Abstract
Glutamate is a regulated molecule in the mammalian testis. Extracellular regulation of glutamate in the body is determined largely by the expression of plasmalemmal glutamate transporters. We have examined by PCR, western blotting and immunocytochemistry the expression of a panel of sodium-dependent plasmalemmal glutamate transporters in the rat testis. Proteins examined included: glutamate aspartate transporter (GLAST), glutamate transporter 1 (GLT1), excitatory amino acid carrier 1 (EAAC1), excitatory amino acid transporter 4 (EAAT4) and EAAT5. We demonstrate that many of the glutamate transporters in the testis are alternately spliced. GLAST is present as exon-3- and exon-9-skipping forms. GLT1 was similarly present as the alternately spliced forms GLT1b and GLT1c, whereas the abundant brain form (GLT1a) was detectable only at the mRNA level. EAAT5 was also strongly expressed, whereas EAAC1 and EAAT4 were absent. These patterns of expression were compared with the patterns of endogenous glutamate localization and with patterns of 𝒹-aspartate accumulation, as assessed by immunocytochemistry. The presence of multiple glutamate transporters in the testis, including unusually spliced forms, suggests that glutamate homeostasis may be critical in this organ. The apparent presence of many of these transporters in the testis and sperm may indicate a need for glutamate transport by such cells.
Keywords: excitatory amino acid transporter, glutamate aspartate transporter, glutamate transporter 1, sperm, splice variant, testis, transporter
Introduction
The role of glutamate and the homeostasis of such in the testis has been the subject of interest for at least 40 years.1, 2 However, much remains to be determined as to the mechanisms by which glutamate, which is abundant in the testis1, 3, 4, 5 and testicular fluids,6 might be compartmentalized in the mammalian testis. Glutamate does not penetrate the blood–testis barrier,2 but does appear to be synthesized locally.7 The need for glutamate homeostasis in the testis derives from the demonstrable roles for this molecule, both as an osmolyte and as an agonist at both N-methyl-𝒹-aspartate and non-N-methyl-𝒹-aspartate-type glutamate receptors.8, 9 Glutamate is the principal free amino acid in the testis, epididymis and semen of most mammalian species; in semen, glutamate is mainly found in the seminal plasma rather than in the spermatozoa.1 Within the testis itself there is limited evidence for roles for glutamate, though it has been shown that both glutamate and an analog of glutamate (𝒹-aspartate) may modify steroidogenic activity.10 In epididymal plasma, a variety of molecules including glutamate are thought to aid in the conversion of gametes into competent, functional cells. Accordingly, glutamate transporters are thought to direct glutamate into the epididymal lumen.11, 12 This transport of glutamate into the lumen is thought to be critical, since transgenic mice that fail to express the glutamate transporter excitatory amino acid carrier 1 (EAAC1; also referred to as excitatory amino acid transporter 3 (EAAT3)) in the epididymis exhibit poor sperm motility. This poor motility has been attributed to anomalous osmolyte balance in the proximal epididymal lumen, which is thought to compromise sperm maturation, including the development of sperm volume-regulatory mechanisms.
Once sperm have exited, the testis glutamate may have a role in directly regulating motility; sperm motion analysis has demonstrated that glutamate in seminal plasma acts to promote motility, this action being mediated via glutamate receptors including N-methyl-𝒹-aspartate receptors.13 Other glutamate receptor classes including metabotropic types8, 14 are also present on sperm and germinal cells and may thus exert biological effects on the sperm if glutamate levels change.
Two key parameters that will influence glutamate homeostasis in a tissue are the (i) presence of a barrier that restricts influx of glutamate from the blood; and (ii) transporters on plasma membranes of cells that remove glutamate from the extracellular space. The current study was initiated because the testis shares clear homologies to the mammalian brain, in the possession of a blood–testis barrier15 that is homologous to, but structurally distinct from, the blood–brain barrier. The blood–testis barrier is predominantly formed by tight junctions between Sertoli cells;16 however, Leydig cells in the interstitial tissues that share antigenic homologies to astrocytes16, 17 may also influence barrier properties. The blood–brain barrier is induced by astrocytes and is constituted by tight junctions between endothelial cells. Astrocytes are the key regulators of extracellular glutamate in the brain due to their expression of plasmalemmal glutamate transporters.18, 19
This study aimed to determine which glutamate transporters were expressed in testis, and whether they were predominantly associated with cells such as Leydig cells that exhibit some glia-like features, or with other cell types. The prior literature in this area is limited; the expression of glutamate transporters in the testis (as opposed to associated structures such as the vas deferens) has been studied by two groups that we are aware of. Thus, Takarada et al.20 have demonstrated the presence of mRNA for the glutamate transporters glutamate aspartate transporter (GLAST; also called EAAT1), glutamate transporter 1 (GLT1; also called EAAT2), EAAC1, EAAT4 and EAAT5 in testis. However, in their study, mRNA species for GLAST and GLT1 were reported (in Northern blots) as being smaller than expected, suggesting that any translated protein would be smaller than the normal full-length form. Takarada et al.20 also demonstrated, using immunocytochemistry, some labeling for GLAST protein in interstitial cells, but labeling was absent in Western blotting, a disparity attributed to differential methodological sensitivities. Conversely, both immunolabeling and western blotting revealed the presence of GLT1, but again it was at a lower molecular weight than the predicted size for full-length GLT1, and EAAT4 was not detectable. The presence or absence of EAAC1 and EAAT5 protein was not investigated. These data accord with the findings of Hu et al.,9 who reported both the presence of mRNA for GLT1 and EAAC1 in testis, and that some glutamate transport in the testis could be inhibited by dihydrokainate, which is a GLT1-selective inhibitor. Using immunocytochemistry, Hu et al.14 also demonstrated the presence of a GLT1 protein in sperm from mouse and humans. Collectively these data would suggest a wider distribution of glutamate transporters in the testis beyond an ‘astrocyte-like' expression in Leydig cells, with the caveat that many of the proteins detected may not be typical full-length versions of the glutamate transporters normally found in astrocytes in the brain, suggesting that they might be exon-skipping or cleaved forms.
In this study we have used PCR analysis of mRNA in conjunction with western blotting and immunocytochemistry to examine glutamate transporter expression in the rat testis. We have used an extensive panel of well-characterized antibodies against the majority of the known plasmalemmal glutamate transporters (including carboxyl-terminal splice variants and exon-skipping forms) for which antibodies are currently available. The distribution of endogenous glutamate, as well as possible endogenous pools of 𝒹-aspartate (a substrate for all known functional glutamate transporters) and exogenously supplied 𝒹-aspartate, was also studied.
Materials and methods
Testes were obtained from 10 adult male dark Agouti rats that had been killed by an overdose of sodium pentobarbital (100 mg kg−1). Tissues were rapidly dissected out and used for different experimental methods as indicated below. Fourteen testes were hemisected and one half of each fixed by immersion with 4% paraformaldehyde in 0.1 mol l−1 phosphate buffer, pH 7.2, for 3 h, while the other half of each was prepared for either western blotting or PCR analysis. The remaining testes were used for 𝒹-aspartate uptake experiments and immunocytochemistry for glutamate as detailed below. Brain tissues and retinae from all the animals were also prepared for western blotting analysis and PCR, to act as internal comparisons for results with the testes.
Total RNA isolation
Dissected rat brain, testis or retinal tissues were washed twice in sterile phosphate-buffered saline (PBS), placed into 10 volumes of Trizol reagent and homogenized using a power homogenizer for 3 min. To allow complete dissociation of nucleoprotein complexes, homogenates were incubated on ice for 5 min. To every 1 ml of homogenate, 0.2 ml chloroform was added followed by vigorous mixing for ∼2 min, and then incubation on ice for 5 min. Samples were centrifuged at 14 000g (4 °C) for 20 min and the upper aqueous phase was removed and transferred to a fresh microcentrifuge tube. To precipitate the RNA, an equal volume of isopropanol was added, followed by incubation at −20 °C for 24 h. Samples were centrifuged at 17 000g for 1 h and the supernatant removed. The RNA pellet was washed twice with ice-cold 75% ethanol and resuspended in sterile RNAase free water.
Reverse transcription (RT)-PCR
Total RNA (5 µg) of each sample was reverse-transcribed into complementary DNA using SuperScript III (Invitrogen, Mulgrave, Victoria, Australia), followed by digestion with ribonuclease H (Invitrogen), according to the manufacturer's instructions. An aliquot of the RT reaction mixture (1 µl) was then used in PCR (final volume 50 µl) consisting of 2 mmol l−1 dNTP, 0.2 µmol l−1 sense and antisense primers, 1.5 mmol l−1 MgCl2, and 2.5 U BIOTAQ DNA polymerase in 1× PCR buffer.
The mRNA expression of GLAST, GLT1a, EAAC1, EAAT4 and EAAT5 in adult rat testis was assessed by RT-PCR analysis using sense and antisense primers (Table 1) that amplified the entire coding region of each glutamate transporter member.
Table 1. Primers used for identification of EAATs in the testis.
| EAAT member | GenBank accession | Forward oligo sequence | Reverse oligo sequence | Product size (bp) |
|---|---|---|---|---|
| GLAST | NM_019225.1 | TGACAAAAAGCAACGGAGAAGAGC | TATCAGAGTAGGGAGGAAAGAGGA | 1743 |
| GLAST1a | AF265360 | CATTGTGGGAATGGCGGC | TATCAGAGTAGGGAGGAAAGAGGA | 1432 |
| GLAST1b | AY954110 | ATGGAGACTCTGACCCGGATC | GGAGGCGGTCCCTTATTGTTAT | 629 |
| GLT1a | X67857.1 | GCTCGTCGCCACTGTCTCCA | CATAAGATACGCTGGGGAGTT | 1824 |
| GLT1b | AF465909.1 | ACTTTGCCTGTCACCTTCCGTTGC | ATGCAGGTCTCGATATCCAGG | 594 |
| GLT1c | AY578981.1 | ACTTTGCCTGTCACCTTCCGTTGC | TAAACCCACGATTGATATTCCACA | 591 |
| EAAC1 | NM_013032.3 | CGCTCCCCGATTCCTCACAAA | CTTCACAGTCCCAGGCATCTA | 1638 |
| EAAT4 | NM_032065.1 | CGCTGACCCGAGGCTGAG | CCGCCCTGGACAAAGAGCTA | 1736 |
| EAAT5 | NM_001108973.1 | ACAACCTACAGTGCATGGAAT | ACTCCTACGCTTAGCCCAAAC | 1763 |
Abbreviations: EAAC, excitatory amino acid carrier; EAAT, excitatory amino acid transporter; GLAST, glutamate aspartate transporter; GLT, glutamate transporter.
