Abstract
The aim of this study was to validate the advantages of the intrafascial nerve-sparing technique compared with the interfascial nerve-sparing technique in extraperitoneal laparoscopic radical prostatectomy. From March 2010 to August 2011, 65 patients with localized prostate cancer (PCa) underwent bilateral intrafascial nerve-sparing extraperitoneal laparoscopic radical prostatectomy. These patients were matched in a 1∶2 ratio to 130 patients with localized PCa who had undergone bilateral interfascial nerve-sparing extraperitoneal laparoscopic radical prostatectomy between January 2008 and August 2011. Operative data and oncological and functional results of both groups were compared. There was no difference in operative data, pathological stages and overall rates of positive surgical margins between the groups. There were 9 and 13 patients lost to follow-up in the intrafascial group and interfascial group, respectively. The intrafascial technique provided earlier recovery of continence at both 3 and 6 months than the interfascial technique. Equal results in terms of continence were found in both groups at 12 months. Better rates of potency at 6 months and 12 months were found in younger patients (age ≤65 years) and overall patients who had undergone the intrafascial nerve-sparing extraperitoneal laparoscopic radical prostatectomy. Biochemical progression-free survival rates 1 year postoperatively were similar in both groups. Using strict indications, compared with the interfascial nerve-sparing technique, the intrafascial technique provided similar operative outcomes and short-term oncological results, quicker recovery of continence and better potency. The intrafascial nerve-sparing technique is recommended as a preferred approach for young PCa patients who are clinical stages cT1 to cT2a and have normal preoperative potency.
Keywords: continence, interfascial nerve-sparing, intrafascial nerve-sparing, laparoscopic radical prostatectomy, potency, prostate cancer (PCa)
Introduction
In men with localized prostate cancer (PCa) and a life expectancy of >10 years, the goal of radical prostatectomy by any approach must be the eradication of disease while preserving continence and, whenever possible, potency. After Walsh and Donker distinguished the locations of the neurovascular bundles (NVB) in relation to the fascial planes around the prostate, the opportunity to provide excellent surgical cancer control with satisfactory continence and potency was presented.1,2
In 1997, the first series describing the transperitoneal laparoscopic radical prostatectomy and the initial experience with extraperitoneal laparoscopic radical prostatectomy (ELRP) were reported by Schuessler et al.3 and Raboy et al.,4 respectively. With the popularization of laparoscopic instruments, based on updated anatomical studies, interfascial nerve-sparing techniques have been applied to improve postoperative continence and potency.5,6,7,8 Stolzenburg et al.9,10 found that the intrafascial nerve-sparing technique enables the dissection of the prostate with limited trauma to the surrounding fascia and the enclosed NVB.
The interfascial nerve-sparing technique was adopted in ELRP for localized PCa at our hospital in 2006. In March 2010, we began to use the intrafascial nerve-sparing technique in ELRP. In this study, we compared operative data, postoperative urinary continence and sexual potency between the intrafascial nerve-sparing techniques and the interfascial nerve-sparing techniques in an attempt to elucidate which technique provides better outcomes.
Materials and methods
Patients selection
In our hospital, candidates for the interfascial nerve-sparing technique were patients with normal preoperative potency, stage T1 or T2 disease, an initial prostate-specific antigen (PSA) of ≤10 ng ml−1 and Gleason scores of ≤7 (3+4). Among them, patients with a clinical stage of cT1 to cT2a and who had ≤3 positive cores on a 12-core prostate biopsy were selected to undergo the intrafascial nerve-sparing technique.
Between March 2010 and August 2011, 65 patients (group A) who underwent the bilateral intrafascial nerve-sparing technique were included in this study. All operations were performed by the same experienced surgeon (XZ). This surgeon performed 329 cases of ELRP before March 2010 and 132 cases of ELRP from March 2010 to August 2011. From patients in this cohort who were under follow-up, we selected 130 cases to serve as the control group in this study (group B). The 130 patients who had undergone bilateral interfascial nerve-sparing ELRP were matched in a 2∶1 ratio to patients who underwent intrafascial nerve-sparing ELRP with respect to age, body mass index, preoperative potency, clinical stage, initial PSA, Gleason score and number of positive cores.
