Abstract
Several reports have promoted the root-derived Korean red ginseng (KRG; Panax ginseng) as alternative treatment for erectile dysfunction (ED), and ginsenosides are known to be the principal active ingredients of ginseng. Recent studies showed that ginseng berries produce more ginsenosides than KRG; thus, we investigated the ability of the Korean ginseng berry extract GB0710 to relax the penile corpus cavernosum smooth muscle (CCSM) in this study. As a comparative control, the results were compared to those obtained using KRG. In addition, possible mechanisms of action for GB0710 were investigated. While KRG and GB0710 both displayed dose-dependent relaxation effects on precontracted rabbit CCSM in vitro, GB0710 was shown to be more potent than KRG. The GB0710-induced relaxation could be partially reduced by removing the endothelium. In addition, pre-treatment with several nitric oxide (NO) inhibitors significantly inhibited the relaxation of muscle strips. Furthermore, administration of GB0710 increased intracavernosal pressure (ICP) in a rat in vivo model in both a dose- and duration-dependent manner. Intracellular NO production in human microvascular endothelial cells could be induced by GB0710 and inhibited by NG-monomethyl-L-arginine. In conclusion, GB0710 had a greater relaxation effect on rabbit CCSM than did KRG extract, and increased ICP in a rat model in both a dose- and a duration-dependent manner. This relaxing effect might be mediated by NO production.
Keywords: corpus cavernosum, erectile dysfunction (ED), ginseng berry, nitric oxide (NO), smooth muscle
Introduction
Erectile dysfunction (ED) is defined as the inability to attain or maintain penile erection sufficient for satisfactory sexual intercourse. ED is a common condition in middle-aged men that has serious adverse effects on quality of life. It has been reported that approximately 50% of men aged 40–80 years suffer from ED to some degree.1
One of the etiological causes of ED is the reduced bioavailability of nitric oxide (NO). This causes insufficient relaxation of smooth muscle in the penile corpus cavernosum, leading to a subsequently insufficient inflow of blood. Several extensive studies have investigated the positive effects of ginseng on ED, thereby implicating the NO pathway as the mechanism of action.2,3 Among these, various reports have promoted the root-derived Korean red gingseng (KRG; Panax ginseng) as alternative treatment for ED, citing the stimulatory effect of KRG on the corpus cavernosum smooth muscle (CCSM) of the penis.4,5,6 The principal active ingredients of ginseng are ginsenosides (also called ginseng saponins), which are amphiphilic molecules comprised of an aglycon backbone (a hydrophobic four-ring steroid-like structure) linked to hydrophilic carbohydrate side chains that consist of monomers, dimers and tetramers.5,7
Ginsenoside is widely and variably distributed thoughout the ginseng plant.8 Kim et al.9 investigated the ginsenoside content of Korean ginseng, indicating ginseng berries (GB) and ginseng roots (which comprise KRG) as the most abundant parts. Specifically, they reported that GB produce more total ginsenosides, with a 4–6 times higher of total ginsenoside content than KRG. Further, when researching the well-established antiglycemic effects of KRG,10 Dey et al.11 suggested that GB exhibit more potent antihyperglycemic activity than the KRG, and that GB are unique in their anti-obesity effects in ob/ob mice. For these reasons, we hypothesized that GB extract may have a stronger relaxation effect on CCSM than KRG extract. To confirm this, we investigated the ability of GB to relax CCSM, using KRG as a comparison. In addition, we evaluated the possible mechanisms of the action of GB extract.
Materials and methods
Animal care and all experimental procedures were carried out with the approval of the Institutional Animal Care and Use Committee of Yonsei University Health System (Seoul, Korea). The in vitro experiments used male New Zealand white rabbits (4–6 months old, 2.5–3.5 kg) and the in vivo experiments used male Sprague Dawley rats (200–250 g).
Preparation of GB extract (GB0710)
Raw GB (Panax ginseng, CA, Meyer) were harvested in July from plants cultivated in the Chungbuk province in Korea, and the seeds were separated and removed. The flesh, juice, and skin of the GB were dried in hot air. The dried GB were first refluxed with 70% ethanol for 10 h, after which the extract was filtered and concentrated under reduced pressure at 45 °C, thus obtaining the GB extract (GB0710).
