Abstract
As the field of assisted reproduction has advanced, many previously untreatable men are now biological fathers. Although finding sperm in men with obstructive azoospermia is not difficult, locating and retrieving spermatozoa in men with non-obstructive azoospermia remains a clinical challenge, largely because sperm production in these men can be patchy or focal in nature. In response to this challenge, strategies such as fine-needle aspiration (FNA) mapping have been developed to find spermatozoa. This review discusses the history, evolution and current clinical utility and findings with FNA mapping for male infertility). Review of the current literature in the English language on FNA (diagnostic or therapeutic) with a keyword focuses on sperm detection, retrieval, safety and complications. FNA was described in human medicine over 100 years ago. Testis FNA was described 45 years ago and FNA ‘mapping' of spermatozoa was described in 1997. This comparative review of the literature on sperm detection and complication rates with FNA and open testis biopsy or microdissection procedures suggests that FNA is highly informative, minimally invasive and is associated with fewer complications than other commonly used approaches to sperm detection in non-obstructive azoospermic patients. FNA mapping has gained considerable traction as an informative, ‘testis sparing' technique for sperm detection in non-obstructive azoospermia. With knowledge of sperm presence and location prior to sperm retrieval, FNA maps can help clinicians tailor sperm retrieval to optimize time, effort and extent of procedures needed to procure spermatozoa in these difficult cases.
Keywords: azoospermia, fine-needle aspiration, hypogonadism, sperm mapping, sperm retrieval
Introduction
As the field of assisted reproduction has advanced, many previously untreatable men have now become biological fathers. Developed in 1992, intracytoplasmic sperm injection (ICSI) was a significant advance that decreased the sperm requirement for egg fertilization from hundreds of thousands with in vitro fertilization (IVF) to one. ICSI also allowed spermatozoa with limited intrinsic fertilizing capacity to reliably fertilize eggs, including ‘immature' spermatozoa derived from the male reproductive tract. Although finding spermatozoa in men with obstruction as a cause of azoospermia is not difficult,1 locating and retrieving spermatozoa in men with non-obstructive azoospermia remains a clinical challenge, largely because sperm production in men with testicular failure can be patchy or focal in nature.1
In response to this challenge, several strategies were developed to find spermatozoa in men with non-obstructive azoospermia. As originally described, open (conventional) testicular sperm extraction (TESE) is performed using single or multiple small incisions in the testicular tunica albuginea.2, 3 Early experience with multibiopsy TESE, however, suggested that as the number of biopsies increased, so too did the concerns of vascular injury resulting in testicular atrophy and hypogonadism due to extensive tissue removal.4, 5 This led to the development of ‘testis-sparing' strategies for finding spermatozoa in these patients. One approach, microdissection TESE, involves a single large, 3–4 cm equatorial or polar incision in the testicular tunica albuginea, followed by physically inverting the testis to expose the entire parenchyma for inspection.6 Subsequently, by the use of operative microscopy, dilated and opaque seminiferous tubules are selectively harvested and evaluated for viable sperm.7 A second ‘testis-sparing' approach, reported several years earlier, involves minimally invasive fine-needle aspiration (FNA) diagnostically to ‘map' the location of spermatozoa in the testis in anticipation of later sperm retrieval.8 Armed with the knowledge of sperm presence and location before egg retrieval for IVF–ICSI, investigators could consider the theoretical possibilities of a more limited or ‘directed' sperm retrieval procedure, employing percutaneous (testicular sperm aspiration (TESA)), open (TESE) or microdissection methods. This review discusses the history, evolution and current clinical utility and findings with FNA mapping for male infertility.
History of FNA mapping
First described by Posner over 100 years ago, FNA has been performed in almost every organ in the body.9 In 1965, the first FNA procedures were reported in the human testes of infertile men.10 In 1988, Schenk and Schill11 first described the cytological features of seminiferous epithelium in detail. Since then, it has been convincingly demonstrated that an excellent correlation exists between testicular cytology by FNA and histology by open testicular biopsy (Table 1). In addition, the ability to interpret histological patterns accurately from cytological findings from FNA procedures is well described.12 Thus, FNA has an established track record of use in the testis and has been considered a reliable and informative procedure for defining male infertility conditions for almost 50 years.
Table 1. Literature correlating testicular FNA cytology to biopsy histology.
| Study | No. of patients | % Agreement |
|---|---|---|
| Persson et al.43 | 42 | 86% |
| Gottschalk-Sabag et al.13 | 47 | 87% |
| Mallidis and Gordon Baker44 | 46 | 94% |
| Craft et al. 199745 | 19 | 84% |
| Odabas et al.46 | 24 | 90% |
| Mahajan et al.47 | 60 | 97% |
| Rammou-Kinia et al.48 | 30 | 87% |
| Meng et al.22 | 87 | 94% |
| Qublan et al.49 | 34 | 96% |
| Arïdoğan et al.41 | 40 | 90% |
| Mehrotra et al.50 | 58 | 94% |
Abbreviation: FNA, fine-needle aspiration.
The concept of ‘mapping' spermatozoa in the testicle using FNA is a more recent and innovative idea that was first suggested by the work of Gottschalk-Sabag and colleagues.13 These investigators reported sampling of the testis in multiple locations, rather than a single location, with few or no complications. Influenced by diagnostic prostatic biopsy strategies that sought to improve cancer detection by increasing sample size, Turek et al.14 further expanded this principle to use FNA systematically and geographically to ‘map' the presence or absence of mature spermatozoa in the testis.
