Abstract
Calcium-binding tyrosine phosphorylation-regulated protein (CABYR) is a highly polymorphic calcium-binding tyrosine- and serine-/threonine-phosphorylated fibrous sheath (FS) protein involved in capacitation. A putative domain (amino acids 12–48) homologous to the regulatory subunit of type II cAMP-dependent protein kinase A (RII) dimerisation and A kinase-anchoring protein (AKAP)-binding domains of protein kinase A at the N-terminus suggests that CABYR may self-assemble and bind to AKAPs. Moreover, there is evidence that CABYR has limited interaction with AKAPs. However, further evidence and new relationships between CABYR and other FS proteins, including AKAPs, will be helpful in understanding the basic physiology of FS. In this study, a new strategy for co-immunoprecipitation of insoluble proteins, as well as the standard co-immunoprecipitation method in combination with mass spectrometry and western blot, was employed to explore the relationship between CABYR, AKAP3 and Ropporin. The results showed that AKAP3 was co-immunoprecipitated with CABYR by the anti-CABYR-A polyclonal antibody, and, conversely, CABYR was also co-immunoprecipitated with AKAP3 by the anti-AKAP3 polyclonal antibody. Another RII-like domain containing protein, Ropporin, was also co-immunoprecipitated with CABYR, indicating that Ropporin is one of CABYR's binding partners. The interactions between CABYR, AKAP3 and Ropporin were confirmed by yeast two-hybrid assays. Further analysis showed that CABYR not only binds to AKAP3 by its RII domain but binds to Ropporin through other regions besides the RII-like domain. This is the first demonstration that CABYR variants form a complex not only with the scaffolding protein AKAP3 but also with another RII-like domain-containing protein in the human sperm FS.
Keywords: AKAP3, CABYR, fibrous sheath, Ropporin, sperm tail, spermatozoa, Western blotting
Introduction
The intact sperm flagellum has four distinct segments: the connecting piece adjacent to the head, the middle piece defined by a tightly packed helical array of mitochondria surrounding the cytoskeletal structures of the flagellum, the principal piece, which constitutes about three quarters of the length of the flagellum and is enclosed by the fibrous sheath (FS), and the short end piece.1 The FS is a unique cytoskeletal structure that underlies the plasma membrane, surrounds the axoneme and outer dense fibres, and defines the extent of the principal piece region of the sperm flagellum. It consists of two longitudinal columns connected by closely arrayed circumferential ribs. The traditional opinion about the FS is that it provides mechanical support for the sperm tail, influences the degree of flexibility by modulating flagellar bending, and defines the plane of flagellar motion and the shape of the flagellar beat.1, 2 However, proteins associated with the FS identified in recent studies indicate that it serves as a scaffold for both glycolytic enzymes and constituents of signalling cascades and plays a role in the regulation of sperm motility.1
More than 20 proteins that are located in or are closely related to the FS of mammalian spermatozoa have been reported. They include A kinase-anchoring protein 3 (AKAP3),3, 4 AKAP4,5, 6 testis-specific, developmentally regulated A-kinase-anchoring protein-80 (TAKAP-80),7 glyceraldehyde phosphate dehydrogenase-S (GAPDS),8, 9 type 1 hexokinase-S (HK1-S),10, 11, 12, 13 glycogen synthase kinase-3β (GSK-3β),14 isoform of aldolase 1 (ALDOA), lactate dehydrogenase-A (LDH-A),15 sperm flagellar energy carrier (SFEC), triose phosphate isomerase, glyceraldehyde 3-phosphate dehydrogenase (GAPDH), pyruvate kinase, lactate dehydrogenase-C (LDH-C), sorbitol dehydrogenase,16 glutathione S-transferase mu 5 (GSTM5),17 FS39,18 Ropporin, Rhophilin,19 sperm autoantigenic protein 17 (SP-17),20, 21, 22 phosphodiesterase 4A (PDE4A),23 fibrous sheath interacting protein 1 (FSIP1), fibrous sheath interacting protein 2 (FSIP2),6 AKAP-associated sperm protein (ASP),22 fibrous sheath calcium-binding tyrosine phosphorylation-regulated protein (CABYR)-binding protein (FSCB)24 and CABYR.25 Very little is known about how the proteins are assembled into the complex unique structure of the FS and how they bind to each other in the FS.
CABYR was initially found by the identification of calcium-binding and proteins phosphorylated by tyrosine kinases using two-dimensional (2D) gel analysis based on a proteomic strategy to identify targets at the intersection of the calcium and protein tyrosine kinase signal transduction pathways in human spermatozoa.25 It is a highly polymorphic, calcium-binding protein that is phosphorylated on tyrosine25 as well as serine or threonine26 during capacitation. Six splice variants of human CABYR have been reported and involve two coding regions, coding region A and B of CABYR gene (CR-A and CR-B). CABYR possesses putative motifs for self-assembly and for binding to AKAP.25 N-terminal amino acids 12–48 of human CABYR (accession no. AF088868) bear a 40% identity and 59% similarity to amino acids 8–44 of human regulatory subunit of type II alpha cAMP-dependent protein kinase A (RIIα). The N-terminal amino acids 12-44 of human Ropporin (accession no. XP_945818) bear a 33% identity and 61% similarity to amino acids 8-44 of human RIIα. Although some evidence has shown that CABYR and Ropporin bind AKAPs,22, 27, 28, 29 looking for further evidence and new interactions between CABYR and other FS proteins, including AKAPs and Ropporin, will be important for understanding the basic physiology of the FS.
Materials and methods
Antibodies and reagents
Human spermatozoa were obtained from student volunteers at the University of Virginia, Charlottesville, VA, USA. Rat polyclonal anti-human CABYR-A (the protein expressed by CR-A), anti-human CABYR-B (the protein expressed by CR-B), and anti-human AKAP serum were prepared by our research group at the University of Virginia, and their specificity has been demonstrated previously.4, 25, 30, 31 Horseradish peroxidase-conjugated goat anti-rat immunoglobulin G was purchased from Sigma-Aldrich (St Louis, MO, USA). An immunoprecipitation kit was purchased from Roche Applied Science (Indianapolis, IN, USA). IPG strips and Criterion 4–15% linear gradient gels were purchased from Bio-Rad (Hercules, CA, USA). Dialysis cassettes were purchased from Pierce (Rockford, IL, USA). Ampholines were purchased from Amersham Pharmacia Biotech (Piscataway, NJ, USA). Human testicular marathon-ready cDNA was purchased from BD Biosciences-Clontech (San Jose, CA, USA). The Matchmaker Gal4 Two-Hybrid System 3 was purchased from Clontech (Palo Alto, CA. USA).