Preparation of red ginseng extract (ginseng root)
Red ginseng (Panax ginseng, CA, Meyer), cultivated and manufactured in Chungbuk province in Korea was added to ethanol and extracted under reflux. The extract was filtered and concentrated under reduced pressure. The concentrations of seven major ginsenosides in GB0710 and KRG extract were analyzed by high-performance liquid chromatography. The results are shown in Figure 1.
Figure 1.
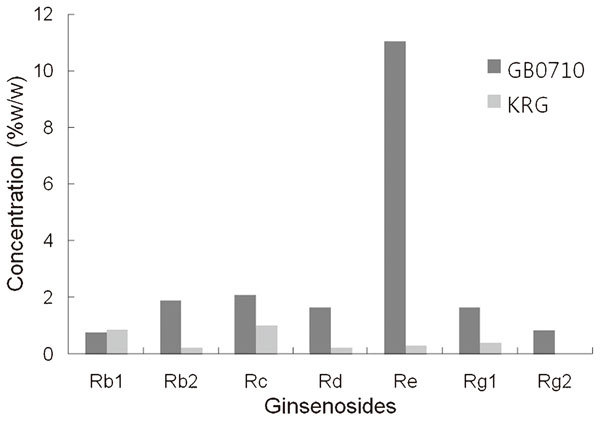
Percentage weight of the seven ginsenosides in GB0710 and KRG extract obtained by high-performance liquid chromatography analysis. KRG, Korean red ginseng; Rb1, ginsenoside-Rb1 (C54H92O23); Rb2, ginsenoside-Rb2 (C53H90O22); Rc, ginsenoside-Rc (C53H90O22); Rd, ginsenoside-Rd (C48H82O18); Re, ginsenoside-Re (C48H82O18); Rg1, ginsenoside-Rg1 (C42H72O14); Rg2, ginsenoside-Rg2 (C42H72O13).
In vitro experiments
Forty-two rabbits were used for the in vitro experiments, and they were anesthetized with phenobarbital sodium (50 mg kg−1). The penis was surgically removed en bloc, with care taken to keep the tunica albuginea intact. The corpus cavernosum was carefully dissected from the surrounding tunica albuginea. Two strips of corpus cavernosum tissue, measuring approximately 2×2×6 mm, were obtained from each rabbit and mounted in 10 ml organ chambers containing Tyrode solution (pH 7.4) at 37°C, equilibrated with 95% oxygen and 5% CO2. The strips were suspended with silk ties to a force–displacement transducer (TSD 105; Biopac Systems, Santa Barbara, CA, USA). Changes in isometric tension were measured using MP100WS (Biopac Systems). The following protocol was used: the prepared strips were stimulated with 5×10−6 mol l−1 phenylephrine (Phe; Sigma-Aldrich, St Louis, MO, USA) every 15 min with stepwise stretching (0.5 g tension/stretch). When the difference of the amplitude of contraction was within 10% of the previous contraction, that tension was considered optimal for isometric contraction.
GB0710 and KRG extract were added to the resting equilibrated muscle strips for the evaluation of their relaxation effects under basal conditions. After the muscle strips were stabilized in contraction with Phe (5×10−6 mol l−1), the effects of various concentrations (0, 10, 20, 50, 100 and 150 mg ml−1) of GB0710 and KRG extract were evaluated. To evaluate the relaxation mechanism, the effects of GB0710 on precontracted strips were also evaluated for strips incubated with either a nitric oxide synthesis (NOS) inhibitor, NG-nitro-L-arginine (L-NNA; 3×10−4 mol l−1), a guanylate cyclase inhibitor, methylene blue (10−4 mol l−1) or 1H-[1,2,4] oxadiazolo-[4, 3-α] quinoxalin-1-one (ODQ; 5×10−6 mol l−1), or the NO scavenger pyrogallol (10−4 mol l−1). L-NNA, methylene blue, ODQ and pyrogallol were purchased from Sigma-Aldrich. Disruption of the endothelium was achieved by rubbing the CCSM strip between the thumb and index finger for approximately 20 s. After rinsing in chilled Tyrode solution, the strip was gently rolled across a dry paper towel to generate shear forces across the endothelial surface of the lacunar space. Removal of the endothelium was confirmed by the absence of the relaxation response to acetyl choline (10−5 mol l−1) or relaxation within 10% of the range in the control state. The effect of the inhibitors was studied by pre-incubation with each inhibitor for 20 min, after which contraction was induced by Phe and the relaxation profiles of GB0710 vs KRG extract were observed. Each strip was used in up to four separate rounds of testing, washing them three times with Tyrode solution and equilibrating for 30 min between rounds.
