Abstract
We investigated the antiproliferative activity of (−)-gossypol on the human prostate cancer cell line PC3 in vitro and in vivo to elucidate its potential molecular mechanisms. Cell growth and viability were evaluated using the 3-(4,5-dimethylthiazol-2-yl)-2,5-diphenyltetrazolium bromide (MTT) assay, and cell apoptosis was detected by flow cytometry, terminal deoxynucleotidyl transferase dUTP nick end labelling (TUNEL) and electron microscopy. Expression of proliferating cell nuclear antigen (PCNA), Bcl-2, CD31, caspase-3 and caspase-8 in tumour tissue was determined by immunohistochemistry. The drug concentration that yielded 50% cell inhibition (IC50 value) was 4.74 μg mL−1. In the PC-3 tumour xenograft study, (−)-gossypol (> 5 mg kg−1) given once a day for 7 days significantly inhibited tumour growth in a dose-dependent manner. Immunohistochemical analysis revealed that (−)-gossypol enhanced caspase-3 and caspase-8 expression and decreased the expression of PCNA, Bcl-2 and CD31 in tumour tissues. It suggested that cell apoptosis and inhibition of angiogenesis might contribute to the anticancer action of (−)-gossypol.
Keywords: apoptosis, electron microscopy, flow cytometry, (−)-gossypol, immunohistochemistry, prostate cancer
Introduction
Prostate cancer (PCa) is the most frequently diagnosed male malignancy and the second leading cause of male cancer death in most industrialized countries 1. Recently, Asian countries, such as Japan and China, have also reported high incidences of PCa and consequent mortality rates. In its early stages, PCa cells depend on androgens for growth and survival, and androgen ablation therapy or surgery at that time is effective in causing tumour regression. Despite the initial efficacy of androgen deprivation therapy, most patients with PCa progress within 2 years from androgen-dependent status to hormone-refractory PCa, for which there is no current effective therapy.
Gossypol is a naturally occurring polyphenolic pigment present in cottonseeds and in cotton plant by-products, such as cottonseed oil and cottonseed meal flour. Gossypol has two enantiomers, (+)-gossypol and (−)-gossypol, the latter being a more potent inhibitor of cancer cell growth 2. Recent studies have focused on the proapoptotic activity of (−)-gossypol. Previously, research on gossypol in relation to cancer has focused mainly on the antiproliferative activity of (±)-gossypol in a variety of cancers in vitro and in vivo, including breast 3, bladder 4, pancreas 5, lung 6, colon 1 and prostate 7 and cancers of the head and neck 8, 9. These anticancer effects have been attributed to the (−)-enantiomer, which is a more potent inhibitor of cancer cell growth 10. (−)-Gossypol is a small-molecule inhibitor of Bcl-2/Bcl-xL/Mcl-1 and potently induces apoptosis in various cancer cell lines 11. Overexpression of the antiapoptotic protein Bcl-2 is observed in 30%–60% of PCas at the time of diagnosis and in nearly 100% of hormone-refractory PCas 12. Moreover, it is believed that gossypol can prevent the metastasis of PCa 13.
This study was designed to investigate the therapeutic potential of (−)-gossypol in human hormone-refractory PCa cells in vivo and to establish the efficacy of (−)-gossypol in inhibiting PC-3 PCa xenograft growth in an athymic nude mouse model.
Materials and methods
Cell culture
Human prostate carcinoma PC-3 cells were purchased from the American Type Culture Collection (ATCC, Manassas, VA, USA). PC-3 cells were routinely cultured in RPMI-1640 medium (Gibco BRL, Grand Island, NY, USA) supplemented with 100 IU mL−1 penicillin G sodium, 100 μg mL−1 streptomycin sulphate and 10% (v/v) fetal bovine serum (Gibco BRL) at 37°C in a humidified incubator with 5% CO2.
(−)-Gossypol preparation
(−)-Gossypol was purified at the College of Life Science and Technology of Xi'an Jiaotong University (Xi'an, China). Its purity was determined by high-pressure liquid chromatography analysis, revealing a chemical purity of > 98% and a chiral purity of > 97%. A 100 μg mL−1 stock solution was prepared by dissolving (−)-gossypol in 100% (v/v) dimethyl sulfoxide (DMSO) on the day of treatment. The working solution was obtained by dilution of the store solution with culture medium.
MTT assay for cell growth and viability
Cell growth and viability were evaluated using the 3-(4,5-dimethylthiazol-2-yl)-2,5-diphenyltetrazolium bromide (MTT) (Sigma-Aldrich, St. Louis, MO, USA) assay. PC-3 cells were seeded (5 × 105 cells per well) in 96-well plates (100 μL per well). After 24 h of growth in RPMI-1640 medium, the cells were treated with various concentrations of (−)-gossypol (2.5–20 μg mL−1) for 72 h. The medium was removed at the end of the treatment, and 0.5 mg mL−1 of MTT was added to the medium. After 4 h, DMSO (200 μL) was added to each well and the optical density was read at 570 nm. Cell sensitivity to drug treatment was expressed as the drug concentration that yielded 50% cell inhibition (IC50). All of the experiments were performed in triplicate.
