm counts. However, it has no significant effect on the morphology of testes.19 One potential mechanism of ornidazole action in infertility models in rats is that spermatogenic cells may be damaged by the increased inhibition of malondialdehyde, while sperm motility may be decreased by inhibiting an energetic transferase or non-protein substance in the epididymis.19 In this study, CNP gene expression in ornidazole-treated rat testes was lower than in the untreated group, but the gene expression of NPR-B was higher than that in the untreated group. This result showed that the variation of CNP/NPR-B mRNA occurred earlier than the morphological differentiation of the seminiferous tubules and was accompanied by a decline of sperm motility in the ornidazole-induced infertile rat model. Therefore, one of the mechanisms through which ornidazole acts in the induction of infertility may be the up-/downregulation of CNP/NPR-B.
Spermatogenesis is controlled by gonadotrophins and testosterones. In males, luteinizing hormon acts on Leydig cells in the testes to produce testosterone, whereas follicle-stimulating hormone acts on the Sertoli cells to maintain their nursemaid function in spermatogenesis. A previous report showed that all three peptides could stimulate testosterone production in vivo, with significant effects at concentrations ≥1×10−8 mol l−1 of ANP, ≥1×10−9 mol l−1 of BNP and ≥1×10−6 mol l−1 of CNP.20 Pereira et al.21 found that ANP stimulated testosterone production in rat testis perfused in vitro but decreased luteinizing hormone-induced testosterone production, which seemed to involve the C receptor. As a local paracrine or autocrine regulator, CNP may be produced by most of the major endocrine glands, including the hypothalamus and the anterior pituitary. Immunocytochemical analysis demonstrated CNP immunoreactivity localized to gonadotroph cells.22 Gonadotrophin-releasing hormone neuronal cell lines also express both CNP and NPR-B.23 These data suggest a paracrine role for CNP in the regulation of gonadotrophin-releasing hormone-secreting hypothalamic neurons and gonadotrophin-releasing hormone-responsive pituitary cells and raise the possibility that CNP may influence the neuroendocrine control of reproduction.24 Importantly, CNP is also a peptide with a distinct role in male reproductive processes because both endocrine functions of the testes and penile erection are regulated by the CNP/NPR-B axis.25 Xia et al.26 reported that adding the synthetic CNP-22 peptide to Sertoli cell cultures perturbed Sertoli cell tight junctions in vitro, causing the disappearance of blood–testes barrier-associated proteins (JAM-A, occludin, N-cadherin and β-catenin) from the cell/cell interface. The blood–testes barrier created by adjacent Sertoli cells and several important proteins (including ABP, INHB and TRF) synthesized by the Sertoli cells, plays a key role in spermatogenesis.27 However, the effect of CNP on Sertoli cell endocrine function (such as effects on ABP, INHB and TRF) is not currently understood. In this study, CNP was used to stimulate Sertoli cells cultured in vitro, and our results showed that CNP could significantly stimulate Sertoli cells to express ABP, INHB and TRF, especially at a dose of 10−7 mol l−1. In conclusion, CNP regulates blood–testes barrier dynamics and also promotes the endocrine function of Sertoli cells.
The biological effects of CNP are mediated by intracellular cGMP accumulation. In the testes, cGMP signal transduction pathways are involved in a variety of local functions, based on autocrine or paracrine effects. In particular, cGMP may influence motility in the spermatozoa, the development of testicular germ cells, the relaxation of peritubular lamina propria cells, testosterone synthesis in the Leydig cells and the dilatation of testicular blood vessels.28 Thus, CNP has a close relationship with male reproductive processes and may be the key factor in spermatogenesis.
Author contributions
HDH and ZH designed the experiment. HDH performed all part of experiments, analysed data and wrote the manuscript; ZSW performed experiments on correlation of CNP with spermatogenic dysfunction, and ZL performed analyses of gene expression of ABP, INHB, and TRF.
Acknowledgments
This investigation was supported by a grant from the research fund of the Tongji Medical College, Huazhong University of Science and Technology (No. 25519004) and the National Key Technologies R&D Program for the Tenth Five-Year Plan, China (No. 2004BA720A33-1).
The authors declare no competing financial interests.