In vivo experiments
A total of 160 rats were used. They were divided into four time groups (1, 2, 3 and 4 weeks; n=40 in each group), and then each time group was subdivided into five dosage groups according to the dosage of GB0710 (0, 20, 40, 100 and 150 mg kg−1 day−1; n=8 in each subgroup). For the administration of GB0710, GB0710 was mixed with physiologic saline and administered daily by mouth, and control group subjects were given equal doses of physiologic saline.
Rats were anaesthetized by intraperitoneal injection of sodium pentobarbital (30–50 mg kg−1), and the bladder and prostate were exposed via transperitoneal midline incision. The pelvic trunk located at the posterolateral wall of the prostate was identified, and the cavernosal nerve was isolated. A platinum electrode was placed on the cavernosal nerve and connected to an electric stimulator (STM100A; Biopac Systems). After incising the penile skin, the corpus cavernosum was isolated. To measure the intracavernosal pressure (ICP), a 26-gauge needle was placed into the corpus cavernosum. To simultaneously monitor systemic blood pressure, a 22-gauge angio-catheter was inserted into the carotid artery and connected to a transducer and polygraph system. The outputs for systemic blood pressure and ICP were connected to a sequential amplifier (DA100; Biopac Systems) via a Sorenson transpac (Abbott Critical Care System, Chicago, IL, USA). Pressure lines and catheters were prevented from clotting by periodic irrigation with heparinized saline. After 1–4 weeks of administering GB0710 (0, 20, 40, 100 and 150 mg kg−1 day−1), the maximal ICP was continuously measured under cavernous nerve stimulation at low voltage (voltage 2 V; frequency 12 Hz; pulse-width 1 ms; duration 1 min), as healthy animals were used in this study.12 To minimize the influence of cavernous nerve stimulation on the blood pressure, which would artificially raise the ICP, the data were presented as the percentage of ICP/systolic blood pressure (SBP). After every ICP study, the tested rats were euthanized.
Intracellular NO production in cell culture
To measure NO production in response to GB0710 administration, human microvascular endothelial cells (HMVECs) were purchased from Lonza Walkersville, Inc. (Walkersville, MD, USA). HMVECs were cultured in complete microvascular endothelial cell growth medium (EGM-2 MV, SingleQuots; Lonza Walkersville, Inc.), in a humidified 5% CO2 incubator at 37°C. The cellular NO level was measured in situ using the NO-specific fluorescent probe 4-amino-5-methylamino-2,7-difluorofluorescein diacetate (DAF-FM/DA; Molecular Probes, Eugene, OR, USA) according to the manufacturer's instructions. The cells were pretreated with or without a NOS inhibitor, NG-monomethyl-L-arginine (L-NMMA, 2 mmol l−1), for 30 min, followed by exposure to GB0710 (200 µg ml−1) or KRG (200 µg ml−1) for 1 h. L-NMMA is a relatively non-selective inhibitor of all NOS isoforms. The cells were incubated in medium containing DAF-FM/DA (10 µmol l−1) for 1 h at 37°C, washed in phenol red-free medium, and fluorescence images were captured from at least 10 randomly selected cells per dish using a confocal laser microscope. The relative level of intracellular NO was determined from the fluorescence of triazole, which is a specific product of the reaction between DAF-FM and NO.
Statistics
All relaxation responses were expressed as a percentage of maximal relaxation, based on the perpendicular vertical distance between the Phe-induced maximal contraction point and the largest downward deflection in the tracing for any given experiment. Inhibitory effects on contractile responses were expressed as percentage of the contraction in the control state. Results were expressed as mean±standard error of the mean, using a Mann–Whitney U test to evaluate the significance of differences in the relaxation effect, ICP and the image density between treatment and control. A Kruskal–Wallis test was applied to determine significant differences among three or more groups at the same concentration of treatment. SPSS for Windows version 12.0 was used for statistical analysis. P values were two-sided, and a P value of less than 0.05 was considered significant.