Ultrastructure of PC-3 cells
Cells (2 × 105 cells mL−1) were seeded in cell culture bottles with 4 mL of cell suspension. After 24 h, diluted (−)-gossypol (4 mL) was added to each bottle in all groups at a final drug concentration of 10 μg mL−1. In parallel, RPMI-1640 culture medium (4 mL) containing the same dose of DMSO was added to the bottles containing the control group. After a 72-h incubation, the cells were trypsinized, washed with serum-free RPMI-1640 medium, centrifuged, fixed with 4% (w/v) paraformaldehyde and then washed twice with phosphate-buffered saline (PBS), fixed with 1% (w/v) osmic acid (precooled to 4°C) for 1 h, dehydrated and embedded in Epon 812. Cell morphology was observed under a JEM-2000EX transmission electron microscope (JEOL, Tokyo, Japan).
Detection of apoptosis by flow cytometry
After 48-h incubation, the cells in the treatment group and control group were harvested, centrifuged, washed twice with PBS and re-suspended in buffer solution. Then, 2 × 105 cells were mixed with 5 μL annexin V-FITC (fluorescein isothiocyanate) (Biovision, Mountain View, CA, USA) and incubated for 10 min at room temperature in the dark. The cells were centrifuged and re-suspended in 190 μL buffer solution supplemented with 10 μL or 20 μg mL−1 propidium iodide (PI) (Sigma-Aldrich). PC-3 cell apoptosis was detected by flow cytometry (FACSAria, Becton Dickinson,San Jose, CA, USA).
TUNEL assay
The TUNEL assay was used to assess apoptotic cell death in PC-3 cells. Adherent cells treated with 10 μg mL−1 (−)-gossypol were fixed, and fragmented DNA in cells undergoing apoptosis was detected using the In Situ Cell Death Detection Kit (Boehringer-Mannheim, Mannheim, Germany) in which strand breaks are end-labelled with fluorescein dUTP by the terminal transferase enzyme. The TUNEL assay was performed according to the manufacturer's instructions. Cells were mounted with Vectashield mounting medium using DAPI and photographed using an Olympus FV1000 laser scanning confocal microscope (Olympus, Tokyo, Japan). A positive control was prepared by treating the sample with DNase I before TUNEL staining. For quantitative analysis, the percentage of TUNEL-positive cells among 200 cancer cells in three fields per section was determined at 200-fold magnification.
Tumour model in nude mice
Male BALB/c nude mice (4 weeks old), weighing 18–22 g, were purchased from the Laboratory Animal Center of the Fourth Military Medical University (Xi'an, China). A total of 40 mice were inoculated subcutaneously on both sides of the lower back above the tail, after alcohol preparation of the skin, using a sterile 22-G needle with 0.1 mL of a cell suspension of 2 × 106 PC-3 cells. Tumour volumes were measured every 3 days using a calliper and calculated according to the formula (L × W2)/2, where L and W are length and width, respectively 14. When the tumours reached an appropriate size (200–250 mm3), the mice were randomized into four groups of 10 mice and then treated with either (−)-gossypol (2.5, 5.0 or 10.0 mg kg−1 i.p. q.d) or DMSO (vehicle) for 7 days. The vehicle control group received the same amount of DMSO as in the treatment groups. Tumour sizes and animal body weights were measured daily by a technician from the Laboratory Animal Center of the Fourth Military Medical University without knowledge of the treatment. All animal experiments were performed according to the protocol approved by Fourth Military Medical University Guidelines for Use and Care of Animals. Tumour growth inhibition (T/C %) values for these four in vivo studies were calculated as described 15.
Histopathological observation
After killing the mice, tumours were removed from the animals and fixed in 10% (v/v) formalin solution. Tissues were paraffin-embedded, and 5-μm-thick sections were stained with hematoxylin and eosin.
Expression of proliferating cell nuclear antigen (PCNA), Bcl-2 and CD31 in tumour tissues
Tumour tissue was dissected, fixed with 40 mg L−1 formaldehyde, embedded with paraffin, deparaffinized with xylene, washed with water and blocked with 300 mL L−1 normal goat serum for 30 min. Then, mouse anti-human monoclonal antibodies for PCNA, Bcl-2 and CD31 (1:50 diluted) (Maixin Biotechnology Co. Ltd., Fuzhou, China) were added. This was followed by incubation at 4°C overnight in a humidified chamber. The next day, the cells were washed thrice with PBS and processed with absorbent paper to remove liquid residue. Donkey anti-mouse IgG antibody labelled with FITC (Santa Cruz Biotechnology Inc., Santa Cruz, CA, USA) was added and incubated at 37°C for 1 h. The cells were washed thrice with PBS, mounted with glycerol buffer and examined under a microscope. A total of 10 high-power fields per section were observed. No fewer than 100 PCNA-positive cells in each visual field were included for observation and analysis. The positive rate was expressed as a percentage. Proliferative index (%) = (number of PCNA-positive cells/total cells) × 100.
Expression of caspase-3 and caspase-8 in tumour tissue
Immunohistochemical staining was performed as described above. A rabbit anti-caspase-3 polyclonal antibody (Zhongshan Golden Bridge Biotechnology Co. Ltd., Beijing, China) and a rabbit anti-caspase-8 polyclonal antibody (Boster Biological Engineering Co., Wuhan, China) (1:100 diluted) were used. The secondary antibody was a goat anti-rabbit IgG labelled with rhodamine (1:100 diluted) (Santa Cruz Biotechnology).