Previous studies have reported the existence of several splice variants for GLAST (GLAST1a and GLAST1b) and GLT1 (GLT1b and GLT1c) in the mammalian brain.21, 22, 23 To study the expression of GLAST1a, GLAST1b, GLT1b and GLT1c in testis, PCR primers specific for each splice variant were synthesized (Table 1).
For amplification of GLAST1a, GLAST1b, GLT1b and GLT1c products, initial denaturation was 95 °C for 2 min followed by 35 cycles of amplification (95 °C for 30 s, 62 °C for 30 s and 72 °C for 1 min). All other PCRs were performed using the following conditions: initial denaturation at 95 °C for 2 min followed by 35 cycles of amplification (95 °C for 30 s, 60 °C for 30 s and 72 °C for 2 min). The reaction products were separated on 1 or 1.5% agarose gels and visualized by staining with 0.5 µg ml−1 ethidium bromide. Sequences of sense and antisense EAAT primers are provided in Table 1.
Total lysate isolation and western blotting
Brain, testis and retina extracts were prepared by homogenization in lysis buffer containing 50 mmol l−1 Tris-HCl (pH 7.4), 150 mmol l−1 NaCl, 1% Nonidet P-40 and protease inhibitor cocktail (Roche Diagnostics, Castle Hill, NSW, Australia). After gentle rotation for 3 h at 4 °C, homogenates were centrifuged at 50 000g for 60 min at 4 °C and the supernatant were collected. Protein lysate (50–100 µg) was dissolved in SDS sample buffer, separated on a 7% SDS polyacrylamide gel and then transferred to nitrocellulose membrane (Pal) by electroblotting. Blots were incubated in blocking buffer (5% non-fat milk, 20 mmol l−1 Tris (pH 7.5), 150 mmol l−1 NaCl and 0.1% Tween-20) for 2 h and then incubated in fresh blocking buffer containing primary antibodies overnight at 4 °C. Following four washes with Tris–NaCl–Tween buffer, blots were incubated for 1 h with horseradish peroxidase-conjugated anti-rabbit immunoglobulin G and washed again. Immunoreactive proteins were detected by enhanced chemiluminescence using the SuperSignal West Dura Extended Duration Substrate Kit (Pierce: Quantum Scientific, Brisbane, Qld, Australia). Samples were always run alongside samples from brain or retinal tissues to ensure the presence of positive controls. Preabsorption of antisera (∼50 µg of antigen peptide per milliliter of diluted antiserum) was used to confirm the specificity of each antiserum (data not shown).
Immunocytochemistry
Immunoperoxidase labeling for the glutamate transporters was performed as previously described using standard methods.22, 24 Briefly, testes were fixed with 4% paraformaldehyde in 0.1 mol l−1 sodium phosphate buffer, then dehydrated through a graded series of water/ethanol solutions, cleared in xylene and embedded in paraffin wax.19 Serial sections (8 µm in thickness) were cut on a Leica rotary microtome and mounted onto silanated microscope slides. Sections were dewaxed with xylene and rehydrated through a graded series of ethanol/water solutions and antigen recovery was performed using Revealit-Ag antigen recovery solution (ImmunoSolution, Newcastle, NSW, Australia). Sections were pre-treated with 3% hydrogen peroxide in methanol for 10 min (during the rehydration process) to inhibit any endogenous peroxidase activity. All sections were blocked in 0.5% bovine serum albumin/0.05% saponin/0.05% sodium azide in 0.1 mol l−1 sodium phosphate buffer for 30 min before primary antibodies were applied. Biotinylated secondary antibodies and streptavidin–biotin–horseradish peroxidase conjugates were subsequently applied at a dilution of 1∶300. Labeling of sections was revealed using 3,3′-diaminobenzidine as a chromogen, and sections were mounted using DePex. Pre-absorption of antisera (50 µg of peptide 1 per milliliter of diluted antiserum) was always used to confirm the specificity of each antiserum (data not shown).
Sperm isolation and immunocytochemistry
Additional labeling was performed on sperm isolated by gentle trituration of the rete testis, to confirm the localization of those proteins initially identified in sperm in histological sections. Sperm were rapidly isolated from ∼10 small (1–2 mm3) portions of the rete testis that were prepared by crude chopping of the rete testis using a scalpel blade in a petri dish in 0.5 ml of PBS (0.9% sodium chloride in 0.1 mol l−1 phosphate buffer, pH 7.2). The tissues were gently triturated using a standard glass Pasteur pipette, the rete testis fragments being triturated for 20–30 s, until the trituration solution became cloudy due to the release of sperm from the rete testis. The cloudy suspension containing sperm was gently centrifuged at room temperature in a 1.5-ml Eppendorf tube at 500g for 30 s using an Eppendorf benchtop microfuge operating at room temperature, to gently pellet the sperm. The pellet containing the sperm was resuspended into 100 µl PBS and 10 µl droplets immediately applied to silanated microscope slides at room temperature for 10–20 min to allow time for the sperm to settle on to, and adhere to the silanated slides. The slides were then quickly washed in PBS for 30 s to remove non-attached sperm and the slides were then fixed with 4% paraformaldehyde for 10 min. Immunocytochemistry was then performed as above.
Glutamate labeling
Immunocytochemical detection of endogenous glutamate was carried out as previously described using well-characterized antibodies against glutamate,25, 26, 27 the tissues being fixed with 2.5% glutaraldehyde in 0.1 mol l−1 sodium cacodylate buffer and then processed for embedding in araldite resin. Semithin (0.5 µm) sections were subsequently cut and immunostained as previously described.
Transport studies
To evaluate which cellular compartments expressed functional glutamate transporters, uptake of the non-metabolizable glutamate analog 𝒹-aspartate was examined. 𝒹-aspartate is widely presumed to be a ligand for all functional glutamate transporters. Detection of 𝒹-aspartate uptake was carried out according to our standard methods.28, 29, 30 Briefly, testes were removed from rats as described and immediately dissected to remove the capsule around the testis. Small (∼2 mm diameter) pieces of tissue were then dissected from each testis and placed into oxygenated physiological media (Ames media; Sigma, Castle Hill, NSW, Australia) or Ames media containing 20 µmol l−1 𝒹-aspartate, at 35 °C for 30 min. Tissues were then removed, washed for 1 min in oxygenated Ames media at 35 °C to remove any free 𝒹-aspartate, and then fixed with 2.5% glutaraldehyde in 0.1 mol l−1 phosphate buffer, pH 7.2 for 1 h. Tissues were subsequently dehydrated, embedded in Araldite resin and immunocytochemistry performed for 𝒹-aspartate using a specific antibody to 𝒹-aspartate, as previously described. Control sections that had not been exposed to 𝒹-aspartate (exposed to normal Ames media alone prior to fixation or fixed immediately after removal from the animal) were also evaluated to determine if any endogenous 𝒹-aspartate could be detected.
Primary antibodies against transporters
The primary antibodies used in this study were all raised in this laboratory and have been extensively characterized in prior publications.31, 32, 33, 34, 35, 36, 37, 38 Figure 1 schematically illustrates the locations of antibody epitopes for normally spliced GLAST and the exon-skipping forms of GLAST (GLAST1a and GLAST1b). Multiple GLT1 antisera were used in this study (Figure 2). They included antisera directed against the alternate splicings of the extreme carboxyl-terminal region of the GLT1 protein (denoted as GLT1a, GLT1b and GLT1c).36 Antisera against an amino-terminal epitope (residues 12–26) and an internal epitope (residues 497–508), both of which should be present in all known forms of GLT1, were also investigated. The EAAC1 antibody was directed against the carboxyl-terminal region of the protein (KSYVNGGFAVDKSDTISFTQTSQF), as was the antibody against EAAT4 (KGASRGRGGNESAM). The EAAT5 antibodies were raised against the amino-terminal region (MVPHTILARGRDVCRRNGLLILSV) or the carboxyl-terminal region (RDEELPAASLNHCTIQISELETNV).
Figure 1.
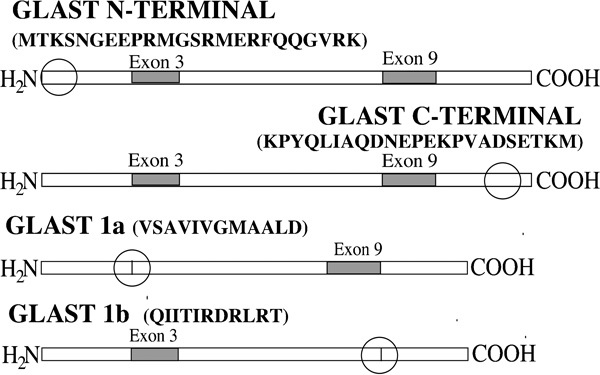
Schematic diagram of the various splice variant forms of GLAST. The sequences that each antibody detects and the region where each epitope is located (circles) are indicated. GLAST, glutamate aspartate transporter.
Figure 2.
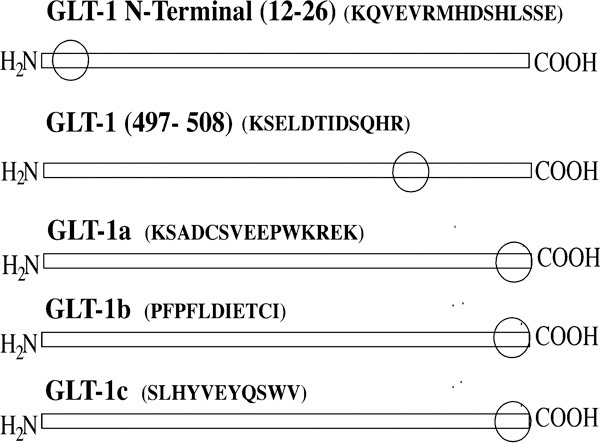
Schematic diagram of the various splice variant forms of GLT1. The sequences that each antibody detects and the region where each epitope is located (circles) are indicated. GLT, glutamate transporter.