Results
In vitro comparison of GB0710 with KRG extract with regard to the relaxation of isolated rabbit CCSM
While the KRG extract caused contraction at low concentrations in the Phe-induced precontracted CCSM, both GB0710 and KRG extract caused relaxation of the CCSM at high concentrations (≥10 mg ml−1 for GB0710, and ≥20 mg ml−1 for KRG). Hence, GB0710 showed a more powerful relaxation effect than KRG extract. For both substances, a concentration-dependent relaxation effect was observed, with the maximal effect at 150 mg ml−1 (96.3%±12.4% in GB0710, and 72.8%±11.8% in KRG) (Figure 2). The half maximal inhibitory concentration of GB0710 was 39.3 mg ml−1, and that of KRG was 111.9 mg ml−1.
Figure 2.
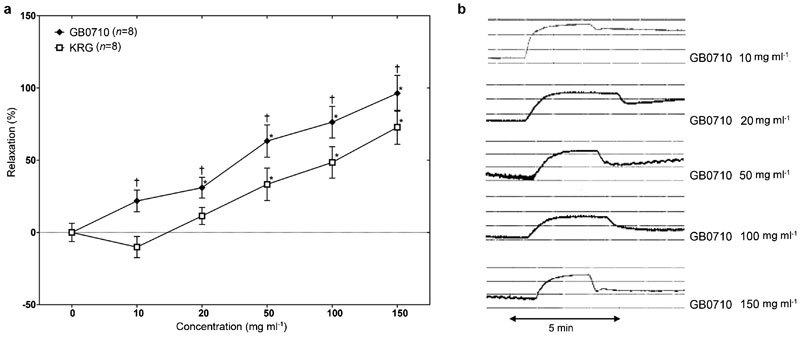
(a) Effects of both GB0710 and KRG extract on the isolated rabbit corpus cavernosum smooth muscle strips submaximally precontracted by phenylephrine (5×10−6 mol l−1). The relaxation effect showed a dose-dependent relaxation, but the relaxation effect of GB0710 was higher than KRG extract. Values are represented as mean±standard error and expressed as percentage of the relaxation. (b) Representative graphs of GB0710 effects on the isolated rabbit corpus cavernosal smooth muscle strip precontracted by phenylephrine (5×10−6 mol l−1). *P<0.05, the difference between treatment and control by Mann–Whitney U test; †P<0.05, the difference between GB0710 treatment and that of KRG treatment at the same concentrations by Mann–Whitney U test. KRG, Korean red ginseng.
GB0710-induced CCSM relaxation in conjunction with the NO pathway
These GB0710-induced relaxation was partially reduced by removing the endothelium (P<0.05; Figure 3). In addition, pre-treatment with any of the NO-inhibitors was able to partially inhibit the relaxation of muscle strips (P<0.05; Figure 3).
Figure 3.
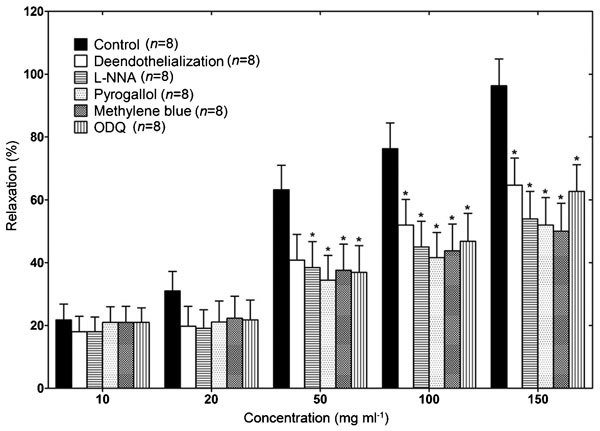
Effects of various treatments on GB0710-induced relaxation in the phenylephrine (5×10−6 mol l−1)-induced precontracted rabbit isolated corpus cavernosum smooth muscle strip. The relaxation effect of GB0710 was partially inhibited by endothelial disruption and by pretreatment with methylene blue (10−4 mol l−1), pyrogallol (10−4 mol l−1), L-NNA (3×10−4 mol l−1), or 1H-[1,2,4] ODQ (5×10−6 mol l−1). Values are represented as mean±standard error and expressed as percentage of the relaxation. *P<0.05, the difference between treatment and control by Mann–Whitney U test. L-NNA, NG-nitro-L-arginine; ODQ, oxadiazolo-[4, 3-α] quinoxalin-1-one.
