Abstract
Previous studies have analyzed the lymphoid and myeloid foci within the gingival mucosa in health and chronic periodontitis (CP); however, the principal APCs responsible for the formation and organizational structure of these foci in CP have not been defined. We show that in human CP tissues, CD1a+ immature Langerhans cells predominantly infiltrate the gingival epithelium, whereas CD83+ mature dendritic cells (DCs) specifically infiltrate the CD4+ lymphoid-rich lamina propria. In vivo evidence shows that exacerbation of CP results in increased levels of proinflammatory cytokines that mediate DC activation/maturation, but also of counterregulatory cytokines that may prevent a Th-polarized response. Consistently, in vitro-generated monocyte-derived DCs pulsed with Porphyromonas gingivalis strain 381 or its LPS undergo maturation, up-regulate accessory molecules, and release proinflammatory (IL-1β, PGE2) and Th (IL-10, IL-12) cytokines. Interestingly, the IL-10:IL-12 ratio elicited from P. gingivalis-pulsed DCs was 3-fold higher than that from Escherichia coli-pulsed DCs. This may account for the significantly (p < 0.05) lower proliferation of autologous CD4+ T cells and reduced release of IFN-γ elicited by P. gingivalis-pulsed DCs. Taken together, these findings suggest a previously unreported mechanism for the pathophysiology of CP, involving the activation and in situ maturation of DCs by the oral pathogen P. gingivalis, leading to release of counterregulatory cytokines and the formation of T cell-DC foci.
The oral mucosa is colonized by commensal and pathogenic flora the mass and diversity of which rival that of the lower bowel (1, 2). This presumably represents a formidable challenge to the host in maintaining immune homeostasis, yet surprisingly little is known of how local immune cells respond to this flora. Specific pathogens within the plaque biofilm induce a strong humoral immune response during periodontitis (3), and this response is apparently derived from both systemic and mucosal immune systems (4). The oral pathogen Porphyromonas gingivalis is uniquely equipped to cause chronic periodontitis (CP),3 infecting and invading through the oral epithelium (1), resisting phagocytosis (5), and infecting dendritic cells (DCs) in vitro and in situ (6). In the rat model of periodontitis, introduction of Ags from another oral pathogen, Actinobacillus actinomycetemcomitans directly into the gingival mucosa induces a rapid and protective humoral immune response in the rat model (7). This suggests that the gingiva may be a significant site of recruitment of cells capable of capturing and presenting Ags locally or in the lymphoid organs. Many studies have addressed the local T cell response in periodontitis (8 –14), but scant evidence exists in humans on how these responses are initiated and regulated.
Of particular significance for initiating the immune response within respiratory (15) and gastrointestinal mucosa (16 –21) are DCs. In their immature state, DCs are efficient Ag capture cells, but as they mature, they undergo phenotypic changes that facilitate their migration toward lymphoid organs and their unique ability to prime naive T cells (22–24). The presence of the proinflammatory cytokines IL-1β and TNF-γ promotes DC migration and maturation (23–25) and also enhances the immune response by promoting clonal expansion and differentiation of Ag-activated CD4+ T cells (26). In contrast, the presence of IL-10 is associated with reduced DC migration (27), immunosuppression (28), conversion of DCs into macrophages (29), and an unfavorable outcome of diseases, such as leprosy, caused by intracellular pathogens (30).
Here we show differential localization of DC subpopulations in gingival mucosa, with immature CD1a+ localized to the epithelium in health and disease and mature CD83+ DCs restricted to the T cell-rich lamina propria (LP) in CP. The presence of elevated levels of cytokines IL-1β, PGE2, and IL-10 in the local milieu of active CP suggests a counterregulatory microenvironment supportive of DC maturation, but inhibitory of an effective cell-mediated response. In vitro evidence indicates that P. gingivalis and its LPS induce DCs to mature but stimulate the release of counterregulatory cytokines (as in vivo) and promote an inefficient T cell response. We propose a novel model for the pathophysiology of CP whereby pathogen- and cytokine-driven DC maturation in situ results in formation of oral lymphoid foci.
Materials and Methods
Human subject use
The Institutional Review Board approved all protocols involving human subjects. Informed consent was obtained from all subjects before commencement of the study. All subjects were in good general health and were not on antibiotic, anti-inflammatory, or anticoagulant therapy during the month preceding the baseline exams. Also excluded from the study were subjects with a history of drug allergies, rheumatic fever or other conditions requiring prophylactic antibiotic treatment, gross caries or other dental pathology, fewer than 20 teeth, and pregnant or nursing females.
Collection and handling of clinical specimens, disease categories, clinical protocols
Gingival tissue specimens from 29 adult subjects, including 8 periodontally healthy, 7 with gingivitis, and 14 with CP, were excised under nerve block anesthesia by sharp dissection. The clinical criteria for these disease categories have been described previously (5, 31, 32). Gingival tissue was properly oriented in OCT medium by inserting a tooth landmark (3-mm strip of filter paper) alongside the tissue specimen, was flash frozen, and then was stored at −80°C. Cryostat sections measuring 7 μm thick were cut and fixed in cold acetone for 10 min. Sections were stained with H&E to confirm the clinical diagnosis by histological means (not shown). The experimental design for the induction of active (n = 12) or “quiescent” (n = 20) gingival inflammation (Table I) in subjects with preexisting mild CP (minimum of three probing pocket depths of ≥ 5 mm, clinical attachment levels of ≥ 5 mm, bleeding on probing, and horizontal bone loss at the same sites), as well as the technique for sampling gingival crevicular fluid (GCF), have been described previously (32). All four periopaper strips from each tooth were placed in one glass screw-capped vial and stored at −80°C.