Results
EAAT mRNA expression in rat testis
Expression of mRNA for GLAST, GLT1a, EAAC1, EAAT4 and EAAT5 in adult rat testis was assessed by RT-PCR analysis using sense and antisense primers (Table 1) that amplified the entire coding region of each glutamate transporter. This analysis produced amplification products for GLAST, GLT1a and EAAT5 but not EAAC1 nor EAAT4 in rat testis (Figure 3a). In control tissues from brain and retina, appropriately sized PCR products for all of the glutamate transporters were present (predicted from the corresponding cDNA sequences), confirming that any lack of PCR product in testis was not due to methodological failure. To examine possible splice variant expression in the testis, PCR was also performed using primers specific for individual splice variants (Figure 3b–e). PCR results were reproducible (three independent experiments using material sourced from three different animals), and indicated that mRNA of GLAST, GLT1a and EAAT5, as well as the splice variants GLAST1a (Figure 3b) GLAST1b (Figure 3c), GLT1b (Figure 3d) and GLT1c (Figure 3e), could be reliably detected by our primers.
Figure 3.
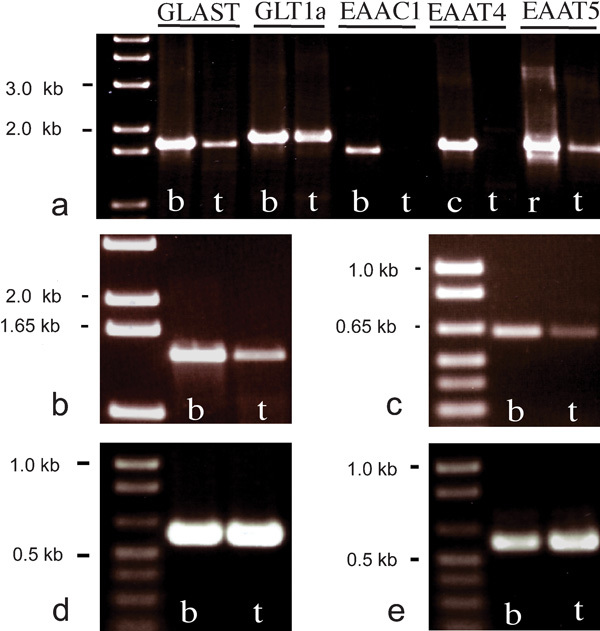
RT-PCR cDNAs prepared from rat whole brain (b), cerebellum (c), retina (r) and testis (t) were used as templates for PCR to amplify the entire coding region of GLAST, GLT1a, EAAC1, EAAT4 and EAAT5 (a). An aliquot of each PCR product was electrophoresed on 1% agarose gel and visualized with ethidium bromide staining. Amplification products of the correct size corresponding to GLAST, GLT1a and EAAT5 (but not EAAC1 and EAAT4) were detected for testis. PCR using primers specific for the transporter splice variants GLAST1a (b), GLAST1b (c) GLT1b (d) and GLT1c (e) produced amplification products of the correct sizes in both brain and testis. EAAC, excitatory amino acid carrier; EAAT, excitatory amino acid transporter; GLAST, glutamate aspartate transporter; GLT, glutamate transporter.
Western blotting for GLAST
Initial studies using our N-terminal-directed GLAST antibody revealed, in testis, a complete lack of labeling in western blotting (Figure 4) despite the presence of strong signals in blots of rat brain at molecular weights corresponding to the monomer (about 55–65 kDa) and dimer (∼130 kDa) of GLAST (Figure 4). By contrast, a modest signal was obtained in western blotting of testis using an antibody directed against the carboxyl-terminal region of GLAST (Figure 4), the apparent mass being typical of monomeric GLAST. The monomeric form of GLAST1a was detected in testis at ∼55 kDa as was the dimer, running at ∼110 kDa. GLAST1b was similarly expressed in both testes, as evinced by a single band at about 55–60 kDa.
Figure 4.
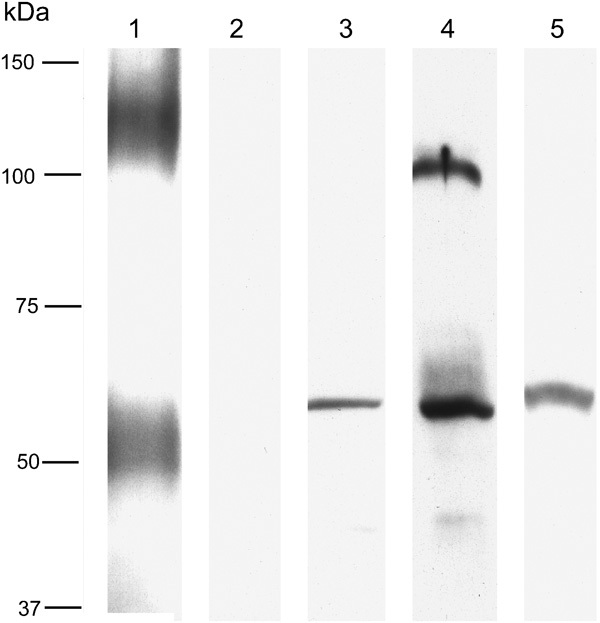
Western blots for the various GLAST epitopes studied. Lane 1 was from brain; lanes 2–5 were testis, labeled for N-terminal GLAST (lane 1, 2), C-terminal GLAST (lane 3), exon-3-skipping GLAST1a (lane 4) and exon-9-skipping GLAST1b (lane 5). N-terminal GLAST labeling was present in the brain (running as a broad monomer band of 50–60 kDa and as a broad dimer band at around 100–150 kDa, but was absent in the testis). C-terminal GLAST labeling was present in the testis (∼55 kDa); GLAST1a was abundant and ran at the expected size (∼55 kDa) and as an ∼110-kDa band, which is thought to represent GLAST1a dimers. A single band of about 55–60 kDa was detected using GLAST1b antibodies. GLAST, glutamate aspartate transporter.
Immunocytochemistry for GLAST
Immunolabeling for N-terminal GLAST resulted in an absence of specific signals in the testis (Figure 5a). In contrast, a clear signal was obtained in the testis with the C-terminal GLAST antibody (Figure 5b and c), labeling being associated with interstitial cells rather than with the seminiferous tubules. This pattern of labeling was almost indistinguishable from that observed with the GLAST1b antibody (Figure 5d). Immunocytochemistry for GLAST1a (Figure 5e and f) revealed abundant labeling in both the interstitial cells (similar to the labeling pattern for the C-terminal epitope of GLAST) and (in those tubules with mature sperm) intense labeling in small rounded compartments that we interpret as being the heads of sperm (Figure 5f).
Figure 5.
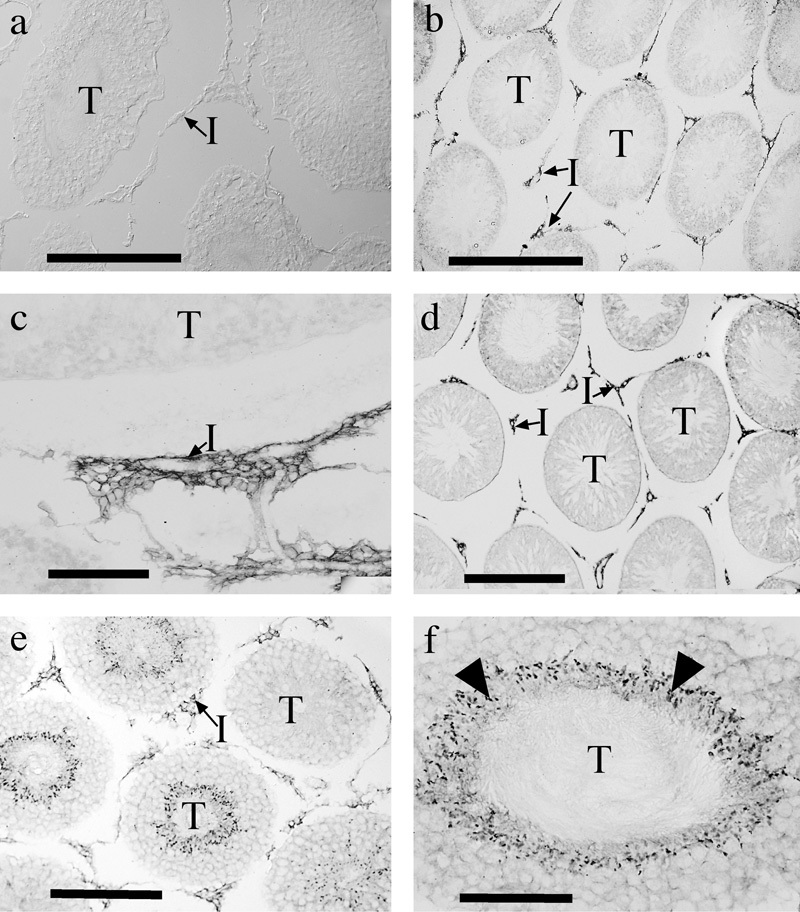
Immunolabeling for various forms of GLAST in the testis. Immunostaining for N-terminal GLAST was absent (a), whereas labeling for C-terminal GLAST (b, c) was present in interstitial cells (I). Labeling for GLAST1b (d) was similarly present in interstitial cells, while labeling for GLAST1a (e, f) was present both in interstitial cells and in a subset of seminiferous tubules (T), where it appeared to be associated with the heads of sperm (arrowheads). Scale bars: a b, d, e=200 µm; c, f=100 µm. GLAST, glutamate aspartate transporter.
Western blotting for GLT1
Initial studies using our pan-GLT antibody Ab12, which is thought to detect all forms of GLT1, revealed in western blots the presence in testis of at least three forms of GLT1, each migrating at slightly differing relative masses, suggesting three different splice forms (Figure 6). These compare well with the wide smear (Figure 6) centered around 60–65 kDa, which is typical of the monomeric form of brain GLT1. By contrast, GLT1a (the predominant brain form of GLT1) was not detected in testis. The band detected by the GLT1b antibody in testis was of a higher molecular weight than expected (∼120 kDa), suggesting that it represented the dimeric form of GLT1b. By contrast, the GLT1c antiserum predominantly detected a band around 55–60 kDa in testis, suggesting that it detected the monomeric form of this protein.