In vivo investigation of the effect of GB0710 on rat CCSM
As reaction to the increased dosage of GB0710, the ICP/SBP (%) increased in a dose-dependent fashion. After 4 weeks of treatment, the ICP/BP (%) was 30.2±4.4, 43.9±4.8, 59.5±5.6, 70.7±3.1 and 73.5±4.0 with GB0710 treatment of 0, 20, 40, 100 and 150 mg kg−1 day−1, respectively. In addition, the maximal ICP/SBP (%) for each group increased in a duration-dependent fashion (Figure 4). When treated with a dose of 150 mg kg−1 day−1 GB0710, ICP/BP (%) was 47.4±4.5, 54.7±4.4, 67.8±4.3 and 73.5±4.0 after 1, 2, 3 and 4 weeks, respectively.
Figure 4.
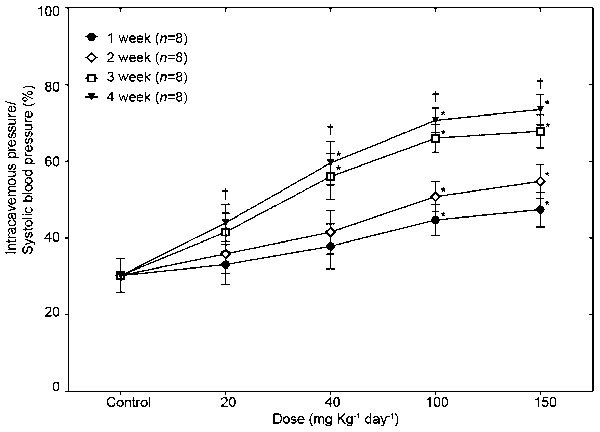
Intracavernosal pressure with cavernosal nerve stimulation after long-term administration of GB0710. After administration of GB0710 at doses of 20, 40, 100 and 150 mg, intracavernosal pressure increased in a dose- and duration-dependent fashion. *P<0.05, the difference between treatment and control by Mann–Whitney U test; †P<0.05, the difference among various groups at the same concentrations of each treatment by Kruskal–Wallis test.
Effect of GB0710 on inducing intracellular NO production in HMVECs
GB0710 increased the intracellular production of NO in HMVECs compared to control cells, and this increase was inhibited to control levels by co-treatment with the NOS inhibitor L-NMMA (Figure 5).
Figure 5.
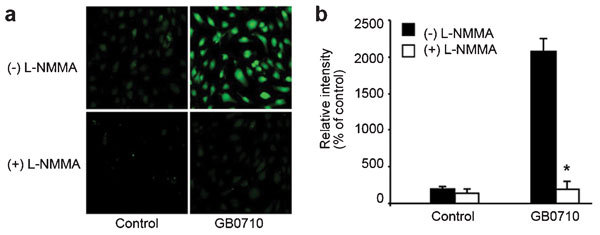
GB0710 induced intracellular NO production in HMVECs. (a) HMVECs were treated with GB0710 (200 µg ml−1) or KRG (200 µg ml−1) in the presence or absence of L-NMMA (2 mmol l−1) for 30 min, followed by incubation with DAF-FM/DA (10 µmol l−1) for 1 h. Intracellular NO levels were determined by confocal microscopy. (b) The relative levels of NO were calculated from fluorescence intensities. *P<0.05, the difference between treatment and control by Mann–Whitney U test. DAF-FM/DA, 4-amino-5-methylamino-2,7-difluorofluorescein diacetate; HMVEC, human microvascular endothelial cell; KRG, Korean red ginseng; L-NMMA, NG-monomethyl-L-arginine; NO, nitric oxide.
Discussion
Many folk remedies claim to improve male ED, including phytochemicals such as extract of Lepidium meyenii, Ferula hermonis, Ginkgo biloba and Aspidosperma ulei, among others.13,14,15 A recent systematic review of randomized clinical trials revealed that Panax ginseng improves glucose metabolism and moderates the immune response.16 KRG is also used as a tonic to maintain physical vitality and for restorative purposes throughout East Asian countries, including Korea and China. Our study demonstrates that GB0710 has a greater relaxation effect on rabbit CCSM than does KRG extract, and that it increases the ICP in an in vivo rat model. We also demonstrated that these positive effects may be due to a relaxing effect mediated by NO production. To the best of our knowledge, this is the first study which has shown that GB extract can enhance erectile capability.