Table I.
List of primary Abs
| Ab | Clones | Working Dilution | Source |
|---|---|---|---|
| CD1a | NA1/34 | 1/100 | DAKO |
| CD4 | 13B8.2 | 1/100 | Immunotech |
| CD8 | DK25 | 1/100 | DAKO |
| CD14 | RM052 | 1/100 | Immunotech |
| CD45RA | 2H4 | 1/100 | Coulter |
| CD45RO | UCHL-1 | 1/50 | DAKO |
| CD83 | HB15A | 1/100 | Immunotech |
| CD19 | SJ25C1 | 1/100 | BD Biosciences |
Analysis of GCF cytokines by ELISA
Sterile saline (400 μl) was added to vials containing strips, and proteins were eluted by placing the vials on a rotator (Roto-Mix; Barnstead/Thermolyne, Dubuque, IA) at 250 ± 10 rpm for 1 h in a 4°C cold room. Initial studies (not shown) established the dilution of test samples that yielded sufficient volume and sensitivity for detection of the four cytokines within the linear range of their respective standard curves (32). Dilutions ranged from 1/2.5 to 1/5 v/v. The diluted samples then were aliquoted into new vials and frozen at −80°C for subsequent analysis. Commercial ELISA kits (R&D Systems, Minneapolis, MN.) were used to analyze the levels of IL-1β, IFN-γ, IL-10, and PGE2 within the GCF samples. All reagents were brought to room temperature, and assay diluent (50 μl) was added to each well. The standard or unknown sample then was added to the wells (200-μl volume), and analysis was performed as described by manufacturer. Color development and intensity of the color were measured with an ELISA plate reader (Molecular Devices, Sunnyvale, CA.). A standard curve was prepared, plotting the optical density vs the concentration of the cytokine expressed as pg/30 s.
Single and double immunohistochemistry (enzyme-linked)
Single immunohistochemistry was performed on prefixed frozen sections by indirect method, as described previously (33). Sections were stained by the biotin-streptavidin-peroxidase method (Vectastain ABC Elite kit, Burlingame, CA). The substrate was 3-3′diaminobenzidine tetrahydrochloride (Vector Laboratories, Burlingame, CA). The primary Abs used are listed (Table II). The specificity was confirmed by substituting the respective isotype controls for the primary Abs. The sections were counterstained with hematoxylin. For double staining, after the first staining, sections were washed and labeled by the biotin-streptavidin-glucose oxidase method (Vectastain ABC GO kit). The sections were counterstained with Vector MethylGreen (Vector Laboratories). The specificity of the secondary Ab was confirmed by substituting the respective isotype control for the secondary Abs. A blinded examiner using light microscopy enumerated the immunoreactive cells. The area of the grid was calculated to obtain the number of cells per square millimeter. Two different areas were randomly selected by a blinded examiner in the epithelium and two in the LP to determine the number of cells per square millimeter. The number of positively stained cells in the epithelium and LP were also compared with each other. Statistical analysis is described below.
Table II.
Active disease results in increased levels of DC-maturating/mobilizing and counterregulatory cytokinesa
| Cytokines (mean pg/30s ± SE) | Disease State
|
|
|---|---|---|
| Active (n = 12) | Quiescent (n = 20) | |
| IL-1β | 224 ± 26b | 80 ± 27 |
| PGE2 | 396 ± 44b | 228 ± 44 |
| IL-10 | 21.34 ± 6b | 8.13 ± 6 |
| IFN-γ | 46 ± 12 | 31 ± 6 |
The experimental protocol is described in Materials and Methods.
Significant increase, as determined by Proc Mixed covariate analysis (p < 0.05).