Figure 6.
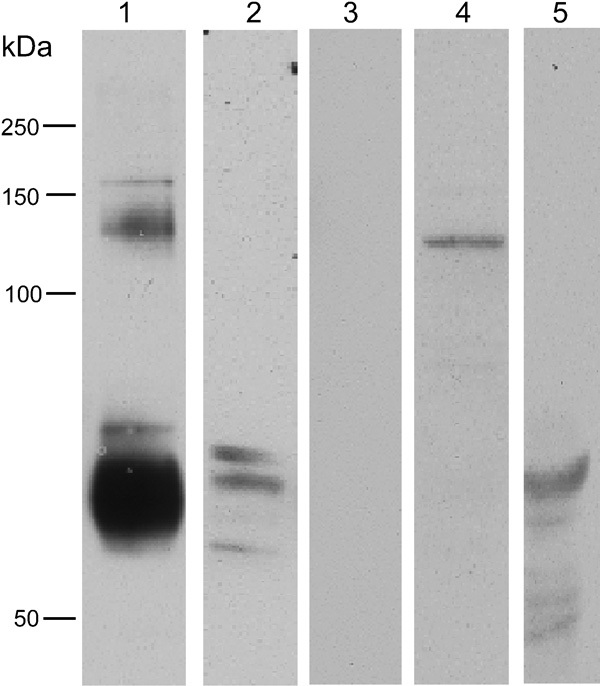
GLT1 western blots. Lane 1 is from the brain; lanes 2–5 are testis, labeled for N-terminal GLT1 (Ab12 common epitope) (lanes 1 and 2), GLT1a (lane 3), GLT1b (lane 4) and GLT1c (lane 5). The common epitope GLT1 antibody labeled two main bands in the brain, at around 60–65 kDa, corresponding to the GLT1 monomer and at around 120–130 kDa, which corresponds to the dimer. In the testis, the antibody revealed three distinct bands at around or slightly smaller than the predicted mass for generic GLT1, suggesting that three possible splice variants might be present. GLT1a was absent in the testis, while GLT1b was present in the testis but at a mass suggestive of it existing as a dimer. GLT1c was present in the testis, with the predominant labeled band at around 55–60 kDa. GLT, glutamate transporter.
Immunolabeling for GLT1
Immunolabeling using both our amino-terminal (Ab12) and internal epitope (Ab497) pan-GLT1 antibodies revealed essentially the same staining patterns (Figure 7), with some strongly labeled seminiferous tubules, and others more weakly labeled. The weak staining was predominantly associated with radial-oriented structures (Figure 7c), which appeared, as per anatomical criteria, to represent the plasma membranes of Sertoli cells that are known to extend long radial processes through the tubules. The more intense but variable staining was attributable to the variable state of sperm maturation in individual tubules, tubules with mature sperm labeling strongly. Dense labeling (Figure 7d) appeared to be associated with anterior compartments of the sperm. Other diffusely distributed puncta were also present in tubules with mature sperm. The pattern of labeling with pan-GLT1 antibodies was compared with that produced by antibodies against GLT1 splice variants. GLT1a (Figure 8a) was not detectable in the testis, but antibodies to GLT1b (Figure 8b) and GLT1c (Figure 8c and d) produced very similar results; each antiserum produced strong labeling of anterior portions of the sperm.
Figure 7.
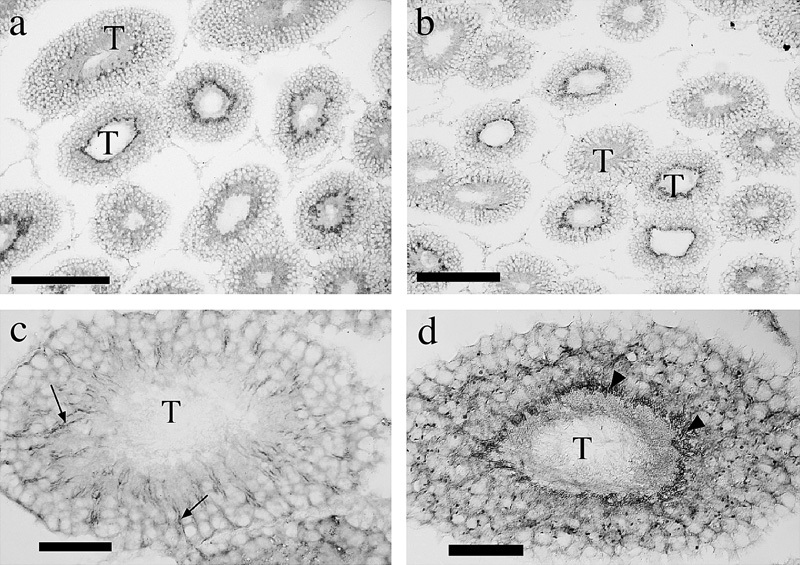
GLT1 immunolabeling using antibodies against the common epitopes including the amino terminus (Ab12; a, c) and an internal epitope (Ab497; b, d). Labeling patterns for the two antisera were indistinguishable (a, b), being variably associated with seminiferous tubules (T), the pattern of labeling appearing to vary with the maturational state of the sperm in individual tubules. The immunolabeling was evident both in radially oriented structures (probably Sertoli cell processes; arrows) and more intensely (d) in elongate puncta (arrowheads), which we interpret as being the anterior portions of sperm. Other diffusely distributed puncta are also present. Scale bars: a, b=200 µm; c, d=50 µm. GLT, glutamate transporter.
Figure 8.
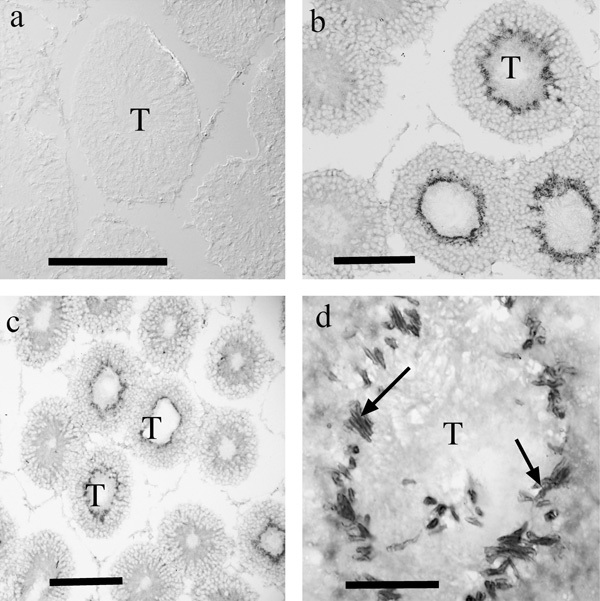
Immunolabeling for GLT1a (a), GLT1b (b) and GLT1c (c, d). GLT1a labeling was absent, but the other GLT1 variants exhibited similar staining patterns. The intense staining in the tubules (T) was in each case associated with tube-like elongate structures (arrows) that appeared to represent anterior parts of the sperm other than the head region. Scale bars=200 µm. GLT, glutamate transporter.
EAAC1 and EAAT4
In accord with the lack of a PCR product for EAAC1, EAAC1 protein was not detectable in the testis by western blotting or immunocytochemistry (data not shown). Similarly, EAAT4 (which was undetectable by PCR in testis) was not detectable in western blots of testis and no immunolabeling was detected (data not shown).
EAAT5 labeling
Western blotting for EAAT5 using our antibody against the C-terminal regions of EAAT5 detected an appropriately sized protein in both retina (where EAAT5 is known to be abundant) and testis (Figure 9). Immunolabeling with either the C-terminal antiserum (Figure 10a and b) or an additional N-terminal antiserum (Figure 10c and d) revealed the same labeling pattern, with conspicuous labeling of small rounded structures that we interpret as being the head regions of sperm (Figure 10b), while weaker but overt labeling was evident in the tails of sperm and in radial processes (Figure 10d), which we interpret as representing processes of Sertoli cells.
Figure 9.
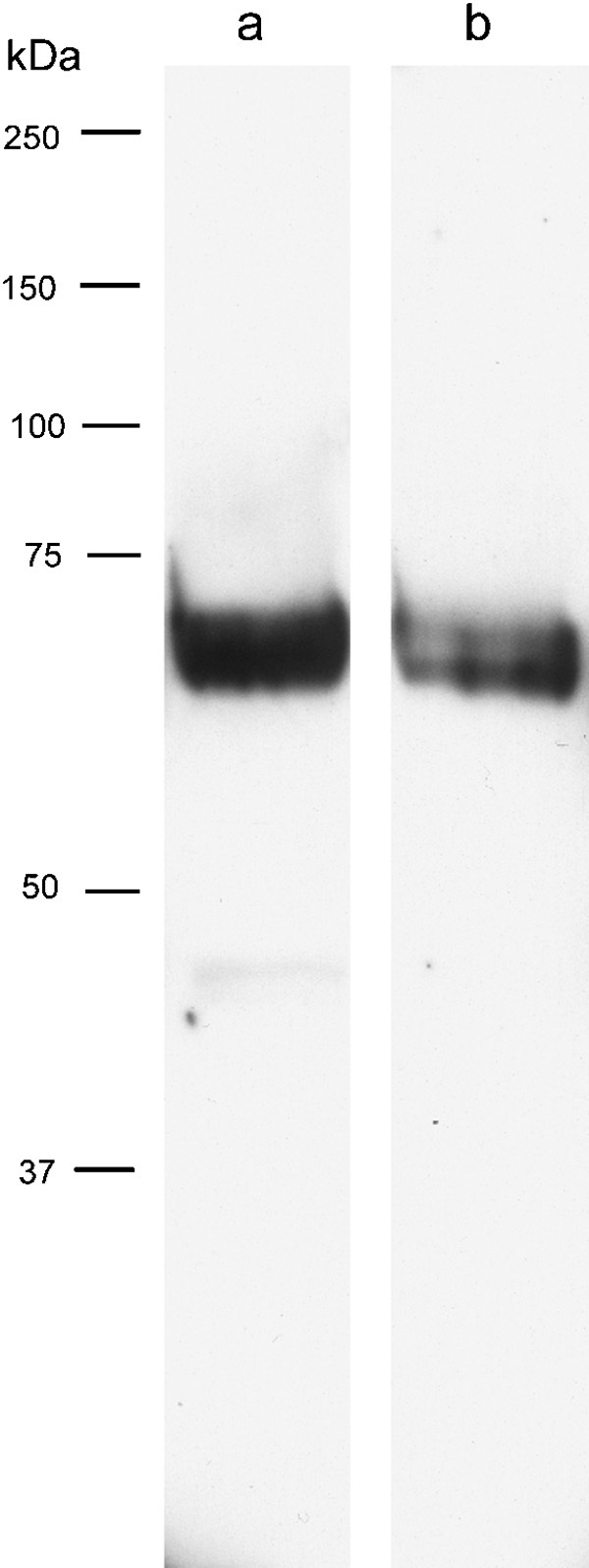
Western blotting for EAAT5 using an antibody to the carboxyl-terminal region of EAAT5 (lanes a and b) using samples from retina (lane a) and testis (lane b). Immunoreactive EAAT5 protein was detected at an appropriate molecular weight in both tissues. EAAT, excitatory amino acid transporter.