All of the patients had been confirmed as having localized PCa by magnetic resonance imaging and transrectal ultrasound-guided biopsy, as well as a negative radionuclide bone scan. The patient characteristics are shown in Table 1.
Table 1. Perioperative data of both groups were compared.
| Group A (bilateral intrafascial) | Group B (bilateral interfascial) | P | |
|---|---|---|---|
| Patients number | 65 | 130 | |
| Age (year) | 65 (56, 70) | 65 (55, 69) | 0.343 |
| Body mass index | 25.92 (23.73, 27.15) | 25.89 (23.45, 27.11) | 0.562 |
| Initial PSA (ng ml−1) | 5.12 (2.90, 7.85) | 5.98 (2.98, 8.06) | 0.437 |
| Biopsy Gleason score | 6 (6, 7) | 6 (6, 7) | 0.359 |
| Number of positive cores | 2 (1, 3) | 2 (1, 3) | 0.482 |
| Time for the anastomosis (min) | 17 (15, 20) | 16 (15, 19) | 0.477 |
| Operative time (min) | 100 (89, 106) | 96 (86, 104) | 0.465 |
| Estimated blood loss (ml) | 94 (81, 98) | 87 (75, 100) | 0.557 |
| Blood transfusion (ml) | no | no | |
| Placement time of the retropubic drain (day) | 3 (3, 4) | 3 (3, 5) | 0.552 |
| Postoperative catheterization time (day) | 7 (6, 8) | 7 (6, 9) | 0.569 |
| Time to oral intake (day) | 2 (2, 3) | 2 (2, 3) | 0.425 |
| Time to ambulation(day) | 4 (3, 5) | 4 (4, 6) | 0.649 |
| Postoperative hospital stay (day) | 8 (8, 9) | 8 (7, 10) | 0.463 |
| Pathological stages, n (%) | 0.424 | ||
| pT2a | 8 (12.3%) | 25 (19.2%) | |
| pT2b | 21 (32.3%) | 41 (31.5%) | |
| pT2c | 27 (41.5%) | 38 (29.2%) | |
| pT3a | 6 (9.2%) | 17 (13.1%) | |
| pT3b | 3(4.6%) | 9 (6.9%) | |
| Positive surgical margin, n (%) | 8 (12.3%) | 21 (16.2%) | 0.529 |
| BPFS (1 year postoperatively) | 51/56 (91.1%) | 102/117 (87.2%) | 0.613 |
Abbreviations: BPFS, biochemical progression-free survival; PSA, prostate-specific antigen.
Median values and the 25th (Q1) and 75th (Q3) percentiles are presented.
All patients approved the use of their clinical data for this study. The Human Ethics Review Committee of Chinese People's Liberation Army General Hospital approved the study protocol.
Surgical technique
The preperitoneal space was prepared using the technique described by Stolzenburg et al.9,10,11 They introduced a specific method of the placement of five trocars. Usually, four trocars were applied in our operations. The first trocar was placed 0.5–1.0 cm below the umbilicus. The second trocar and the third trocar were lateral to the rectus muscle approximately 2 finger-breadths below the umbilicus on the right side and the left side, respectively. The fourth trocar was placed approximately 2 finger-breadths inside the right anterior superior iliac spine.
As a staging procedure, bilateral pelvic lymph node dissection should be performed when indicated: enlarged lymph nodes found on magnetic resonance imaging, PSA of >10 ng ml−1 and Gleason scores of >7 (4+3). None of the patients in this study had undergone pelvic lymph node dissection.