Semi-quantitative fluorescence intensity determination and microvessel density (MVD) counting
The fluorescence intensity for Bcl-2, caspase-3 and caspase-8 were semi-quantitatively analysed by ImagePro Plus 6.0 software (Media Cybernetics, Silver Spring, MD, USA). Five visual fields in each section were chosen for the determination of the integrated fluorescence intensity and area. The expression intensity was represented by the integrated fluorescence intensity per unit area. MVD counting was conducted as follows: five visual fields in each section under a × 200 magnification objective lens were chosen for MVD, in which the most abundant microvessel in the most intensively tumour-infiltrated areas stained green. The mean value was used for the MVD counts.
Statistical analyses
SPSS version 11.0 for Windows (SPSS, Chicago, IL, USA) was used for all statistical analyses. All the data are presented as mean ± SD. Statistical analysis was performed using the unpaired t-test. P < 0.05 was considered as statistically significant.
Results
Inhibitory effect of (−)-gossypol on the growth of PC3 cells
(−)-Gossypol inhibited the growth of PC-3 cells in a dose-dependent manner, as shown in Figure 1. At 72 h, the survival rate was 85.1% ± 4.1%, 45.2% ± 3.2%, 28.2% ± 2.2% and 16.3% ± 2.5% in the 2.5, 5.0, 10.0 and 20.0 μg mL−1 (−)-gossypol-treated groups, respectively. The IC50 value of (−)-gossypol was 4.74 μg mL−1.
Figure 1.
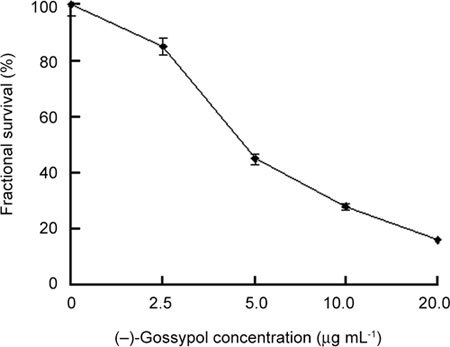
Dose-dependent activity of (−)-gossypol on PC-3 cells. Cells were treated with (−)-gossypol (2.5–20.0 μg mL−1) for 72 h.
Ultrastructural changes in PC3 cells induced by (−)-gossypol
Prostate cancer-3 cells possess characteristic microvilli on their surface, with euchromatin-dominant nuclei and clear nucleoli (Figure 2A). On treatment with 10 μg mL−1 (−)-gossypol for 72 h, surface microvilli disappeared, alveolar processes appeared on the cell surface, nucleus chromatin became condensed, vacuoles and apoptotic bodies were observed, suggesting that these cells were undergoing apoptosis (Figure 2B).
Figure 2.
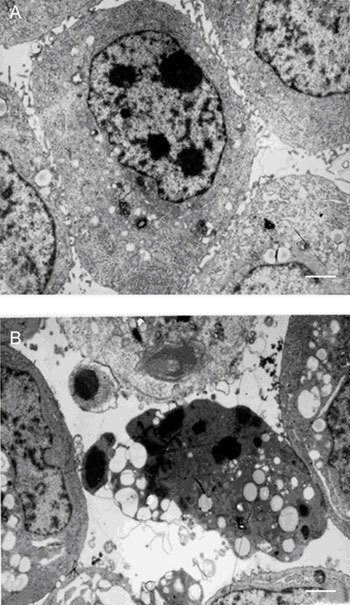
Ultrastructure of PC-3 cells treated with or without (−)-gossypol. (A): Untreated PC-3 cell; (B): PC-3 cell treated with 10.0 μg mL−1 (−)-gossypol for 72 h. Bar = 1 μm.
(−)-Gossypol induces apoptosis in PC3 cells
As evident in Figure 3, about 10% of the cells exposed to 2.5 μg mL−1 of (−)-gossypol (the effect of vehicle control was subtracted, as with other doses) were apoptotic; with 5 μg mL−1 of (−)-gossypol, about 15% of the cells were apoptotic; with 10 μg mL−1 of (−)-gossypol, 30% of the cells were apoptotic, and cells exposed to 20 μg mL−1 of gossypol increased the portion of apoptotic cells to about 55%. This suggests that (−)-gossypol induces apoptosis in PC-3 cells in a dose-dependent manner.
Figure 3.
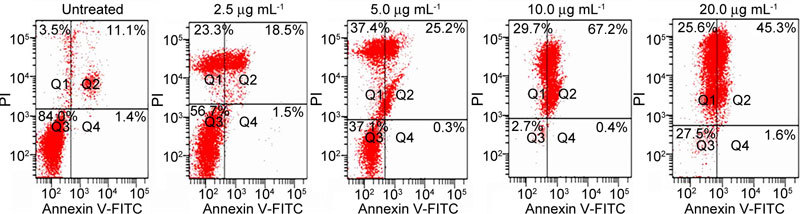
Dose-dependent induction of apoptosis by (−)-gossypol in PC-3 cells. PC-3 cells (105) were treated with different concentrations of (−)-gossypol for 48 h. Cells were then stained with annexin V-FITC and propidium iodide and analysed using flow cytometry. The results are representative of three independent experiments. PI, propidium iodide.