Figure 10.
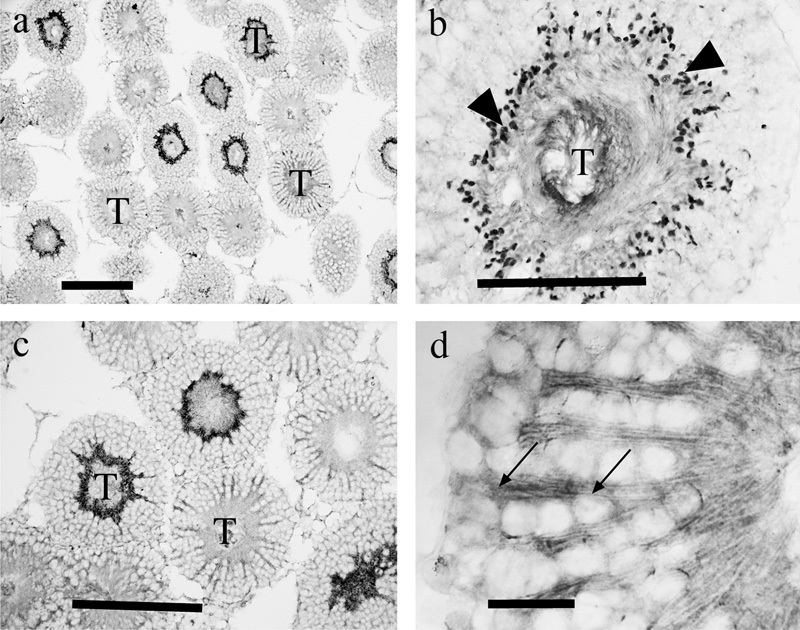
EAAT5 immunolabeling using a C-terminal directed antiserum (a, b) or one directed against the N-terminal (c, d). The pattern of labeling varied with the maturational state of the sperm in individual tubules (T), the most intense labeling (b) being in puncta (arrowheads), which we interpreted as being the head regions of the sperm, and less intensely (d) in radially oriented structures (probably Sertoli cell processes; arrows). Scale bars: a, c=200 µm; b=100 µm; d=25 µm. EAAT, excitatory amino acid transporter; T, tubules.
Immunolabeling of isolated sperm
Immunolabeling for each EAAT in sections of testis revealed that some tubules displayed little labeling, while others displayed intense labeling, due at least in part to the differing maturational stages of the sperm. Accordingly, isolated sperm were evaluated to determine the distribution of glutamate transporters in more mature isolated sperm (Figure 11a–g).
Figure 11.
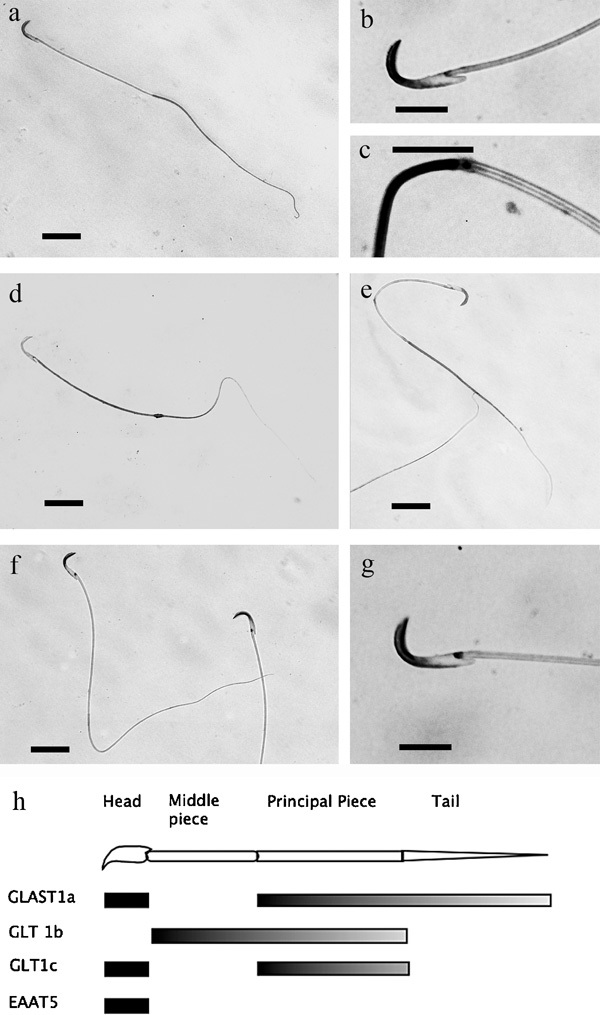
Immunolabeling of isolated sperm for GLAST1a (a–c), GLT1b (d), GLT1c (e) and EAAT5 (f, g). The abrupt change in labeling for GLAST1a between the head and the mid-piece, and between the mid-piece and tail is depicted at high magnification (b, c). Similarly, the localization of EAAT5 in the head is depicted at high magnification (g). (h) Schematic summary of localizations. Scale bars: a, d, e, f=25 µm; b, c, g=10 µm. EAAT, excitatory amino acid transporter; GLAST, glutamate aspartate transporter; GLT, glutamate transporter.
Each of the glutamate transporters detected in sperm in sections of testis was again present in the isolated sperm. However, differences in distribution, which we interpret as representing further maturation of the sperm as they exit the testis, were evident. Curiously, each glutamate transporter exhibited a unique pattern of distribution within each individual sperm, which is summarized graphically in Figure 11h; thus, GLAST1a antibodies, which label the heads of sperm in the sectioned material, also labeled the principal piece and tail regions of the isolated sperm (Figure11a–c). By contrast, GLT1B antibodies labeled the middle and principal piece of the sperm (Figure 11d), GLT1C antibodies predominantly labeled the head and principal piece (Figure 11e), while EAAT5 antibodies labeled only the heads of the sperm (Figure 11f and g). Control experiments for each antibody revealed no labeling (data not shown).
Distribution of endogenous glutamate
Immunocytochemistry on glutaraldehyde-fixed specimens of testis revealed a heterogeneous pattern of staining for glutamate (Figure 12a and b) with little or no labeling in most interstitial cells, or in spermatogonia at the outer surface of the tubules. Conversely, labeling was intense in many cells in deeper layers of the tubules, though unlabeled radial processes of what we believe to be Sertoli cells were weakly labeled or unlabeled. In the central parts of the tubules, an unlabeled zone was present. In the luminal regions of the tubules, parts of the sperm were strongly labeled (Figure 12c). Amorphous deposits seen in the luminal regions of some tubules (Figure 12c) were interpreted as representing the localization of glutamate in seminal fluids that has become immobilized during the fixation process.
Figure 12.
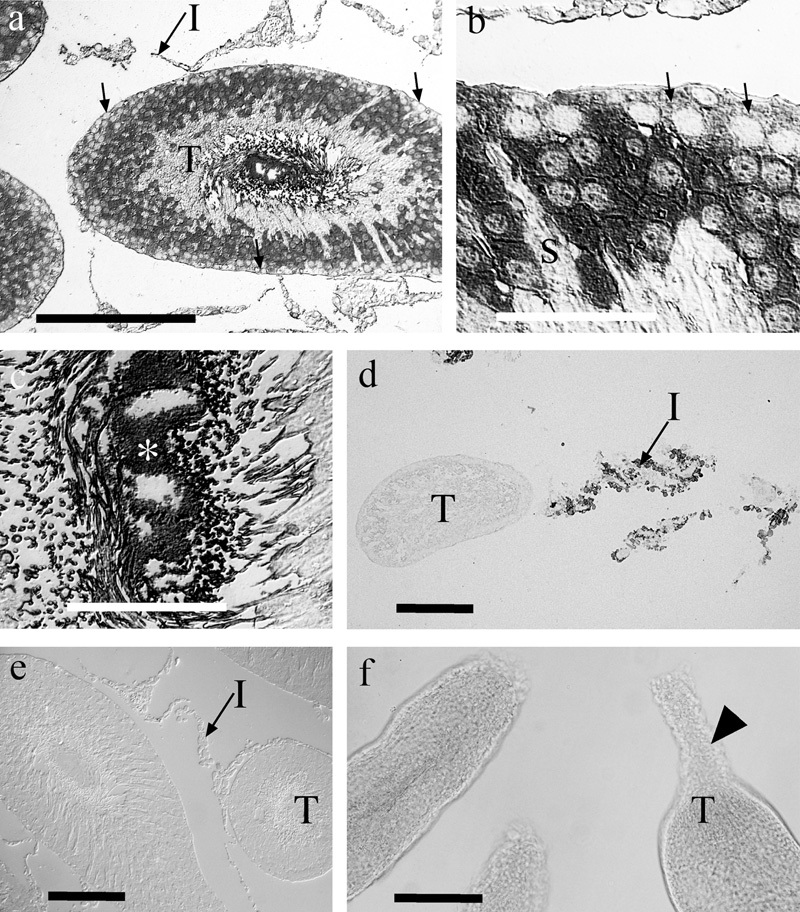
Immunocytochemistries for endogenous glutamate and for 𝒹-aspartate. Glutamate immunoreactivity (a–c) was associated with somata in the outer two-thirds of the tubules (T), though the most superficial cells (arrows), presumed to be spermatogonia, tended to be weakly labeled. Radially oriented Sertoli cells (S) were frequently unlabeled or very weakly labeled. Strong labeling of the lumen of the tubules suggested that high levels of glutamate were present in the sperm and in amorphous deposits (*), which we interpret as representing glutamate that was fixed in the luminal fluid. Very little glutamate labeling was present in interstitial cells (I). 𝒹-aspartate uptake (d) was evident in interstitial cells (I) but not in the tubules (T). Conversely, in control sections (e) not exposed to 𝒹-aspartate, the interstitial cells were unlabeled. Panel f demonstrates that the cut ends of the isolated tubules were typically sealed (arrowhead) due to constriction of the tubules, thereby preventing ingress of 𝒹-aspartate. Scale bars: a, d, e, f=100 µm; b, c=25 µm.