An early feasibility study of FNA mapping was performed in a cohort of infertile, azoospermic men.14 All men had an open testicular biopsy, a testicular biopsy touch imprint and a single FNA from identical, matched testicular sites, and all samples were assessed for the presence of mature spermatozoa. Adequacy criteria for FNA specimens were strictly defined, and formal 2×2 table correlation of the techniques was performed (Figure 1). Among 34 paired biopsy–FNA sites, FNA was observed to be more sensitive than, and equally specific as, testicular biopsy for sperm detection. When compared to the biopsy touch imprint, FNA was equally sensitive and specific. The conclusion that a single FNA sample was at least as informative as a testis biopsy for sperm detection, yet much less invasive, led the way for the development of a relatively safe, templated, multisample FNA procedure for sperm detection in azoospermic men that is popular today.
Figure 1.
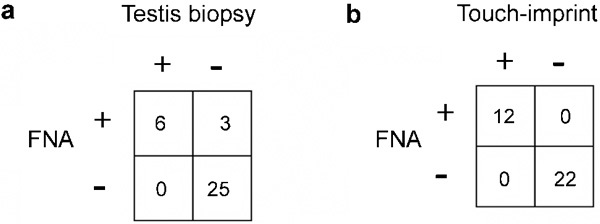
Correlation of sperm detection results between FNA and (a) testis biopsy at site-matched locations in the testis (n=34 sites) and (b) touch imprint at site-matched locations in the testis (n=34 sites). Note: ‘+' denotes spermatozoa present and ‘−' denotes spermatozoa absent8 (adapted with permission). FNA, fine-needle aspiration.
Currently, two kinds of FNA maps are performed on azoospermic men. A simple map consists of four or fewer aspirations and is generally employed as a diagnostic test to confirm the clinical expectation of sperm production in men whose testes may be obstructed.1 A compound map consists of >4 aspirations/testis and is used to locate spermatozoa in failing testes.1, 14, 15, 16 Currently, compound maps with as many as 18 sample sites are commonly performed in NOA patients (Figure 2).
Figure 2.
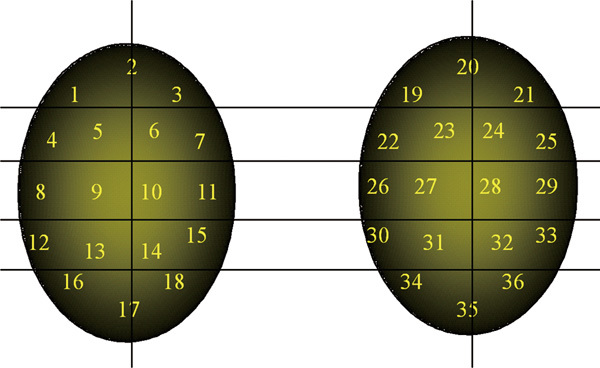
A templated, compound FNA map showing 18 aspiration sites on each testicle. FNA, fine-needle aspiration.
FNA mapping technique
Testicular FNA mapping is performed by a classic FNA technique.17 Under local anesthesia in the office, the testis and scrotal skin are fixed relative to each other with a gauze wrap posteriorly (Figure 3). The ‘testicular wrap' of gauze is a convenient handle to manipulate the testis and also fixes the scrotal skin over the testicle for the procedure. Percutaneous aspiration sites are marked on the scrotal skin, 5 mm apart according to a template (Figure 3). The number of aspiration sites varies with testis size and ranges from 4 (to confirm obstruction) to 18 per testis (for non-obstructive azoospermia). FNA is performed with a sharp-beveled, 23-gauge, 1-inch needle using the established suction cutting technique.17 Precise, gentle, in-and-out movements, varying from 5 to 8 mm, are used to aspirate tissue fragments. Ten to 20 needle excursions are made at each site. Suction is released, and the tissue fragments were expelled onto a slide, gently smeared and immediately fixed in 95% ethyl alcohol. Pressure is applied to each mapped site for hemostasis. A routine Papanicolaou stain is performed on the smeared slide. Smears are reviewed by experienced cytologists for specimen adequacy (defined as at least 12 clusters of testicular cells or at least 2000 well-dispersed testicular cells) and the presence or absence of mature spermatozoa with tails.14 For immediate interpretation, fixed slides are stained with undiluted toluidine blue and read with bright-field microscopy after 15 s. Patients take an average of two pain pills after the procedure and a return to normal activity is allowed after 24 h. Complications are extremely rare (<0.1%) and include hematospermia, hematocele and prolonged post-operative pain (>3 days).
Figure 3.
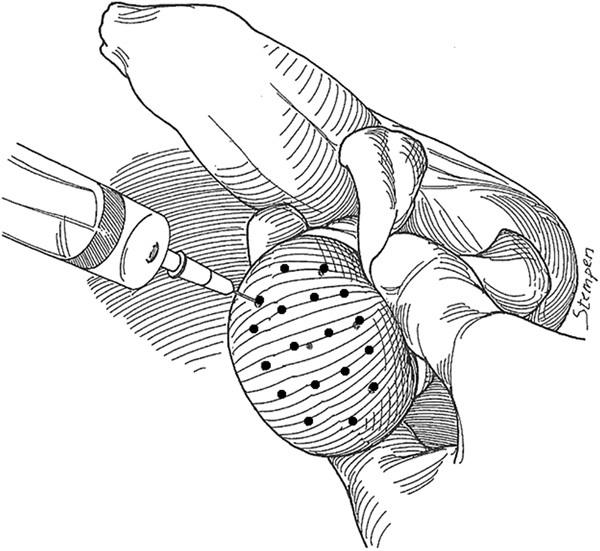
A schematic drawing of FNA mapping showing the testis ‘wrap' of gauze for fixation and sampling template marked on the scrotum8 (adapted with permission). FNA, fine-needle aspiration.