Co-immunoprecipitation of human sperm proteins by polyclonal anti-CABYR-A or anti-AKAP3 serum
To optimize co-immunoprecipitation of CABYR and AKAP3 from human spermatozoa, two different methods were used: (i) method 1: semen specimens were obtained by masturbation from normal, healthy young men. Only ejaculates with normal values of semen parameters defined by WHO in 199232 were used in this study. Individual semen samples were allowed to liquefy at room temperature (normally for 1 h, range: 0.5–3 h). The motile spermatozoa were harvested by the swim-up method of Bronson and Fusi.33 Immunoprecipitations were performed on 2×108 spermatozoa and 20 µl of rat polyclonal anti-CABYR-A or pre-immune serum following the manufacturer's instructions. The protein G -agarose was pelleted at 12 000 g for 20 s at room temperature. Immune complexes were dissociated in 200 µl Celis buffer (9.8 mol l−1 urea, 2% (v/v) Nonidet P-40, 100 mmol l−1 dithiothreitol (DTT) with a protease inhibitor mixture (Roche Applied Science) at 4 °C for 20 min with gentle shaking and then separated by 2D gel electrophoresis, followed by silver staining or western blotting; (ii) method 2: this method was used for immunoprecipitations of less-soluble proteins than are possible using method 1. AKAP3 protein was found to be very insoluble and could not be well dissolved or immunoprecipitated by the lysis buffer above. Here, we used a novel modified immunoprecipitation strategy for insoluble or less-soluble proteins. Spermatozoa (8×108) were resuspended in 4 ml Celis buffer containing the complete protease inhibitor cocktail, but lacking DTT, and then incubated for 0.5–1 h at 4 °C on a rocking platform. The suspension was centrifuged at 4 °C, 12 000 g in a table-top microfuge for 10 min to remove debris. The supernatant was transferred to a dialysis cassette with 10-kDa cutoff and dialysed against 0.1× phosphate-buffered saline (PBS) (one-tenth strength) for 24 h at 4 °C with two changes of PBS. The dialysed suspension was centrifuged at 4 °C and 6000g in a table-top microfuge for 10 min to sediment the precipitated pellet. The suspension was transferred evenly to four 1.5-ml tubes, and immunoprecipitation was performed as described in method 1. The immunoprecipitate was then retrieved by eluting the agarose pellet with 200 µl Celis buffer or with 50 µl 2× Laemmli sample buffer. Protein G-agarose was then removed by centrifugation at 12 000 g for 20 s at 15–25 °C in a microfuge. The supernatant was transferred to a fresh tube for 2D gel electrophoresis.
2D Isoelectric focusing (IEF)–sodium dodecyl sulphate polyacrylamide gel electrophoresis (SDS-PAGE) of human sperm proteins
Human sperm proteins immunoprecipitated by different antibodies were applied as the first electrophoretic dimension after adding 2% (v/v) ampholines (pH 3.5–10). IEF was performed with a Protean IEF Cell (Bio-Rad). Nonlinear strips (11 cm, pH 3–10) were rehydrated at 50 V for at least 12 h at a sample loading volume of 200 µl. IEF was then performed using a linear ramp to 8000 V for a total of 30 000 Vh. The current was limited to 50 mA per strip, and the temperature was maintained at 20 °C. For SDS-PAGE, the IPG strips were incubated for 20 min in equilibration buffer containing 37.5 mmol l−1 Tris-HCl (pH 8.8), 6 mol l−1 urea, 4% (w/v) SDS, 20% (v/v) glycerol and 100 mmol l−1 DTT. Equilibrated IPG strips were then transferred for the second dimension SDS-PAGE onto Criterion 4–15% linear gradient gels. Electrophoresis was carried out at room temperature.
Immunoblotting
Proteins were transferred from unstained gels to polyvinylidine fluoride membranes with a Bio-Rad Trans Blot Electrophoretic Transfer Cell according to the manufacturer's instructions. Membranes were blocked with 5% (w/v) non-fat milk in PBS for 1 h at room temperature, washed three times with PBS–Tween (0.05% (v/v) Tween-20 in PBS), and then incubated overnight at 4 °C with 15 ml of the previously determined working dilution of rat pre-immune and immune sera (anti-CABYR-A serum in 1:3000; anti-AKAP3 serum in 1:2000). After being washed again, the membranes were incubated with horseradish peroxidase-conjugated goat anti-rat immunoglobulins (Sigma-Aldrich). The signal was detected by enhanced chemiluminescence (Amersham Pharmacia Biotech) or developed with 3,3′,5,5′-tetramethylbenzidine (Kirkegaard and Perry Labs, Gaithersburg, MD, USA). Then each experiment was performed for three additional times and each blot was probed with specific antibody or a control immunoglobulin G to immunoprecipitate sperm proteins for exactly the same time.
Tandem mass spectrometry peptide sequencing
Potential co-immunoprecipitating positive spots were cut out of the 2D gel and microsequenced by tandem mass spectrometry at the W.M. Keck Biomedical Mass Spectrometry Laboratory of the University of Virginia. Briefly, spots were digested in-gel by trypsin overnight at 37 °C before analysis. The data were analysed by comparison with a database, the Sequest search algorithm against the NCBI non-redundant database.
Yeast two-hybrid assay
Vectors, yeast and major reagents were supplied as part of the Matchmaker Gal4 Two-Hybrid System 3. All gene segments used were obtained by PCR using 0.2 ng cloned human testicular marathon-ready cDNA as the template. Detailed information with respect to the design of primers is shown in Table 1. The full-length open reading frame of CR-A (49-1527), the deletion construct of CR-A without the RII-like domain (199-1527) (TCR-A) and the full-length open reading frame of CR-B (1546-2142) were cloned into pGADT7. The full-length open-reading frame of AKAP3 (230-2788) and Ropporin (1-636) were cloned into pGBKT7 vectors using standard cloning procedures. The yeast strain AH109 was simultaneously cotransformed with two recombinant plasmids having different selection markers using LiAc-mediated yeast transformation, as described in the Yeast Protocols Handbook (PT3024-1; Clontech). In this two-hybrid system, GAL4 binding domain binds to the GAL upstream activating sequence, and if the fusion proteins interact, the activating domain is brought into proximity with the promoters of four reporter genes (HIS3, ADE2, MEL1 and lacZ), thereby activating transcription and permitting growth on selection medium (His– and Ade–) and the expression of α-galactosidase (MEL1 product) and β-galactosidase (lacZ product). Cotransformed yeast cells were isolated by growth on SD/–Leu/–Trp plates at 30 °C for 3 days. For high stringency or medium stringency selection, cells were then transferred to SD/–Ade/–His/–Leu/–Trp or SD/–His/–Leu/–Trp plates, supplemented with 20 g ml−1 X-Gal (5-bromo-4-chloro-3-indolyl-α-𝒹-galactopyranoside), and allowed to grow at 30 °C for 3–5 days to select for colonies that expressed interacting proteins.