References
- Potter LR, Yoder AR, Flora DR, Antos LK, Dickey DM. Natriuretic peptides: their structures, receptors, physiologic functions and therapeutic applications. Handb Exp Pharmacol. 2009;191:341–66. doi: 10.1007/978-3-540-68964-5_15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Woodard GE, Rosado JA. Natriuretic peptides in vascular physiology and pathology. Int Rev Cell Mol Biol. 2008;268:59–93. doi: 10.1016/S1937-6448(08)00803-4. [DOI] [PubMed] [Google Scholar]
- Potter LR, Abbey-Hosch S, Dickey DM. Natriuretic peptides, their receptors, and cyclic guanosine monophosphate-dependent signaling functions. Endocr Rev. 2006;27:47–72. doi: 10.1210/er.2005-0014. [DOI] [PubMed] [Google Scholar]
- Walther T, Stepan H. C-type natriuretic peptide in reproduction, pregnancy and fetal development. J Endocrinol. 2004;180:17–22. doi: 10.1677/joe.0.1800017. [DOI] [PubMed] [Google Scholar]
- Chusho H, Tamura N, Ogawa Y, Yasoda A, Suda M, et al. Dwarfism and early death in mice lacking C-type natriuretic peptide. Proc Natl Acad Sci USA. 2001;98:4016–21. doi: 10.1073/pnas.071389098. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tamura N, Doolittle LK, Hammer RE, Shelton JM, Richardson JA, et al. Critical roles of the guanylyl cyclase B receptor in endochondral ossification and development of female reproductive organs. Proc Natl Acad Sci USA. 2004;101:17300–5. doi: 10.1073/pnas.0407894101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huang H, Acuff CG, Steinhelper ME. Isolation, mapping, and regulated expression of the gene encoding mouse C-type natriuretic peptide. Am J Physiol. 1996;271:H1565–75. doi: 10.1152/ajpheart.1996.271.4.H1565. [DOI] [PubMed] [Google Scholar]
- Acuff CG, Huang H, Steinhelper ME. Estradiol induces C-type natriuretic peptide gene expression in mouse uterus. Am J Physiol. 1997;273:H2672–7. doi: 10.1152/ajpheart.1997.273.6.H2672. [DOI] [PubMed] [Google Scholar]
- Chrisman TD, Schulz S, Potter LR, Garbers DL. Seminal plasma factors that cause large elevations in cellular cyclic GMP are C-type natriuretic peptides. J Biol Chem. 1993;268:3698–703. [PubMed] [Google Scholar]
- Nielsen SJ, Gøtze JP, Jensen HL, Rehfeld JF. ProCNP and CNP are expressed primarily in male genital organs. Regul Pept. 2008;146:204–12. doi: 10.1016/j.regpep.2007.09.022. [DOI] [PubMed] [Google Scholar]
- Huang DH, Zhao H, Li HG, Tian YH, Ding XF, et al. Gene expression changes of urokinase plasminogen activator and urokinase receptor in rat testes at postnatal stages. Asian J Androl. 2007;9:679–83. doi: 10.1111/j.1745-7262.2007.00272.x. [DOI] [PubMed] [Google Scholar]
- Huang DH, Zhao H, Tian YH, Xiong CL, Wang L. Isolation, purification and identification of Sertoli cells from rat testes. Acta Anatom Sin. 2007;28:246–9. [Google Scholar]
- Vigueras-Villasenor RM, Moreno-Mendoza NA, Reyes-Torres G, Molina-Ortiz D, Leon MC, et al. The effect of estrogen on testicular gonocyte maturation. Reprod Toxicol. 2006;22:513–20. doi: 10.1016/j.reprotox.2006.03.008. [DOI] [PubMed] [Google Scholar]
- Killian J, Pratis K, Clifton RJ, Stanton PG, Robertson DM, et al. 5alpha-reductase isoenzymes 1 and 2 in the rat testes during postnatal development. Biol Reprod. 2003;68:1711–8. doi: 10.1095/biolreprod.102.009142. [DOI] [PubMed] [Google Scholar]
