Abstract
Defining the molecular characteristics of seminal plasma proteins is essential for understanding their function in physiological and pathological conditions. Starting from the predicted importance of human seminal plasma gelatin-binding proteins, comprising fibronectin (FN) and FN-related molecules, for male fertility, this study aims at gaining insight into their immuno-glycobiochemical properties. Human seminal plasma from subjects with normal semen parameters were separated on a gelatin–Sepharose column and analyzed by sodium dodecyl sulfate-polyacrylamide gel electrophoresis and immunoblotting using antibodies against distinct FN forms. Heterogeneity of the isolated molecular species was examined by protein chip arrays combined with surface-enhanced laser desorption/ionization time of flight mass spectrometry, on normal, metal and hydrophobic surfaces. Carbohydrate composition was investigated using mannose-, fucose- and sialic acid-specific plant lectins and galectin-1. The results obtained indicated a pattern of isolated proteins corresponding to that of known FN fragments, as confirmed by immunoreactivity. Among them heparin-binding ability was preferentially associated with low molecular mass species. As for posttranslational modifications, phosphorylation and glycosylation of distinct fragments were revealed. Lectin binding to fragments containing the gelatin-binding domain, particularly with Ricinus communis agglutinin I, was stronger than to fragments containing the cell-binding site of FN. A low level of sialylation and distinctive concanavalin A- and Lens culinaris agglutinin-reactive species were also observed. Galectin-1 did not interact with the isolated preparation. Resolving the molecular heterogeneity of normal human seminal plasma FN and gaining initial insight into possible similarities/differences with known FN molecular species may be considered a prerequisite step preceding challenging the clinical usefulness of these molecular properties.
Keywords: fibronectin, gelatin-binding, glycosylation, heparin-binding, human seminal plasma
Introduction
Seminal fluid is a nutritive and protective medium for spermatozoa, that is, it has an essential role in their survival and passage through the female reproductive tract. It consists of a complex mixture of sugars, lipids, salts, metal ions, basic amines, amino acids, hormones, enzymes, etc. 1. Introducing proteomic analysis of human seminal fluid has led to more detailed analysis and has indicated a large number of extracellular proteins, proteases and other proteins secreted by testes, prostate and other male accessory glands 2, 3, 4, 5. However, the structure-function relationship of seminal plasma proteins at the molecular level and the relation of more basic questions to particular clinical end points is still insufficiently explored.
Fibronectin (FN) and proteins containing FN-type II domain are a group of human seminal plasma proteins that are supposed to be involved in capacitation and sperm-egg interaction. FN is a modular multidomain protein that exhibits a distinct and complex pattern of molecular forms differing with respect to amino acid and oligosaccharide composition; in humans, there are potentially 20 different forms of FN 6, 7. All FN modules are highly conserved and present in many diverse proteins. A total of 12 FN-type I repeats are involved in fibrin and collagen binding. Two FN-type II repeats are involved in binding collagen, whereas 15 FN-type III repeats contain cell-, integrin- and heparin-binding sites 8. By combining particular FN-related domains into a mosaic structure in different molecules, the distinct collagen-binding, that is, gelatin-binding, as well as heparin-binding activities are shared in common. In biological systems, gelatin/collagen-binding is important for cell adhesion, morphology and surface architecture. Thus, FN acts as a general cell adhesion molecule by anchoring cells to collagen and organizes cell-cell and cell-extracellular matrix (ECM) binding 6, 7. FN-associated activities are responsible for interaction with various proteins and surface markers, bearing important consequences for maintaining integrity of intra- and extracellular proteins/structures in health and disease. Thus, FN binds gelatin, heparin, integrins, actin, DNA, fibrin, hyaluronic acid, proteoglycans/gangliosides and thrombospondin, and is consequently involved in cellular migration, adhesion, morphology and spreading, cytoskeletal organization, oncogenic transformation, phagocytosis, hemostasis/thrombosis, embryonic differentiation, etc. 6, 7, 8.
Although the role of FN in the male reproductive system has not been elucidated, it is thought that sperm function can be modified by distinct FN species. Sperm membrane-incorporated FN exhibits changes in regional antigenic expression during sperm maturation, whereas secreted FN is a product of male accessory sex glands and can be attached to sperm tails during ejaculation 9, 10. Some studies on the effects of exogenously added FN or estimation of its concentration in relation to sperm parameters have indicated that it can influence sperm motility and sperm-egg interaction 11, 12, 13, 14. Thus, anti-FN antibodies (Abs) were shown to increase sperm motility, whereas in the presence of exogenously added FN, the number of motile spermatozoa was significantly reduced 15. In addition, FN-derived peptides can competitively inhibit sperm-oolemmal adherence, whereas FN present during bovine in vitro fertilization strongly inhibits sperm penetration 15, 16, 17. The possibility of impairment of the fertilization process by adherence of microorganisms to the equatorial FN of human sperm is also proposed 18.
So far, only a few FN-related structural studies have been performed using non-fractionated human seminal plasma samples without detailed glycobiochemical and functional characterization. These studies suggested the absence of intact FN, difference in FN concentration and patterns of molecular masses and sialylation in relation to semen parameters 19, 20.
As for proteins containing two tandemly repeated FN-type II modules, they have been isolated from the seminal plasma of various animal species, and among them, the family collectively called bovine seminal plasma (BSP) proteins, are the most known and characterized 21, 22. The presence of homologous proteins in human seminal plasma is suggested 23, but their antigenic similarity to FN is poorly characterized. In addition to these, proteins containing four tandemly repeated FN-type II modules were recently identified 24. They are highly conserved and the expression is related to the epididymus. Their function is not known, but available data indicate a role in cell volume control during sperm maturation.
Starting from human seminal plasma gelatin-binding proteins as molecules with predicted importance for fertilization, fertility and as markers of prostate and testicular physiology 25, this study for the first time examines their immunological and glycobiochemical properties. This primarily experimental study was specifically focused on the structural characterization of seminal plasma FN. The possible clinical significance of a single particular glycoprotein could emerge from their marker potential, that is, defining pathology-related changes in comparison with normal physiology or modulation of functions by defining related biomimetics or neutralizing agents. Resolving the intrinsic heterogeneity of the protein and glycan parts of human seminal plasma FN expressed under normal physiological conditions and gaining initial insight into possible similarities/differences with known FN molecular species may be considered as a prerequisite step before challenging the clinical usefulness of the molecular properties.
Materials and methods
Reagents
Monoclonal anti-plasma FN Ab, clone P1H11 and human recombinant galectin-1 (gal-1) were purchased from R&D Systems Inc. (Minneapolis, MN, USA). Monoclonal anti-cellular FN Ab 1940 (specific for extra domain A, EDA) was obtained from Chemicon International (Temecula, CA, USA). Elite Vectastain ABC kit, biotinylated anti-mouse IgG, DAB (3,3′-diaminobenzidine) substrate kit and biotinylated lectins, Ricinus communis agglutinin I (RCA I), concanavalin A (ConA), Lens culinaris agglutinin (LCA), Sambucus nigra agglutinin (SNA) and Maackia amurensis agglutinin (MAA), were from Vector Laboratories (Burlingame, CA, USA). Gelatin was from ICN Biochemicals (Cleveland, OH, USA). CNBr-activated Sepharose was from Amersham Biosciences (Uppsala, Sweden). ColorBurst Electrophoresis Markers were from Sigma (St. Louis, MO, USA). Roti Black silver staining kit was purchased from Carl Roth GmbH+Co. (Karlsruhe, Germany). Immobilon-P–polyvinylidene fluoride (PVDF) membrane was from Millipore (Bedford, MA, USA). ProteinChip NP20 (normal phase) array, ProteinChip IMAC30 (immobilized metal-affinity capture) array, ProteinChip H50 (hydrophobic/reversed phase) array, ProteinChip PS20 (preactivated surface), sinapinic acid and ProteinChip All-in-one protein Standards II were from BioRad (Hercules, CA, USA). All other chemicals were reagent grade.