Sperm detection by FNA mapping
The ability of any technique to determine the presence of spermatozoa in the testis depends to a large degree on the intensity of sampling, as judged by the higher yield of spermatozoa by microdissection TESE than by simple TESE in most reported series.6, 7, 18, 19 Similarly, several studies corroborate this trend with FNA mapping. Weiss et al.20 reported a 35.7% sperm detection rate by using three FNA sites per testis. This compares favorably to a sperm detection rate of 30% when a single biopsy is performed on men with non-obstructive azoospermia.21 Turek and colleagues 1, 22 observed a 47% sperm detection rate among 96 consecutive infertile non-obstructive azoospermic patients receiving a mean of 7.6 FNA sites per testicle and a 52% detection rate in 87 men who received a mean of 14 FNA sites/testis. In a study done by Lewin et al.15 on 85 azoospermic men with non-obstructed testis, testicular FNA identified mature spermatozoa in 58.8% from a mean of 15 aspiration sites (range 10–20) per testicle. Finally, a recent analysis of over 100 recent FNA mapping procedures suggests that a sperm detection rate of 60% is possible by using 18 sites/testicle (unpublished data). Therefore, as FNA sample size is increased, so are sperm detection rates.
Further analysis of the literature on FNA sampling intensity and sperm detection reveals another interesting trend. It appears that the rate of increase in sperm detection as sample intensity increases approaches an asymptotic limit (Figure 4). This suggests that there may be an FNA sample size above which sperm detection rates do not increase meaningfully. However, that sample size is currently unknown.
Figure 4.
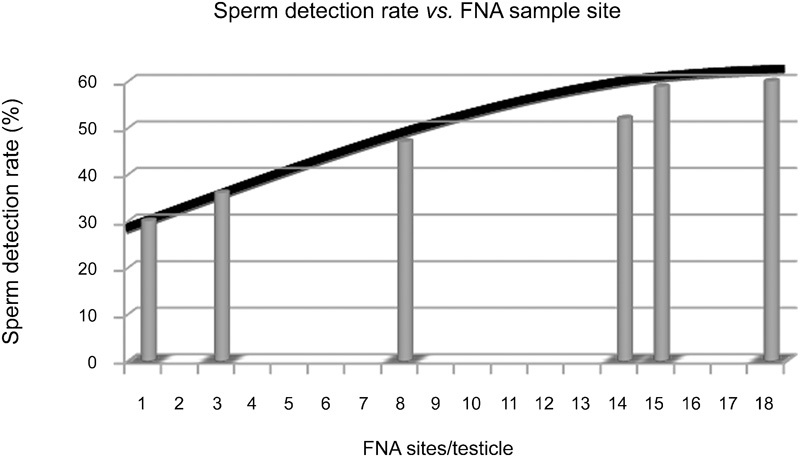
The asymptotic curve of testis sampling versus sperm detection. A summary of the reported literature on the chance of finding spermatozoa by number of biopsies or FNA. Each bar represents a separate published report.1, 15, 20, 21, 22 FNA, fine-needle aspiration.
FNA mapping has also been used to describe the geographic variation, or heterogeneity, of spermatozoa within non-obstructive azoospermic testes. From a comprehensive study of 118 consecutive azoospermic, infertile men whose testes underwent FNA mapping, obvious differences in the sperm detection rates were described between men with obstructive and non-obstructive azoospermia.1 In men with obstructive azoospermia, spermatozoa were found in 100% of sites and in 100% of FNA map locations. However, in men with non-obstructive azoospermia, intratesticular variation (site-to-site variability within the same testis) of spermatozoa was observed in 25% of cases, along with an intertesticular discordance (side-to-side variation in the same individual) rate of 19%. This is consistent with the findings from other reports and suggests that bilateral examinations are crucial to informing men with non-obstructive azoospermia fully about opportunities for fatherhood.15, 23 In the subgroup of men with no spermatozoa on a prior testicular biopsy, FNA maps (mean: 7.6 sites/testis) revealed spermatozoa in 27% of cases. FNA mapping results have also been analyzed to determine whether particular geographical sites in the testis are more likely to have spermatozoa than others.1. For this study, individual testis maps from each testicular side in 96 non-obstructive azoospermic patients were pooled. It was observed that all FNA sites showed spermatozoa at the same frequency without a suggestion of sperm ‘hot spots' in non-obstructive testes. Thus, FNA mapping has defined a wealth of phenotypic information about sperm production in non-obstructive azoospermia.
Most recently, FNA mapping findings in patients after sterilizing chemotherapy and even bone marrow transplantation with total body irradiation for cancer have been reported.24, 25 Another recent study used FNA mapping to help define which testicle is more likely to contain spermatozoa in men with non-obstructive azoospermia and unilateral testis pathology such as varicocoele or undescended testis.26 The goal of that study was to define associations between several common unilateral testicular pathologies and the sperm findings from bilateral testis FNA in non-obstructive azoospermia (Table 2). The density of spermatozoa detected by FNA mapping in each testis was analyzed to discern sperm lateralization patterns in affected and unaffected testes. A total of 1098 FNA sites from 56 men with varicocoele, cryptorchidism, epididymo-orchitis, mumps orchitis and testicular torsion were analyzed. Overall, 38 patients (68%) had spermatozoa detected in at least one testis. Most men (68%) had equal proportions of FNA sites showing spermatozoa in both testes, 29% had more spermatozoa on the unaffected testis and 3% had more spermatozoa on the affected testis. Significantly fewer sperm-positive sites were observed on the affected (272/752) than unaffected sides (164/346) (P<0.0001). Thus, when assessed by FNA mapping, most men with non-obstructive azoospermia and known unilateral testis pathology will have equal proportions of spermatozoa in both testes. However, when sperm production differs between sides, the unaffected side is more likely to have spermatozoa.