The first phosphodieterase-5 inhibitor has been available on the market since 1998, and several phosphodieterase-5 inhibitors have become the first line therapy for men with ED. Although evidence-based sexual drug treatment has increased tremendously over the past two decades, and despite effective well-established treatment options for ED, many patients are still dissatisfied.17 Dropout rates for current medical therapies are high, and overall satisfaction is below expectation due to drug inefficiency and adverse events such as headache and hot flashes. Further, while ED can generally be treated successfully using current treatment options, it cannot be cured. For these reasons, phytotherapy remains an important alternative treatment. A recent systematic review provided evidence for the effectiveness of red ginseng in the treatment of ED from six randomized clinical trials.18 Although there were some limitations in the sample sizes and the methodological qualities of the primary studies,18 this meta-analysis found a significant effect of the tested herbal medicine (n=349, risk ratio=2.40, 95% CI=1.65–3.51, P<0.00001, heterogeneity: τ2=0.05, χ2=6.42, P=0.27, I2=22%). Subgroup analyses also showed beneficial effects of red ginseng in psychogenic ED (n=135, risk ratio=2.05; 95% CI=1.33–3.16, P=0.001, heterogeneity: χ2=0.08, P=0.96, I2=0%).
As aforementioned, several researchers showed that red ginseng might induce relaxation of the smooth muscles of the corpus carvernosum via the NO pathway, and ginsenosides are thought to be the principle active constituents of red ginseng. The content and composition of ginsenosides vary in the ginseng roots, leaves and berries, and the pharmacologically active components of ginseng remain unclear despite several trials to identity them. Although KRG extract prepared from the root of the plant has been most commonly used for herbal medicine, GB can be used similarly to treat diseases and sexual dysfunction, as well as for aphrodisiac purposes.11,19 Using GB also has economic advantages over KRG, because KRG is made from 6-year-old ginseng plants, whereas GB can be harvested yearly. Moreover, our analysis revealed markedly higher ginsenoside concentration in GB0710 than in KRG as shown in Figure 1. Notably, the concentrations of most ginsenosides (ginsenosides-Rb2, Rc, Rd, Re, Rg1 and Rg2) were higher in GB0710 than in KRG extract. Further studies are required to elucidate the exact mechanism of action of GB0710.
Ginsenosides have been shown in a rabbit model to cause a dose-dependent relaxation of the CCSM by increasing the release of NO.5,20,21 In a previous study, we described several candidate pathways involved in KRG-induced relaxation.5 One of those pathways involves NO, a potent relaxant for peripheral vascular smooth muscle, whose action is mediated by cyclic GMP. Acetylcholine may stimulate the formation of an endothelium derived relaxing factor, which has subsequently been identified as either a NO, or a chemically unstable NO precursor. We show here that the relaxation effect of GB0710 on CCSM was partially reduced by various NO inhibitors. In addition, we found that GB0710 increased intracellular NO production in cultured HMVEC cells, which was attenuable by co-treatment with the NOS inhibitor L-NMMA. These findings confirm that the relaxation effect of GB0710 is mediated through the NO pathway, which is in accordance with several studies by others.22,23
Alternatively, Sung et al.7 demonstrated that ginsenoside activates large-conductance K(Ca) channels, which induce CCSM relaxation. Meanwhile, it has been suggested that the KRG-mediated protection from ED is linked to its ability to scavenge oxygen radicals in a rat model of non-insulin-dependent diabetes mellitus. Oxidative stress is an important factor in vascular complications of diabetes.24 In addition, a recent study by Kim et al.25 suggested that KRG may improve erectile function in a rat model of metabolic syndrome with erectile dysfunction. It showed that the peak ICP and cGMP level of the placebo-treated group was markedly decreased compared to the KRG-treated group. We used healthy animals in this study before introducing a pathological condition such as diabetes mellitus or hypercholesterolemia, therefore further research using an animal model of ED is desirable. While future clinical trials are needed to determine the efficacy and safety of GB0710 for human use, our results suggest that GB0710 is a viable alternative to current therapeutic options for ED.