Table 1. Construction of Gal4 fusions of human genes for yeast two-hybrid assay.
| Construction | Accession No. in database | Nucleotides | Primers used in PCR | Designed restrictive enzymes |
|---|---|---|---|---|
| pGADT7-CR-A | AF088868 | 49-1527 | 5′-CGGAATTCatgatttcttcaaagcccagac-3′ | EcoR I |
| 5′-CGGGATCCTTATTCAGCTGTTGATTCCCCT-3′ | BamH I | |||
| pGADT7-TCR-A | AF088868 | 199-1527 | 5′-CGGAATTCactactatggatataaaagatc-3′ | EcoR I |
| 5′-CGGGATCCTTATTCAGCTGTTGATTCCCCTT-3′ | BamH I | |||
| pGADT7-CR-B | AF088868 | 1546-2142 | 5′-CGGAATTCgcaatggcaacaagt gaacgagg-3′ | EcoR I |
| 5′-CCGCTCGAGTCAGTTTTCAGTTTCTGCTTTGC-3′ | Xho I | |||
| Pgbkt7-akap3 | NM_006422 | 230-2788 | 5′-ggaattccatatgtcagaaaaggttgactggtt-3′ | Nde I |
| 5′-acgcgtcgacTTACAGGTTCACCATCAGCCAGT-3′ | Sal I | |||
| pGBKT7-ropporin | AF303889 | 1-636 | 5′-catgccatggctcaga cagataagccaacat-3′ | Nco I |
| 5′-cggaattcCTCCAGCTGAACCCTGGGGT-3′ | EcoR I |
Quantitative α-galactosidase assay
SD/–Leu/–Trp or SD/–His/–Leu/–Trp cultures were inoculated with a single, fresh yeast colony and incubated overnight at 30 °C. The absorbance at 600 nm of culture solution was measured, and the supernatant was harvested by centrifugation at 14 000 g for 2 min. Supernatant of 40 µl was combined with 120 µl fresh assay buffer (2:1 ratio of 0.5 mol l−1 sodium acetate, pH 4.5, to 100 mmol l−1 p-nitrophenyl α-𝒹-galactopyranoside (Sigma-Aldrich) and incubated at 30 °C for 60 min. The reaction was stopped by adding 840 µl 0.1 mol l−1 Na2CO3. The absorbance was measured at 410 nm in a 1.5-ml cuvette, and α-galactosidase units were calculated with the following formula: [milliunits/(ml×cell)]=A410×1000 µl×1000/[16.9 (ml/µmol)×60 min×40 µl×A600] (Yeast Protocols Handbook). Each yeast colony was assayed in triplicate.
Results
Identification of CABYR binding to AKAP3 in human spermatozoa by co-immunoprecipitation
To determine the location of AKAP3 on the 2D gels, the gels of human sperm Celis extracts were probed with AKAP3 polyclonal antibody (Figure 1a) or treated with pre-immune serum (Figure 1b). These located AKAP3 at ∼110 kDa. Next, the 2D gels of human sperm extracts immunoprecipitated with method 2 by anti-CABYR-A polyclonal antibody (Figure 1c) or pre-immune serum (Figure 1d) were probed with anti-AKAP3 polyclonal antibody. An AKAP3-positive spot indicated by the arrow (Figure 1c) was only present in the human sperm immunoprecipitate by the anti-CABYR-A polyclonal antibody but not in that precipitated with pre-immune serum (Figure 1d). Similarly, the 2D gels of human sperm proteins immunoprecipitated by the anti-CABYR-B polyclonal antibody (Figure 1e), but not the pre-immune serum (Figure 1f), produced an AKAP3-positive spot at the same location. To verify these co-immunoprecipitation results, the role of antibodies as precipitating or labelling was reversed using method 2. The 2D gels of human sperm proteins immunoprecipitated by anti-AKAP3 polyclonal antibody (Figure 2a) or pre-immune serum (Figure 2b) were probed by anti-CABYR-A polyclonal antibody and showed a CABYR-A-positive spot indicated by the arrow in Figure 2a located at the position of the 86-kDa CABYR-A variant. Additionally, the 2D gels of human sperm extracts immunoprecipitated with the anti-AKAP3 polyclonal antibody (Figure 2c) or pre-immune serum (Figure 2d) probed by the anti-CABYR-B polyclonal antibody also showed a string of CABYR-B positive spots indicated by the arrow in Figure 2c.
Figure 1.

AKAP3 was immunoprecipitated with CABYR by anti-CABYR-A or anti-CABYR-B antibody. (a, b) 2D gels of human sperm extracts probed by anti-AKAP3 polyclonal antibody (a) or pre-immune serum (b). The spot located at 110 kDa with pI 7.0 is the AKAP3. (c, d) 2D gels of human sperm immunoprecipitated by anti-CABYR-A polyclonal antibody (c) or pre-immune serum (d) probed by the anti-AKAP3 polyclonal antibody. The spot indicated by the arrow (c) is the co-immunoprecipitated AKAP3. (e, f) 2D Western blots of human sperm extracts immunoprecipitated by anti-CABYR-B polyclonal antibody (e) or pre-immune serum (f) probed by the anti-AKAP3 polyclonal antibody. The spot indicated by an arrow (e) is the co-immunoprecipitated AKAP3. AKAP, A kinase-anchoring protein; CABYR, calcium-binding tyrosine phosphorylation-regulated protein; 2D, two-dimensional; MW, molecular weight; pI, isoelectric point.
Figure 2.
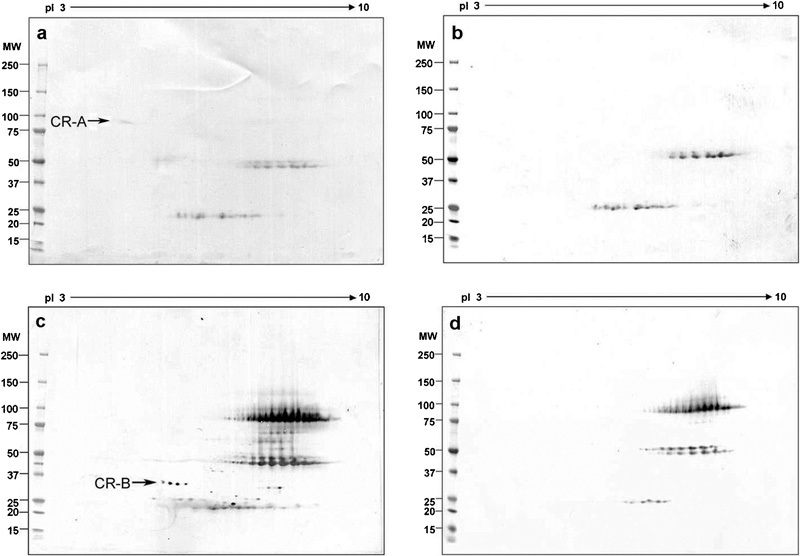
The 86-kDa CABYR-A-only variant and CABYR-B-containing variants were co-immunoprecipitated with AKAP3 by anti-AKAP3 antibody. (a, b) 2D gels of human sperm extracts immunoprecipitated with anti-AKAP3 polyclonal antibody (a) and pre-immune serum (b) probed by the anti-CABYR-A polyclonal antibody. The spot indicated by an arrow (a) is the co-immunoprecipitated 86-kDa CABYR-A-only variant. (c, d) 2D gels of human sperm extracts immunoprecipitated with anti-AKAP3 polyclonal antibody (c) and pre-immune serum (d) probed by the anti-CABYR-B polyclonal antibody. The spot indicated by an arrow (c) is the co-immunoprecipitated CABYR-B-containing variant. AKAP, A kinase-anchoring protein; CABYR, calcium-binding tyrosine phosphorylation-regulated protein; MW, molecular weight; pI, isoelectric point.