Apoptotic cell death in PC-3 cells
We used TUNEL labelling to examine the level of apoptosis in different experimental groups (Figure 4). TUNEL-positive nuclei are present as cyan nuclei, whereas DAPI staining of normal cell nuclei is indicated by blue colour. Compared with the control (Figure 4A), there was an obvious increase in the number of TUNEL-positive nuclei in PC-3 cells (Figure 4B) treated with 10 μg mL−1 (−)-gossypol. The percentage of apoptotic cells in control PC-3 cells was 1.2% ± 0.1%, but in the (−)-gossypol treatment group, this percentage increased to 35.7% ± 1.6% (P < 0.01).
Figure 4.
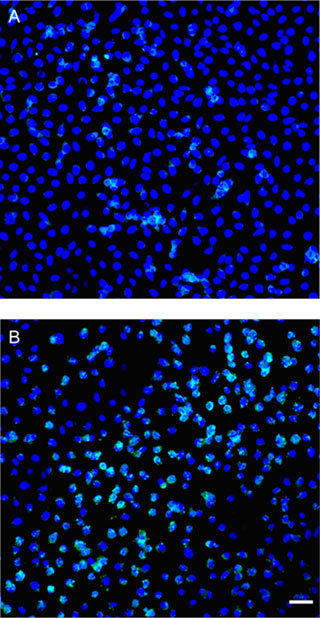
TUNEL labelling of PC-3 cells. (A): Control PC-3 cells; (B): 10 μg mL−1 (−)-gossypol treatment. Bar = 50 μm.
Tumour growth of PC3 xenografts after (−)-gossypol treatment
Tumour volumes were recorded every 2 days until the animals were killed on day 7. On day 4, tumour volumes were decreased in the 5.0 mg kg−1 and 10.0 mg kg−1 body weight groups, as compared with the control group (P < 0.05). From day 1 to day 7, tumour volumes in the control group were increased by 6.5-fold, whereas tumour volumes in the (−)-gossypol treatment groups were elevated by 2.5-fold (10.0 mg kg−1), 3.7-fold (5.0 mg kg−1) and 4.8-fold (2.5 mg kg−1) (Figure 5).
Figure 5.
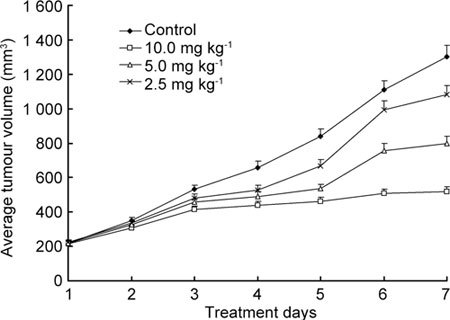
Tumour growth of PC3 xenografts treated with (−)-gossypol.
(−)-Gossypol was given by i.p. injection once a day for seven consecutive days at 2.5 mg kg−1, 5.0 mg kg−1 or 10.0 mg kg−1 body weight. The positive control group was given i.p. injections of vehicle once a day for seven consecutive days.
The tumour growth inhibition (T/C %) values for these four in vivo studies were calculated as described 15 and are summarized in Table 1. According to National Cancer Institution (NCI) criteria, T/C % < 42% is considered significant antitumour activity; T/C % < 10% is considered to indicate highly significant antitumour activity and is the level used by the NCI to justify a clinical trial (DN-2 level activity; see Alessandri et al. 14). The concentration of 10 mg kg−1(−)-gossypol achieved a T/C % of 39.9%, indicating that it possesses significant antitumour activity.
Table 1. Comparison of tumour growth inhibition (T/C %) versus starting tumour sizes in the four in vivo experiments.
| Group | Tumour size at start (mm3) | T/C % |
|---|---|---|
| Control | 220 | 100 |
| 2.5 mg kg−1 | 225 | 83.0 |
| 5.0 mg kg−1 | 220 | 61.4 |
| 10.0 mg kg−1 | 214 | 39.9 |
When the median tumour size in the control group reached 1 300 mm3, tumour growth inhibition (T/C %) was calculated as the percentage median tumour size in the treatment group versus the control group.
Histopathological features of transplanted tumours in (−)-gossypol-treated mice
When a tumour was poorly differentiated, tumour cells organized into solid sheets and microacini. Areas of necrosis were not observed in the control group. However, the percentage of cells undergoing necrosis in the three (−)-gossypol groups (2.5, 5.0 and 10.0 mg kg−1 body weight) was 3.2% ± 1.5%, 10.3% ± 2.2% and 32.5% ± 4.3% of total nucleated cells, respectively (Figure 6).
Figure 6.
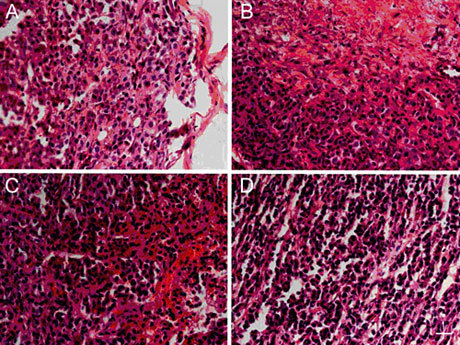
The effect of (−)-gossypol on the pathological features of transplanted tumours. (A): Control; (B): 10.0 mg kg−1 treatment group; (C): 5.0 mg kg−1 treatment group; (D): 2.5 mg kg−1 treatment group. Bar = 50 μm.
Expression of PCNA in tumour tissues treated with (−)-gossypol
Differences in PCNA expression in tumour cells and control cells were obvious. With the exception of the 2.5 mg kg−1 group, PCNA expression was reduced to different degrees. (−)-Gossypol regulated the expression of PCNA in a dose-dependent manner (Figures 7 and 8).