DC cultures, multiparameter flow cytometry analysis
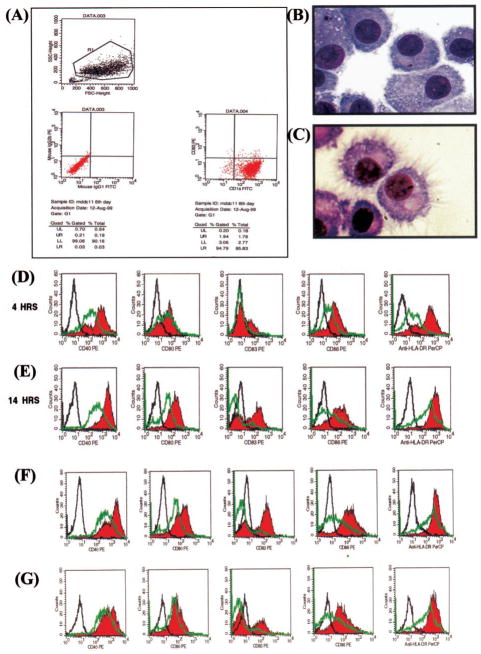
Monocyte-derived DCs (MDDCs) were generated as described by Palucka et al. (34). Briefly, monocytes were isolated from mononuclear fractions of peripheral blood by negative selection and seeded in the presence of GM-CSF and IL-4 (1–2 × 105 cells/ml) for 6– 8 days, after which flow cytometry (see Fig. 3) was performed to confirm the immature DC phenotype (CD14−CD83−CD1a+). Cell surface markers of DCs were evaluated by four-color immunofluorescence staining with the following mAbs: CD1a-FITC (BioSource International, Camarillo, CA); CD40-PE (Coulter, Seattle, WA and Coulter-Immunotech, Westbrook, ME); CD80-PE (BD Biosciences, Mountain View, CA); CD83-PE (Immunotech); CD86-PE (BD PharMingen, San Diego, CA); HLA-DR-PerCP (BD Biosciences); and CD14 APC (Caltag Laboratories, Burlingame, CA). After 30 min at 4°C and washing with staining buffer (PBS, pH 7.2, 2 mM EDTA, and 2% FBS), cells were fixed in 1% paraformaldehyde. Analysis was performed with FACSCalibur (BD Biosciences). Marker expression was analyzed as the percentage of positive cells in the relevant population defined by forward scatter and side scatter characteristics. Expression levels were evaluated by assessing mean fluorescence intensity indices calculated by relating mean fluorescence intensity noted with the relevant mAb to that with the isotype control mAb for samples labeled in parallel and acquired by using the same setting.
FIGURE 3.
The oral mucosal pathogen P. gingivalis or its LPS activate DCs to undergo maturation and up-regulate costimulatory molecule expression in vitro. Day 6 immature MDDCs were generated as described in Materials and Methods. A, Immature MDDC phenotype (CD1a+, CD83+), as confirmed by multiparameter flow cytometry analysis. CD14 expression (not shown) was typically 80–95% negative. B, Immature MDDCs stained by H&E. C, LPS-matured MDDCs stained by H&E. D and E, FACS histograms showing up-regulation (red histogram) of, from left to right, CD40, CD80, CD83, CD86, and HLA-Dr on MDDCs after coculture with P. gingivalis whole cells for 4 and 14 h, respectively. F, MDDCs cocultured with P. gingivalis LPS (100 ng/ml) for 14 h. G, MDDCs cocultured with E. coli LPS (100 ng/ml) for 14 h. Green histograms are DC controls and black histograms are isotype controls. The primary mAbs used are given in Table II and in Materials and Methods.
Purification of autologous CD4+ cells
CD4+ cells were purified from PBMC of the same donor that were used for DC generation (autologous), as described previously (34). Cells bearing CD4 Ag were isolated from mononuclear fraction through positive selection with anti-CD4 mAb and goat anti-mouse IgG-coated microbeads (Miltenyi Biotec, Gladbach, Germany). Isolation of CD4+ cells was achieved with Minimacs separation columns (Miltenyi Biotec) as described by the manufacturer. In all of the experiments, the isolated cells were 80–90% CD4+ as determined by staining with FITC-conjugated anti- CD4 mAb followed by flow cytometry analysis (not shown).
LPS purification
The methodology for isolation and purification of LPS from P. gingivalis 381 and E. coli American Type Culture Collection (ATCC, Manassas, VA) type strain 25922 was as described previously in our laboratory (35). Briefly, whole cell pellets were subjected to hot-phenol water extraction; the aqueous phase was subjected to extensive dialysis against distilled water, followed by lyophilization and then isopyknic density gradient centrifugation. The LPS-containing fractions were dialyzed extensively against distilled water, lyophilized, and subjected to biochemical analysis for purity (34).
Cytokines from DCs and T cells
To study cytokines produced by MDDCs, culture supernatants were collected at 24 h from DC pulsed with P. gingivalis 381, E. coli ATCC type strain 25922 (25:1 bacteria-to-DC ratio), or 100 ng/ml of P. gingivalis or E. coli LPS. For T cell cytokines (IFN-γ), culture supernatants were collected after DCs were cocultured with autologous CD4+ T cells for 5 days. The production of cytokines (IL-10, IL-12 p70, IL-1β, PGE2, and IFN-γ) was determined in triplicate by using commercial ELISA Kits (R&D Systems) and by following the manufacturer’s instructions. The values shown in Table III represent the mean of 10 separate analyses.
Table III.
Cytokine release and autologous T cell proliferation by DCs pulsed with P. gingivalis (Pg) A7436 or E. coli 25922 and their LPSa
| Groups | IL-1β (pg/ml) ±SE | PGE2 (pg/ml) ±SE | IL-10 (pg/ml) ±SE | IL-12 p70 (pg/ml) ±SE | IFN-γ (pg/ml) ±SE | T Cell Proliferation (incorporation of [3H]thymidine into CD4+ autologous T cells) |
|---|---|---|---|---|---|---|
| DC + Pg | 48 ± 1.8b | 358 ± 34b | 88 ± 54b | 12 ± 7b | 35 ± 35 | 4,964 ± 2,328b |
| DC + E. coli | 272 ± 7.2 | 863 ± 9.8 | 600 ± 208 | 267 ± 98 | 1,031 ± 941 | 12,985 ± 2,328 |
| DC + PgLPS | NDc | ND | 137 ± 78 | 30 ± 11 | 42 ± 42 | 3,451 ± 1,927 |
| DC + E. coli LPS | ND | ND | 178 ± 95 | 30 ± 15 | 19 ± 111 | 10,133 ± 3,516 |
| DC | 0d | 42 ± 23 | 0d | 0d | 0d | 142 ± 30 |
| PHA | 68,336 ± 34,193 |
Means ± SE of 10 separate experiments. Cytokine levels from DCs (IL-1β, PGE2, IL-10, and IL-12) and T cells (IFN-γ) were analyzed by ELISA as described in Materials and Methods.