Table 2. Clinical characteristics and FNA findings in azoospermic men with associated unilateral testicular pathology26 (adapted with permission).
| Varicocoele (n=32) | Cryptorchidism (n=16) | Epididymo-orchitis (n=3) | Mumps orchitis (n=2) | Torsion (n=3) | |
|---|---|---|---|---|---|
| Mean age (years) | 38.6 | 36.8 | 36.0 | 43.5 | 38.3 |
| Mean volume of affected testis (ml) | 15.2 | 12.0 | 18.5 | 15.3 | 15.3 |
| Mean volume of unaffected testis (ml) | 15.9 | 8.8 | 16 | 20 | 23 |
| No. of aspiration sites | 656 | 288 | 69 | 33 | 52 |
| Mean FSH (mIU ml−1) | 19.1 | 24.2 | 11.7 | 17.9 | 12.5 |
| Men with genetic findings (n) | 7 | 1 | 0 | 0 | 0 |
| Detection of sperm n (%) | 20 (63%) | 11 (69%) | 3 (100%) | 2 (100%) | 2 (67%) |
Abbreviations: FNA, fine-needle aspiration; FSH, follicle-stimulating hormone.
In addition to sperm detection, FNA mapping has detected early testicular tumors before they are palpable and has been employed to improve male infertility phenotyping for research.27, 28, 29 As an example of the latter, FNA mapping may increase our ability to dissect out and study relatively subtle genetic events that may underlie human infertility. For example, it is known that mutations in genes required for DNA repair (PMS2 and Mlh1) in mice lead to infertility characterized by meiotic or maturation arrest.30 On the basis of the testicular phenotype reported in mice, we studied a cohort of infertile human testes with a similar global pattern of maturation arrest determined by FNA mapping to answer the question whether defects in genes involved in DNA repair cause infertility in men as they do in mice.28 Indeed, mapped patients with maturation arrest had significantly more mutations in testicular DNA with dinucleotide (microsatellite) repeats than did obstructed men. Since they are diagnostic procedures, maps can be archived and applied to improve phenotype–genotype correlations in genetic research.
FNA mapping: guide to sperm retrieval
Soon after its description in 1997, systematic, diagnostic FNA mapping was used to identify in whom, and exactly where, spermatozoa are present in men with non-obstructive azoospermia, in order to guide sperm retrieval during IVF–ICSI. In the first 21 consecutive IVF–ICSI cycles reported, sufficient spermatozoa for all oocytes were retrieved in 20 sperm retrieval attempts (95%).31 A mean of 3.1 testis biopsies/patient was required with an average biopsy size of 72 mg. Mean operative time for sperm retrieval procedures was 88 min, including placement of local anesthesia until the final laboratory determination of sufficient spermatozoa at retrieval. In these 21 cycles, the overall two-pronuclear fertilization rate was 66% and continuing clinical pregnancies were obtained in 48% of initiated cycles. The early experience with this technique suggested that FNA-derived testicular ‘maps' are accurate enough to ensure excellent and reliable sperm retrieval rates from fresh testicular spermatozoa.
In a larger clinical series, investigators also examined the timing of sperm retrieval after FNA mapping. From ultrasound-based reports of tissue changes after open testis biopsy, it is clear that sperm retrieval should be postponed at least 6 months.5 In a study of 46 patients, the timing and success of post-FNA sperm retrieval or reconstructive procedures were examined to determine if there was a window of time in which FNA mapping might affect negatively the ability to retrieve spermatozoa later.1 The mean time to sperm retrieval or vasectomy reversal after FNA was 4.9 months (range: 0.5–13 months) with no decrease in sperm retrieval rates as time to sperm retrieval decreased. Further experience has also shown that spermatozoa can be successfully found at retrieval up to 13 years after FNA mapping.
FNA mapping has also influenced how sperm retrievals are approached and executed. Advanced knowledge of sperm distribution in the testis obtained through FNA mapping has led to a ‘tailoring' of the most appropriate and least invasive procedure at sperm retrieval. In general, if the FNA map shows a global and uniformly distributed sperm pattern, then TESA procedures suffice for sperm retrieval. If, however, only a few sites of spermatozoa are found (>2) and sperm distribution is not globally distributed, then an open testis biopsy (TESE) is directed to the active sites of spermatozoa on the map. Finally, if only a single mapped site shows spermatozoa, a microdissection TESE is generally needed to acquire sufficient spermatozoa. From a cohort of 159 cases of non-obstructive azoospermic men who underwent FNA mapping before sperm retrieval, it was found that 44% of patients required sperm retrieval by TESA, 33% by TESE and 23% by microdissection TESE (Figure 5).32 The majority (>80%) of sperm retrieval cases also required only unilateral procedures to provide sufficient spermatozoa for IVF–ICSI and the overall rate of retrieving sufficient spermatozoa for all oocytes retrieved was maintained at 95% of cases. Lastly, prior knowledge of the complexity of the sperm retrieval procedure guides the planning, effort and efficiency of the andrology laboratory for sperm retrieval (Table 3). Thus, knowledge of sperm location with FNA mapping can simplify and streamline subsequent sperm retrieval procedures on many levels in difficult cases of azoospermia. Given reports of TESE procedures requiring 20 biopsies or more per patient, the description of complete organ devascularization after sperm retrieval certainly lends support to this ‘testis-sparing' approach to sperm retrieval.5, 33
Figure 5.