Interfascial nerve-sparing technique
The fatty and areolar tissue were swept gently from the endopelvic fascia, the anterior surface of the bladder neck and the prostate, respectively. The endopelvic fascia was incised, and the fibrous tissue between the apex of the prostate and the levator ani muscle was separated fully side by side. The puboprostatic ligament was dissected. The dorsal venous complex (DVC) was ligated by a figure-of-eight suture with a 15-cm, 2-zero Vicryl suture (Ethicon Inc., Somerville, NJ, USA).
The bladder neck was identified by palpation with the ultrasonic scalpel and repeated traction on the catheter. A transverse incision was made at the 12 o'clock position, and then blunt and sharp dissections were performed bilaterally in the plane between the bladder neck and the prostate. After dissection of the posterior bladder neck, bilateral deferent ducts were exposed and disconnected. The seminal vesicles were mobilized completely and sharply transected carefully. The posterior layer of the Denonvilliers' fascia was opened horizontally. Blunt dissection down to the apex of the prostate was carefully performed between the prostatic fascia and the endopelvic fascia (Figure 1). The vascular pedicles were controlled using Hem-O-Lok clips (Teleflex Medical, Research Triangle Park, NJ, USA) and sharply transected, and the NVB were preserved as much as possible. The use of coagulation was avoided in the interfascial dissection.
Figure 1.
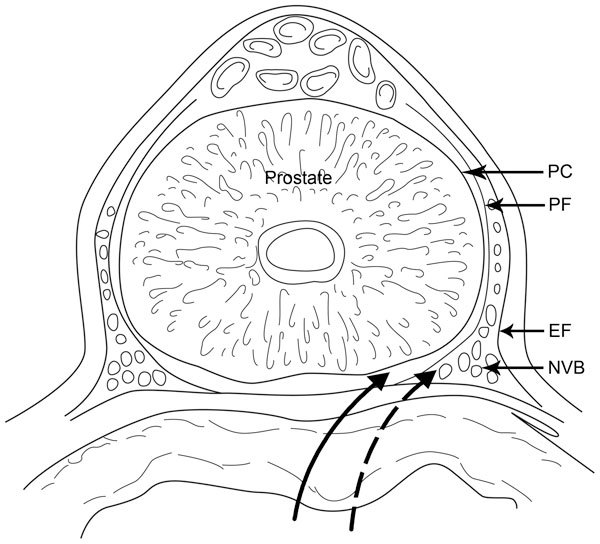
The solid line indicates the direction of the intrafascial dissection between the prostatic capsule and the prostatic fascia. The dashed line indicates the direction of the interfascial dissection between the prostatic fascia and the endopelvic fascia. EF, endopelvic fascia; NVB, neurovascular bundles; PC, prostatic capsule; PF, prostatic fascia.
After the prostate was completely mobilized anteriorly, laterally and posteriorly, the ligated DVC was disconnected close to the prostate. The urethra was separated and transected with sharp scissors at the apex of the prostate and then was maintained as long as possible. The prostate was completely detached and inserted into a specimen bag, which was usually removed at the end of the operation.
A running urethrovesical anastomosis using a 25-cm, 2-zero Monocryl suture with a UR-6 tape needle (Ethicon Inc.) was performed.12 To test the integrity of the urinary reconstruction, the bladder was filled with 200 ml saline. Any saline extravasation identified during this manoeuvre could be controlled with additional interrupted stitches.
The specimen bag was removed via the subumbilical incision. A retropubic drain was placed through the right lateral 5-mm port. After removing of all trocars, the skin wounds were closed. The subumbilical incision and the wound from the 12-mm trocar required deep fascial sutures to prevent wound herniation.
Intrafascial nerve-sparing technique
There were some differences between the intrafascial and interfascial techniques. During the intrafascial nerve-sparing technique, the endopelvic fascia was not incised, the puboprostatic ligament was not dissected and DVC was not ligated. After the fatty and areolar tissue were swept gently from the endopelvic fascia, the anterior surface of the bladder neck and the prostate, the bladder neck was incised. This was followed by the dissection of the posterior bladder neck, and then the bilateral deferent ducts and the seminal vesicles were exposed and transected.