Statistically significant differences as assessed by Kruskall-Wallis test (p < 0.05).
ND, not done. DC and T cell viability was determined by trypan blue exclusion and did not differ between the groups (not shown).
Not detectable.
T cell proliferation assay
The ability of bacteria-pulsed DCs to stimulate autologous T cell proliferation was performed as described previously (36). Day 6 MDDCs were pulsed with either P. gingivalis (25:1 multiplicity of infection (MOI)); E. coli (25:1 MOI); 100 ng/ml P. gingivalis LPS; or 100 ng/ml E. coli LPS, for 24 h at 37°C. DCs in complete RPMI with no bacteria or LPS were used as controls. DCs were washed extensively and cultured at graded doses (5000 DC/200 μl, 1000 DC/200 μl, and 300 DC/200 μl) in complete RPMI 1640 medium with 10% human AB serum with autologous CD4+ T cells (50,000 cells/200 μl). Peak proliferative response was achieved with 1000 DC, so this number was used for subsequent assays. Proliferation was determined after 5 days by uptake of tritiated thymidine (1 μCi/well for the last 16 h). Assay was repeated 10 times on separate days, and the mean results are shown in Table III.
Statistical analyses
Descriptive statistics, means, and SE for the numbers of immunoreactive cells with each cell surface marker in healthy, gingivitis, and CP tissues (see Fig. 2) were calculated and analyzed for statistical significance by Tukey’s multiple comparisons test (p < 0.05; Minitab, State College, PA). Differences within epithelium and LP for each individual cell marker were analyzed by Student’s t test (p < 0.05). Descriptive statistics, means, and SE of the clinical indices (not shown) and GCF cytokine levels (Table I) were calculated by using SAS 6.12 (Cary, NC) and Proc Mixed with experimental group and genotype as the factors and the baseline levels as the covariate, as described previously (31). Proc Mixed covariate analysis was used to determine which means were statistically significant, with the output being least squares means and least significant difference. Differences in IL-10, IL-1β, PGE2, and IFN-γ between-group means were declared only if the p value for the F statistic in the analysis of covariance was ≤0.05, and the least significant difference also was significant at ≤0.05. Results of in vitro cytokine levels and T cell proliferation (Table III) were analyzed by Kruskall-Wallis test (p < 0.05; Minitab).
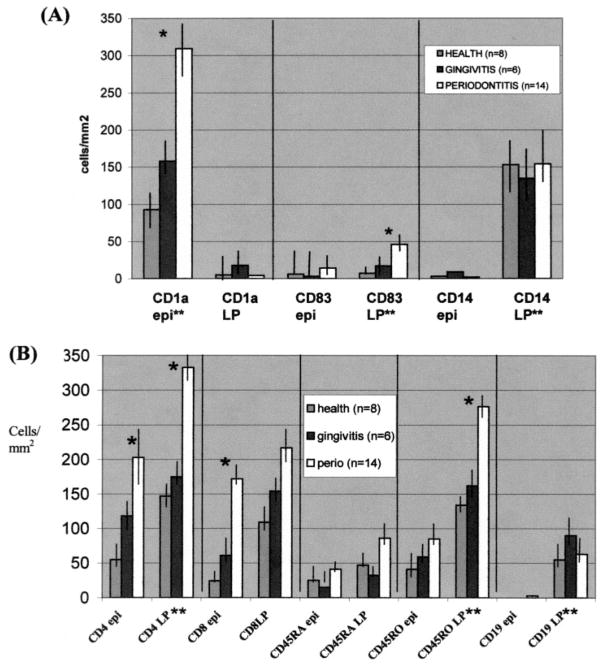
FIGURE 2.
Increased numbers of CD83+ mature DCs infiltrate the T cell-rich LP of diseased mucosa. Shown are the mean numbers (+ SE) of immunoreactive DC/myeloid subpopulations (A) and lymphoid subpopulations (B) in the epithelium (epi) and LP per square millimeter of gingival tissue from subjects in each clinical category. *, Statistically significant differences (p < 0.05) relative to gingivitis and health (Tukey’s multiple comparisons test). **, Statistically significant differences (p < 0.05), comparing epithelium to connective tissue compartment or vice versa (Student’s t test). Data are representative of at least three serial sections from each subject, which were stained as described in Materials and Methods.