Immunolabeling for 𝒹-aspartate
Uptake of 𝒹-aspartate into interstitial cells was readily demonstrated (Figure 12d). Surprisingly, however, no uptake into tubules was observed despite the presence of many glutamate transporters. Labeling for 𝒹-aspartate in testes that had been fixed without prior exposure to exogenous 𝒹-aspartate resulted in no specific signals, suggesting that if any 𝒹-aspartate was present it was at levels below our detection threshold (Figure 12e). Careful scrutiny of the preparations that were being exposed to 𝒹-aspartate revealed the reason for the lack of uptake; we had assumed that the blood–testis barrier at the surface of the tubules would prevent 𝒹-aspartate uptake and this appeared to be correct. Conversely, entry of 𝒹-aspartate into the tubules via the lumen (where the blood–testis barrier does not exist) was anticipated. However, we observed (Figure 12f) that the tubules contracted within 1–5 s of being cut open, effectively sealing the lumen of the tubule, thus preventing 𝒹-aspartate entry.
Discussion
This study demonstrates that multiple forms of glutamate transporters are expressed in the rat testis at both the mRNA and protein level. The study strongly supports the prior studies and conclusions of Takarada et al.,20 who showed that GLAST and GLT1 were detectable but that these proteins were typically smaller than those in brain. Similarly, our study supports the prior finding of functional GLT1 in testis.9, 14 The current study demonstrates that multiple splice variants of these two transporters are present and that some of these splicings result in smaller proteins of a size commensurate with those detected by Takarada et al.20 A more fine-grained comparison of results is unfortunately difficult because of limited information as to the precise epitopes being detected by commercial antibodies used in prior studies. Thus, the GLT1 antibody from Chemicon (Temecula, CA, USA), used by Takarada et al.,20 is directed against an undisclosed epitope in the last 30 amino acids of the carboxyl-terminal region, rendering it potentially able to detect GLT1a, GLT1b and/or GLT1c. The Chemicon antibody has been shown, in western blotting, to detect brain proteins varying between 42 and 71 kDa,39, 40 a size range that precludes immediate distinction of the forms of GLT1 protein detected. Similarly, the Santa Cruz GLT1 antibody used by Hu et al.14 detects an undisclosed internal epitope, suggesting that it may have the potential to detect multiple different splice variants. We suggest that the forms of GLT1 present in testis, and detected by earlier studies, would predominantly include GLT1b and GLT1c and possibly a C-terminal cleaved form of GLT1a.
The commercial GLAST antibody used by Takarada et al.20 is directed against the sequence QLIAQDNEPEKPVADSETKM, which corresponds to the extreme carboxyl-terminal region of rat GLAST (information kindly communicated to the authors by Millipore, Billerica, MA, USA). Thus, the results would accord with our data, which shows a small pool of C-terminal-immunoreactive GLAST. Our finding that this pool of C-terminal-immunoreactive GLAST migrates in western blots at comparable size to GLAST1a and GLAST1b suggests that the C-terminal GLAST we detect is also potentially alternately spliced at exon-3 or exon-9. The limited amount of C-terminal-immunoreactive GLAST that we describe in the testis might explain why it was apparently not detected in western blots by Takarada et al..20 Our ability to detect uptake of 𝒹-aspartate into interstitial cells that express immunoreactivity for C-terminal GLAST/GLAST1a/GLAST1b suggests that these proteins may be functional transporters. The lack of detectable amino-terminal GLAST in the tissue, despite being consistently detected in brain tissues in our hands,19, 24, 40, 41 suggests that the amino-terminus may be modified or cleaved in the testis. Susarla et al.42 have noted that increased transport activity by GLAST can be associated with a paradoxical loss of immunoreactivity for N- and C-terminal epitopes of GLAST, a phenomenon previously noted as indicating the modification or cleavage of these regions. Similarly, our previous studies have shown that the amino-terminal region of GLAST1b is typically absent.23 We conclude that much of the GLAST in the testis is the exon-3- and exon-9-skipping forms, but with the additional caveat that these splice variants also lack the amino-terminal region, and mostly lack the carboxyl-terminal region.
The lack of EAAC1 detected in this study at the mRNA and protein level contrasts with the prior observations of Takarada et al.,20 who detected a PCR product. The reasons for this disparity are unclear but cannot represent methodological failure, as we are readily able to detect appropriate PCR products from brain tissues as shown in this study, and can similarly detect protein in the brain.43 There may be several reasons for this disparity; thus, Jin et al.44 have previously described an alternate promoter for EAAC1, which yields a smaller EAAC1 protein. The involvement of multiple promoters in driving transcription of EAAC1 may occur in different tissue types and produce transcripts that display demonstrable heterogeneity at the 5′ UTR. This suggestion is based on a substantial number of reports linking multiplicity of promoters with heterogeneity at the 5′ UTR.45, 46, 47 In the current study, the PCR was conducted using primers designed in the 5′ and 3′ UTR of EAAC1; it is plausible that use of an alternate promoter might generate an alternate UTR region that would therefore not be complementary to our primer sequences. This possibility remains the subject of future investigations.
In this study, PCR products for EAAT4 were not detected and EAAT4 protein was not detected in western blotting or by immunocytochemistry. This accords with the lack of protein found by Takarada et al.20 and lack of signals in their in situ hybridization experiments, but is again in contrast to their finding of a PCR product. Similar reasons to those advanced with respect to lack of signal for EAAC1 PCR may be applicable. Other variables such as numbers of amplification cycles (35 in this study versus 40 cycles in the Takarada et al.'s study) or other factors remains to be determined, but even if very limited amounts of mRNA encoding EAAC1 or EAAT4 are present, such mRNA is unlikely to play subsequent biological roles. We suggest that the lack of detectable EAAC1 and EAAT4 protein in the Takarada et al.'s20 study and in this study strongly indicates that these two proteins are absent.
By contrast, the abundance of EAAT5 protein that we detect by both of our antibodies at the appropriate molecular weight and the detection of abundant mRNA by PCR strongly suggest that this transporter is important in the testis, for reasons that are discussed later.
Are the glutamate transporters that we detect functional?
In any immunocytochemical analysis of the expression of transporters, there is always room for doubt as to whether they are functional.37 In this study, we have demonstrated that at the cellular level, cells expressing GLAST/GLAST1a are capable of transporting 𝒹-aspartate, which is a non-metabolizable analog of glutamate. Thus, this transporter is by inference, capable of transporting glutamate. The contraction of the myoid cells48 precluded entry of 𝒹-aspartate into the lumen of the seminal tubules, preventing an examination of the transport properties of cells within the tubules. However, the lack of labeling did allow us to confirm that the Sertoli cells (which form the blood–testis barrier) did not express functional transporters at least on their outer surface, and that the blood–testis barrier was impermeable to 𝒹-aspartate. This accords with the earlier observations of Setchell et al.,2 who described the barrier as being impermeable to glutamate.
To bypass the barriers presented by the Sertoli cells and the rapid sealing of the tubules when cut, it would be technically simple to disassociate the tubules, but such an approach would then reduce the probability that we could resolve transport properties at the identified cell level. Accordingly, we have not adopted this approach.
Full-length glutamate transporters including carboxyl-terminal splice variants such as GLT1b and GLT1c are undoubtedly capable of transporting glutamate in the brain, and at least some of the GLT1 in the testis can transport glutamate as assessed biochemically.9 It has been reported that GLAST1a is a functional transporter in bone,49 and so, in accord with our prior comments, is probably also functional in the testis. The role of GLAST1b is unclear, since although it is abundant in the brain, especially after insults,23 there are suggestions that it is not a functional glutamate transporter, but may interfere with trafficking of glutamate by normally spliced forms of GLAST.50
Maturation of glutamate transporter expression
An overt feature of our immunocytochemical studies was the presence of maturational differences in immunolabeling characteristics between sperm in different tubules, and a further maturation of this in more mature sperm isolated from the rete testis. The mechanisms for this shift in expression patterns are unclear as is its significance, but it is noteworthy that each transporter ultimately adopts a distinct distribution pattern in the sperm, possibly suggestive of regional variation in the significance and characteristics of glutamate transport within individual compartments of the sperm. The differential compartmentation of glutamate transporters within very small domains of sperm is comparable to the microcompartmentation of such proteins in the brain and retina.21, 38
Roles of glutamate and possible functional consequences of glutamate transporter expression
It has previously been shown that mice which lack EAAC1 in the epididymis (due to the knockout of c-ros)11 exhibit swollen spermatozoa.12 This has been interpreted as an example of the role of glutamate as an osmolyte, which in this instance would be dys-regulated due to impaired epididymal supply of the osmolyte (glutamate) or its premature loss.51
Other less overt roles for glutamate transporters are also possible; thus, EAAT5, which is present in the sperm, exhibits a large chloride conductance that is co-associated with glutamate transport. Evoking such a conductance by exposing sperm to glutamate would have the effect of hyperpolarizing the sperm and could thus contribute to the hyperpolarization-mediated activation of sperm.52 Moreover, it is known that extracellular glutamate concentration rises in a proximal–distal manner in the epididymis,53 as does sperm motility.54 Accordingly, it is possible that glutamate-evoked EAAT5-mediated chloride fluxes may have a role in modifying sperm motility.