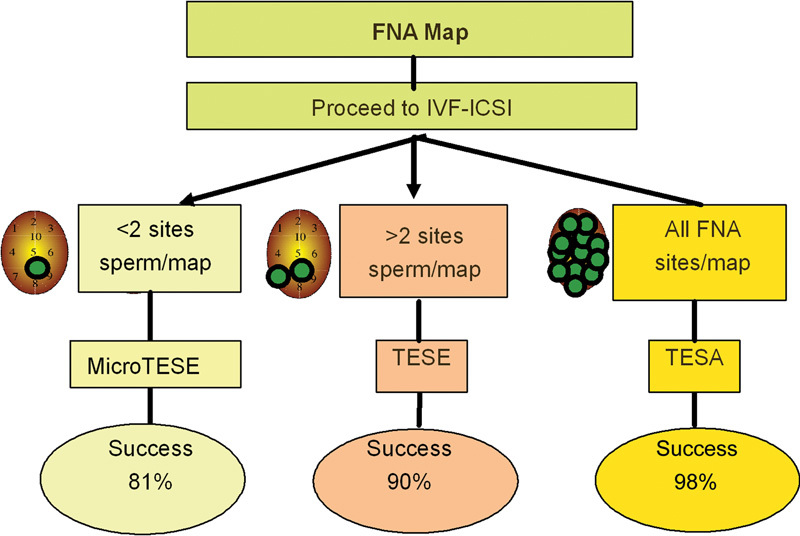
The clinical pathway involving FNA mapping for non-obstructive azoospermia. The extent and type of sperm retrieval can be tailored to the finding on the FNA map. The percentage of success at the bottom of each column represents the chance that enough spermatozoa will be found for all eggs at the time of sperm retrieval. FNA, fine-needle aspiration; ICSI, intracytoplasmic sperm injection; IVF, in vitro fertilization; TESA, testicular sperm aspiration; TESE, testicular sperm extraction.
Table 3. Guidelines regarding andrology laboratory effort for successful sperm retrieval in non-obstructive azoospermia51.
| Sperm search man-hours required (h) | |
|---|---|
| TESA | 1–2 |
| Simple TESE | 2–4 |
| Microdissection TESE | 4–6+ |
Abbreviations: h, hour; TESA, testicular sperm aspiration; TESE, testicular sperm extraction.
FNA mapping: safety
The wider geographical testicular sampling achieved by FNA mapping can also be achieved by multiple open biopsies.34 However, the safety profiles of these approaches differ considerably. The complications of multiple open biopsies (including microdissection TESE) are hematomas, infection, hypogonadism and devascularization of the testis resulting in testicular atrophy.4, 5, 15, 35, 36 In a study by Schlegel and Su,5 64 patients were evaluated by ultrasound after TESE procedures. At 3 months, 82% of patients had intratesticular abnormalities on ultrasound that were suggestive of persistent hematoma or inflammation or both. At 6 months, the acute inflammatory changes typically resolved leaving linear scars. Two patients had impaired testicular blood flow and one had complete devascularization of the testis. Finally, a recent analysis of microdissection TESE in an animal model has demonstrated contralateral alterations in spermatogenesis in unilaterally dissected cases.37 Although in the initial series of FNA-mapped patients with non-obstructive azoospermia, Turek et al.8 found no intratesticular hematomas 30 min after the procedure using a 23-gauge needle, Lewin et al.15 demonstrated sonographic evidence of bleeding in 4/58 (7%) of patients with percutaneous biopsies made by an 18-gauge biopsy needle. The lack of any significant effect of FNA on testicular ultrasonography, testicular size or semen quality in a canine model also confirms the clinical findings in humans.38 Thus, by ultrasound comparison, there appears to be a significant difference in potential testis injury between open testis biopsy and FNA.
A significant safety issue in men with non-obstructive azoospermia is hypogonadism after sperm retrieval procedures. This complication may lead to temporary or permanent testosterone replacement after sperm retrieval, and represents a significant medical burden for men of reproductive age. It is generally agreed that unselected, multiple, open testicular biopsies present the largest risk of hypogonadism in the infertile and often atrophic testis.5 It has also been reported that fewer testicular biopsies in TESE procedures result in a less pronounced postoperative testosterone decrease.36 The increased precision of tissue dissection offered with microTESE is also associated with measurable effects on androgen balance in many hands.35, 36, 39 In a study by Takada et al.,36 69 patients were followed after microdissection TESE. Serum testosterone concentrations recovered to only 50–84% of preoperative levels after 1 year in patients with various causes of non-obstructive azoospermia. Endocrinological findings of similar magnitude after microdissection have been reported by other investigators.35, 39 Thus, the magnitude of the effect of open testicular biopsies on testosterone production and testicular atrophy is well defined and has stimulated the development of further ‘testis-sparing' strategies for sperm retrieval.
The effect of FNA on testicular atrophy and serum hormone levels has also been evaluated. Westerland et al.40 noted no significant changes in follicle-stimulating hormone, testosterone or testicular volumes 3 months after testicular sperm aspiration with a 19-gauge needle. In a study of FNA-mapped patients, Turek et al.1 examined 88 men at a mean of 3 months (range: 0.5–13 months) postprocedure and observed no testicular atrophy by routine orchidometry in any patient. In addition, no clinical or surgical complications were observed. Among 85 azoospermic men who underwent testicular FNA in another series, no hematomas or infections were reported.15 However, three patients in this series had pain that lasted 3 days after the procedure; in these patients, testicular ultrasound was normal. The lack of complications after FNA procedures has also been confirmed by other investigators41, 42 and suggests that FNA certainly qualifies as a ‘testis-sparing' strategy for the planning and execution of sperm retrieval.