Another difference between the intrafascial and interfascial techniques was that the Denonvilliers' fascia was not incised (Figure 2). Blunt dissection was performed along the prostatic capsule and toward the apex of the prostate in the intrafascial plane, which was between the prostatic capsule and the prostatic fascia (Figure 1). The vascular pedicles were controlled using Hem-O-Lok clips and sharply transected. The periprostatic fascia was dissected at the 3 o'clock position and 9 o'clock position, and the NVB were completely separated from the prostate. The DVC was separated along the surface of the prostate.
Figure 2.
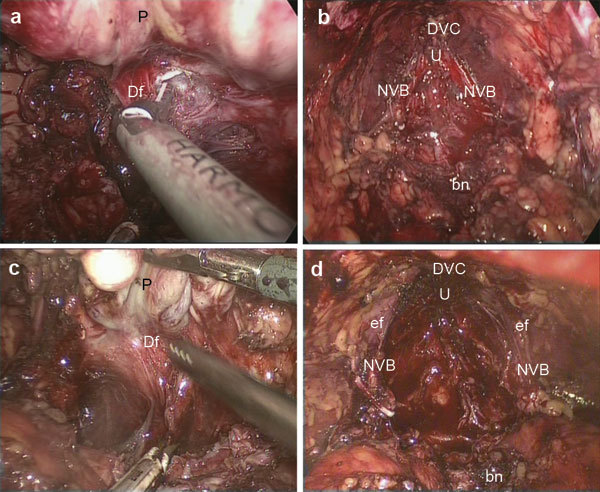
In the interfascial dissection, the Denonvilliers' fascia was opened horizontally (a). In the interfascial dissection, the endopelvic fascia was incised, the puboprostatic ligament was dissected and the DVC was ligated (b). In the intrafascial dissection, the Denonvilliers' fascia was not opened. Along the prostatic capsule, blunt dissection was performed between the prostatic capsule and the prostatic fascia (c). In the intrafascial dissection, the endopelvic fascia was not incised, the puboprostatic ligament was not dissected and the DVC was not ligated (d). bn, bladder neck; Df, Denonvilliers' fascia; DVC, Dorsal venous complex; ef, endopelvic fascia; NVB, neurovascular bundles; U, urethra; P, prostate.
The remainder of the procedure was identical to the interfascial nerve-sparing ELRP.
Postoperative treatment and follow-up
The Foley catheter was normally left in place for at least 5 days. All specimens were examined and diagnosed according to the TNM 2009 classification by an experienced pathologist.
Pre- and postoperative evaluation of continence and potency for all patients was performed using a modified symptom questionnaire and the Sexual Health Inventory for Men (SHIM) questionnaire.13 Patients not requiring any pads or those who did require 1 pad for safety were defined as continent. A requirement of 2–3 pads per day in patients with normal physical activity (walking) was considered ‘mild incontinence' (stress incontinence), and more than 3 pads daily was considered ‘incontinence.' Potency for the current patient series was defined as total scores of ≥22 in the SHIM questionnaire. In this study, all patients had a normal preoperative potency with SHIM scores ≥22. Patients were contacted by telephone, and the answers to the above questions were recorded individually. Questionnaires were mailed to patients without a telephone interview.
Summary statistics are presented as the median and the 25th (Q1) and 75th (Q3) percentiles. Groups were compared using chi-square analysis, Fisher's exact test, and the Mann–Whitney U test, as appropriate. Statistical significance was set at a P value less than 0.05, and all reported P values were two-sided. All data were analyzed using SPSS (Statistical Package for the Social Sciences) 18.0 software.
Results
There were no blood transfusions, no conversions between surgical procedures and no re-interventions in either group. Similar perioperative data were observed in both groups (Table 1). The median of operative time and estimated blood loss in the intrafascial group were slightly higher than in the interfascial group, but the differences were not statistically significant (P=0.465 and P=0.557, the Mann–Whitney U test).