Human seminal plasma
Human semen samples were obtained from consenting normal fertile subjects seen at INEP-Zemun, Serbia, according to local ethical standards. Samples with sperm parameters within the normal range for numbers, morphology and motility, according to the recommended criteria of the World Health Organization 1999 were selected 26. Sperm volume was > 2 mL, liquefaction occurred in < 30 min, the total number of spermatozoa was > 20 × 106, spermatozoa of normal morphology formed > 70% and the total number of mobile spermatozoa was > 50% in each sample. Sperm cells and other debris were removed from the ejaculate by centrifugation at 900 × g for 20 min. Twenty samples were randomly divided into two groups and two pools (n = 10 each) were formed. These pools were used immediately or stored at −20°C, until processed.
Isolation of gelatin-binding proteins
Gelatin-binding proteins were isolated from pools of human seminal plasma using the standard method for isolation of FN and other proteins that contain a gelatin-binding domain, that is, affinity chromatography on gelatin–Sepharose 4B column (7 mL) according to Ruoslahti et al. 27. Gelatin was bound to CNBr-activated Sepharose according to the manufacturer's instructions. Pooled seminal plasma (8 mL) was applied on the gelatin–Sepharose 4B column equilibrated with 0.05 mol L−1 phosphate-buffered saline (PBS, pH 7.2) for 3 h. The column was washed with equilibration buffer and subsequently with 1 mol L−1 NaCl till the absorbance baseline was reached. The bound fraction was then eluted with 4 mol L−1 urea at a flow rate of 1 mL min−1. Fractions (3 mL) were collected and the elution was monitored by measuring the optical density at 280 nm on a CE594 double beam spectrophotometer (CECIL Instruments, Cambridge, UK). Fractions of maxima were pooled, extensively dialyzed against 0.05 mol L−1 PBS (pH 7.2) and concentrated by ultrafiltration using Microcon YM-10 Centrifugal Filter Devices (Millipore).
Sodium dodecyl sulfate-polyacrylamide gel electrophoresis (SDS-PAGE)
SDS-PAGE was performed on 4%–20% gradient gel or on 6% and 20% separating gels and on 3.75% stacking gel according to Laemmli 28. The gel was stained using the Roti Black silver staining kit (Carl Roth GmbH+Co.), according to the manufacturers' instructions. The gel was calibrated with ColorBurst Electrophoresis Markers (8–220 kDa) (Sigma).
Immuno- and lectin blotting
Proteins were transferred on to Immobilon-P–PVDF membrane by semi-dry blotting using a Multiphor II Nova Blot Unit (Pharmacia LKB, Uppsala, Sweden). The conditions were as follows: transfer buffer, 25 mmol L−1 Tris, containing 192 mmol L−1 glycine and 20% methanol (pH 8.3); 1.2 mA cm−2 for 1 h. The membrane was blocked with 1% casein in 0.05 mol L−1 PBS (pH 7.2), for 1 h at 4°C.
For immunoblotting, the membrane was incubated with monoclonal anti-plasma FN Ab or monoclonal EDA-specific anti-cellular FN Ab (both at 0.5 μg mL−1 in 1% casein 0.05 mol L−1 PBS [pH 7.2]) overnight at 4°C, washed three times in 0.05% Tween in 0.05 mol L−1 PBS (pH 7.2) and then incubated with biotinylated anti-mouse IgG Ab for 30 min at room temperature (RT). After repeating the washing procedure, avidin/biotinylated horseradish peroxidase (HRPO) from Elite Vectastain ABC kit (prepared according to the manufacturer's instructions) was added followed by incubation for 30 min at RT. The membrane was then washed again three times and proteins were visualized using substrate solution containing hydrogen peroxide solution (H2O2) and DAB according to the manufacturers' instructions.
For lectin blot, membranes were incubated with biotinylated lectins (10 μg mL−1) for 1 h at RT, washed three times in 0.05% Tween in 0.05 mol L−1 PBS (pH 7.2) and incubated with avidin/biotin–HRPO for 30 min at RT. After washing, proteins were subsequently visualized using the DAB substrate kit for peroxidase.
The corresponding negative controls (omitting the primary Ab/binding in the presence of lectin-competing sugars) were also included and gave no visible reactions.
Hemagglutination assay
The assays were carried out in 96-well microtitre plates at 37°C. Two-fold serial dilutions of gal-1 in 20 mmol L−1 PBS (pH 7.2) containing 2 mmol L−1 β-mercaptoethanol and 4 mmol L−1 EDTA were mixed with equal volumes of 2% trypsin-treated rabbit erythrocytes. The hemagglutination titer was determined after 1 h. The inhibiting effect of human seminal plasma gelatin-binding proteins was examined by preincubation with gal-1 at 37°C for 30 min, followed by hemagglutination assay.
ProteinChip analysis of isolated gelatin-binding proteins
ProteinChip analysis implies application of the sample to different chromatographic surfaces of protein chips, which provides selective retention of molecules, depending on their affinity to the given surface. Retained molecules are mixed with the matrix, ionized and desorbed by laser, followed by surface-enhanced laser desorption/ionization time of flight mass spectrometry (SELDI-TOF MS). This technique allows sample analysis in small volumes, with high sensitivity and resolution, and is especially suitable for analysis of low molecular mass proteins 29.
Profiling on normal phase, NP20 ProteinChip array
A 5-μL aliquot of affinity-purified proteins was applied on an NP20 ProteinChip array spot and allowed to air-dry at RT. The spots were then washed twice with 5 μL 0.01 mol L−1 HEPES (pH 7.5) followed by 5 μL of deionized water. After complete drying of spots, 1 μL of 50% sinapinic acid (in acetonitrile/dH2O/trifluoroacetic acid [50%/49.9%/0.1%]) was added to each spot, dried and then reapplied. All probes were done in duplicate.
Affinity capture on preactivated surface, PS20 ProteinChip array
A 5-μL sample of protein (corresponding FN-Ab, gal-1, heparin or gelatin) was added to each spot and incubated in a humid chamber overnight. The spots were washed with 5 μL of 0.05 mol L−1 PBS (pH 7.2), twice for 2 min at RT followed by blocking with 0.5 mol L−1 Tris-HCl (pH 8.0) buffer for 1 h at RT. After repeating the washing step, 5 μL of isolated preparation was added, per spot, and incubation continued for 3.5 h at RT. For analysis of isolated preparations on protein chips coated with gal-1, we used the preparation alone or in combination with 0.2 mol L−1 lactose (1:1) as a control for specificity of reaction.
The spot was then washed again with 0.05 mol L−1 PBS (pH 7.2), followed by 5 μL 0.01 mol L−1 HEPES and finally with 5 μL of deionized water. All procedures included shaking (0.04 × g). After complete drying of the spot, 1 μL of 50% sinapinic acid was added to each spot, dried and then reapplied. All probes were done in duplicate.