The future of mapping: metabolomics
Since sperm detection in non-obstructive azoospermia is correlated with sampling intensity, the ideal diagnostic technique would be to sample at higher density than current technologies and be non-invasive and non-surgical in nature. Recently, nuclear magnetic resonance and magnetic resonance imaging scanning have been combined into metabolomic scanning, which fits this diagnostic ideal very well. On the basis of the concept that the vast majority of cellular activity in the testis is associated with sperm production, investigators sought to define the unique chemical ‘signatures' associated with various histological states of spermatogenesis in 27 infertile men by the use of quantitative high resolution-magic angle spinning (proton magnetic resonance spectroscopy) spectroscopy.29 In that study, metabolic sampling of ex vivo testicular biopsies was used to ‘read' histological patterns accurately and a predictive model for sperm presence with proton magnetic resonance spectroscopy was developed, on the basis of tissue phosphatidylcholine concentrations. In addition to the potential of this technology to define the chemical signature for sperm presence in the testis, it may be able to metabolically ‘sample' the testis in hundreds of regions non-invasively, making it the ultimate ‘mapping' technique. Lastly, this technology has the potential to identify chemical signatures of testicular tumors and quantify the effects of drugs and other interventions (i.e., varicocoele repair) on testicular function.
The authors declare to have no competing financial interests.
References
- Turek PJ, Ljung BM, Cha I, Conaghan J. Diagnostic findings from testis fine needle aspiration mapping in obstructed and non-obstructed azoospermic men. J Urol. 2000;163:1709–16. [PubMed] [Google Scholar]
- Mulhall JP, Burgess CM, Cunningham D, Carson R, Harris D, et al. Presence of mature sperm in testicular parenchyma of men with nonobstructive azoospermia: prevalence and predictive factors. Urology. 1997;49:91–6. doi: 10.1016/S0090-4295(96)00356-1. [DOI] [PubMed] [Google Scholar]
- Schlegel PN, Palermo GD, Goldstein M, Menendez S, Zaninovic N, et al. Testicular sperm extraction with intracytoplasmic sperm injection for nonobstructive azoospermia. Urology. 1997;49:435–40. doi: 10.1016/S0090-4295(97)00032-0. [DOI] [PubMed] [Google Scholar]
- Ramasamy R, Yagan N, Schlegel P. Structural and functional changes to the testis after conventional versus microdissection testicular sperm extraction. Urology. 2005;65:1190–4. doi: 10.1016/j.urology.2004.12.059. [DOI] [PubMed] [Google Scholar]
- Schlegel PN, Su LM. Physiological consequences of testicular sperm extraction. Hum Reprod. 1997;12:1688–92. doi: 10.1093/humrep/12.8.1688. [DOI] [PubMed] [Google Scholar]
- Schlegel PN. Testicular sperm extraction: microdissection improves sperm yield with minimal tissue excision. Hum Reprod. 1999;14:131–5. doi: 10.1093/humrep/14.1.131. [DOI] [PubMed] [Google Scholar]
- Okada H, Dobashi M, Yamazaki T, Hara I, Fujisawa M, et al. Conventional versus microdissection testicular sperm extraction for nonobstructive azoospermia. J Urol. 2002;168:1063–7. doi: 10.1016/S0022-5347(05)64575-2. [DOI] [PubMed] [Google Scholar]
- Turek PJ, Cha I, Ljung BM. Systematic fine-needle aspiration of the testis: correlation to biopsy and results of organ “mapping” for sperm in azoospermic men. Urology. 1997;49:743–8. doi: 10.1016/S0090-4295(97)00154-4. [DOI] [PubMed] [Google Scholar]
- Posner C. Die diagnostische Hodenpunktion. Berl Klin Wochenschr. 1905;42:1119–21. [Google Scholar]
- Obrant KO, Persson PS. Cytological study of the testis by aspiration biopsy in the evaluation of fertility. Urol Int. 1965;20:176–89. doi: 10.1159/000279375. [DOI] [PubMed] [Google Scholar]
- Schenk U, Schill WB. Cytology of the human seminiferous epithelium. Acta Cytol. 1988;32:689–96. [PubMed] [Google Scholar]
- Meng MV, Cha I, Ljung BM, Turek PJ. Testicular fine needle aspiration in infertile men: correlation of cytologic pattern with histology on biopsy. Am J Surg Pathol. 2001;25:71–9. doi: 10.1097/00000478-200101000-00008. [DOI] [PubMed] [Google Scholar]
- Gottschalk-Sabag S, Glick T, Weiss DB. Fine needle aspiration of the testis and correlation with testicular open biopsy. Acta Cytol. 1993;37:67–72. [PubMed] [Google Scholar]
- Turek PJ, Cha I, Ljung BM. Systematic fine-needle aspiration of the testis: Correlation to biopsy and results of organ “mapping” for mature sperm in azoospermic men. Urology. 1997;49:743–8. doi: 10.1016/S0090-4295(97)00154-4. [DOI] [PubMed] [Google Scholar]