Identification of CABYR binding with Ropporin
CABYR is a soluble protein. It can be partly dissolved in gentle detergents such as NP-40, Triton-X 100 and Tween 20, though the majority of CABYR is present in the insoluble fraction (data not shown). Therefore, the regular standard co-immunoprecipitation with the gentle detergent was also employed to identify the potential binding partners of CABYR. Here the strategy is to examine silver-stained gels of human sperm extracts immunoprecipitated by the anti-CABYR-A polyclonal antibody (Figure 3a) and pre-immune sera (Figure 3b) and to identify the unique spots identified in the gel by anti-CABYR-A polyclonal antibody by microsequencing in order to identify the potential binding proteins of CABYR. The candidate spots indicated by the arrows (Figure 3a) were removed for mass spectrometric analysis. The peptide sequences and corresponding proteins are shown in Table 2.
Figure 3.
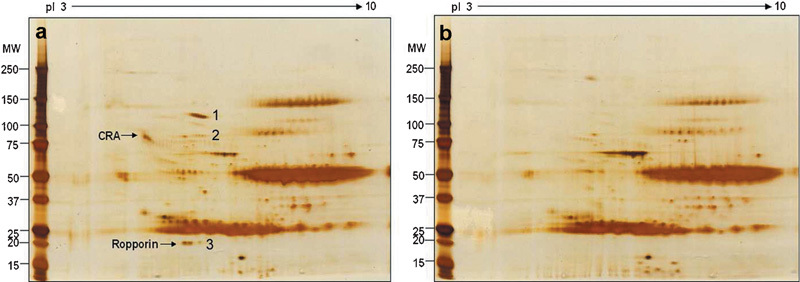
2D silver stain gels of human sperm extracts immunoprecipitated by rat anti-CABYR-A polyclonal antibody (a) and pre-immune serum (b). Three potential positive spots were removed from gels and microsequenced by mass spectrometry. Spot 1 is inter-alpha-inhibitor H4 heavy chain [Rattus norvegicus] (accession no. AAH89806.1); it should come from the rat antiserum; spot 2 contains inter-alpha-inhibitor H4 heavy chain [Rattus norvegicus] and HSP90AA1 protein (accession no. AAH23006.2) sequences; Spot 3 is human Ropporin (accession no. AAG27712.2.). The CABYR-A spot is indicated by the upper arrow. CABYR, calcium-binding tyrosine phosphorylation-regulated protein; 2D, two-dimensional; HSP90, heat-shock protein 90; MW, molecular weight; pI, isoelectric point.
Table 2. Peptide sequences' corresponding proteins found by mass spectrometry.
| No. of spots | Accession no. in database | Name of the proteins | Source of species | MS peptides |
|---|---|---|---|---|
| 1 | AAH89806.1 | Inter-alpha-inhibitor H4 heavy chain | Rattus norvegicus | FAHTVVTSR |
| ADTVQEATFQVELPR | ||||
| GESAGLVK | ||||
| KTEQFEVSVNVAPGSK | ||||
| RLGMYELLLKVRPEQLVK | ||||
| AHIQFKPTLSQQRKSQNEQDTVLDGDFTVR | ||||
| NVLFVIDK | ||||
| EALIK | ||||
| AVDYASKIPAQGGTNINKAVLSAVELLDKSNQAELLPSK | ||||
| LALDNGGLAR | ||||
| YNFQHHFKGSEMVVAGKLRDQGPDVLLAK | ||||
| EFQGPKYIFHNFMERLWALLTIQQQLEQR | ||||
| YNFVTPLTHMVVTK | ||||
| LLTSR | ||||
| LGDGLVGSRQYMPPPGLPGPPGLPGPPGPPGHPHFASSIDYGR | ||||
| VVEQEGTTPEESPNPDHPRAPTIILPLPGSGVDQLCVDILHSEKPMKLFVDINQGLEVVGK | ||||
| KTLFSVLPGLK | ||||
| TGLLQLSGPDKVTISLLSLDDPQR | ||||
| VLGIDYPATR | ||||
| LSYQDGFPGTEISCWTVK | ||||
| 2 | AAH23006.2 | HSP90AA1 protein | Homo sapiens | ADLINNLGTIAK |
| EDQTEYLEER | ||||
| YIDQEELNK | ||||
| NPDDITNEEYGEFYK | ||||
| HFSVEGQLEFRALLFVPR | ||||
| HIYYITGETKDQVANSAFVER | ||||
| AAH89806.1 | Inter-alpha-inhibitor H4 heavy chain | Rattus norvegicus | FAHTVVTSR | |
| ADTVQEATFQVELPR | ||||
| QYTAAVGRGESAGLVK | ||||
| KTEQFEVSVNVAPGSK | ||||
| RLGMYEL | ||||
| LLKVRPEQLVK | ||||
| AHIQFKPTLSQQRKSQNEQDTVLDGDFTVR | ||||
| NVLFVIDK | ||||
| EALIK | ||||
| IPAQGGTNINKAVLSAVELLDKSNQAELLPSK | ||||
| LALDNGGLAR | ||||
| YNFQHHFKGSEMVVAGKLRDQGPDVLLAK | ||||
| YIFHNFMER | ||||
| LGDGLVGSRQYMPPPGLPGPPGLPGPPGPPGHPHFASSIDYGR | ||||
| 3 | AAG27712.2 or NP_060048.2 | Ropporin | Homo sapiens | AELTPELLKILHSQVAGR |
| AEELAQMWKVVNLPTDLFNSVMNVGRFTEEIEWLK | ||||
| IPFSTFQFLYTYIAKVDGEISASHVSR |
Abbreviation: MS, mass spectrometry.
Further analysis of these mass spectrometric results indicated that the origin of the inter-alpha-inhibitor H4 heavy chain is the rat, and careful observation of the silver-stained gel showed that spot 1 was also found (faintly) in the pre-immune control. Ropporin and HSP90AA1 protein are the most likely candidates to be the binding partners of CABYR. Compared with HSP90AA1 of spot 2, Ropporin of spot 3 is abundant, clear and reproducible. These experiments were performed at least three times. The silver stain gel shown here is a representative example.