Results
Mature DCs specifically infiltrate the T cell-rich LP in CP
We have enumerated immature CD1a+ Langerhans cells (LCs) and mature CD83+ DCs by using immunohistochemistry (Fig. 1) within epithelium and LP of the gingiva in health, gingivitis, and CP (Fig. 2A). Macrophage/myeloid cells (CD14+) and lymphocyte subsets also were enumerated within these same tissue compartments for comparison (Fig. 2B).
FIGURE 1.
Source of clinical specimens, representative stainings. A, GCF was sampled from the gingival sulcus, as described in Materials and Methods. Gingival tissue was surgically excised, snap frozen, and serial sectioned (0.7μm) with a cryostat. Quantitation of immunoreactive cells in epithelium (epi) and LP was performed by light microscopy (×40 objective) with an ocular grid (see circles), as described in Materials and Methods. Shown is representative immunoenzyme staining (arrows) achieved using: the mAb NA1/34 (anti-CD1a) on a healthy (B) and CP (C) specimen and the mAb HB15A (anti-CD83) on a healthy (D) and CP (E) specimen. Isotype controls run in parallel revealed no staining (not shown).
Microscopic analysis of healthy epithelium revealed the presence of large numbers of CD1a+ LCs, but only a few CD83+ mature DCs or CD14+ cells (Fig. 2A). In gingivitis, there was a slight increase in the numbers of CD1a+-labeled cells in the epithelium. However, the transition from health to CP was associated with a significant increase (over 3-fold increase, from 90 cells/mm2 to 312 cells/mm2; p < 0.05) in numbers of LCs in the epithelium. Moreover, the transition from health to CP was associated with a significant increase (6-fold increase, from 8 cells/mm2 to 48 cells/mm2; p < 0.05) in numbers of mature DCs in the LP.
In healthy epithelium, lymphocytes also comprise a large number of cells (Fig. 2B) and, relative to the epithelium, the LP contains a denser resident population of lymphocytes. Transition from health to CP was marked by a significant increase (p < 0.05) in numbers of CD4+ T cells and CD45RO+ T cells, both of which were predominantly present in LP, the zone of mature DC infiltration. CD8+ T cells also increased in CP, but this increase was only significant (p < 0.05) in the epithelium, where LCs reside. B cells were present in large numbers (≅150 cells/mm2) at all times and always localized to the LP, similar to the pattern observed by CD14+ myeloid cells.
Increased levels of DC-mobilizing/maturing cytokines are released in active periodontitis in vivo
We next analyzed the levels of cytokines that might influence DC redistribution/maturation and T cell activation within this tissue. We designed a clinical study in human subjects with preexisting mild CP to determine the level of these cytokines in the GCF in active vs quiescent disease. The results (Table I) indicate that disease exacerbation is accompanied by significant changes in the local cytokine microenvironment, including significant (p < 0.05) increases in the levels of IL-1β, PGE2, and IL-10. IFN-γ was present in the GCF of all CP subjects but did not increase significantly in active disease.
The oral mucosal pathogen P. gingivalis or its LPS activates DCs to undergo maturation and up-regulate costimulatory molecule expression in vitro
Recent in situ studies of periodontitis in rats (7) and humans (37) suggest that costimulatory molecule expression on gingival APCs might play a role in alveolar bone loss and the local T cell response, respectively. Here, human immature MDDCs (Fig. 3A) were pulsed with the oral pathogen P. gingivalis for from 4 to 14 h. Previous studies from our laboratory (6) as well as unpublished observations established the optimum MOI (25:1) for internalization of P. gingivalis by DCs, as confirmed by trifluorochrome phagocytosis microassay (5) and electron microscopy (data not shown). As our previous studies indicate that the LPS of P. gingivalis is an immunodominant Ag capable of inducing a strong IgG response in human subjects with CP (31), MDDCs were also pulsed with LPS (100 ng/ml) from P. gingivalis. The gut commensal E. coli ATCC type strain 25922 and its purified LPS served as the controls. Our results show that P. gingivalis (Fig. 3, D and E), its LPS (Fig. 3F), or LPS of E. coli (Fig. 3G) induced DCs to undergo maturation in a time-dependent manner, as evidenced by up-regulation of CD83. Moreover, expression of costimulatory molecules required for efficient Ag presentation, including CD80, CD86, CD40, and HLA-Dr also were up-regulated. The morphologic changes as immature DCs become matured by LPS also are shown (Fig. 3, B and C).
P. gingivalis-matured DCs release relevant cytokines and stimulate a limited T cell response
Based on in situ, in vivo, and in vitro correlates detailed above, we had reason to expect that DCs matured by the oral pathogen P. gingivalis would be potent at stimulating T cell responses, as determined by IFN-γ production and proliferation. However, we had reservations in this regard because of the GCF evidence that IFN-γ did not increase significantly in active CP. This was possibly attributable to the presence of counterregulatory Th2-biasing cytokines, such as IL-10 (27) and PGE2 (38). Therefore, we analyzed the supernatants from the DCs pulsed as above with P. gingivalis, E. coli and their LPS moieties for proinflammatory cytokines (IL-1β, PGE2), the Th2-biasing cytokine IL-10, and the Th1-biasing cytokine IL-12. DCs pulsed with either P. gingivalis or E. coli release IL-1β, PGE2, IL-10, and IL-12, although P. gingivalis-pulsed DCs released significantly less (p < 0.05) of all four cytokines (Table III). The ratio of IL-10:IL-12 elicited by P. gingivalis was 3-fold higher than that by E. coli (7:1 vs 2:1, respectively). This elevation in IL-10:IL-12 ratio induced by P. gingivalis may be reflected in the significantly (p < 0.05) lower IFN-γ levels from CD4+ T cells cocultured with DC-P. gingivalis, relative to DC-E. coli (35 vs 1031 pg/ml, respectively) and in the limited proliferation of autologous T cells elicited by P. gingivalis-pulsed DCs as compared with E. coli-pulsed DCs (Table III).