- Lewin A, Reubinoff B, Porat-Katz A, Weiss D, Eisenberg V, et al. Testicular fine needle aspiration: the alternative method for sperm retrieval in non-obstructive azoospermia. Hum Reprod. 1999;14:1785–90. doi: 10.1093/humrep/14.7.1785. [DOI] [PubMed] [Google Scholar]
- Cohen A, Vazquez J, del Sordo M, Muro F, Nataskin H, et al. Correlation of testis biopsy histology and fine needle aspiration in nonobstructive azospermia. The Buenos Aires 3 years experience. Fert Steril. 2003;80:235. [Google Scholar]
- Ljung BM.Techniques of aspiration and smear preparationIn: Koss LG, Woyke S, Olszewski W, editors.Aspiration Biopsy: Cytologic Interpretation and Histological Bases. 2nd ed New York: Igaku-Shoin; 199212–34. [Google Scholar]
- Turunc T, Gul U, Haydardedeoglu B, Bal N, Kuzgunbay B, et al. Conventional testicular sperm extraction combined with the microdissection technique in nonobstructive azoospermic patients: a prospective comparative study. Fertil Steril. 2010;94:2157–60. doi: 10.1016/j.fertnstert.2010.01.008. [DOI] [PubMed] [Google Scholar]
- Tsujimura A, Matsumiya K, Miyagawa Y, Tohda A, Miura H, et al. Conventional multiple or microdissection testicular sperm extraction: a comparative study. Hum Reprod. 2002;17:2924–9. doi: 10.1093/humrep/17.11.2924. [DOI] [PubMed] [Google Scholar]
- Weiss DB, Gottschalk-Sabag S, Bar-On E, Zukerman Z, Gat Y, et al. Seminiferous tubule cytological pattern in infertile, azoospermic men in diagnosis and therapy. Harefuah. 1997;132:614–8. [PubMed] [Google Scholar]
- Kim ED, Gilbaugh JH, 3rd, Patel VR, Turek PJ, Lipshultz Testis biopsies frequently demonstrate sperm in men with azoospermia and significantly elevated follicle-stimulating hormone levels. J Urol. 1997;157:144–6. [PubMed] [Google Scholar]
- Meng MV, Cha I, Ljung BM, Turek PJ. Relationship between classic histological pattern and sperm findings on fine needle aspiration map in infertile men. Hum Reprod. 2000;15:1973–7. doi: 10.1093/humrep/15.9.1973. [DOI] [PubMed] [Google Scholar]
- Samli M, Sariyuce O, Basar M, Evrenkaya T.Evaluation of nonobstructive azoospermia with bilateral testicular biopsy J Urol 199916134210037435 [Google Scholar]
- Damani MN, Master V, Meng MV, Turek PJ, Oates RM. Post-chemotherapy ejaculatory azoospermia: fatherhood with sperm from testis tissue using intracytoplasmic sperm injection. J Clin Oncol. 2002;20:930–6. doi: 10.1200/JCO.2002.20.4.930. [DOI] [PubMed] [Google Scholar]
- Ignatov AP, Eisenberg MS, Turek PJ. Paternity after directed collection of testicular sperm for in vitro fertilization after BMT for hematological malignancies. Bone Marrow Transplant. 2010;45:1474–6. doi: 10.1038/bmt.2009.372. [DOI] [PubMed] [Google Scholar]
- Shefi S, Kaplan K, Turek PJ. Analysis of spermatogenesis in non-obstructive azoospermic and virtually azoospermic men with known testicular pathology. Reprod Biomed Online. 2009;18:460–4. doi: 10.1016/s1472-6483(10)60120-4. [DOI] [PubMed] [Google Scholar]
- Freedland SJ, Cha I, Turek PJ. Nonpalpable Leydig's cell tumors diagnosed by fine needle aspiration. J Urol. 1997;158:543–4. [PubMed] [Google Scholar]
- Nudell D, Castillo M, Turek PJ, Pera RR. Increased frequency of mutations in DNA from infertile men with meiotic arrest. Hum Reprod. 2000;15:1289–94. doi: 10.1093/humrep/15.6.1289. [DOI] [PubMed] [Google Scholar]
- Aaronson DS, Iman R, Walsh TJ, Kurhanewicz J, Turek PJ. A novel application of 1H magnetic resonance spectroscopy: non-invasive identification of spermatogenesis in men with non-obstructive azoospermia. Hum Reprod. 2010;25:847–52. doi: 10.1093/humrep/dep475. [DOI] [PubMed] [Google Scholar]
- Baker SM, Bronner CE, Zhang L, Plug AW, Robatzek M, et al. Male mice defective in the DNA mismatch repair gene PMS2 exhibit abnormal chromosome synapsis in meiosis. Cell. 1995;82:309–19. doi: 10.1016/0092-8674(95)90318-6. [DOI] [PubMed] [Google Scholar]
- Turek PJ, Givens C, Schriock ED, Meng MV, Pederson RA, et al. Testis sperm extraction and intracytoplasmic sperm injection guided by prior fine needle aspiration mapping in nonobstructive azoospermia. Fertil Steril. 1999;71:552–7. doi: 10.1016/s0015-0282(98)00499-3. [DOI] [PubMed] [Google Scholar]
- Arredondo S, Shen SH, Conaghan J, Turek PJ. A clinical care pathway for nonobstructive azoospermia based on testis biopsy and mapping data. Fertil Steril. 2004;82:S86. [Google Scholar]
- Jha R, Sayami G. Testicular fine needle aspiration in evaluation of male infertility. J Nepal Med Assoc. 2009;48:78–84. [PubMed] [Google Scholar]