Immobilized metal-affinity capture on IMAC30 ProteinChip array
IMAC30 spots were charged with 5 μL of 0.1 mol L−1 ferric sulfate for 5 min at RT. Spots were then washed with 5 μL of deionized water for 1 min and neutralized with 5 μL 0.1 mol L−1 sodium acetate buffer (pH 4.0) for 5 min.
After another rinsing with water and subsequently with 5 μL of binding buffer (0.1 mol L−1 sodium phosphate, 0.5 mol L−1 NaCl [pH 7.2]) for 5 min at RT, 5 μL of the sample was added to each spot and incubated for 30 min in a humid chamber at RT. All procedures included shaking (0.04 × g). After complete drying of the spots, 1 μL of 50% sinapinic acid was added to each spot, dried and then reapplied. All probes were done in duplicate.
Reversed phase/hydrophobic capture on H50 ProteinChip array
Spots were prerinsed with 50% methanol for 5 min, air-dried and then prewetted with 5 μL of 10% acetonitrile/0.1% trifluoroacetic acid for 2 min. After repeating the procedure once, 5 μL of sample was added to each spot and incubated for 30 min in a humid chamber at RT. Each spot was washed twice with 5 μL of 10% acetonitrile/0.1% trifluoroacetic acid for 2 min, and then with 5 μL deionized water. All procedures included shaking (0.04 × g). After complete drying of the spots, 1 μL of 50% sinapinic acid was added to each spot, dried and then reapplied. All probes were done in duplicate.
SELDI-TOF MS
Protein chip arrays were analyzed by SELDI-TOF MS using the ProteinChip Reader, Series 4000, Personal edition (BioRad). All spectra were acquired in 25 kV positive ion acquisition mode, mass range of 2.5–250 kDa and with 8 815 laser shots per spot of 6 000 nJ laser energy. Mass calibration was performed with the ProteinChip all-in-one protein standards II. All spectra were analyzed using CiphergenExpress Software 3.0 (BioRad).
Results
Molecular pattern and FN immunoreactivity of gelatin-binding proteins from human seminal plasma
Representative SDS-PAGE of human seminal plasma proteins isolated by affinity chromatography on a column, with immobilized gelatin as the ligand, is shown in Figure 1. They comprise a complex pattern of differently abundant molecular mass species ranging from 8–200 kDa. Taking advantage of on-chip profiling combined with MS detection particularly suitable for analysis of low molecular mass proteins, a more comprehensive profile was obtained (Figure 2). Thus, in the low molecular mass region of 8–20 kDa, several peaks in the proximity of 10, 12, 13, 14 and 15 kDa, appearing broad and heterogeneous, were consistently observed (Figure 2A). In the region of 20–80 kDa, sharp and more homogenous peaks were detected. They comprised two main peaks of 42 and 54 kDa, and five less abundant ones of 21, 27, 30, 67 and 74 kDa (Figure 2B and C). In the high molecular mass region, peaks of 96, 101, 108, 138, 152, 194 and 203 kDa were found (Figure 2D).
Figure 1.
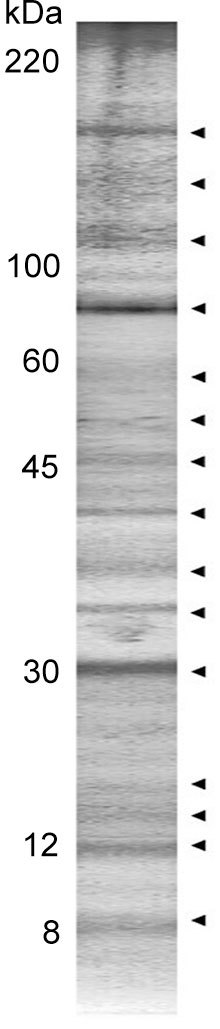
Sodium dodecyl sulfate-polyacrylamide gel electrophoresis (SDS-PAGE) of affinity-purified gelatin-binding proteins from human seminal plasma. Pooled human seminal plasma from subjects with normal semen parameters was applied to gelatin–Sepharose 4B column, and the bound fraction was eluted with 4 mol L−1 urea. The isolated preparation was subjected to SDS-PAGE on a 4%–20% gradient gel under reducing conditions. The proteins were stained with silver. The numbers indicate molecular mass (kDa) of standard proteins used for gel calibration. The electrophoretic pattern is indicated by arrowheads.
Figure 2.
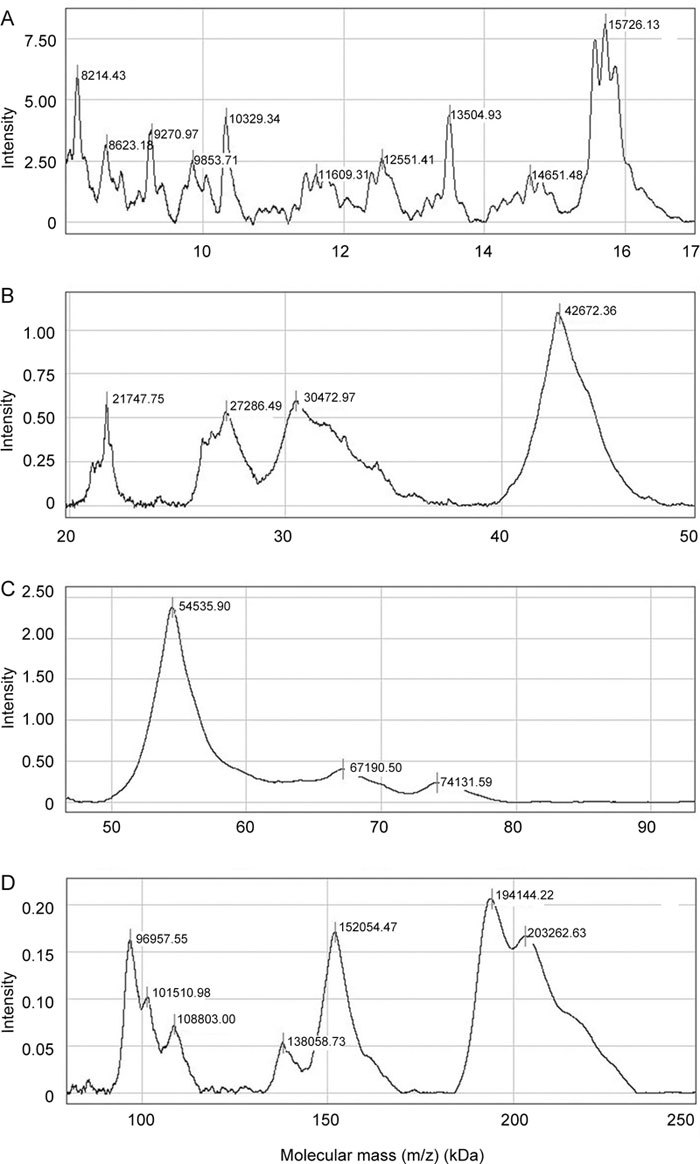
Normal phase (NP20) protein chip profiling of affinity-purified gelatin-binding proteins from human seminal plasma. Affinity-purified gelatin-binding proteins from human seminal plasma were applied to NP20 ProteinChip array and processed as indicated in Materials and methods. A surface-enhanced laser desorption/ionization-time of flight (SELDI-TOF) mass spectrum was acquired in ion-positive mode at 25 kV and analyzed using CiphergenExpress Software 3.0 (BioRad, Hercules, CA, USA). Calibration was performed with ProteinChip all-in-one protein standards II. Molecular mass ranges are as follows: (A) 8–17 kDa, (B) 20–50 kDa, (C) 50–90 kDa and (D) 90–250 kDa.