Verification of CABYR binding to AKAP3 and Ropporin in human spermatozoa by the yeast two-hybrid system
On the basis of the potential functional domains in CABYR,25 three different CABYR gene segments were used for the construction of recombinant pGADT7 to verify the interaction between CABYR and AKAP3 or Ropporin and to further examine the role of the RII-like domain of CABYR in binding to AKAP3 or Ropporin (Figure 4). Full-length open reading frames of AKAP3 and Ropporin were used for the construction of recombinant pGBKT7. The ability of the cotransformants to grow on selective SD/–Leu/–Trp plates (Figure 5a and c) and SD/–Ade/–His/–Leu/–Trp plates (Figure 5b) or on SD/–His/–Leu/–Trp plates (Figure 5d) was analysed. The results showed that transformants with CR-A+AKAP3 grew normally in selective SD/–Ade/–His/–Leu/–Trp medium, as did the positive control. More importantly, when X-α-gal was spread on top of the medium, the transformants gradually became blue just like the positive control after 3–5 days in culture at 30 °C (Figure 5b1). This result indicated that all reporter genes were activated by the interaction between CABYR-A and AKAP3. By contrast, the transformant with TCR-A+AKAP3 (Figure 5b2) could not grow in this medium. The transformants with CR-B+AKAP3 (Figure 5b3) also grew in the selective SD/–Ade/–His/–Leu/–Trp medium, but their growth was slower, and the cells were more dispersed. This cotransformant also turned a blue colour. The colour was not as intense as that of the positive control, suggesting that there is a relatively weak interaction between CABYR-B and AKAP3 compared with that of CABYR-A and AKAP3. No cotransformants with CR-A+Ropporin, TCR-A+Ropporin or CR-B+Ropporin could grow in high-stringency SD/–Ade/–His/–Leu/–Trp medium, but the first two could grow in medium-stringency SD/–His/–Leu/–Trp (Figure 5d1 and 2) medium, as could the positive control (Figure 5d4). However, the transformant with CR-B+Ropporin (Figure 5d3) did not grow in the same way as the negative control (Figure 5d5). These results suggest that there is a small and weak interaction between CABYR-A and Ropporin as well as between TCABYR-A and Ropporin, but not between CABYR-B and Ropporin. Unlike in the interaction between CABYR-A and AKAP3, the deletion of the CR-A RII domain did have an effect on, but did not totally abolish, the interaction between CABYR-A and Ropporin.
Figure 4.
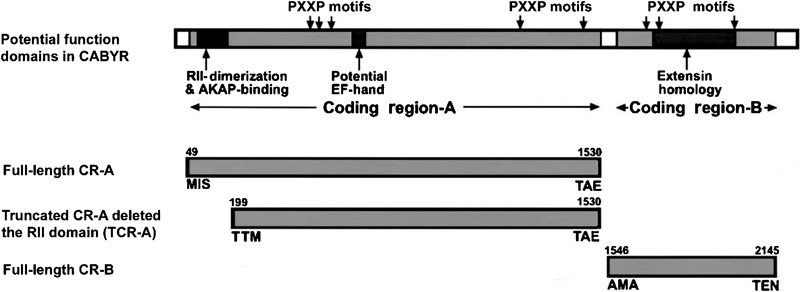
A schematic map of CABYR adopted with permission from Naaby-Hansen et al.25 illustrates the positions of different hypothetical domains and the CABYR gene segments used for the construction of transformants. The numbers refer to the aa positions. aa, amino acid; CABYR, calcium-binding tyrosine phosphorylation-regulated protein; CR, coding region.
Figure 5.
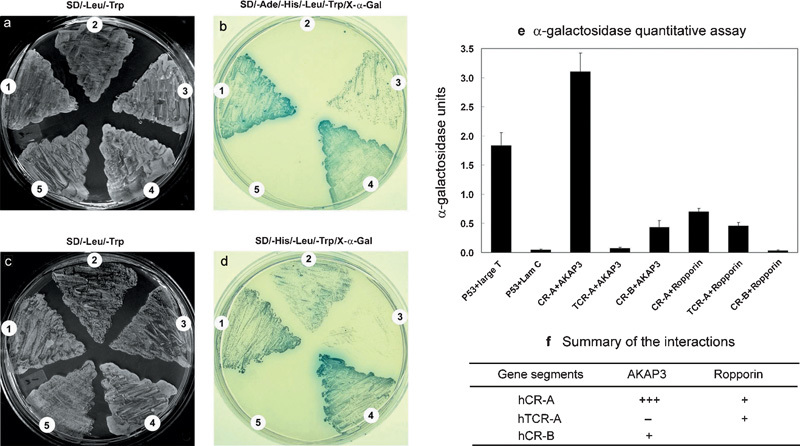
Verification of the interaction between CABYR and AKAP3 or Ropporin by the yeast two-hybrid assay. The yeast strain AH109 was cotransformed with pGADT7-hCR-A, pGADT7-hTCR-A or pGADT7-hCR-B and pGBKT7-AKAP3, pGBKT7-Ropporin (cotransformed with pGADT7-T and pGBKT7-53 as a positive control; pGADT7-T and pGBKT7-Lam as a negative control). The abilities of the transformants to grow on selective SD/–Leu/–Trp plates (a, c), SD/–Ade/–His/–Leu/–Trp plates (b) or SD/–His/–Leu/–Trp plates (d) were analysed. (a, b) The analysis of the interaction between CABYR and AKAP3: 1. CR-A+AKAP3; 2. TCR-A+AKAP3; 3. CR-B+AKAP3; 4. p53+Large T (positive control); 5. p53+Lam C (negative control). (c, d) The analysis of the interaction between CABYR and Ropporin: 1. CR-A +Ropporin; 2. TCR-A+Ropporin; 3. CR-B+Ropporin; 4. p53+Large T (positive control); 5. p53+Lam C (negative control). (e) α-galactosidase quantitative data are plotted with error bars corresponding to three separate experiments. The α-galactosidase activity of the transformant bearing CR-A+AKAP3 is much higher than that the transformant bearing the P53+SV40 large T. TCR-A+AKAP3 has very low α-galactosidase activity similar to that of p53+Lam C. (f) A summary of the interaction between CABYR and AKAP3 or Ropporin. AKAP, A kinase-anchoring protein; CABYR, calcium-binding tyrosine phosphorylation-regulated protein; CR, coding region; TCR, truncated coding region.