Discussion
Our results show a marked change in the tissue localization of DCs from health to CP. Indeed, immature CD1a+ DCs (by definition, Ag capture cells) increased significantly in numbers in the diseased epithelium, whereas CD83+ mature DCs (by definition, APCs) increased in numbers within the lymphoid-rich diseased LP. Interestingly, when the data for all the markers was expressed as a percentage of change from health to CP (not shown), the following cells changed by >100%: mature DCs in LP (↑164%); immature DCs in LP (↓198%); and CD8+ cells in epithelium (↑169%).
Although these percentages were not tested statistically, we have interpreted the increase in mature DCs and decrease in immature DCs in the same tissues as evidence for local redistribution of LCs to LP, and subsequent maturation in situ (Fig. 4); however, the actual source of mature DCs is unknown presently. Our results further show that although certain cytokines released in this microenvironment (i.e., IL-1β and PGE2) could promote in situ DCs activation/maturation and T cell expansion, the counterregulatory cytokine IL-10 also is released. Finally, the oral pathogen P. gingivalis is able to induce cultured MDDCs to undergo maturation and to release relevant proinflammatory (i.e., IL-1β and PGE2) and Th cytokines (IL-10 and IL-12), but elicits a limited T cell response compared with E. coli-pulsed DCs.
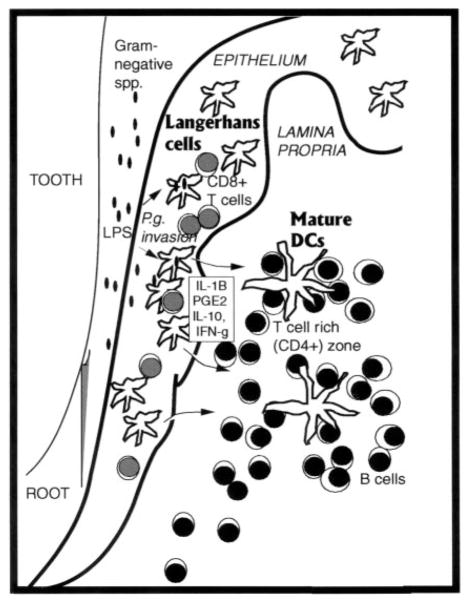
FIGURE 4.
Working model of DC/lymphoid foci and local milieu in CP.
Based on these results, we have developed a novel working model for the pathophysiology of CP involving the formation of what we have termed “oral lymphoid follicles” or OLF (Fig. 4) within the LP around the dentition. We acknowledge that the term “follicle” might imply a proliferating B cell lymphoid organ and hence may be misleading, but nonetheless propose this terminology. We propose that the development of OLF is initiated in gingivitis by LCs homing to the gingival epithelium in response to bacteria/Ags in the oral biofilm (39), as well as a source of the earliest signals for lymphoid/myeloid trafficking, i.e., epithelium/keratinocytes (40). That the CD1a+ cells enumerated in this study were in fact LCs was confirmed by scanning laser confocal microscopy and double immunofluorescence with CD1a and the LC-specific marker of Birbeck granules, LAG (41), as well as single immunoenzyme staining with langerin (Ref. 43; data not shown). Earlier studies have shown the dynamics of LCs trafficking in the respiratory mucosa in response to bacterial Ags (15), as well as LCs in oral mucosa/gingiva in response to plaque/allergen accumulation (10, 39, 42, 44, 45). One study identified increased numbers of LCs in the gingival epithelium of CP patients and also identified P. gingivalis (i.e., Bacteroides gingivalis) and Actinobacillus actinomycetemcomitans within the same tissues by immunohistochemistry (47). Our previous studies have shown by scanning laser confocal microscopy that LCs are infected with P. gingivalis in situ (6). In this same study, the pathophysiology of chronic gingivitis/mild CP was equated with a bacterially induced contact hypersensitivity response (46), wherein Ag (i.e., P. gingivalis)-sensitized gingival epithelial LCs engage CD8+ T cells homing to the gingival epithelium (6). This supposition is consistent with the cellular infiltrates within the epithelium but is too simplistic to account for all of our findings, particularly those observed within the LP. The foci of myeloid and lymphoid cells within the LP of the diseased periodontium have been characterized by other investigators (8–14). Some have interpreted the predominance of CD4+ activated/memory T cells and B cells within the LP as evidence that the CP “lesion” is in fact a tertiary lymphoid organ (48). It is necessary to differentiate, within OLF, expression of primary immune response markers from those consistent with recall responses expected within a tertiary lymphoid organ.