There were five temporary urinary leakages in the intrafascial group requiring prolonged catheterization to 14 days. Three cases of an anterior urethral stricture in the interfascial group were found at 3 months postoperatively and successfully treated with endoscopic urethrotomy. No rectal injuries, lymphoceles, incisional infections or incisional hernias were found in either group.
The pathological stages and overall positive surgical margin (PSM) rates in both groups were similar. Though a higher incidence of pT3 disease was observed in group B, the difference was not statistically significant.
There were 9 and 13 patients lost to follow-up in the intrafascial group and interfascial group, respectively. Biochemical progression-free survival (BPFS) at 1 year postoperatively in both groups was 91.1% and 87.2%. The difference was not statistically significant (Table 1).
Continence and potency results in both groups are shown in Table 2. At 3 months, 80.4%, 14.3% and 5.3% of the patients in group A were defined as continent (0–1 pad per day), mildly incontinent (2–3 pads per day) and incontinent (>3 pads per day), respectively. The percentages for group B were 59.8%, 25.6% and 14.5%, respectively. The differences between the two groups were statistically significant (P=0.025, Fisher's exact test). At 6 months, the percentage of patients who were continent, mildly incontinent and incontinent in both groups were 87.5%, 8.9%, 3.6% and 70.1%, 17.9%, 12.0%, respectively. The differences were statistically significant (P=0.042, Fisher's exact test). At 12 months, the differences between both groups were not statistically significant (P=1.000, Fisher's exact test).
Table 2. Postoperative continence and potency rates.
| Group A ( Bilateral Intrafascial) | Group B ( Bilateral Interfascial) | P | ||
|---|---|---|---|---|
| Continence | Pad Usage | |||
| 3 months | 0–1 pad | 80.4% (45/56) | 59.8% (70/117) | 0.025 |
| 2–3 pads | 14.3% (8/56) | 25.6% (30/117) | ||
| >3 pads | 5.3% (3/56) | 14.5% (17/117) | ||
| 6 months | 0–1 pad | 87.5% (49/56) | 70.1% (82/117) | 0.042 |
| 2–3 pads | 8.9% (5/56) | 17.9% (21/117) | ||
| >3 pads | 3.6% (2/56) | 12.0% (14/117) | ||
| 12 months | 0–1 pad | 96.6% (53/56) | 94.0% (110/117) | 1.000 |
| 2–3 pads | 3.6% (2/56) | 4.3% (5/117) | ||
| >3 pads | 0% (0/56) | 1.7% (2/117) | ||
| Potency | Age | |||
| 6 months | Overall | 46.4% (26/56) | 24.8% (29/117) | 0.005 |
| ≤65 years | 53.6% (15/28) | 28.8% (17/59) | 0.033 | |
| >65 years | 39.3% (11/28) | 20.7% (12/58) | 0.076 | |
| 12 months | Overall | 67.9% (38/56) | 42.7% (50/117) | 0.002 |
| ≤65 years | 78.6% (22/28) | 50.8% (30/59) | 0.019 | |
| >65 years | 57.1% (16/28) | 34.5% (20/58) | 0.062 |
The median age of both groups was 65. The patients in both groups were divided into two subgroups by their age (greater or less than 65 years old). At 6 months, 46.4% patients in group A and 24.8% patients in group B were potent, and this difference was statistically significant (P=0.005, chi-square analysis). In younger patients (≤65 years), the difference in potency rates was statistically significant (53.6% vs. 28.8%, P=0.033, chi-square analysis). However, in older patients (>65 years) the difference was not statistically significant (39.3% vs. 20.7%, P=0.076, chi-square analysis). At 12 months, better potency rates were found in overall patients in group A (P=0.002, chi-square analysis). In younger patients (≤65 years), the potency rates in group A was better (P=0.002, chi-square analysis). In older patients (>65 years) the difference of potency rates was not statistically significant (57.1% vs. 34.5%, P=0.062, chi-square analysis).