The quantitative α-galactosidase assay also confirmed the findings above. The α-galactosidase activity of the transformant bearing CR-A+AKAP3 was higher than that of P53+SV40 large T (positive control). By contrast, the transformant bearing TCR-A+AKAP3, in which the TCR-A construct deleted the RII-like domain, had very low activity similar to that of the P53+Lam C (negative control). These results strongly indicate that the interaction between CABYR-A and AKAP3 is mediated by the RII-like domain. The transformants bearing CR-B+AKAP3, CR-A+Ropporin and TCR-A+Ropporin exhibit distinctly lower activities than those of the positive control but clearly higher activities than those of the negative control, supporting the finding above that there is a relatively weak interaction between CABYR-B and AKAP3 and between CABYR-A and Ropporin or between TCABYR-A and Ropporin. Because the transformant bearing TCR-A+Ropporin had a much lower activity than did the one bearing CR-A+Ropporin, the weak interaction between CABYR-A and Ropporin may be mediated not only by the RII domain but also by some other domain. The transformant bearing CR-B+Ropporin had very low α-galactosidase activity, similar to that of P53+Lam C (negative control) (Figure 5e). The data from the quantitative α-galactosidase assay here are consistent with the findings from the growth assay of the transformants on selective medium. The interaction between CABYR and AKAP3 or Ropporin is summarized in Figure 5f.
Discussion
The interaction between CABYR and AKAP3
Previous bioinformatic analysis of CABYR suggests that the domain in CABYR-A of CABYR, like that of Ropporin and sperm autoantigenic protein 17, shares a strong sequence similarity with the conserved RIIα domain of protein kinase A.25 The binding experiments cannot be performed with native AKAP3 because this protein is highly insoluble.28 The difficulty of identifying the protein–protein interaction in the FS by co-immunoprecipitation lay in the impossibility of dissolving any of the related proteins using the regular lysis buffer. Most of the intrinsic FS proteins, including AKAPs, are rather insoluble proteins.1, 34 So far, most studies of the interactions between AKAPs and their binding partners have used recombinant transfected cells, such as COS-7,23 HEK293,35 bacteria12, 36 or the yeast two-hybrid system.6, 22, 35 Early in the present study, the standard co-immunoprecipitation method was tried but was revealed not to resolve AKAPs or make them immunoprecipitate (data not shown). To dissolve all proteins in sufficient amounts and to identify the potential binding partners of CABYR, a novel co-immunoprecipitation strategy for less insoluble and insoluble proteins was employed and produced satisfactory results. The data here showed that at least some of the protein–protein interactions could be maintained in the Celis buffer without DTT and β-ME and that the interaction could be recovered after the extract was renatured by dialysis against PBS. The present study, in which both co-immunoprecipitation and yeast two-hybrid analysis were used, confirmed that there does exist an interaction between CABYR and AKAP3. Not only was it shown that the native proteins of CABYR and AKAP3 could be co-immunoprecipitated with each other by either one of their polyclonal antibodies, but it was also shown that the recombinant CABYR and AKAP3 proteins significantly interacted in the yeast two-hybrid assay. Here, the cotransformed transformants bearing constructs expressing both recombinant CABYR and AKAP3 grew normally in the selective SD/–Ade/–His/–Leu/–Trp medium, suggesting that the interaction between CABYR and AKAP3 activates the expression of the reporter genes ADE2 and HIS3; the MEL1 reporter gene is also activated as demonstrated by the quantitative α-galactosidase assays. This result is not consistent with a previous report showing the interaction between CABYR and AKAPs to be much more limited.29 Conversely, the strength of this interaction was much stronger than that between P53 and SV40 large T. Importantly, when one of the constructs expressed the truncated CR-A in which the RII domain had been deleted, the cotransformed AH109 with the construct expressing the full-length AKAP3 could not grow in selective SD/–Ade/–His/–Leu/–Trp medium, and the α-galactosidase activity of this cotransformant was as low as in the negative control. This provides direct evidence that the interaction between CABYR and scaffolding protein AKAP3 in the FS of human sperm is mediated by the RII domain on the N-terminus of CABYR. Because the anchoring of RII of protein kinase A to AKAP requires RII dimerisation, it could be that the dimerisation of CABYR between different variants is necessary for mediating its binding to AKAP3. As CABYR is a calcium-binding, tyrosine- as well as serine- or threonine-phosphorylated protein, its binding to AKAP3 may reflect a functional need for Ca2+ signalling transduction, and it may play a role in motility at sites along the FS.
The interaction between CABYR and Ropporin
The ultrastructural location of Ropporin is mainly on the inner surface of the FS.19 This specific location is close to that of CABYR, which is present within the FS, including the surface of the longitudinal column and ribs, as well as over electron-dense material lying between the FS and the outer dense fibre.25 Biochemical characterisation has shown that Ropporin, unlike the intrinsic components of the FS, which are known to be resistant to 6 mol l−1 urea,30 is present in both Triton X-100-soluble and -insoluble fractions and was almost completely dissolved in 4 mol l−1 urea, suggesting that Ropporin is essentially a soluble protein and binds to the FS. Similarly, our experiments showed that CABYR is present in both Triton X-100-soluble and -insoluble fractions, and so it should be a soluble protein that binds to the surface of FS as does Ropporin. Furthermore, the N-terminal sequences of both CABYR and Ropporin are highly homologous to the dimerisation motif of RIIα of protein kinase A. On the basis of the similar pattern of localisation, biochemical characteristics and molecular structure of CABYR and Ropporin, we primarily pursued evidence for interaction between CABYR and AKAP3, as found for the interaction between Ropporin and AKAP3,22 and revealed an interaction between CABYR and AKAP3. The present study showed that Ropporin was co-immunoprecipitated with CABYR by an anti-CABYR-A polyclonal antibody by regular co-immunoprecipitation methods involving gentle lysis. Reproducible experiments showed that a short string of spots with molecular weights of about 20 kDa and isoelectric point (pI) 5–6 were always present on the silver stain gels of an anti-CABYR-A polyclonal antibody immunoprecipitate, and microsequencing indicated that they were Ropporin. Proteins from mouse sperm immunoprecipitated with anti-mouse CABYR-A polyclonal antibody also have the same Ropporin spots, identified by mass spectrometry.37 A yeast two-hybrid assay in which constructs expressed the full-length Ropporin protein, different CABYR segments, truncated CABYR-A in which the RII domain had been deleted or CABYR-B confirmed these findings. Unlike the findings for AKAP3 and CABYR, both full-length and truncated CABYR-A could bind to Ropporin. The interaction between CABYR-A and Ropporin was weaker than that between CABYR-A and AKAP3, and CABYR-B did not interact with Ropporin. Obviously, CABYR not only dimerizes with its different variants but also forms heterodimers or oligomers with its binding partner Ropporin. This result is also supported indirectly by the finding that Ropporin forms a heterodimer with sperm autoantigenic protein 17 in vitro.19
The yeast two-hybrid assay not only verified the interactions between CABYR and AKAP3 or Ropporin, but also indicated that the RII-domain of CABYR is critical for its binding with AKAP3 but may not be the only domain for its binding to Ropporin. CABYR-B of CABYR also participates in the binding to AKAP3, albeit weakly, but not in the binding to Ropporin. The interaction between CABYR-A and AKAP3 is much stronger than that of P53 and SV40 large T according to the quantitative α-galactosidase data.