Distribution and possible roles of glutamate in the testis
Endogenous glutamate was present in a large number of cells in the testis; notable exceptions included the interstitial cells that accumulate 𝒹-aspartate and express GLAST1a but contain little detectable glutamate. This may be attributable to the glia-like phenotype of these cells, since they express high levels of glutamine synthetase, an enzyme that rapidly metabolizes any available glutamate to glutamine.16, 55 We do not believe that this disparity is attributable to delays in fixation of the testis, since such delays in fixation normally cause a rise in glutamate levels in tissues that express glutamine synthetase.26 It might be expected that other cells that express the glutamate-synthesizing enzyme glutamate dehydrogenase, such as the Sertoli cells,56 might contain detectable pools of glutamate. The conspicuous lack of glutamate that was observed in the Sertoli cells is readily explainable; the data suggest that the glutamate is either rapidly metabolized to other compounds, or, plausibly (since glutamate dehydrogenase is reversible), may be driving the catabolism of glutamate back to α-ketoglutarate.
The roles of endogenous glutamate pools in the testis remain unclear. Multiple roles for glutamate have been proposed in addition to putative actions as a ‘neurotransmitter' and as an osmolyte. These roles include the production of gamma-aminobutyric acid and glutathione. Since gamma-aminobutyric acid appears to be synthesized in both sperm57 and interstitial glia-like Leylig cells,58 this would be compatible with the view that GLAST1a and GLAST1b might supply some glutamate for this synthetic process in Leydig cells, while multiple glutamate transporters might contribute to substrate provision in sperm. Similarly, high levels of glutathione and the synthetic machinery to produce it are present in the testis,59 particularly in cells such as spermatogonia, spermatocytes and Sertoli cells. Other glutamate-like compounds such as citryl-ℓ-glutamic acid are known to be highly enriched in the testis,60 but it is unclear where they are localized and how they relate metabolically to glutamate.
Conclusions
A surprisingly wide range of glutamate transporters are present in the testis and many are probably functional. The prior evidence for synthesis of glutamate in the testis,7 the possession of a blood–testis barrier and the evidence for multiple transporters in the testis indicate that glutamate homeostasis in the testis is a complex phenomenon. Determining if co-expressed glutamate transporters have discrete or overlapping roles remains the subject for future investigations.
Author contributions
Aven Lee performed PCR and western blotting experiments for forms of GLT-1. Ashley Anderson performed western blotting for other forms of glutamate transporters. Amanda Barnett performed immunolabeling of isolated sperm preparations. Anthony Chan performed all the critical histology. David Pow performed immunocytochemistry of tissue sections and 𝒹-aspartate uptake studies. All authors contributed intellectually to the study design, interpretation and MS preparation.
Acknowledgments
This study was supported by grants from NHMRC (Australia). DVP is supported by an NHMRC fellowship. We thank Ms Lauren Macnab for her skilled technical assistance in preliminary parts of the study.
The authors declare no competing financial interests.
References
- Mann T. The Biochemistry of Semen and the Male Reproductive Tract. London: Methuen; 1964. [Google Scholar]
- Setchell BP, Hinks NT, Voglmayr JK, Scott TW. Amino acids in ram testicular fluid and semen and their metabolism by spermatozoa. Biochem J. 1967;105:1061–5. doi: 10.1042/bj1051061. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Härkönen M, Suvanto O, Kormano M. Glutamate in post-natal rat testis. J Reprod Fertil. 1970;21:533–6. doi: 10.1530/jrf.0.0210533. [DOI] [PubMed] [Google Scholar]
- Mushahwar IK, Koeppe RE. Free amino acids of testes—concentrations of free amino acids in the testes of several species and the precursors of glutamate and glutamine in rat testes in vivo. Biochem J. 1973;132:353–9. doi: 10.1042/bj1320353. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kochakian CD. Free amino acids of sex organs of the mouse: regulation by androgen. Am J Physiol. 1975;228:1231–5. doi: 10.1152/ajplegacy.1975.228.4.1231. [DOI] [PubMed] [Google Scholar]
- Hinton BT. The testicular and epididymal luminal amino acid microenvironment in the rat. J Androl. 1990;11:498–505. [PubMed] [Google Scholar]
- Setchell BP, Voglmayr JK, Waites GM. A blood–testis barrier restricting passage from blood into rete testis fluid but not into lymph. J Physiol. 1969;200:73–85. doi: 10.1113/jphysiol.1969.sp008682. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Storto M, Sallese M, Salvatore L, Poulet R, Condorelli DF, et al. Expression of metabotropic glutamate receptors in the rat and human testis. J Endocrinol. 2001;170:71–8. doi: 10.1677/joe.0.1700071. [DOI] [PubMed] [Google Scholar]
- Hu JH, Yang N, Ma YH, Jiang J, Zhang JF, et al. Identification of glutamate transporters and receptors in mouse testis. Acta Pharmacol Sin. 2004;25:366–71. [PubMed] [Google Scholar]
- Nagata Y, Homma H, Matsumoto M, Imai K. Stimulation of steroidogenic acute regulatory protein StAR gene expression by 𝒹-aspartate in rat Leydig cells. FEBS Lett. 1999;454:317–20. doi: 10.1016/s0014-5793(99)00840-6. [DOI] [PubMed] [Google Scholar]
- Wagenfeld A, Yeung CH, Lehnert W, Nieschlag E, Cooper TG. Lack of glutamate transporter EAAC1 in the epididymis of infertile c-ros receptor tyrosine-kinase deficient mice. J Androl. 2002;23:772–82. [PubMed] [Google Scholar]
- Cooper TG, Wagenfeld A, Cornwall GA, Hsia N, Chu ST, et al. Gene and protein expression in the epididymis of infertile c-ros receptor tyrosine kinase-deficient mice. Biol Reprod. 2003;69:1750–62. doi: 10.1095/biolreprod.103.017566. [DOI] [PubMed] [Google Scholar]
- Froman DP, Wardell JC, Feltmann AJ. Sperm mobility: deduction of a model explaining phenotypic variation in roosters Gallus domesticus. Biol Reprod. 2006;74:487–91. doi: 10.1095/biolreprod.105.046755. [DOI] [PubMed] [Google Scholar]
- Hu JH, Yang N, Ma YH, Jiang J, Zhang JF, et al. Identification of glutamate receptors and transporters in mouse and human sperm. J Androl. 2004;25:140–6. doi: 10.1002/j.1939-4640.2004.tb02769.x. [DOI] [PubMed] [Google Scholar]
- Waites GM, Gladwell RT. Physiological significance of fluid secretion in the testis and blood–testis barrier. Physiol Rev. 1982;62:624–71. doi: 10.1152/physrev.1982.62.2.624. [DOI] [PubMed] [Google Scholar]
- Holash JA, Harik SI, Perry G, Stewart PA. Barrier properties of testis microvessels. Proc Natl Acad Sci USA. 1993;90:11069–73. doi: 10.1073/pnas.90.23.11069. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Davidoff MS, Middendorff R, Köfüncü E, Müller D, Jezek D, et al. Leydig cells of the human testis possess astrocyte and oligodendrocyte marker molecules. Acta Histochem. 2002;104:39–49. doi: 10.1078/0065-1281-00630. [DOI] [PubMed] [Google Scholar]
- Danbolt NC. Glutamate uptake. Prog Neurobiol. 2001;65:1–105. doi: 10.1016/s0301-0082(00)00067-8. [DOI] [PubMed] [Google Scholar]
- Williams SM, Sullivan RK, Scott HL, Finkelstein DI, Colditz PB, et al. Glial glutamate transporter expression patterns in brains from multiple mammalian species. Glia. 2005;49:520–41. doi: 10.1002/glia.20139. [DOI] [PubMed] [Google Scholar]
- Takarada T, Hinoi E, Balcar VJ, Taniura H, Yoneda Y. Possible expression of functional glutamate transporters in the rat testis. J Endocrinol. 2004;181:233–44. doi: 10.1677/joe.0.1810233. [DOI] [PubMed] [Google Scholar]
- Reye P, Sullivan R, Scott H, Pow DV. Distribution of two splice variants of the glutamate transporter GLT1 in rat brain and pituitary. Glia. 2002;38:246–55. doi: 10.1002/glia.10059. [DOI] [PubMed] [Google Scholar]
- Sullivan R, Rauen T, Fischer F, Wiessner M, Grewer C, et al. Cloning, transport properties, and differential localization of two splice variants of GLT-1 in the rat CNS: implications for CNS glutamate homeostasis. Glia. 2004;45:155–69. doi: 10.1002/glia.10317. [DOI] [PubMed] [Google Scholar]
- Sullivan SM, Macnab LT, Björkman ST, Colditz PB, Pow DV. GLAST1b, the exon-9 skipping form of the glutamate-aspartate transporter EAAT1 is a sensitive marker of neuronal dysfunction in the hypoxic brain. Neuroscience. 2007;149:434–45. doi: 10.1016/j.neuroscience.2007.08.011. [DOI] [PubMed] [Google Scholar]
- Pow DV, Sullivan R, Scott H. Antibody production and immunocytochemical localization of amino acid transporters. Methods Mol Biol. 2003;227:213–44. doi: 10.1385/1-59259-387-9:213. [DOI] [PubMed] [Google Scholar]
- Pow DV, Crook DK. Extremely high titre polyclonal antisera against small neurotransmitter molecules: rapid production, characterisation and use in light- and electron-microscopic immunocytochemistry. J Neurosci Methods. 1993;48:51–63. doi: 10.1016/s0165-0270(05)80007-x. [DOI] [PubMed] [Google Scholar]