- Tournaye H, Liu J, Nagy PZ, Camus M, Goossens A, et al. Correlation between testicular histology and outcome after intracytoplasmic sperm injection using testicular spermatozoa. Hum Reprod. 1996;11:127–32. doi: 10.1093/oxfordjournals.humrep.a019004. [DOI] [PubMed] [Google Scholar]
- Ishikawa T, Yamaguchi K, Chiba K, Takenaka A, Fujisawa M. Serum hormones in patients with nonobstructive azoospermia after microdissection testicular sperm extraction. J Urol. 2009;182:1495–9. doi: 10.1016/j.juro.2009.06.029. [DOI] [PubMed] [Google Scholar]
- Takada S, Tsujimura A, Ueda T, Matsuoka Y, Takao T, et al. Androgen decline in patients with nonobstructive azoospermia after microdissection testicular sperm extraction. Urology. 2008;72:114–8. doi: 10.1016/j.urology.2008.02.022. [DOI] [PubMed] [Google Scholar]
- Oliveira Filho AB, Souza RS, Azeredo-Oliveira MT, Peruquetti RL, et al. Microdissection testicular sperm extraction causes spermatogenic alterations in the contralateral testis. Genet Mol Res. 2010;9:1405–13. doi: 10.4238/vol9-3gmr860. [DOI] [PubMed] [Google Scholar]
- Gouletsou P, Galatos A, Leontides L, Sideri A.Impact of fine- or large-needle aspiration on canine testes: clinical, in vivo ultrasonographic and seminological assessment Reprod Domest Anime-pub ahead of print 1 December 2010; doi: 10.1111/j.1439-0531.2010.01734.x [DOI] [PubMed]
- Everaert K, de Croo I, Kerckaert W, Dekuyper P, Dhont M, et al. Long term effects of micro-surgical testicular sperm extraction on androgen status in patients with nonobstructive azoospermia. BMC Urol. 2006;6:9–12. doi: 10.1186/1471-2490-6-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Westlander G, Erling E, Seth G, Nils L, Lars N, et al. Serial ultrasonography, hormonal profile and antisperm antibody response after testicular sperm aspiration. Hum Reprod. 2001;16:2621–7. doi: 10.1093/humrep/16.12.2621. [DOI] [PubMed] [Google Scholar]
- Arïdoğan IA, Bayazït Y, Yaman M, Ersöz C, Doran S. Comparison of fine-needle aspiration and open biopsy of testis in sperm retrieval and histopathologic diagnosis. Andrologia. 2003;35:121–5. doi: 10.1046/j.1439-0272.2003.00547.x. [DOI] [PubMed] [Google Scholar]
- Carpi A, Menchini Fabris FG, Palego P, Di Coscio G, Romani R, et al. Fine-needle and large-needle percutaneous aspiration biopsy of testicles in men with nonobstructive azoospermia: safety and diagnostic performance. Fertil Steril. 2005;83:1029–33. doi: 10.1016/j.fertnstert.2004.09.027. [DOI] [PubMed] [Google Scholar]
- Persson PS, Ahern C, Obrant KO. Aspiration biopsy smear of testis in azoospermia. Scand J Urol Nephrol. 1971;5:22–6. doi: 10.3109/00365597109133568. [DOI] [PubMed] [Google Scholar]
- Mallidis C, Gordon Baker HW. Fine needle tissue aspiration biopsy of the testis. Fertil Steril. 1994;61:367–75. [PubMed] [Google Scholar]
- Craft I, Tsirigotis M, Courtauld E, Farrer-Brown G. Testicular needle aspiration as an alternative to biopsy for the assessment of spermatogenesis. Hum Reprod. 1997;12:1483–7. doi: 10.1093/humrep/12.7.1483. [DOI] [PubMed] [Google Scholar]
- Odabas Ö, Urgas S, Aydin S, Yilmaz Y, Kemal Atilla M. Assessment of testicular cytology by fine-needle aspiration and the imprint technique: are they reliable diagnostic modalities. Br J Urol. 1997;79:445–8. doi: 10.1046/j.1464-410x.1997.00024.x. [DOI] [PubMed] [Google Scholar]
- Mahajan AD, Imdad Ali N, Walwalkar SJ, Rege JD, Pathak HR. The role of fine-needle aspiration cytology of the testis in the diagnostic evaluation of infertility. Br J Urol. 1999;84:485–8. doi: 10.1046/j.1464-410x.1999.00169.x. [DOI] [PubMed] [Google Scholar]
- Rammou-Kinia R, Anagnostopoulou I, Tassiopoulos F, Lykourinas M. Fine needle aspiration of the testis. Correlation between cytology and histology. Acta Cytol. 1999;43:991–8. doi: 10.1159/000331384. [DOI] [PubMed] [Google Scholar]
- Qublan HS, Al-Jader KM, Al-Kaisi NS, Alghoweri AS, Abu-Khait SA, et al. Fine needle aspiration cytology compared with open biopsy histology for the diagnosis of azoospermia. J Obstet Gynaecol. 2002;22:527–31. doi: 10.1080/0144361021000003690. [DOI] [PubMed] [Google Scholar]
- Mehrotra R, Chaurasia D. Fine needle aspiration cytology of the testis as the first-line diagnostic modality in azoospermia: a comparative study of cytology and histology. Cytopathology. 2008;19:363–8. doi: 10.1111/j.1365-2303.2007.00485.x. [DOI] [PubMed] [Google Scholar]
- Turek PJ.Sperm retrieval techniquesIn: Carrell DT, Pederson CM, editors.The Practice of Reproductive Endocrinology and Infertility: The Practical Clinic and Laboratory New York: Springer; 2010. Chap. 29, 453–67. [Google Scholar]