The human seminal plasma gelatin-binding preparation was subsequently characterized immunologically by employing monoclonal Abs against distinct epitopes of human FN. Immunoblot analysis using anti-human plasma FN Ab revealed 54, 74, 96, 150, 190 and 200 kDa bands (Figure 3Aa). Among them, the 74, 150, 190 and 200 kDa bands were reactive with monoclonal anti-cellular FN Ab (EDA-specific) (Figure 3Ab). When the on-chip immobilized assay was used, results comparable with Western blot (WB) were obtained in the 50–200 kDa region (data not shown), except for additional faint but detectable peaks of 8–12 kDa recognized by the EDA-specific Ab (Figure 3B, spectrum b) and peaks of 12–15 kDa reactive to anti-plasma FN Ab (Figure 3B, spectrum a).
Figure 3.
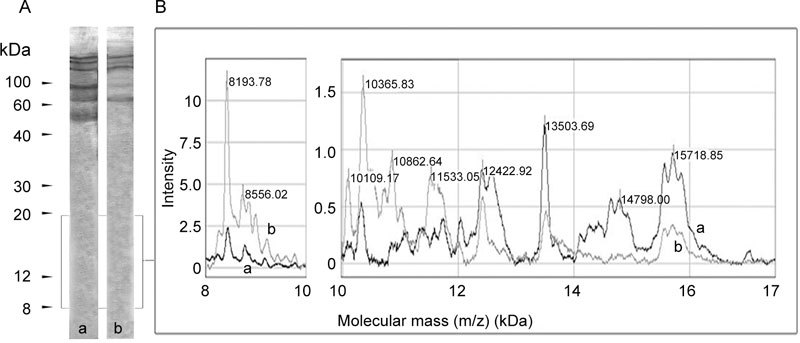
Fibronectin (FN)-immunoreactivity of affinity-purified gelatin-binding proteins from human seminal plasma. (A): Immunoblot analysis: affinity-purified gelatin-binding proteins from human seminal plasma were resolved by SDS-PAGE, blotted onto Immobilon-P–PVDF membrane and probed with monoclonal anti-plasma FN antibody (Ab) (a) and monoclonal anti-cellular FN Ab (b). Bound antibody was visualized using biotinylated anti-mouse IgG Ab followed by avidin/biotinylated-HRPO mixture and by addition of DAB substrate solution. Numbers indicate molecular mass (kDa). (B): On-chip immunoaffinity profiling: monoclonal anti-plasma FN antibody (a) or monoclonal anti-cellular FN antibody (b) were immobilized on preactivated surface (PS20) ProteinChip array and allowed to react with affinity-purified gelatin-binding proteins, as described in Materials and methods. On-chip immunoaffinity captured molecules were detected by SELDI-TOF mass spectrometry in ion-positive mode at 25 kV and analyzed using CiphergenExpress Software 3.0 ( (BioRad, Hercules, CA, USA)). Calibration was performed with ProteinChip all-in-one protein standards II. The mass region of 8–17 kDa is shown.
Heparin-binding activity
Affinity-purified human seminal plasma proteins were tested for binding to immobilized heparin (Figure 4, spectrum A). Considering the intensity of gelatin binding as arbitrary (Figure 4, spectrum B), the 54-kDa fraction appeared to bind gelatin preferentially, the 63-, 66- and 74-kDa species bound gelatin and heparin equally, whereas low molecular mass species (10, 11, 12, 13, 14 and 15 kDa) preferentially bound heparin.
Figure 4.
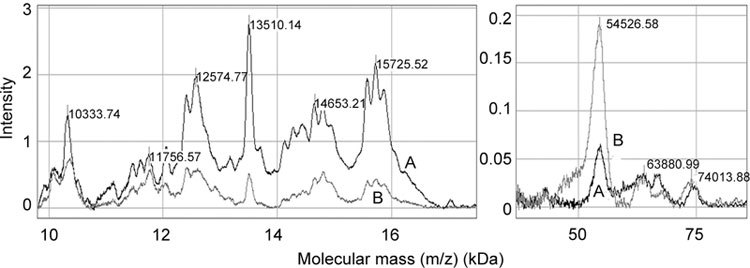
Heparin-binding pattern of affinity-purified gelatin-binding proteins from human seminal plasma. Heparin (spectrum A) or gelatin (spectrum B) were immobilized on preactivated surface (PS20) ProteinChip array and allowed to react with affinity-purified gelatin-binding proteins, as described in Materials and methods. On-chip ligand-captured molecules were detected by SELDI-TOF mass spectrometry in ion-positive mode at 25 kV and analyzed using CiphergenExpress Software 3.0 (BioRad, Hercules, CA, USA). Calibration was performed with ProteinChip all-in-one protein standards II.
Hydrophobic and metal-binding properties
Hydrophobic surface capture of isolated human seminal plasma gelatin-binding proteins allowed separation of the main heterogeneous 8-, 11-, 13- and 14-kDa peaks, in addition to several smaller peaks in the molecular mass region of 8–17 kDa. In the region of 20–70 kDa, a main peak of 66 kDa was detected (Figure 5).
Figure 5.
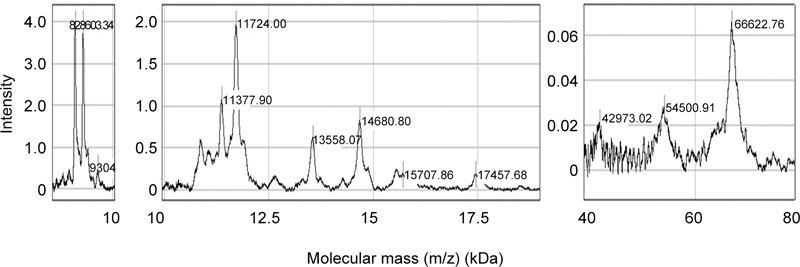
Hydrophobic properties of affinity-purified gelatin-binding proteins from human seminal plasma. H50 ProteinChip array was prepared and incubated with affinity-purified gelatin-binding proteins, as indicated in Materials and methods. The bound proteins were detected by SELDI-TOF mass spectrometry in ion-positive mode at 25 kV and analyzed using CiphergenExpress Software 3.0 (BioRad, Hercules, CA, USA). Calibration was performed with ProteinChip all-in-one protein standards II.
When the same preparation was captured on the metal-affinity surface, main peaks of 10, 11, 13, 15, 42 and 54-kDa were revealed (Figure 6).
Figure 6.
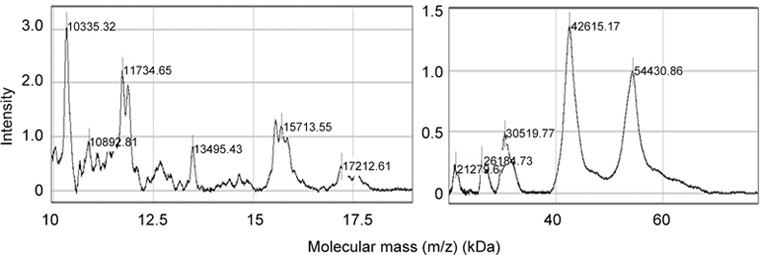
Metal-binding properties of affinity-purified gelatin-binding proteins from human seminal plasma. IMAC30 ProteinChip array charged with Fe3+ ions was prepared and incubated with affinity-purified gelatin-binding proteins, as indicated in Materials and methods. The bound proteins were detected by SELDI-TOF mass spectrometry in ion-positive mode at 25 kV and analyzed using CiphergenExpress Software 3.0 (BioRad, Hercules, CA, USA). Calibration was performed with ProteinChip all-in-one protein standards II.