Figure 7.
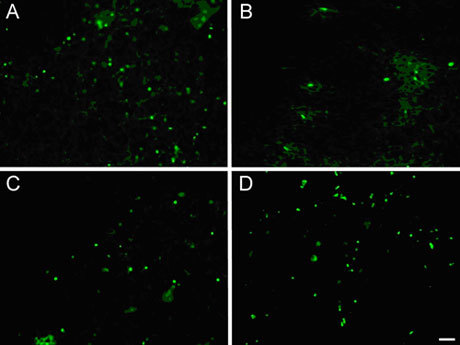
Expression of PCNA in PC-3 transplanted tumour tissues. (A): Control; (B): 10.0 mg kg−1 treatment group; (C): 5.0 mg kg−1 treatment group; (D): 2.5 mg kg−1 treatment group. Bar = 20 μm.
Figure 8.
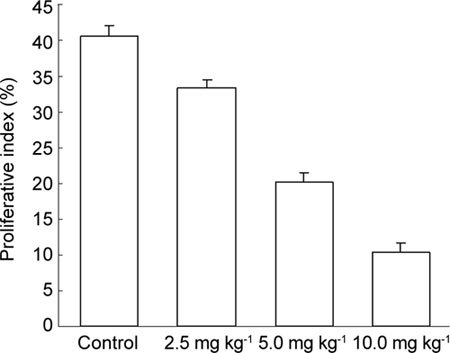
Proliferative index (%) of proliferating cell nuclear antigen (PCNA) in tumour tissues. PC-3 xenograft was treated with (−)-gossypol (2.5, 5.0 and 10.0 mg kg−1) for 7 days. The proliferative index was calculated by the ratio of positive cells to the total number of tumour cells counted.
Expression of Bcl-2, caspase-3 and caspase-8
A significantly higher percentage of tumour cells expressed Bcl-2 in the control group. Different doses of (−)-gossypol caused a decrease in Bcl-2 protein expression in tumour tissues by varying degrees, implying that (−)-gossypol induces PC-3 tumour xenograft apoptosis (Figure 9, Table 2).
Figure 9.
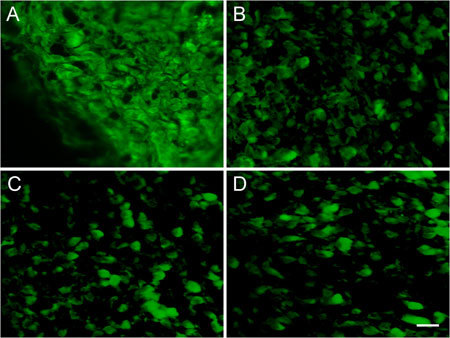
Immunofluorescence of Bcl-2 in PC-3 transplanted tumour tissues. The PC-3 xenograft was treated with (−)-gossypol (2.5–10.0 mg kg−1) for 7 days. (A): Control; (B): 10.0 mg kg−1 treatment group; (C): 5.0 mg kg−1 treatment group; (D): 2.5 mg kg−1 treatment group. Bar = 20 μm.
Table 2. Effect of (˜−)-gossypol on the expression of Bcl-2, caspase-3 and caspase-8.
| Groups | Bcl-2 | Caspase-3 | Caspase-8 |
|---|---|---|---|
| Control | 0.93 ± 0.16 | 0.50 ± 0.02 | 0.75 ± 0.10 |
| 2.5 mg kg−1 | 0.56 ± 0.06a | 0.55 ± 0.02a | 1.39 ± 0.12b |
| 5.0 mg kg−1 | 0.56 ± 0.04a | 0.58 ± 0.01a | 1.44 ± 0.12b |
| 10.0 mg kg−1 | 0.43 ± 0.03b | 0.69 ± 0.01a | 1.49 ± 0.13b |
aP < 0.05, bP < 0.01, compared with control.
The expressions of caspase-3 and caspase-8 were very weak in the control group, but they were strong in the groups treated with different doses of (−)-gossypol and displayed a dose-dependant relationship. This finding suggested that (−)-gossypol could induce tumour cell apoptosis by inducing caspase activation (Figures 10 and 11, Table 2).
Figure 10.
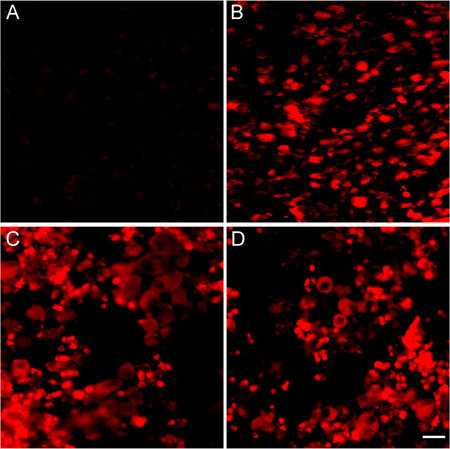
Expression of caspase-3 in tumour tissues. The PC3 xenograft was treated with (−)-gossypol (2.5–10.0 mg kg−1) for 7 days. (A): Control; (B): 10.0 mg kg−1 treatment group; (C): 5.0 mg kg−1 treatment group; (D): 2.5 mg kg−1 treatment group. Bar = 20 μm.
Figure 11.