Cumulatively, we found that GB0710 had a greater relaxation effect on rabbit CCSM than KRG. Further, GB0710 increased the ICP in a rat model in both a dose- and a duration-dependent manner. According to our in vitro findings, GB0710 likely exerts its effect through the NO pathway. Further studies are required to elucidate the exact mechanism of action of GB0710, and its possible role in the treatment of ED.
Author contributions
KSC, CWP and YDC participated in the design and concept formation of this study. CWP, CKK, HYJ, SJL and WGK performed the experiments and acquisition of the data. CWP, CKK and JYL carried out the data analysis. KSC, CWP, CKK, HYJ and JYL drafted the manuscript. WGK, SJL and YMK did the major revisions of the manuscript. KSC and YDC approved the manuscript finally.
Acknowledgments
This work was supported by a grant of the Korea Healthcare Technology R&D Project, Ministry for Health, Welfare & Family Affairs, Republic of Korea (No. A084120) and by Amorepacific Co. (Seoul, Korea).
The authors declare no competing financial interests.
References
- Feldman HA, Goldstein I, Hatzichristou DG, Krane RJ, McKinlay JB. Impotence and its medical and psychosocial correlates: results of the Massachusetts Male Aging Study. J Urol. 1994;151:54–61. doi: 10.1016/s0022-5347(17)34871-1. [DOI] [PubMed] [Google Scholar]
- Azadzoi KM, Schulman RN, Aviram M, Siroky MB. Oxidative stress in arteriogenic erectile dysfunction: prophylactic role of antioxidants. J Urol. 2005;174:386–93. doi: 10.1097/01.ju.0000161209.39959.67. [DOI] [PubMed] [Google Scholar]
- Moore CR, Wang R. Pathophysiology and treatment of diabetic erectile dysfunction. Asian J Androl. 2006;8:675–84. doi: 10.1111/j.1745-7262.2006.00223.x. [DOI] [PubMed] [Google Scholar]
- Tamaoki J, Nakata J, Kawatani K, Tagaya E, Nagai A. Ginsenoside-induced relaxation of human bronchial smooth muscle via release of nitric oxide. Br J Pharmacol. 2000;130:1859–64. doi: 10.1038/sj.bjp.0703511. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Choi YD, Xin ZC, Choi HK. Effect of Korean red ginseng on the rabbit corpus cavernosal smooth muscle. Int J Impot Res. 1998;10:37–43. doi: 10.1038/sj.ijir.3900300. [DOI] [PubMed] [Google Scholar]
- Ham WS, Kim WT, Lee JS, Ju HJ, Kang SJ, et al. [Efficacy and Safety of Red Ginseng Extract Powder in Patients with Erectile Dysfunction: Multicenter, Randomized, Double-Blind, Placebo-Controlled Study] Korean J Urol. 2009;50:159–64. [Google Scholar]
- Sung HH, Chae MR, So I, Jeon JH, Park JK, et al. Effects of ginsenoside on large-conductance K(Ca) channels in human corporal smooth muscle cells. Int J Impot Res. 2011;23:193–9. doi: 10.1038/ijir.2011.25. [DOI] [PubMed] [Google Scholar]
- Attele AS, Wu JA, Yuan CS. Ginseng pharmacology: multiple constituents and multiple actions. Biochem Pharmacol. 1999;58:1685–93. doi: 10.1016/s0006-2952(99)00212-9. [DOI] [PubMed] [Google Scholar]
- Kim YK, Yoo DS, Xu H, Park NI, Kim HH, et al. Ginsenoside content of berries and roots of three typical Korean ginseng (Panax ginseng) cultivars. Nat Prod Commun. 2009;4:903–6. [PubMed] [Google Scholar]
- Jeon WJ, Oh JS, Park MS, Ji GE. Anti-hyperglycemic effect of fermented ginseng in type 2 diabetes mellitus mouse model. Phytother Res. 2013;27:166–72. doi: 10.1002/ptr.4706. [DOI] [PubMed] [Google Scholar]
- Dey L, Xie JT, Wang A, Wu J, Maleckar SA, et al. Anti-hyperglycemic effects of ginseng: comparison between root and berry. Phytomedicine. 2003;10:600–5. doi: 10.1078/094471103322331908. [DOI] [PubMed] [Google Scholar]