Glycosylation assessment
Glycosylation of isolated gelatin-binding proteins was assessed using the following plant lectins: RCA I (specific for Galβ1,4GlcNAc), ConA (specific for the mannosyl core of high mannose-, hybrid- and biantennary complex-type of N-glycans), LCA (specific for α1,6 core fucose), SNA (specific for sialic acid α2,6 linked Gal/GalNAc) and MAA (specific for sialic acid α2,3 linked Gal/GalNAc).
The RCA I-, ConA- and LCA-binding patterns were similar and revealed the main 42- and 54-kDa bands, as well as several others in the range of 100–200 kDa (Figure 7). Differences were found with respect to the 96-kDa band, which was recognized by RCA I only, and the 15-kDa band, which was bound by RCA I and LCA, but not by ConA. As for SNA, it reacted more weakly than RCA I, ConA and LCA, whereas MAA gave a barely detectable reaction (Figure 7).
Figure 7.
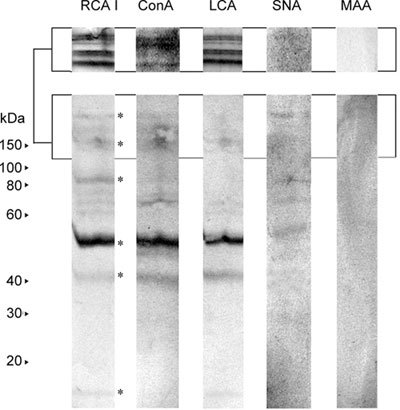
Lectin blot analysis of affinity-purified gelatin-binding proteins from human seminal plasma. Affinity-purified gelatin-binding proteins from human seminal plasma were resolved by 12.5% SDS-PAGE under reducing conditions, blotted onto Immobilon-P–PVDF membrane and probed with biotinylated lectins: RCA I (Ricinus communis agglutinin I), ConA (lectin from Canavalia ensiformis), LCA (Lens culinaris agglutinin), SNA (Sambucus nigra agglutinin) and MAA (Maackia amurensis agglutinin). Bound lectins were visualized using a mixture of avidin and biotinylated-HRPO followed by addition of H2O2/3,3′-diaminobenzidine. Numbers indicate molecular mass (kDa). Lectin binding to higher molecular mass species was more noticeable when the sample was resolved on 20% gel (upper panel) because of the generally known diffuse appearance of glycoproteins on SDS-PAGE. Asterisks indicate main lectin-reactive bands.
Besides plant lectins, human gal-1 was also used to analyze the oligosaccharide composition of isolated gelatin-binding proteins. These proteins did not inhibit the hemagglutination activity of gal-1 or bind to it in the chip affinity capture assay in a lactose-dependent manner (data not shown).
Discussion
The results obtained for an affinity-purified preparation from human seminal plasma indicated the existence of structurally diverse gelatin-binding species differing in molecular mass, glycosylation and the capacity to interact with endogenous ligands. Molecular mass pattern of isolated proteins corresponded to known FN fragments, often reported as a result of in vivo or in vitro proteolytic digestion 30, 31, 32, 33, 34, and this was confirmed by the immunoreactivity with distinct monoclonal anti-human FN Abs. Thus, protein bands in the region of 50–200 kDa were identified as the main human plasma FN immunoreactive fragments and, specifically, in the high molecular mass region, anti-EDA reactivity typical of cellular FN was also detected. In addition to main immunoreactive fragments, low but detectable and distinct FN immunoreactivity of 8–15 kDa protein bands was observed. This pattern of low molecular mass species resembles that of the acidic gelatin-binding proteins as the main proteins present in the seminal plasma of different animals 21, 35, 36, 37. As already mentioned, they contain an FN-type II domain, but there are no data on their possible antigenic relatedness with FN. FN and these FN-related gelatin-binding proteins, as well as human seminal plasma proteins homologous to boar spermadhesins comprising at least 12 distinct species of 10–18 kDa 38, 39 share the heparin-binding ability in common. In view of this property, the affinity-purified human seminal plasma preparation was probed with heparin as a ligand. The results obtained indicated that the greatest binding capacity was associated with low molecular mass species in the range of 10–17 kDa.
Thus, under normal physiological conditions, FN is present in human seminal plasma in fragmented form, probably due to the action of proteolytic enzymes. FN fragmentation is, however, known as general sign of different pathophysiological conditions, such as wound healing, arthritis, bladder and gastrointestinal cancer, head and neck cancers, etc. 32, 40, 41, 42, 43.
Our human seminal plasma gelatin-binding preparation was further characterized by hydrophobic chromatography. Generally, FN is known as a sticky protein, and it has the ability to adhere to different hydrophilic and hydrophobic surfaces, which is important in relation to its role in cell adhesion and cell–matrix interaction 44. The results obtained indicated several molecular species whose molecular mass pattern partly corresponded to that reported for porcine plasma FN. Thus, the available data indicated differences in the hydrophobicities of particular porcine plasma FN fragments, among which the 14-kDa moiety had the highest and the 140-kDa fraction the lowest 45.
It is known that FN can be posttranslationally modified by phosphorylation and glycosylation 7, 46. These modifications are thought to be very important for its structural and functional properties, and characteristically change during normal as well as pathological conditions 47, 48.
The results of metal-affinity chromatography point to several human seminal plasma FN-related fragments in the region of 10–60 kDa as possibly phosphorylated. This may be correlated with the data on normal- and transformed cell line-derived FNs, indicating 40-, 10- and 12-kDa fragments as phosphorylated 46. Phosphorylation of FN is claimed to be highly specific and conserved, and it is reported that FN from both normal and tumor cells are phosphorylated only on serine residues 46. Generally, phosphorylation can influence cellular morphology and it was suggested that changes in FN phosphorylation can contribute to decreased retention at the surface of transformed cells.
Concerning glycosylation, FN is reported to contain both N- and O-glycans and carbohydrates account for 5% of its molecular mass 7. FN from various sources differs in the degree of sialylation and the type of linkage of sialic acid, as well as in the absence or presence of fucose 47, 49, 50, 51, 52, 53, 54. Thus, the N-glycans of plasma FN are complex-type biantennary oligosaccharides, largely sialylated with predominance of the sialic acid α2,6 Gal linkage, whereas fetal/oncofetal FN, which is upregulated during development/transformation, is core fucosylated and generally not or poorly sialylated, with predominance of the sialic acid α2,3 Gal linkage.
The results obtained for human seminal plasma gelatin-binding species indicated the strongest binding by RCA and accordingly a low level of sialylation (based on SNA and MAA reactivity), with both α2,3 Gal and α2,6 Gal containing glycans. N-linked glycans (based on ConA reactivity) was found to be modified by α1,6 core fucosylation (based on LCA-reactivity). Taken together, distinct lectin-binding patterns of isolated preparation in relation to molecular masses was in agreement with the available data indicating that FN glycosylation is mostly associated with gelatin-binding domain and also cell-binding domain 7, 55.