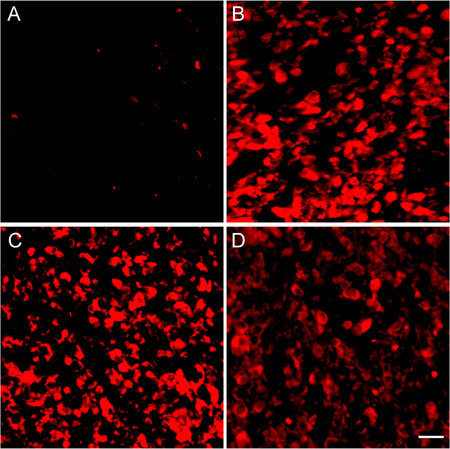
Expression of caspase-8 in tumour tissues. The PC-3 xenografts were treated with (−)-gossypol (2.5–10.0 mg kg−1) for 7 days. (A): Control; (B): 10.0 mg kg−1 treatment group; (C): 5.0 mg kg−1 treatment group; (D): 2.5 mg kg−1 treatment group. Bar = 20 μm.
Expression of CD31
In the control group, significant expression of CD31 was found in tumour tissue, which formed a network-like structure. The expression of CD31 in tumours treated with different doses of (−)-gossypol was decreased in a dose-dependent manner. The MVD in the 2.5, 5.0 and 10.0 mg kg−1 groups was 43.2% ± 9.5%, 24.6% ± 6.9% and 9.4% ± 3.2%, respectively. Compared with the control group (53.2% ± 3.5%), the MVD counts decreased significantly (P < 0.05 or P < 0.01) (Figures 12 and 13). This observation suggested that (−)-gossypol inhibited angiogenesis in PC-3 xenograft tumours.
Figure 12.
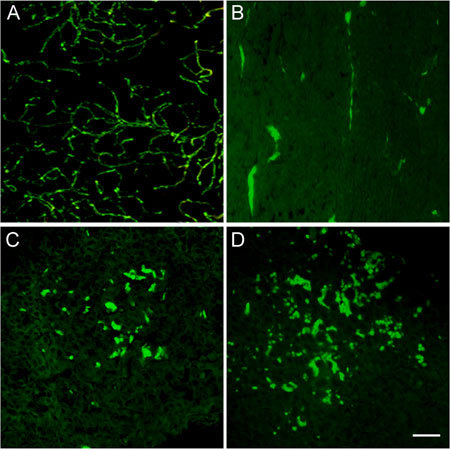
Expression of CD31 in tumour tissues. The PC-3 xenograft was treated with (−)-gossypol (2.5–10.0 mg kg−1 body weight) for 7 days. (A): Control; (B): 10.0 mg kg−1 treatment group; (C): 5.0 mg kg−1 treatment group; (D): 2.5 mg kg−1 treatment group. Bar = 20 μm.
Figure 13.
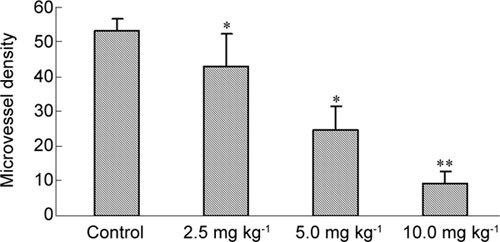
Microvessel density (MVD) in tumour tissues. The PC-3 xenograft was treated with (−)-gossypol (2.5–10.0 mg kg−1) for 7 days. *P < 0.05, **P < 0.01, compared with control.
Discussion
(−)-Gossypol has garnered much attention owing to its potential as an antitumour drug 16, 17. (−)-Gossypol can downregulate the expression of proteins that inhibit apoptosis, such as Bcl-xL and Mcl-1, while upregulating the expression of proapoptotic proteins, such as Bim, Puma, Noxa and Bax, and mediating apoptosis by activating caspase-3, caspase-8 and caspase-9.
Bcl-2 inhibits apoptosis and prolongs the lifespan of a variety of cell types. Overexpression of Bcl-2 can inhibit a variety of stimuli-induced apoptosis events. There is a hydrophobic groove on the surface of the antiapoptotic Bcl-2 family proteins that can bind BH3 domains exposed on the surface of proapoptotic proteins, forming heterodimers. Bcl-2 and BH3 interact to regulate apoptosis. Small molecules that mimic the BH3 domain can effectively inhibit the antiapoptotic action of Bcl-2 family proteins and induce the release of proapoptotic proteins such as Bax and Bak. In this study, we showed that (−)-gossypol can effectively inhibit the expression of Bcl-2 in tumour tissue, which in turn promotes apoptosis in PCa cells. This result is consistent with previous in vitro studies 7, 18.
Aspartate-specific cysteine protease (caspase) is critical for apoptosis. It was clear from the results of this study that (−)-gossypol can activate caspase-3 and caspase-8 through death receptors derived from the cell surface or through cytochrome c released from the mitochondria. This finding is also consistent with the results of previous studies performed in vitro 4, 18. Huang et al. 7 found that apoptosis caused by (−)-gossypol is mediated by the regulation of the Bcl-2 and caspase family in androgen-independent DU-145 PCa cells. Our data also suggest that (−)-gossypol may have the same effect in PC-3 cells.