- Bivalacqua TJ, Deng W, Kendirci M, Usta MF, Robinson C, et al. Mesenchymal stem cells alone or ex vivo gene modified with endothelial nitric oxide synthase reverse age-associated erectile dysfunction. Am J Physiol Heart Circ Physiol. 2007;292:H1278–90. doi: 10.1152/ajpheart.00685.2006. [DOI] [PubMed] [Google Scholar]
- Benvindo OD, Nascimento NR, Santos CF, Fonteles MC, Silveira ER, et al. Relaxant effect and possible mechanism of 17-nor-subincanadine E in rabbit corpora cavernosa. Asian J Androl. 2011;13:747–53. doi: 10.1038/aja.2011.41. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim JJ, Han DH, Lim SH, Kim TH, Chae MR, et al. Effects of Ginkgo biloba extracts with mirodenafil on the relaxation of corpus cavernosal smooth muscle and the potassium channel activity of corporal smooth muscle cells. Asian J Androl. 2011;13:742–6. doi: 10.1038/aja.2010.184. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hadidi KA, Aburjai T, Battah AK. A comparative study of Ferula hermonis root extracts and sildenafil on copulatory behaviour of male rats. Fitoterapia. 2003;74:242–6. doi: 10.1016/s0367-326x(03)00032-7. [DOI] [PubMed] [Google Scholar]
- Shergis JL, Zhang AL, Zhou W, Xue CC.Panax ginseng in randomised controlled trials: a systematic review. Phytother Rese-pub ahead of print 12 September 2012; doi: 10.1002/ptr.4832. [DOI] [PubMed]
- Ho CC, Tan HM. Rise of herbal and traditional medicine in erectile dysfunction management. Curr Urol Rep. 2011;12:470–8. doi: 10.1007/s11934-011-0217-x. [DOI] [PubMed] [Google Scholar]
- Jang DJ, Lee MS, Shin BC, Lee YC, Ernst E. Red ginseng for treating erectile dysfunction: a systematic review. Br J Clin Pharmacol. 2008;66:444–50. doi: 10.1111/j.1365-2125.2008.03236.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Attele AS, Zhou YP, Xie JT, Wu JA, Zhang L, et al. Antidiabetic effects of Panax ginseng berry extract and the identification of an effective component. Diabetes. 2002;51:1851–8. doi: 10.2337/diabetes.51.6.1851. [DOI] [PubMed] [Google Scholar]
- Choi YD, Rha KH, Choi HK. In vitro and in vivo experimental effect of Korean red ginseng on erection. J Urol. 1999;162:1508–11. [PubMed] [Google Scholar]
- Kim HJ, Woo DS, Lee G, Kim JJ. The relaxation effects of ginseng saponin in rabbit corporal smooth muscle: is it a nitric oxide donor. Br J Urol. 1998;82:744–8. doi: 10.1046/j.1464-410x.1998.00811.x. [DOI] [PubMed] [Google Scholar]
- Chen X, Lee TJ. Ginsenosides-induced nitric oxide-mediated relaxation of the rabbit corpus cavernosum. Br J Pharmacol. 1995;115:15–8. doi: 10.1111/j.1476-5381.1995.tb16313.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gillis CN. Panax ginseng pharmacology: a nitric oxide link. Biochem Pharmacol. 1997;54:1–8. doi: 10.1016/s0006-2952(97)00193-7. [DOI] [PubMed] [Google Scholar]
- Ryu JK, Lee T, Kim DJ, Park IS, Yoon SM, et al. Free radical-scavenging activity of Korean red ginseng for erectile dysfunction in non-insulin-dependent diabetes mellitus rats. Urology. 2005;65:611–5. doi: 10.1016/j.urology.2004.10.038. [DOI] [PubMed] [Google Scholar]
- Kim SD, Kim YJ, Huh JS, Kim SW, Sohn DW. Improvement of erectile function by Korean red ginseng (Panax ginseng) in a male rat model of metabolic syndrome. Asian J Androl. 2013;15:395–9. doi: 10.1038/aja.2012.159. [DOI] [PMC free article] [PubMed] [Google Scholar]