Glycosylation was found to be important for FN stability and could influence the interaction with cells 7, 56. Thus, the presence of N-linked polylactosamine chains is found to decrease the affinity for gelatin and influence cell-spreading potency of FN 57. In relation to this, the carbohydrate composition of human seminal plasma gelatin-binding molecules was assessed by interaction with human gal-1, which is specific for beta-galactoside and reported to have a role in fertilization 58. So far, tissue FN was reported to be an endogenous ligand for gal-1 and this was related to expression of polylactosamine chains on the FN molecule 59. Although the results of assessment of carbohydrate composition partly suggested oncofetal type of glycosylation, gal-1 did not interact with isolated preparation in lactose-inhabitable manner.
As a consequence of the extreme heterogeneity of glycoproteins, a major area of concern is that the related structural knowledge is generally still limited. Male reproductive physiology involves various processes mediated by multiple complementary receptor–ligand systems and the available literature data indicate particular roles for protein–carbohydrate interactions 60. Thus, it is desirable to develop a knowledge base for biological systems that operate through oligosaccharide recognition, which is a significant component for human seminal plasma proteins. Species-specific recognition and binding between spermatozoa and the oocyte can be modulated by various monosaccharides, polysaccharides or glycoproteins and they can also affect sperm motility 17, 60. Heparin was found to be the only carbohydrate that significantly increased the penetration rate. In contrast to this, FN caused marked reduction in sperm motility parameters, whereas exogenously added mannose, fucoidan, dextran sulfate and FN were the most potent inhibitors of oocyte penetration. In this respect, structural knowledge about human seminal plasma FN as a multifunctional glycoprotein seems to be an inevitable part of defining the molecular requirements of gamete interaction and the expression of species specificity during early events of fertilization.
Our study provides data on the glycoimmunological properties of soluble human seminal plasma FN in normal fertile subjects. They are not clinical data, but they can be a base for creating a biomedical strategy to modify signal and recognitive properties of FN molecules as ligands in carbohydrate-dependent or protein–protein interactions. Carbohydrate-based drugs or vaccines, already on the market in various phases of clinical trials, are promising candidates for therapeutic agents and in contemporary medicinal glycoscience, the control and modification of cell motility, one reliable indicator of fertility, are of special interest 61. The results obtained indicate human seminal plasma FN as the cellular-type molecule containing an extra domain A. Thus, it is known that cellular FN differs in activity from plasma FN, that is, it has significantly higher agglutinating activity and an influence on cell morphology 6, 62. As for the FN fragmentation observed, this can differently influence its activity. Our previous investigation indicated that cancer-derived FN fragments with altered glycans exhibit significantly lower potency for promoting cell adhesion than normal intact FN 63. The importance of defining the structure of FN fragments is documented for the process of controlling prostate cancer cell invasion and tumor progression, resulting in the development of a prostate cancer vaccine 64, 65. Thus, on the one hand, FN and some fragments possess cell-interaction/binding properties and on the other hand, they can elicit different responses in terms of production of biologically active mediators 66, 67, 68, 69.
The described heterogeneity and predicted biosignalling properties might be of importance as reference molecular markers of origin and a guide for establishing a network of molecules among which interactions can have biomedical consequences. Defining the molecular properties of human seminal plasma FN expressed under normal physiological conditions sets the stage for further exploration of protein modifications for versatile functions.
Acknowledgments
This work was supported by the Ministry for Science and Technological Development of the Republic of Serbia (No. 143048).
References
- Owen DH, Katz DF. A review of the physical and chemical properties of human semen and the formulation of a semen stimulant. J Androl. 2005;26:459–69. doi: 10.2164/jandrol.04104. [DOI] [PubMed] [Google Scholar]
- Fung KY, Glode LM, Green S, Duncan MW. A comprehensive characterization of the peptide and the protein constituents of human seminal fluid. Prostate. 2004;61:171–81. doi: 10.1002/pros.20089. [DOI] [PubMed] [Google Scholar]
- Pilch B, Mann M. Large-scale and high-confidence proteomic analysis of human seminal plasma. Genome Biol. 2006;7:R40. doi: 10.1186/gb-2006-7-5-r40. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Duncan MW, Thompson HS. Proteomics of semen and its constituents. Proteomics Clin Appl. 2007;1:861–75. doi: 10.1002/prca.200700228. [DOI] [PubMed] [Google Scholar]
- Wang J, Wang J, Zhang HR, Shi HJ, Ma D, et al. Proteomic analysis of seminal plasma from asthenozoospermia patients reveals proteins that affect oxidative stress responses and semen quality. Asian J Androl. 2009;11:484–91. doi: 10.1038/aja.2009.26. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hynes RO, Yamada KM. Fibronectins: multifunctional modular glycoproteins. J Cell Biol. 1982;95:369–77. doi: 10.1083/jcb.95.2.369. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pankov R, Yamada KM. Fibronectin at a glance. J Cell Sci. 2002;115:3861–3. doi: 10.1242/jcs.00059. [DOI] [PubMed] [Google Scholar]
- Fibronectin. Available from http://www.copewithcytokines.de/cope.cgi?key=Fibronectin
- Fusi FM, Bronson RA. Sperm surface fibronectin. Expression following capacitation. J Androl. 1992;13:28–35. [PubMed] [Google Scholar]
- Glander HJ, Herrmann K, Haustein UF. The equatorial fibronectin band (EFB) on human spermatozoa–a diagnostic help for male fertility. Andrologia. 1987;19:456–9. doi: 10.1111/j.1439-0272.1987.tb02327.x. [DOI] [PubMed] [Google Scholar]
- Miranda PV, Tezon JG. Characterization of fibronectin as a marker for human epididymal sperm maturation. Mol Reprod Dev. 1992;33:443–50. doi: 10.1002/mrd.1080330411. [DOI] [PubMed] [Google Scholar]
- Pinke LA, Swanlund DJ, Hensleigh HC, McCarthy JB, Roberts KP, et al. Analysis of fibronectin on human sperm. J Urol. 1997;158:936–41. doi: 10.1097/00005392-199709000-00075. [DOI] [PubMed] [Google Scholar]
- Wennemuth G, Schiemann PJ, Krause W, Gressner AM, Aumüller G. Influence of fibronectin on the motility of human spermatozoa. Int J Androl. 1997;20:10–6. doi: 10.1046/j.1365-2605.1997.00005.x. [DOI] [PubMed] [Google Scholar]
- Glander HJ, Schaller J, Rohwedder A, Henkel R. Adhesion molecules and matrix proteins on human spermatozoa. Andrologia. 1998;30:289–96. doi: 10.1111/j.1439-0272.1998.tb01173.x. [DOI] [PubMed] [Google Scholar]
- Xu F, Cui X, Yang F. Effect of anti-fibronectin-serum on fertilization capacity of human spermatozoa. Hua Xi Yi Ke Da Xue Xue Bao. 1994;25:422–5. [PubMed] [Google Scholar]
- Thys M, Nauwynck H, Maes D, Hoogewijs M, Vercauteren D, et al. Expression and putative function of fibronectin and its receptor (integrin alpha(5)beta(1)) in male and female gametes during bovine fertilization in vitro. Reproduction. 2009;138:471–82. doi: 10.1530/REP-09-0094. [DOI] [PubMed] [Google Scholar]