We propose that OLF are not fully developed unless and until sufficient antigenic challenge is provided by specific pathogens/commensals in the subgingival flora such as P. gingivalis and Fusobacterium nucleatum (1, 2, 40). In like manner, lymphoid follicles in Peyer’s patches do not develop normally in germ-free animals, but are restored when animals are monoinfected (49, 50). Both P. gingivalis and F. nucleatum have been shown to stimulate human gingival epithelial cells in vitro to produce β-defensins, proinflammatory cytokines, and chemokines in vitro that are required for leukocyte recruitment (40). We further propose that although T cell expansion and IFN-γ production (Table I) occurs in OLF, the development of a protective cell-mediated response is limited by the presence of elevated IL-10 and PGE2 levels in vivo (Table III).
Our in vitro results indicate that DCs matured by P. gingivalis are able to stimulate a very limited autologous CD4+T cell response, as compared with DCs matured by E. coli. Our efforts are now being directed to understanding the mechanism(s) of this disparity. One possibility being investigated in the human and murine (51) systems is that P. gingivalis or its LPS induce a Th2-biased response in DCs. This is supported by the data in Table III showing an elevation in IL-10:IL-12 ratio from P. gingivalis-pulsed DCs, relative to E. coli-pulsed DCs (7:1 vs 2:1), but confirmation of a Th2-biased response requires more rigorous investigation.
In conclusion, our results support the role of DCs in the pathophysiology of CP through their activation and in situ maturation in the T cell-rich LP under the influence of oral pathogens and a cytokine milieu that may be counterregulatory to a protective T cell-mediated response.
Footnotes
This study was supported by Public Health Service Grants DE14160-01 and DE13154-01 (to C.W.C. and R.J.) from the National Institute of Dental and Craniofacial Research.
Abbreviations used in this paper: CP, chronic periodontitis; DC, dendritic cells; GCF, gingival crevicular fluid; LP, lamina propria; LC, Langerhans cell; MDDC, monocyte-derived DC; MOI, multiplicity of infection; OLF, oral lymphoid follicles.
References
- 1.Cutler CW, Kalmar JR, Genco CA. Pathogenic strategies of the oral anaerobe. Porphyromonas gingivalis Trends Microbiol. 1995;3:45. doi: 10.1016/s0966-842x(00)88874-5. [DOI] [PubMed] [Google Scholar]
- 2.Dahlen G. Role of suspected periodontopathogens in microbiological monitoring of periodontitis. Adv Dent Res. 1993;7:163. doi: 10.1177/08959374930070020701. [DOI] [PubMed] [Google Scholar]
- 3.Califano JV, Schifferle RE, Gunsolley JC, Best AM, Schenkein HA, Tew JG. Antibody reactive with Porphyromonas gingivalis serotypes K1– 6 in adult and generalized early-onset periodontitis. J Periodontol. 1999;70:730. doi: 10.1902/jop.1999.70.7.730. [DOI] [PubMed] [Google Scholar]
- 4.Kinane DF, Lappin DF, Koulouria O, Buckley A. Humoral immune responses in periodontal disease may have mucosal and systemic immune features. Clin Exp Immunol. 1999;115:534. doi: 10.1046/j.1365-2249.1999.00819.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Cutler CW, Arnold RR, Schenkein HA. Inhibition of C3 and IgG proteolysis enhances phagocytosis ofPorphyromonas gingivalis. J Immunol. 1993;151:7016. [PubMed] [Google Scholar]
- 6.Cutler CW, Jotwani R, Palucka KA, Davoust J, Bell D, Banchereau J. Evidence and a novel hypothesis for the role of dendritic cells and Porphyromonas gingivalis in adult periodontitis. J Periodont Res. 1999;34:406. doi: 10.1111/j.1600-0765.1999.tb02274.x. [DOI] [PubMed] [Google Scholar]
- 7.Kawai T, Eisen-Lev R, Seki M, Eastcott JW, Wilson ME, Taubman MA. Requirement of B7 costimulation for Th1-mediated inflammatory bone resorption in experimental periodontal disease. J Immunol. 2000;164:2102. doi: 10.4049/jimmunol.164.4.2102. [DOI] [PubMed] [Google Scholar]
- 8.Yamazaki K, Nakajima T, Ohsawa Y, Tabeta K, Yoshie H, Sakurai K, Seymour GJ. Selective expansion of T cells in gingival lesions of patients with chronic inflammatory periodontal disease. Clin Exp Immunol. 2000;120:154. doi: 10.1046/j.1365-2249.2000.01179.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Gemmell E, Winning TA, Bird PS, Seymour GJ. Cytokine profiles of lesional and splenic T cells in Porphyromonas gingivalis infection in a murine model. J Periodontol. 1998;69:1131. doi: 10.1902/jop.1998.69.10.1131. [DOI] [PubMed] [Google Scholar]