Discussion
PCa is the second most frequently diagnosed cancer and was the sixth leading cause of cancer death in males worldwide in 2008.14 In China, the incidence of PCa was 1.71/100 000 in 1993, 3.40/100 000 in 1997, 3.90/100 000 in 2005, 4.24/100 000 in 2006, 4.39/100 000 in 2007 and 4.57/100 000 in 2008.15,16 Although the incidence of PCa in China is not as high as in Europe and North America, it is increasing rapidly.
Our initial nerve-sparing ELRP in 2003 was performed using the conventional interfascial technique. In 2006, the intrafascial nerve-sparing technique became possible in our institution. Until now, the intrafascial nerve-sparing technique has been a technique of very advanced surgeons.9,10,17,18,19,20,21,22,23,24
In the intrafascial nerve-sparing ELRP, the dissection of the prostate was performed between the prostatic capsule and the prostatic fascia and left virtually no periprostatic tissue overlying the prostate. Theoretically, this approach may lead to a higher incidence of PSM due to a dissection that is closer to the prostate gland. Potdevin et al.17 found that there was a high rate of PSM in patients with pT3 disease who underwent the intrafascial nerve-sparing technique during robot-assisted laparoscopic radical prostatectomy. However, Shikanov et al.18, Neill et al.19 and Khoder et al.20 reported that the bilateral interfascial nerve-sparing technique does not result in higher rates of PSM in low-risk patients.
In our study, the pathological stages, overall PSM rates and postoperative BPFS 1 year of both groups were similar. In the short term, there appeared to be no compromise in cancer control with the intrafascial technique for carefully selected patients. Active surveillance and very long follow-up would be necessary to detect any differences between the groups.
The advantage of the intrafascial technique for postoperative potency and continence was another question investigated by many urologists. Potdevin et al.17 found that the intrafascial technique greatly improved potency rates and shortened the time to the return of continence following robot-assisted laparoscopic radical prostatectomy. Shikanov et al.18 reported that men with bilateral intrafascial nerve-sparing procedures demonstrated better sexual function. Neill et al.19 found an earlier return to continence after intrafascial nerve-sparing laparoscopic radical prostatectomy. In June 2010, Stolzenburg et al.21 compared outcomes for intrafascial and interfascial nerve-sparing radical prostatectomies. They divided their patients into three subgroups, <55 years old, 55–65 years old and >65 years old. They found that the intrafascial technique provided better potency and continence in younger patients.
In this study, we divided the patients into two subgroups based on an age greater or less than 65 years and attempted to validate the above conclusions. The intrafascial technique provided an earlier recovery of continence at 3 and 6 months and equally continent results at 12 months. Better potency rates at 6 months and 12 months were found in younger patients and overall in patients who had undergone the intrafascial nerve-sparing ELRP. During the intrafascial technique, the endopelvic fascia and the puboprostatic ligament were all preserved. Reduced dissection of periurethral structures would be helpful in the early recovery of continence.19 During the intrafascial technique, a nerve-sparing procedure was performed between the prostatic capsule and the prostatic fascia, and the use of coagulation was avoided so that the NVB could be more completely maintained. This may be the reason for the quicker recovery of potency in our study.
A limitation of our study is that the sample size was small, and this was a retrospective analysis with a short postoperative follow-up period, which allowed a potential selection bias. Further large, prospective investigations and long-term follow-up are required to evaluate the oncological and functional results of the intrafascial and interfascial nerve-sparing techniques.
In summary, our study confirmed that using strict indications, the intrafascial nerve-sparing technique provides similar short-term oncological results, quicker recovery of continence and better potency than the interfascial nerve-sparing technique. The intrafascial nerve-sparing technique is recommended as a preferred approach for young PCa patients with clinical stages of cT1 to cT2a and normal preoperative potency.