In summary, direct evidence for the interaction between CABYR and AKAP3 or Ropporin in human spermatozoa was demonstrated by both co-immunoprecipitation and the yeast two-hybrid interaction assay. Deletion of the RII domain abolished the interaction between CABYR and AKAP3 but did not entirely abolish the interaction between CABYR and Ropporin. Thus, CABYR binds to AKAP3 by its RII domain but also binds to Ropporin by the RII domain and other domains. These interactions between CABYR and AKAP3 or Ropporin could be involved in the assembly of signalling complexes in the FS.
Author contributions
YFL and WH contributed equally to this work. YFL and WH conceived the study, designed and carried out the experiments, and drafted the manuscript. AM and YHK prepared the antibodies and participated in part of the co-immunoprecipitation study. LD and KK gave technical support and beneficial advice. CJF participated in the design of the study and helped to draft the manuscript. JCH administered the experiment, participated in its design and coordination, and helped to draft the manuscript. All authors read and approved the final manuscript.
Acknowledgments
This work was supported by D43 TW/HD 000654 from the Fogarty International Center, NIH, USA.
The author declare no competing financial interests.
References
- Eddy EM, Toshimori K, O'Brien DA. Fibrous sheath of mammalian spermatozoa. Microsc Res Tech. 2003;61:103–15. doi: 10.1002/jemt.10320. [DOI] [PubMed] [Google Scholar]
- Fawcett DW. The mammalian spermatozoon. Dev Biol. 1975;44:394–436. doi: 10.1016/0012-1606(75)90411-x. [DOI] [PubMed] [Google Scholar]
- Vijayaraghavan S, Liberty GA, Mohan J, Winfrey VP, Olson GE, et al. Isolation and molecular characterization of AKAP110, a novel, sperm-specific protein kinase A-anchoring protein. Mol Endocrinol. 1999;13:705–17. doi: 10.1210/mend.13.5.0278. [DOI] [PubMed] [Google Scholar]
- Mandal A, Naaby-Hansen S, Wolkowicz MJ, Klotz K, Shetty J, et al. FSP95, a testisspecific 95-kilodalton FS antigen that undergoes tyrosine phosphorylation in capacitated human spermatozoa. Biol Reprod. 1999;61:1184–97. doi: 10.1095/biolreprod61.5.1184. [DOI] [PubMed] [Google Scholar]
- Carrera A, Gerton GL, Moss SB. The major FS polypeptide of mouse sperm: structural and functional similarities to the A-kinase anchoring proteins. Dev Biol. 1994;165:272–84. doi: 10.1006/dbio.1994.1252. [DOI] [PubMed] [Google Scholar]
- Brown PR, Miki K, Harper DB, Eddy EM. A-kinase anchoring protein 4 binding proteins in the FS of the sperm flagellum. Biol Reprod. 2003;68:2241–8. doi: 10.1095/biolreprod.102.013466. [DOI] [PubMed] [Google Scholar]
- Mei X, Singh IS, Erlichman J, Orr GA. Cloning and characterization of a testis-specific, developmentally regulated A-kinase-anchoring protein (TAKAP-80) present on the FS of rat sperm. Eur J Biochem. 1997;246:425–32. doi: 10.1111/j.1432-1033.1997.t01-1-00425.x. [DOI] [PubMed] [Google Scholar]
- Welch JE, Schatte EC, O'Brien DA, Eddy EM. Expression of a glyceraldehydes 3-phosphate dehydrogenase gene specific to mouse spermatogenic cells. Biol Reprod. 1992;46:869–78. doi: 10.1095/biolreprod46.5.869. [DOI] [PubMed] [Google Scholar]
- Bunch DO, Welch JE, Magyar PL, Eddy EM, O'Brien DA. Glyceraldehyde 3-phosphate dehydrogenase-S protein distribution during mouse spermatogenesis. Biol Reprod. 1998;58:834–41. doi: 10.1095/biolreprod58.3.834. [DOI] [PubMed] [Google Scholar]
- Mori C, Welch JE, Fulcher KD, O'Brien DA, Eddy EM. Unique hexokinase messenger ribonucleic acids lacking the porin-binding domain are developmentally expressed in mouse spermatogenic cells. Biol Reprod. 1993;49:191–203. doi: 10.1095/biolreprod49.2.191. [DOI] [PubMed] [Google Scholar]
- Visconti PE, Olds-Clarke P, Moss SB, Kalab P, Travis AJ, et al. Properties and localization of a tyrosine phosphorylated form of hexokinase in mouse sperm. Mol Reprod Dev. 1996;43:82–93. doi: 10.1002/(SICI)1098-2795(199601)43:1<82::AID-MRD11>3.0.CO;2-6. [DOI] [PubMed] [Google Scholar]
- Mori C, Nakamura N, Welch JE, Gotoh H, Goulding EH, et al. Mouse spermatogenic cellspecific type 1 hexokinase (mHk1-s) transcripts are expressed by alternative splicing from the mHk1 gene and the HK1-S protein is localized mainly in the sperm tail. Mol Reprod Dev. 1998;49:374–85. doi: 10.1002/(SICI)1098-2795(199804)49:4<374::AID-MRD4>3.0.CO;2-K. [DOI] [PubMed] [Google Scholar]
- Travis AJ, Foster JA, Rosenbaum NA, Visconti PE, Gerton GL, et al. Targeting of a germ cell-specific type 1 hexokinase lacking a porin-binding domain to the mitochondria as well as to the head and FS of murine spermatozoa. Mol Biol Cell. 1998;9:263–76. doi: 10.1091/mbc.9.2.263. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hsu HC, Lee YL, Cheng TS, Howng SL, Chang LK, et al. Characterization of two nontestis-specific CABYR variants that bind to GSK3b with a proline-rich extension-like domain. Biochem Biophys Res Commun. 2005;329:1108–17. doi: 10.1016/j.bbrc.2005.02.089. [DOI] [PubMed] [Google Scholar]
- Krisfalusi M, Miki K, Magyar PL, O'Brien DA. Multiple glycolytic enzymes are tightly bound to the FS of mouse spermatozoa. Biol Reprod. 2006;75:270–78. doi: 10.1095/biolreprod.105.049684. [DOI] [PubMed] [Google Scholar]