- Tanghe S, Van Soom A, Duchateau L, Nauwynck H, de Kruif A. Carbohydrates and glycoproteins involved in bovine fertilization in vitro. Mol Reprod Dev. 2004;68:492–9. doi: 10.1002/mrd.20095. [DOI] [PubMed] [Google Scholar]
- Glander HJ, Herrmann K, Haustein UF. Impairment of the fertilization process by adherence of microorganism to the equatorial fibronectin band (EFB) of human sperm. A pathogenic hypothesis. Int Urol Nephrol. 1990;22:483–6. doi: 10.1007/BF02549782. [DOI] [PubMed] [Google Scholar]
- Katnik-Prastowska I, Przybysz M, Chełmońska-Soyta A. Fibronectin fragments in human seminal plasma. Acta Biochim Pol. 2005;52:557–60. [PubMed] [Google Scholar]
- Katnik-Prastowska I, Kratz EM, Faundez R, Chełmońska-Soyta A. Lower expression of the alpha2,3-sialylated fibronectin glycoform and appearance of the asialo-fibronectin glycoform are associated with high concentrations of fibronectin in human seminal plasma with abnormal semen parameters. Clin Chem Lab Med. 2006;44:1119–25. doi: 10.1515/CCLM.2006.193. [DOI] [PubMed] [Google Scholar]
- Manjunath P, Sairam MR. Purification and biochemical characterization of three major acidic proteins (BSP-A1, BSP-A2 and BSP-A3) from bovine seminal plasma. Biochem J. 1987;241:685–92. doi: 10.1042/bj2410685. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Manjunath P, Sairam MR, Uma J. Purification of four gelatin-binding proteins from bovine seminal plasma by affinity chromatography. Biosci Rep. 1987;7:231–8. doi: 10.1007/BF01124794. [DOI] [PubMed] [Google Scholar]
- Leblond E, Desnoyers L, Manjunath P. Phosphorylcholine-binding proteins from the seminal fluids of different species share antigenic determinants with the major proteins of bovine seminal plasma. Mol Reprod Dev. 1993;34:443–9. doi: 10.1002/mrd.1080340414. [DOI] [PubMed] [Google Scholar]
- Sahin E, Petrunkina AM, Ekhlasi-Hundrieser M, Hettel C, Waberski D, et al. Fibronectin type II-module proteins in the bovine genital tract and their putative role in cell volume control during sperm maturation. Reprod Fertil Dev. 2009;21:479–88. doi: 10.1071/rd08209. [DOI] [PubMed] [Google Scholar]
- Wennemuth G, Meinhardt A, Mallidis C, Albrecht M, Krause W, et al. Assessment of fibronectin as a potential new clinical tool in andrology. Andrologia. 2001;33:43–6. doi: 10.1046/j.1439-0272.2001.00370.x. [DOI] [PubMed] [Google Scholar]
- World Health Organization. WHO laboratory Manual for the Examination of Human Semen and Sperm-Cervical Mucus Interaction4th ed. Cambridge, United Kingdom: Cambridge University Press1999
- Ruoslahti E, Hayman EG, Pierschbacher M, Engvall E. Fibronectin: purification, immunochemical properties, and biological activities. Methods Enzymol. 1982;82:803–31. doi: 10.1016/0076-6879(82)82103-4. [DOI] [PubMed] [Google Scholar]
- Laemmli U. Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature. 1970;227:680–5. doi: 10.1038/227680a0. [DOI] [PubMed] [Google Scholar]
- Merchant M, Weinberger SR. Recent advancements in surface-enhanced laser desorption/ionization-time of flight-mass spectrometry. Electrophoresis. 2000;21:1164–77. doi: 10.1002/(SICI)1522-2683(20000401)21:6<1164::AID-ELPS1164>3.0.CO;2-0. [DOI] [PubMed] [Google Scholar]
- Zardi L, Carnemolla B, Balza E, Borsi L, Castellani P, et al. Elution of fibronectin proteolytic fragments from a hydroxyapatite chromatography column. A simple procedure for the purification of fibronectin domains. Eur J Biochem. 1985;146:571–9. doi: 10.1111/j.1432-1033.1985.tb08690.x. [DOI] [PubMed] [Google Scholar]
- Castellani P, Siri A, Rosellini C, Infusini E, Borsi L, et al. Transformed human cells release different fibronectin variants than do normal cells. J Cell Biol. 1986;103:1671–7. doi: 10.1083/jcb.103.5.1671. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grinnell F, Zhu M. Identification of neutrophil elastase as the proteinase in burn wound fluid responsible for degradation of fibronectin. J Invest Dermatol. 1994;103:155–61. doi: 10.1111/1523-1747.ep12392625. [DOI] [PubMed] [Google Scholar]
- Ffrench-Constant. Alternative splicing of fibronectin-many different proteins but few different functions Exp Cell Res 1995221261–71. [DOI] [PubMed] [Google Scholar]
- Labat-Robert J. Fibronectin in malignancy. Semin Cancer Biol. 2002;12:187–95. doi: 10.1016/S1044-579X(02)00022-6. [DOI] [PubMed] [Google Scholar]
- Villemure M, Lazure C, Manjunath P. Isolation and characterization of gelatin-binding proteins from goat seminal plasma. Reprod Biol Endocrinol. 2003;1:39–49. doi: 10.1186/1477-7827-1-39. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Boisvert M, Bergeron A, Lazure C, Manjunath P. Isolation and characterization of gelatin-binding bison seminal vesicle secretory proteins. Biol Reprod. 2004;70:656–61. doi: 10.1095/biolreprod.103.023069. [DOI] [PubMed] [Google Scholar]
- Bergeron A, Villemure M, Lazure C, Manjunath P. Isolation and characterization of the major proteins of ram seminal plasma. Mol Reprod Dev. 2005;71:461–70. doi: 10.1002/mrd.20310. [DOI] [PubMed] [Google Scholar]
- Kraus M, Tichá M, Jonáková V. Heparin-binding proteins of human seminal plasma homologous with boar spermadhesins. J Reprod Immunol. 2001;51:131–44. doi: 10.1016/s0165-0378(01)00072-9. [DOI] [PubMed] [Google Scholar]
- Kraus M, Tichá M, Zelezná B, Peknicová J, Jonáková V. Characterization of human seminal plasma proteins homologous to boar AQN spermadhesins. J Reprod Immunol. 2005;65:33–46. doi: 10.1016/j.jri.2004.10.001. [DOI] [PubMed] [Google Scholar]
- Barilla ML, Carsons SE. Fibronectin fragments and their role in inflammatory arthritis. Semin Arthritis Rheum. 2000;29:252–65. doi: 10.1016/s0049-0172(00)80012-8. [DOI] [PubMed] [Google Scholar]
- Guo JM, Zhang XY, Chen HL, Wang GM, Zhang YK. Structural alterations of sugar chains in urine fibronectin from bladder cancer patients and its enzymatic mechanism. J Cancer Res Clin Oncol. 2001;127:512–9. doi: 10.1007/s004320100245. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hegele A, Heidenreich A, Kropf J, von Knobloch R, Varga Z, et al. Plasma levels of cellular fibronectin in patients with localized and metastatic renal cell carcinoma. Tumour Biol. 2004;25:111–6. doi: 10.1159/000079142. [DOI] [PubMed] [Google Scholar]
- Warawdekar UM, Zingde SM, Iyer KS, Jagannath P, Mehta AR, et al. Elevated levels and fragmented nature of cellular fibronectin in the plasma of gastrointestinal and head and neck cancer patients. Clin Chim Acta. 2006;372:83–93. doi: 10.1016/j.cca.2006.03.023. [DOI] [PubMed] [Google Scholar]
- Grinnell F, Feld MK. Fibronectin adsorption on hydrophilic and hydrophobic surfaces detected by antibody binding and analyzed during cell adhesion in serum-containing medium. J Biol Chem. 1982;257:4888–93. [PubMed] [Google Scholar]
- Hayashi-Nagai A, Kitagaki-Ogawa H, Matsumoto I, Hayashi M, Seno N. Hydrophobic properties of porcine fibronectin and its functional domains. J Biochem. 1991;109:83–8. [PubMed] [Google Scholar]
- Ali IU, Hunter T. Structural comparison of fibronectins from normal and transformed cells. J Biol Chem. 1981;256:7671–7. [PubMed] [Google Scholar]
- Köttgen E, Hell B, Müller C, Kainer F, Tauber R. Developmental changes in the glycosylation and binding properties of human fibronectins. Characterization of the glycan structures and ligand binding of human fibronectins from adult plasma, cord blood and amniotic fluid. Biol Chem Hoppe Seyle. 1989;370:1285–94. doi: 10.1515/bchm3.1989.370.2.1285. [DOI] [PubMed] [Google Scholar]
- Murayama K, Nichols EJ, Levery SB, Carter WG, Hakomori S. The carbohydrate structure of human fibronectins: a comparison between normal embryonic lung fibroblasts WI-38 and the SV40 virus transformed cell line VA13. Glycoconj J. 1984. pp. 155–69.