Proliferating cell nuclear antigen is a nuclear protein synthesized in the G1/S phase and has an important role in DNA replication. PCNA expression is low in non-dividing cells, but it increases greatly in proliferating cells and transformed cells. The expression level of PCNA is closely related to the cell state, which means that the level of PCNA expression correlates to the degree of malignancy, invasion and the metastasis of cancer cells. Thus, PCNA is an important evaluative marker for tumour growth and prognosis 19. The results of this study showed that (−)-gossypol inhibits the expression of PCNA in a dose-dependent manner, indicating that this compound might mediate the repair and synthesis of DNA 20. Although DNA synthesis and repair may influence tumour cell proliferation, our results confirmed the role of decreased PCNA in (−)-gossypol-treated mice.
CD31 is an endothelial cell marker expressed in both endothelial cells of blood vessels and the lymphatic system. The intensity and density of CD31 expression reflects changes in MVD, which is closely related to tumour growth and metastasis 21. However, the prognostic significance of MVD in PCa is controversial. Whether MVD is correlated with stage/grade, recurrence, metastasis or disease-specific survival is not clear 22, 23, 24, 25, 26, 27, 28, 29. The results of this study showed that (−)-gossypol inhibits the expression of CD31 and augments the necrosis of tumours, suggesting that (−)-gossypol has a role in the injury to tumour vascular endothelial cells. These results are in agreement with a previous report 30.
In conclusion, (−)-gossypol inhibits the growth of PCa tumours and induces apoptosis. (−)-Gossypol displays greater toxicity at high doses. Thus, (−)-gossypol represents a potential new therapy for androgen-independent PCa.
Acknowledgments
This study was supported in part by grants from National Natural Science Foundation of China (No. 30570494 and No. 30772658). We thank Dr Xing-Bin Hu (The Second Department of Blood Transfusion, Xijing Hospital, Xi'an, China) for assisting writing this manuscript.
References
- Zhang M, Liu H, Guo R, Ling Y, Wu X, et al. Molecular mechanism of gossypol-induced cell growth inhibition and cell death of HT-29 human colon carcinoma cells. Biochem Pharmacol. 2003;66:93–103. doi: 10.1016/s0006-2952(03)00248-x. [DOI] [PubMed] [Google Scholar]
- Jiang J, Sugimoto Y, Liu S, Chang HL, Park KY, et al. The inhibitory effects of gossypol on human prostate cancer cells-PC3 are associated with transforming growth factor beta1 (TGFβ1) signal transduction pathway. Anticancer Res. 2004;24:91–100. [PubMed] [Google Scholar]
- Ligueros M, Jeoung D, Tang B, Hochhauser D, Reidenberg MM, et al. Gossypol inhibition of mitosis, cyclin D1 and Rb protein in human mammary cancer cells and cyclin-D1 transfected human fibrosarcoma cells. Br J Cancer. 1997;76:21–8. doi: 10.1038/bjc.1997.330. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Macoska JA, Adsule S, Tantivejkul K, Wang S, Pienta KJ, et al. (−)-Gossypol promotes the apoptosis of bladder cancer cells in vitro. Pharmacol Res. 2008;58:323–31. doi: 10.1016/j.phrs.2008.09.005. [DOI] [PubMed] [Google Scholar]
- Mohammad RM, Wang S, Banerjee S, Wu X, Chen J, et al. Nonpeptidic small-molecule inhibitor of Bcl-2 and Bcl-XL, (-)-Gossypol, enhances biological effect of genistein against BxPC-3 human pancreatic cancer cell line. Pancreas. 2005;31:317–24. doi: 10.1097/01.mpa.0000179731.46210.01. [DOI] [PubMed] [Google Scholar]
- Kilic A, Schuchert MJ, Luketich JD, Landreneau RJ, El-Hefnawy T. Efficacy of signal pathway inhibitors alone and in combination with Cisplatin varies between human non-small cell lung cancer lines. J Surg Res. 2009;154:9–12. doi: 10.1016/j.jss.2008.05.022. [DOI] [PubMed] [Google Scholar]
- Huang YW, Wang LS, Chang HL, Ye W, Dowd MK, et al. Molecular mechanisms of (-)-gossypol-induced apoptosis in human prostate cancer cells. Anticancer Res. 2006;26:1925–33. [PubMed] [Google Scholar]
- Wolter KG, Wang SJ, Henson BS, Wang S, Griffith KA, et al. (-)-Gossypol inhibits growth and promotes apoptosis of human head and neck squamous cell carcinoma in vivo. Neoplasia. 2006;8:163–72. doi: 10.1593/neo.05691. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bauer JA, Trask DK, Kumar B, Los G, Castro J, et al. Reversal of cisplatin resistance with a BH3 mimetic, (-)-gossypol, in head and neck cancer cells: role of wild-type p53 and Bcl-xL. Mol Cancer Ther. 2005;4:1096–104. doi: 10.1158/1535-7163.MCT-05-0081. [DOI] [PubMed] [Google Scholar]