- Kim YH, Haidl G, Schaefer M, Egner U, Mandal A, et al. Compartmentalization of a unique ADP/ATP carrier protein SFEC (sperm flagellar energy carrier, AAC4) with glycolytic enzymes in the fibrous sheath of the human sperm flagellar principal piece. Dev Biol. 2007;302:463–76. doi: 10.1016/j.ydbio.2006.10.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fulcher KD, Welch JE, Klapper DG, O'Brien DA, Eddy EM. Identification of a unique mclass gluthathione S-transferase in mouse spermatogenic cells. Mol Reprod Dev. 1995;42:415–24. doi: 10.1002/mrd.1080420407. [DOI] [PubMed] [Google Scholar]
- Catalano RD, Hillhouse EW, Vlad M. Developmental expression and characterization of FS39, a testis complementary DNA encoding an intermediate filament-related protein of the sperm FS. Biol Reprod. 2001;65:277–87. doi: 10.1095/biolreprod65.1.277. [DOI] [PubMed] [Google Scholar]
- Fujita A, Nakamura K, Kato T, Watanabe N, Ishizaki T, et al. Ropporin, a sperm-specific binding protein of rhophilin, that is localized in the FS of sperm flagella. J Cell Sci. 2000;113:103–12. doi: 10.1242/jcs.113.1.103. [DOI] [PubMed] [Google Scholar]
- Kong M, Richardson RT, Widgren EE, O'Rand MG. Sequence and localization of the mouse sperm autoantigenic protein, Sp17. Biol Reprod. 1995;53:579–90. doi: 10.1095/biolreprod53.3.579. [DOI] [PubMed] [Google Scholar]
- Frayne J, Hall L. A re-evaluation of sperm protein 17 (Sp17) indicates a regulatory role in an A-kinase anchoring protein complex, rather than a unique role in sperm–zona pellucida binding. Reproduction. 2002;124:767–74. doi: 10.1530/rep.0.1240767. [DOI] [PubMed] [Google Scholar]
- Carr DW, Fujita A, Stentz CL, Liberty GA, Olsen GE, et al. Identification of sperm specific proteins that interact with A-kinase anchoring proteins in a manner similar to the type II regulatory subunit of PKA. J Biol Chem. 2001;276:17332–8. doi: 10.1074/jbc.M011252200. [DOI] [PubMed] [Google Scholar]
- Bajpai M, Fiedler SE, Huang Z, Vijayaraghavan S, Olson GE, et al. AKAP3 selectively binds PDE4A isoforms in bovine spermatozoa. Biol Reprod. 2006;74:109–18. doi: 10.1095/biolreprod.105.043588. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li YF, He W, Jha KN, Klotz K, Kim YH, et al. FSCB, a novel protein kinase Aphosphorylated calcium-binding protein, is a CABYR-binding partner involved in late steps of fibrous sheath biogenesis. J Biol Chem. 2007;282:34104–19. doi: 10.1074/jbc.M702238200. [DOI] [PubMed] [Google Scholar]
- Naaby-Hansen S, Mandal A, Wolkowicz MJ, Sen B, Westbrook VA, et al. CABYR, a novel calcium-binding tyrosine phosphorylation-regulated fibrous sheath protein involved in capacitation. Dev Biol. 2002;242:236–54. doi: 10.1006/dbio.2001.0527. [DOI] [PubMed] [Google Scholar]
- Ficarro S, Chertihin O, Westbrook VA, White F, Jayes F, et al. Phosphoproteome analysis of capacitated human sperm. Evidence of tyrosine phosphorylation of AKAP 3 and valosin containing protein/P97 during capacitation. J Biol Chem. 2003;278:11579–89. doi: 10.1074/jbc.M202325200. [DOI] [PubMed] [Google Scholar]
- Fiedler SE, Bajpai M, Carr DW. Identification and characterization of RHOA-interacting proteins in bovine spermatozoa. Biol Reprod. 2007;78:184–92. doi: 10.1095/biolreprod.107.062943. [DOI] [PubMed] [Google Scholar]
- Lea IA, Widgren EE, O'Rand MG. Association of sperm protein 17 with A-kinase anchoring protein 3 in flagella. Reprod Biol Endocrinol. 2004;2:57. doi: 10.1186/1477-7827-2-57. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hanlon Newell AE, Fiedler SE, Ruan JM, Pan J, Wang PJ, et al. Protein kinase A RII-like (R2D2) proteins exhibit differential localization and AKAP interaction. Cell Motil Cytoskel. 2008;65:539–552. doi: 10.1002/cm.20279. [DOI] [PubMed] [Google Scholar]
- Kim YH, Jha KN, Mandal A, Vanage G, Farris E, et al. Translation and assembly of CABYR coding region B in fibrous sheath and restriction of calcium binding to coding region A. Dev Biol. 2005;286:46–56. doi: 10.1016/j.ydbio.2005.07.005. [DOI] [PubMed] [Google Scholar]
- Luconi M, Porazzi I, Ferruzzi P, Marchiani S, Forti G, et al. Tyrosine phosphorylation of the A kinase anchoring protein 3 (AKAP3) and soluble adenylate cyclase are involved in the increase of human sperm motility by bicarbonate. Biol Reprod. 2005;72:22–32. doi: 10.1095/biolreprod.104.032490. [DOI] [PubMed] [Google Scholar]
- World Health Organization . WHO Laboratory Manual for the Examination of Human Semen and Semen–Cervical Mucus Interaction. 3rd ed. Cambridge: Cambridge University Press; [Google Scholar]
- Bronson RA, Fusi F. Sperm–oolemmal interaction: role of the Arg–Gly–Asp (RGD) adhesion peptide. Fertil Steril. 1990;54:527–529. doi: 10.1016/s0015-0282(16)53775-3. [DOI] [PubMed] [Google Scholar]
- Brito M, Figueroa J, Maldonado EU, Vera JC, Burzio LO. The major component of the rat sperm FS is a phosphoprotein. Gamete Res. 1989;22:205–17. doi: 10.1002/mrd.1120220208. [DOI] [PubMed] [Google Scholar]
- Carlson CR, Ruppelt A, Taskén K. A kinase anchoring protein (AKAP) interaction and dimerization of the RIa and RIb regulatory subunits of protein kinase A in vivo by the yeast two hybrid system. J Mol Biol. 2003;327:609–18. doi: 10.1016/s0022-2836(03)00093-7. [DOI] [PubMed] [Google Scholar]
- Niu J, Vaiskunaite R, Suzuki N, Kozasa T, Carr DW, et al. Interaction of heterotrimeric G13 protein with an A-kinase anchoring protein 110 (AKAP110) mediates cAMP independent PKA activation. Curr Biol. 2001;11:1686–90. doi: 10.1016/s0960-9822(01)00530-9. [DOI] [PubMed] [Google Scholar]
- Li YF, He W, Kim YH, Mandal A, Digilio L, et al. CABYR isoforms expressed in late steps of spermiogenesis bind with AKAPs and ropporin in mouse sperm fibrous sheath Reprod Biol Endocrinol 20108101doi:10.1186/1477-7827-8-101. [DOI] [PMC free article] [PubMed] [Google Scholar]