- Yamaguchi Y, Isemura M, Kosakai A, Sato M, Suzuki M, et al. Characterization of fibronectin from fetal human plasma in comparison with adult plasma fibronectin. Biochem Biophys Acta. 1984;790:53–60. doi: 10.1016/0167-4838(84)90331-5. [DOI] [PubMed] [Google Scholar]
- Krusius T, Fukuda M, Dell A, Ruoslahti E. Structure of the carbohydrate units of human amniotic fluid fibronectin. J Biol Chem. 1985;260:4110–6. [PubMed] [Google Scholar]
- Carsons S, Lavietes BB, Slomiany A, Diamond HS, Berkowitz E. Carbohydrate heterogeneity of fibronectins. J Clin Invest. 1987;80:1342–9. doi: 10.1172/JCI113211. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Takamoto M, Endo T, Isemura M, Kochibe N, Kobata A. Structures of asparagine-linked oligosaccharides of human placental fibronectin. J Biochem (Tokyo) 1989;105:742–50. doi: 10.1093/oxfordjournals.jbchem.a122738. [DOI] [PubMed] [Google Scholar]
- Takamoto M, Endo T, Isemura M, Yamaguchi Y, Okamura K, et al. Detection of bisected biantennary form in the asparagine-linked oligosaccharides of fibronectin isolated from human term amniotic fluid. J Biochem (Tokyo) 1989;106:228–35. doi: 10.1093/oxfordjournals.jbchem.a122837. [DOI] [PubMed] [Google Scholar]
- Tajiri M, Yoshida S, Wada Y. Differential analysis of site-specific glycans on plasma and cellular fibronectins: application of a hydrophilic affinity method for glycopeptide enrichment. Glycobiology. 2005;15:1332–40. doi: 10.1093/glycob/cwj019. [DOI] [PubMed] [Google Scholar]
- Nichols EJ, Fenderson BA, Carter WG, Hakomori S. Domain specific distribution of charbohydrates in human fibronectins and the transformation-dependent translocation of branched type 2 chain defined by monoclonal antibody C6. J Biol Chem. 1986;261:11295–301. [PubMed] [Google Scholar]
- Bernard BA, Yamada KM, Olden K. Carbohydrates selectively protect a specific domain of fibronectin against proteases. J Biol Chem. 1982;257:8549–54. [PubMed] [Google Scholar]
- Jones GE, Arumugham RG, Tanzer ML. Fibronectin glycosylation modulates fibroblast adhesion and spreading. J Cell Biol. 1986;103:1663–70. doi: 10.1083/jcb.103.5.1663. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Leffler H, Carlsson S, Hedlund M, Qian Y, Poirier F. Introduction to galectins. Glycoconj J. 2004;19:433–40. doi: 10.1023/B:GLYC.0000014072.34840.04. [DOI] [PubMed] [Google Scholar]
- Ozeki Y, Matsui T, Yamamoto Y, Funahashi M, Hamako J, et al. Tissue fibronectin is an endogenous ligand for galectin-1. Glycobiology. 1995;5:255–61. doi: 10.1093/glycob/5.2.255. [DOI] [PubMed] [Google Scholar]
- Sinowatz F, Topfer-Petersen E, Calvete J.Glycobiology of fertilizationIn: Gabius HJ and Gabius S, editors. WeinheimChapman & Hall, 1997p595
- Guo Z, Boons GJ.Carbohydrate-based Vaccines and ImmunotherapiesNew Jersey: John Wiley & Sons Inc., 2009
- Manabe R, Ohe N, Maeda T, Fukuda T, Sekiguchi K. Modulation of cell-adhesive activity of fibronectin by the alternatively spliced EDA segment. J Cell Biol. 1997;139:295–307. doi: 10.1083/jcb.139.1.295. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Janković MM, Kosanović MM. Fibronectin pattern in benign hyperplasia and cancer of the prostate. Dis Markers. 2008;25:49–58. doi: 10.1155/2008/308420. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Livant DL, Brabec RK, Pienta KJ, Allen DL, Kurachi K, et al. Anti-invasive, antitumorigenic, and antimetastatic activities of the PHSCN sequence in prostate carcinoma. Cancer Res. 2000;60:309–20. [PubMed] [Google Scholar]
- van Golen KL, Bao L, Brewer GJ, Pienta KJ, Kamradt JM, et al. Suppression of tumor recurrence and metastasis by a combination of the PHSCN sequence and the antiangiogenic compound tetrathiomolybdate in prostate carcinoma. Neoplasia. 2002;4:373–9. doi: 10.1038/sj.neo.7900258. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ruoslahti E, Hayman EG, Engvall E, Cothran WC, Butler WT. Alignment of biologically active domains in the fibronectin molecule. J Biol Chem. 1981;256:7277–81. [PubMed] [Google Scholar]
- Wachtfogel YT, Abrams W, Kucich U, Weinbaum G, Schapira M, et al. Fibronectin degradation products containing the cytoadhesive tetrapeptide stimulate human neutrophil degranulation. J Clin Invest. 1988;81:1310–6. doi: 10.1172/JCI113456. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sekiguchi K, Hakomori S, Funahashi M, Matsumoto I, Seno N. Binding of fibronectin and its proteolytic fragments to glycosaminoglycans. Exposure of cryptic glycosaminoglycan-binding domains upon limited proteolysis. J Biol Chem. 1983;258:14359–65. [PubMed] [Google Scholar]
- Shiota S, Takano K, Nakagawa H. A 10-kDa fragment of fibronectin type III domain is a neutrophil chemoattractant purified from conditioned medium of rat granulation tissue. Biol Pharm Bull. 2001;24:835–7. doi: 10.1248/bpb.24.835. [DOI] [PubMed] [Google Scholar]