- Pow DV, Crook DK. Rapid postmortem changes in the cellular localisation of amino acid transmitters in the retina as assessed by immunocytochemistry. Brain Res. 1994;653:199–209. doi: 10.1016/0006-8993(94)90390-5. [DOI] [PubMed] [Google Scholar]
- Pow DV, Robinson SR. Glutamate in some retinal neurons is derived solely from glia. Neuroscience. 1994;60:355–66. doi: 10.1016/0306-4522(94)90249-6. [DOI] [PubMed] [Google Scholar]
- Barnett NL, Pow DV. Antisense knockdown of GLAST, a glial glutamate transporter, compromises retinal function. Invest Ophthalmol Vis Sci. 2000;41:585–91. [PubMed] [Google Scholar]
- Pow DV, Barnett NL. Changing patterns of spatial buffering of glutamate in developing rat retinae are mediated by the Müller cell glutamate transporter GLAST. Cell Tissue Res. 1999;297:57–66. doi: 10.1007/s004410051333. [DOI] [PubMed] [Google Scholar]
- Williams SM, Macnab LT, Pow DV. Cryptic expression of functional glutamate transporters in the developing rodent brain. Neuron Glia Biol. 2006;2:199–215. doi: 10.1017/S1740925X06000263. [DOI] [PubMed] [Google Scholar]
- Macnab LT, Williams SM, Pow DV. Expression of the exon 3 skipping form of GLAST, GLAST1a, in brain and retina. Neuroreport. 2006;17:1867–70. doi: 10.1097/WNR.0b013e328010b898. [DOI] [PubMed] [Google Scholar]
- Macnab LT, Pow DV. Expression of the exon 9-skipping form of EAAT2 in astrocytes of rats. Neuroscience. 2007;150:705–11. doi: 10.1016/j.neuroscience.2007.09.049. [DOI] [PubMed] [Google Scholar]
- Macnab LT, Pow DV. Central nervous system expression of the exon 9 skipping form of the glutamate transporter GLAST. Neuroreport. 2007;18:741–5. doi: 10.1097/WNR.0b013e3280c143b0. [DOI] [PubMed] [Google Scholar]
- Pow DV, Barnett NL. Developmental expression of excitatory amino acid transporter 5 a photoreceptor and bipolar cell glutamate transporter in rat retina. Neurosci Lett. 2000;280:21–4. doi: 10.1016/s0304-3940(99)00988-x. [DOI] [PubMed] [Google Scholar]
- Pow DV, Cook DG. Neuronal expression of splice variants of “glial” glutamate transporters in brains afflicted by Alzheimer's disease: unmasking an intrinsic neuronal property. Neurochem Res. 2009;34:1748–57. doi: 10.1007/s11064-009-9957-0. [DOI] [PubMed] [Google Scholar]
- Rauen T, Wiessner M, Sullivan R, Lee A, Pow DV. A new GLT1 splice variant cloning and immunolocalization of GLT1c in the mammalian retina and brain. Neurochem Int. 2004;45:1095–106. doi: 10.1016/j.neuint.2004.04.006. [DOI] [PubMed] [Google Scholar]
- Pow DV, Barnett NL, Penfold P. Are neuronal transporters relevant in retinal glutamate homeostasis. Neurochem Int. 2000;37:191–8. doi: 10.1016/s0197-0186(00)00022-x. [DOI] [PubMed] [Google Scholar]
- Reye P, Sullivan R, Fletcher EL, Pow DV. Distribution of two splice variants of the glutamate transporter GLT1 in the retinas of humans, monkeys, rabbits, rats, cats, and chickens. J Comp Neurol. 2002;445:1–12. doi: 10.1002/cne.10095. [DOI] [PubMed] [Google Scholar]
- Boudaba C, Linn DM, Halmos KC, Tasker JG. Increased tonic activation of presynaptic metabotropic glutamate receptors in the rat supraoptic nucleus following chronic dehydration. J Physiol. 2003;551:815–23. doi: 10.1113/jphysiol.2003.042739. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Diaz CM, Macnab LT, Williams SM, Sullivan RK, Pow DV. EAAT1 and 𝒹-serine expression are early features of human retinal development. Exp Eye Res. 2007;84:876–85. doi: 10.1016/j.exer.2007.01.008. [DOI] [PubMed] [Google Scholar]
- Shin JW, Nguyen KT, Pow DV, Knight T, Buljan V, et al. Distribution of glutamate transporter GLAST in membranes of cultured astrocytes in the presence of glutamate transport substrates and ATP. Neurochem Res. 2009;34:1758–66. doi: 10.1007/s11064-009-9982-z. [DOI] [PubMed] [Google Scholar]
- Susarla BT, Seal RP, Zelenaia O, Watson DJ, Wolfe JH, et al. Differential regulation of GLAST immunoreactivity and activity by protein kinase C—evidence for modification of amino and carboxyl termini. J Neurochem. 2004;291:1151–63. doi: 10.1111/j.1471-4159.2004.02791.x. [DOI] [PubMed] [Google Scholar]
- Duerson K, Woltjer RL, Mookherjee P, Leverenz JB, Montine TJ, et al. Detergent-insoluble EAAC1/EAAT3 aberrantly accumulates in hippocampal neurons of Alzheimer's disease patients. Brain Pathol. 2009;19:267–78. doi: 10.1111/j.1750-3639.2008.00186.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jin XP, Peng JB, Huang F, Zhu YN, Fei J, et al. An mRNA molecule encoding truncated excitatory amino acid carrier 1 (EAAC1) protein (EAAC2) is transcribed from an independent promoter but not an alternative splicing event. Cell Res. 2002;12:257–62. doi: 10.1038/sj.cr.7290132. [DOI] [PubMed] [Google Scholar]
- Nakamuta M, Oka K, Krushkal J, Kobayashi K, Yamamoto M, et al. Alternative mRNA splicing and differential promoter utilization determine tissue-specific expression of the apolipoprotein B mRNA editing protein (Apobec) gene in mice. Structure and evaluation of Apobec and related nucleotide deaminases. J Biol Chem. 1995;270:13042–56. doi: 10.1074/jbc.270.22.13042. [DOI] [PubMed] [Google Scholar]
- Rajagopalan S, Wan DF, Habib GM, Sepulveda AR, McLeod MR, et al. Six mRNA with different 5′ ends are encoded by a single γ-glutammyltransferase gene in the mouse. Proc Natl Acad Sci USA. 1993;90:6179–83. doi: 10.1073/pnas.90.13.6179. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schibler U, Sierra F. Alternative promoters in developmental gene expression. Annu Rev Genet. 1987;21:237–57. doi: 10.1146/annurev.ge.21.120187.001321. [DOI] [PubMed] [Google Scholar]
- Maekawa M, Kamimura K, Nagano T. Peritubular myoid cells in the testis, their structure and function. Arch Histol Cytol. 1996;59:1–13. doi: 10.1679/aohc.59.1. [DOI] [PubMed] [Google Scholar]
- Huggett J, Vaughan-Thomas A, Mason D. The open reading frame of the Na+-dependent glutamate transporter GLAST-1 is expressed in bone and a splice variant of this molecule is expressed in bone and brain. FEBS Lett. 2000;485:13–18. doi: 10.1016/s0014-5793(00)02175-x. [DOI] [PubMed] [Google Scholar]
- Vallejo-Illarramendi A, Domercq M, Matute C. A novel alternative splicing form of excitatory amino acid transporter 1 is a negative regulator of glutamate uptake. J Neurochem. 2005;95:341–8. doi: 10.1111/j.1471-4159.2005.03370.x. [DOI] [PubMed] [Google Scholar]
- Cooper TG, Barfield JP. Utility of infertile male models for contraception and conservation. Mol Cell Endocrinol. 2006;250:206–11. doi: 10.1016/j.mce.2005.12.047. [DOI] [PubMed] [Google Scholar]
- Gatti JL, Billard R, Christen R. Ionic regulation of the plasma membrane potential of rainbow trout (Salmo gairdneri) spermatozoa: role in the initiation of sperm motility. J Cell Physiol. 1990;143:546–54. doi: 10.1002/jcp.1041430320. [DOI] [PubMed] [Google Scholar]
- Pruneda A, Yeung CH, Bonet S, Pinart E, Cooper TG. Concentrations of carnitine, glutamate and myo-inositol in epididymal fluid and spermatozoa from boars. Anim Reprod Sci. 2007;97:344–55. doi: 10.1016/j.anireprosci.2006.01.013. [DOI] [PubMed] [Google Scholar]
- van der Horst G, Seier JV, Spinks AC, Hendricks S. The maturation of sperm motility in the epididymis and vas deferens of the vervet monkey, Cercopithecus aethiops. Int J Androl. 1999;22:197–207. doi: 10.1046/j.1365-2605.1999.00171.x. [DOI] [PubMed] [Google Scholar]
- van Straaten HW, He Y, van Duist MM, Labruyère WT, Vermeulen JL, et al. Cellular concentrations of glutamine synthetase in murine organs. Biochem Cell Biol. 2006;84:215–31. doi: 10.1139/o05-170. [DOI] [PubMed] [Google Scholar]
- Spanaki C, Zaganas I, Kleopa KA, Plaitakis A. Human GLUD2 glutamate dehydrogenase is expressed in neural and testicular supporting cells. J Biol Chem. 2010;285:16748–56. doi: 10.1074/jbc.M109.092999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Persson H, Pelto-Huikko M, Metis M, Soder O, Brene S, et al. Expression of the neurotransmitter-synthesizing enzyme glutamic acid decarboxylase in male germ cells. Mol Cell Biol. 1990;10:4701–11. doi: 10.1128/mcb.10.9.4701. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Geigerseder C, Doepner R, Thalhammer A, Frungieri MB, Gamel-Didelon K, et al. Evidence for a GABAergic system in rodent and human testis—local GABA production and GABA receptors. Neuroendocrinology. 2003;77:314–23. doi: 10.1159/000070897. [DOI] [PubMed] [Google Scholar]
- Castellón EA. Glutathione and gamma-glutamyl cycle enzymes in rat testis during sexual maturation. Arch Androl. 2003;33:179–85. doi: 10.3109/01485019408987822. [DOI] [PubMed] [Google Scholar]
- Miyake M, Kume S, Kakimoto Y. Correlation of the level of beta-citryl-ℓ-glutamic acid with spermatogenesis in rat testes. Biochim Biophys Acta. 1982;719:495–500. doi: 10.1016/0304-4165(82)90238-0. [DOI] [PubMed] [Google Scholar]