Author contributions
TZ participated in the design of the study and some operations as the first assistant and wrote this manuscript. XZ designed this study and performed all of the operations. XM and HZL participated in the design of the study and some operations as the first assistant. JPG, WC, JD, GFC, BJW and TPS participated in some operations as the first assistant. ELS performed the follow-up. WHC and QBH participated in the statistical analysis. All authors read and approved the final manuscript.
Acknowledgments
This work was supported by the 50th General Financial Grant from China Postdoctoral Science Foundation (Grant No. 201150M1535), China.
The authors have no competing financial interests.
References
- Walsh PC. Anatomic radical prostatectomy: evolution of the surgical technique. J Urol. 1998;160:2418–24. doi: 10.1097/00005392-199812020-00010. [DOI] [PubMed] [Google Scholar]
- Walsh PC, Donker PJ. Impotence following radical prostatectomy: insight into etiology and prevention. J Urol. 1982;128:492–7. doi: 10.1016/s0022-5347(17)53012-8. [DOI] [PubMed] [Google Scholar]
- Schuessler WW, Schulam PG, Clayman RV, Kavoussi LR. Laparoscopic radical prostatectomy: initial short-term experience. Urology. 1997;50:854–57. doi: 10.1016/S0090-4295(97)00543-8. [DOI] [PubMed] [Google Scholar]
- Raboy A, Ferzli G, Albert P. Initial experience with extraperitoneal endoscopic radical retropubic prostatectomy. Urology. 1997;50:849–53. doi: 10.1016/S0090-4295(97)00485-8. [DOI] [PubMed] [Google Scholar]
- Fromont G, Baumert H, Cathelineau X, Rozet F, Validire P, et al. Intraoperative frozen section analysis during nerve sparing laparoscopic radical prostatectomy: feasibility study. J Urol. 2003;170:1843–6. doi: 10.1097/01.ju.0000092081.71167.34. [DOI] [PubMed] [Google Scholar]
- Tewari A, Peabody JO, Fischer M, Sarle R, Vallancien G, et al. An operative and anatomic study to help in nerve sparing during laparoscopic and robotic radical prostatectomy. Eur Urol. 2003;43:444–54. doi: 10.1016/s0302-2838(03)00093-9. [DOI] [PubMed] [Google Scholar]
- Rhee HK, Tuerk IA. Radical nerve-sparing laparoscopic prostatectomy. BJU Int. 2004;94:449–74. doi: 10.1111/j.1464-410X.2004.05026.x. [DOI] [PubMed] [Google Scholar]
- Rassweiler J, Wagner AA, Moazin M, Gozen AS, Teber D, et al. Anatomic nerve-sparing laparoscopic radical prostatectomy: comparison of retrograde and antegrade techniques. Urology. 2006;68:587–91; discussion 91–2. doi: 10.1016/j.urology.2006.03.082. [DOI] [PubMed] [Google Scholar]
- Stolzenburg JU, Rabenalt R, Tannapfel A, Liatsikos EN. Intrafascial nerve-sparing endoscopic extraperitoneal radical prostatectomy. Urology. 2006;67:17–21. doi: 10.1016/j.urology.2005.09.052. [DOI] [PubMed] [Google Scholar]
- Stolzenburg JU, Rabenalt R, Do M, Schwalenberg T, Winkler M, et al. Intrafascial nerve-sparing endoscopic extraperitoneal radical prostatectomy. Eur Urol. 2008;53:931–40. doi: 10.1016/j.eururo.2007.11.047. [DOI] [PubMed] [Google Scholar]
- Stolzenburg JU, Do M, Pfeiffer H, Konig F, Aedtner B, et al. The endoscopic extraperitoneal radical prostatectomy (EERPE): technique and initial experience. World J Urol. 2002;20:48–55. doi: 10.1007/s00345-002-0265-4. [DOI] [PubMed] [Google Scholar]