- Shelley MD, Hartley L, Fish RG, Groundwater P, Morgan JJ, et al. Stereo-specific cytotoxic effects of gossypol enantiomers and gossypolone in tumour cell lines. Cancer Lett. 1999;135:171–80. doi: 10.1016/s0304-3835(98)00302-4. [DOI] [PubMed] [Google Scholar]
- Wang G, Nikolovska-Coleska Z, Yang CY, Wang R, Tang G, et al. Structure-based design of potent small-molecule inhibitors of anti-apoptotic Bcl-2 proteins. J Med Chem. 2006;49:6139–42. doi: 10.1021/jm060460o. [DOI] [PubMed] [Google Scholar]
- Xu L, Yang D, Wang S, Tang W, Liu M, et al. (-)-Gossypol enhances response to radiation therapy and results in tumor regression of human prostate cancer. Mol Cancer Ther. 2005;4:197–205. [PubMed] [Google Scholar]
- Huang YW, Wang LS, Dowd MK, Wan PJ, Lin YC. (-)-Gossypol reduces invasiveness in metastatic prostate cancer cells. Anticancer Res. 2009;29:2179–88. [PubMed] [Google Scholar]
- Alessandri G, Filippeschi S, Sinibaldi P, Mornet F, Passera P, et al. Influence of gangliosides on primary and metastatic neoplastic growth in human and murine cells. Cancer Res. 1987;47:4243–7. [PubMed] [Google Scholar]
- Corbett TH, Polin L, Roberts BJ.editors. Transplantable syngeneic rodent tumorsTotowa: Humana Press; 2002
- Shi J, Yu J, Pohorly JE, Kakuda Y. Polyphenolics in grape seeds-biochemistry and functionality. J Med Food. 2003;6:291–9. doi: 10.1089/109662003772519831. [DOI] [PubMed] [Google Scholar]
- Blackstaffe L, Shelley MD, Fish RG. Cytotoxicity of gossypol enantiomers and its quinone metabolite gossypolone in melanoma cell lines. Melanoma Res. 1997;7:364–72. doi: 10.1097/00008390-199710000-00002. [DOI] [PubMed] [Google Scholar]
- Zhang M, Liu H, Tian Z, Griffith BN, Ji M, et al. Gossypol induces apoptosis in human PC-3 prostate cancer cells by modulating caspase-dependent and caspase-independent cell death pathways. Life Sci. 2007;80:767–74. doi: 10.1016/j.lfs.2006.11.004. [DOI] [PubMed] [Google Scholar]
- Tanaka S, Haruma K, Tatsuta S, Hiraga Y, Teixeira CR, et al. Proliferating cell nuclear antigen expression correlates with the metastatic potential of submucosal invasive colorectal carcinoma. Oncology. 1995;52:134–9. doi: 10.1159/000227444. [DOI] [PubMed] [Google Scholar]
- Essers J, Theil AF, Baldeyron C, van Cappellen WA, Houtsmuller AB, et al. Nuclear dynamics of PCNA in DNA replication and repair. Mol Cell Biol. 2005;25:9350–9. doi: 10.1128/MCB.25.21.9350-9359.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Raspollini MR, Amunni G, Villanucci A, Baroni G, Boddi V, et al. Prognostic significance of microvessel density and vascular endothelial growth factor expression in advanced ovarian serous carcinoma. Int J Gynecol Cancer. 2004;14:815–23. doi: 10.1111/j.1048-891X.2004.014514.x. [DOI] [PubMed] [Google Scholar]
- Lissbrant IF, Lissbrant E, Damber JE, Bergh A. Blood vessels are regulators of growth, diagnostic markers and therapeutic targets in prostate cancer. Scand J Urol Nephrol. 2001;35:437–52. doi: 10.1080/003655901753367532. [DOI] [PubMed] [Google Scholar]
- Borre M, Offersen BV, Nerstrom B, Overgaard J. Microvessel density predicts survival in prostate cancer patients subjected to watchful waiting. Br J Cancer. 1998;78:940–4. doi: 10.1038/bjc.1998.605. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weidner N. Tumor angiogenesis: review of current applications in tumor prognostication. Semin Diagn Pathol. 1993;10:302–13. [PubMed] [Google Scholar]
- Bostwick DG, Iczkowski KA. Microvessel density in prostate cancer: prognostic and therapeutic utility. Semin Urol Oncol. 1998;16:118–23. [PubMed] [Google Scholar]
- Gettman MT, Pacelli A, Slezak J, Bergstralh EJ, Blute M, et al. Role of microvessel density in predicting recurrence in pathologic Stage T3 prostatic adenocarcinoma. Urology. 1999;54:479–85. doi: 10.1016/s0090-4295(99)00202-2. [DOI] [PubMed] [Google Scholar]
- Halvorsen OJ, Haukaas S, Hoisaeter PA, Akslen LA. Independent prognostic importance of microvessel density in clinically localized prostate cancer. Anticancer Res. 2000;20:3791–9. [PubMed] [Google Scholar]
- Weidner N, Carroll PR, Flax J, Blumenfeld W, Folkman J. Tumor angiogenesis correlates with metastasis in invasive prostate carcinoma. Am J Pathol. 1993;143:401–9. [PMC free article] [PubMed] [Google Scholar]
- Navone NM, Logothetis CJ, von Eschenbach AC, Troncoso P. Model systems of prostate cancer: uses and limitations. Cancer Metastasis Rev. 1998;17:361–71. doi: 10.1023/a:1006165017279. [DOI] [PubMed] [Google Scholar]
- Karaca B, Kucukzeybek Y, Gorumlu G, Erten C, Gul MK, et al. Profiling of angiogenic cytokines produced by hormone- and drug-refractory prostate cancer cell lines, PC-3 and DU-145 before and after treatment with gossypol. Eur Cytokine Netw. 2008;19:176–84. doi: 10.1684/ecn.2008.0139. [DOI] [PubMed] [Google Scholar]