- Jahnukainen K, Chrysis D, Hou M, Parvinen M, Eksborg S, et al. Increased apoptosis occurring during the first wave of spermatogenesis is stage-specific and primarily affects midpachytene spermatocytes in the rat testes. Biol Reprod. 2004;70:290–6. doi: 10.1095/biolreprod.103.018390. [DOI] [PubMed] [Google Scholar]
- Sudoh T, Minamino N, Kenji K, Matsuo H. C-type natriuretic peptide (CNP): a new member of natriuretic peptide family identified in porcine brain. Biochem Biophys Res Commun. 1990;168:863–70. doi: 10.1016/0006-291x(90)92401-k. [DOI] [PubMed] [Google Scholar]
- Ellis PJ, Furlong RA, Wilson A, Morris S, Carter D, et al. Modulation of the mouse testes transcriptome during postnatal development and in selected models of male infertility. Mol Hum Reprod. 2004;10:271–81. doi: 10.1093/molehr/gah043. [DOI] [PubMed] [Google Scholar]
- Ming Y, Shang XJ, Xiong CL, Pang XB, Xiong F.Urokinase-type plasminogen activator improves the reproductive function of male rats. Zhonghua Nan Ke Xue 200612963–7.Chinese. [PubMed] [Google Scholar]
- Pang XB, Zhu Y, Li HG, Zhou H, Zhu JW, et al. Effect of ornidazole on sperm in rats and its mechanism of action. Zhonghua Nan Ke Xue 20051126–28.Chinese. [PubMed] [Google Scholar]
- El-Gehani F, Tena-Sempere M, Ruskoaho H, Huhtaniemi I. Natriuretic peptides stimulate steroidogenesis in the fetal rat testes. Biol Reprod. 2001;65:595–600. doi: 10.1095/biolreprod65.2.595. [DOI] [PubMed] [Google Scholar]
- Pereira VM, Costa AP, Rosa-E-Silva AA, Vieira MA, Reis AM. Regulation of steroidogenesis by atrial natriuretic peptide (ANP) in the rat testis: differential involvement of GC-A and C receptors. Peptides. 2008;29:2024–32. doi: 10.1016/j.peptides.2008.08.005. [DOI] [PubMed] [Google Scholar]
- McArdle CA, Olcese J, Schmidt C, Poch A, Kratzmeier M, et al. C-type natriuretic peptide (CNP) in the pituitary: is CNP an autocrine regulator of gonadotropes. Endocrinology. 1994;135:2794–801. doi: 10.1210/endo.135.6.7988473. [DOI] [PubMed] [Google Scholar]
- Middendorff R, Paust HJ, Davidoff MS, Olcese J. Synthesis of C-type natriuretic peptide (CNP) by immortalized LHRH cells. J Neuroendocrinol. 1997;9:177–82. doi: 10.1046/j.1365-2826.1997.00563.x. [DOI] [PubMed] [Google Scholar]
- Fowkes RC, McArdle CA. C-type natriuretic peptide: an important neuroendocrine regulator. Trends Endocrinol Metab. 2000;11:333–8. doi: 10.1016/s1043-2760(00)00288-5. [DOI] [PubMed] [Google Scholar]
- Kuthe A, Reinecke M, Uckert S, Becker A, David I, et al. Expression of guanylyl cyclase B in the human corpus cavernosum penis and the possible involvement of its ligand C-type natriuretic polypeptide in the induction of penile erection. J Urol. 2003;169:1918–22. doi: 10.1097/01.ju.0000055602.35858.8f. [DOI] [PubMed] [Google Scholar]
- Xia W, Mruk DD, Cheng CY. C-type natriuretic peptide regulates blood–testes barrier dynamics in adult rat testes. Proc Natl Acad Sci USA. 2007;104:3841–6. doi: 10.1073/pnas.0610100104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yan HH, Mruk DD, Wong EW, Lee WM, Cheng CY. An autocrine axis in the testes that coordinates spermiation and blood–testes barrier restructuring during spermatogenesis. Proc Natl Acad Sci USA. 2008;105:8950–5. doi: 10.1073/pnas.0711264105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Middendorff R, Davidoff MS, Behrends S, Mewe M, Miethens A, et al. Multiple roles of the messenger molecule cGMP in testicular function. Andrologia. 2000;32:55–9. [PubMed] [Google Scholar]