- Zhang X, Ju Z, Wang C, Ai X, Ma X, et al. The single needle method for urethrovesical anastomosis with strengthened posterior fixation during laparoscopic radical prostatectomy. J Huazhong Univ Sci Technolog Med Sci. 2009;29:745–49. doi: 10.1007/s11596-009-0615-1. [DOI] [PubMed] [Google Scholar]
- Cappelleri JC, Rosen RC. The Sexual Health Inventory for Men (SHIM): a 5-year review of research and clinical experience. Int J Impot Res. 2005;17:307–19. doi: 10.1038/sj.ijir.3901327. [DOI] [PubMed] [Google Scholar]
- Jemal A, Bray F, Center MM, Ferlay J, Ward E, et al. Global cancer statistics. CA Cancer J Clin. 2011;61:69–90. doi: 10.3322/caac.20107. [DOI] [PubMed] [Google Scholar]
- Zhao P, Chen W. Beijing; Peking Union Medical College Press; 2004; Chinese cancer registry annual report 2004; p. 61. [Google Scholar]
- He J, Zhao P, Chen W. Beijing; Military Medical Science Press; 2011; Chinese cancer registry annual report 2011; p. 74. [Google Scholar]
- Potdevin L, Ercolani M, Jeong J, Kim IY. Functional and oncologic outcomes comparing interfascial and intrafascial nerve sparing in robot-assisted laparoscopic radical prostatectomies. J Endourol. 2009;23:1479–84. doi: 10.1089/end.2009.0369. [DOI] [PubMed] [Google Scholar]
- Shikanov S, Woo J, Al-Ahmadie H, Katz MH, Zagaja GP, et al. Extrafascial versus interfascial nerve-sparing technique for robotic-assisted laparoscopic prostatectomy: comparison of functional outcomes and positive surgical margins characteristics. Urology. 2009;74:611–6. doi: 10.1016/j.urology.2009.01.092. [DOI] [PubMed] [Google Scholar]
- Neill MG, Louie-Johnsun M, Chabert C, Eden C. Does intrafascial dissection during nerve-sparing laparoscopic radical prostatectomy compromise cancer control. BJU Int. 2009;104:1730–3. doi: 10.1111/j.1464-410x.2009.08670.x. [DOI] [PubMed] [Google Scholar]
- Khoder WY, Buchner A, Siegert S, Stief CG, Schlenker B. Oncological and functional results of open intrafascial radical prostatectomy. Urologe A. 2011;50:1106–9. doi: 10.1007/s00120-011-2635-2. [DOI] [PubMed] [Google Scholar]
- Stolzenburg JU, Kallidonis P, Do M, Dietel A, Hafner T, et al. A comparison of outcomes for interfascial and intrafascial nerve-sparing radical prostatectomy. Urology. 2010;76:743–8. doi: 10.1016/j.urology.2010.03.089. [DOI] [PubMed] [Google Scholar]
- Stewart GD, El-Mokadem I, McLornan ME, Stolzenburg JU, McNeill SA. Functional and oncological outcomes of men under 60 years of age having endoscopic surgery for prostate cancer are optimal following intrafascial endoscopic extraperitoneal radical prostatectomy. Surgeon. 2011;9:65–71. doi: 10.1016/j.surge.2010.07.005. [DOI] [PubMed] [Google Scholar]
- Greco F, Wagner S, Hoda MR, Kawan F, Inferrera A, et al. Laparoscopic vs open retropubic intrafascial nerve-sparing radical prostatectomy: surgical and functional outcomes in 300 patients. BJU Int. 2010;106:543–7. doi: 10.1111/j.1464-410X.2009.09157.x. [DOI] [PubMed] [Google Scholar]
- Greco F, Hoda MR, Wagner S, Reichelt O, Inferrera A, et al. Bilateral vs unilateral laparoscopic intrafascial nerve-sparing radical prostatectomy: evaluation of surgical and functional outcomes in 457 patients. BJU Int. 2011;108:583–7. doi: 10.1111/j.1464-410X.2010.09836.x. [DOI] [PubMed] [Google Scholar]