- 10.Lundqvist C, Baranov V, Teglund S, Hammarstrom S, Hammarstrom M-L. Cytokine profile and ultrastructure of intraepithelial γδ T cell in a chronically inflamed human gingiva suggest a cytotoxic effector function. J Immunol. 1994;153:2302. [PubMed] [Google Scholar]
- 11.Lundqvist C, Hammarstrom ML. T-cell receptor γδ-expressing intraepithelial lymphocytes are present in normal and chronically inflamed gingiva. Immunology. 1993;79:38. [PMC free article] [PubMed] [Google Scholar]
- 12.Okada H, Kida T, Yamagami H. Identification and distribution of immunocompetent cells in inflamed gingiva of human chronic periodontitis. Infect Immun. 1983;41:365. doi: 10.1128/iai.41.1.365-374.1983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Seymour GJ, Taubman MA, Eastcott JW, Gemmell E, Smith DJ. CD29 expression on CD4+ gingival lymphocytes supports migration of activated memory T lymphocytes to diseased periodontal tissue. Oral Microbiol Immunol. 1997;12:129. doi: 10.1111/j.1399-302x.1997.tb00368.x. [DOI] [PubMed] [Google Scholar]
- 14.Takeichi O, Haber J, Smith DJ, Moro I, Taubman MA. Cytokine profiles of T lymphocytes from gingival tissues with pathological pocketing. J Dent Res. 2000;79:1548. doi: 10.1177/00220345000790080401. [DOI] [PubMed] [Google Scholar]
- 15.McWilliam AS, Napoli S, Marsh AM, Pemper FL, Nelson DJ, Pimm CL, Stumbles PA, Wells TNC, Holt PG. Dendritic cells are recruited to airway epithelium by a broad spectrum of stimuli. J Exp Med. 1996;184:2429. doi: 10.1084/jem.184.6.2429. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Cook DN, Prosser DM, Foster R, Zhang J, Kuklin NA, Abbondanzo SJ, Niu XD, Chen SC, Manfra DJ, Wiekowski MT, et al. CCR6 mediates dendritic cell localization, lymphocyte homeostasis and immune responses in mucosal tissue. Immunity. 2000;12:495. doi: 10.1016/s1074-7613(00)80201-0. [DOI] [PubMed] [Google Scholar]
- 17.Lefevre ME, Joel DD, Schidlovsky G. Retention of ingested latex particles in Peyer’s patches of germfree and conventional mice. Proc Soc Exp Biol Med. 1985;179:522. doi: 10.3181/00379727-179-42133. [DOI] [PubMed] [Google Scholar]
- 18.Farstad IN, Halstensen TS, Fausa O, Brantzaeg P. Heterogeneity or M cell-associated B and T cells in human Peyer’s patches. Immunol. 1994;83:457. [PMC free article] [PubMed] [Google Scholar]
- 19.Iwasaki A, Kelsall B. Freshly isolated Peyers patch, but not spleen, dendritic cells produce interleukin 10 and induce the differentiation of T helper type 2 cells. J Exp Med. 1999;190:229. doi: 10.1084/jem.190.2.229. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Kelsall BL, Strober W. Distinct populations of dendritic cells are present in the subepithelial dome and T cell regions of the murine Peyer’s patch. J Exp Med. 1996;183:237. doi: 10.1084/jem.183.1.237. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Mayer L, Panja A, Li Y, Siden E, Pizzimenti A, Gerardi F, Chandswang N. Unique features of antigen presentation in the intestine. Ann NY Acad Sci. 1992;664:39. doi: 10.1111/j.1749-6632.1992.tb39747.x. [DOI] [PubMed] [Google Scholar]
- 22.Neutra MR. Current concepts in mucosal immunity. V. Role of M cells in transepithelial transport of antigens and pathogens to the mucosal immune system. Am J Physiol. 1998;274:G785. doi: 10.1152/ajpgi.1998.274.5.G785. [DOI] [PubMed] [Google Scholar]
- 23.Steinman RM, Pack M, Inaba K. Dendritic cells in the T-cell areas of lymphoid organs. Immunol Rev. 1997;156:25. doi: 10.1111/j.1600-065x.1997.tb00956.x. [DOI] [PubMed] [Google Scholar]
- 24.Banchereau J, Steinman RM. Dendritic cells and the control of immunity. Nature. 1998;392:245. doi: 10.1038/32588. [DOI] [PubMed] [Google Scholar]
- 25.Cutler CW, Jotwani R, Pulendran B. Dendritic cells: immune saviors or Achilles’ heel? Infect Immun. 2001;69:4703. doi: 10.1128/IAI.69.8.4703-4708.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Pape KA, Khoruts A, Mondino A, Jenkins MK. Inflammatory cytokines enhance the in vivo clonal expansion and differentiation of antigen-activated CD4+ T cells. J Immunol. 1997;159:591. [PubMed] [Google Scholar]
- 27.Wang B, Zhuang L, Fujisawa H, Shinder GA, Feliciani C, Shivji GM, Suzuki H, Amerio P, Toto P, Sauder DN. Enhanced epidermal Langerhans cell migration in IL-10 knockout mice. J Immunol. 1999;162:277. [PubMed] [Google Scholar]
- 28.D’Andrea A, Aste-Amezaga M, Valiente NM, Ma X, Kubin M, Trinchieri G. Interleukin-10 (IL-10) inhibits human lymphocyte interferon γ-production by suppressing natural killer stimulatory factor/IL-12 synthesis in accessory cells. J Exp Med. 1993;178:1041. doi: 10.1084/jem.178.3.1041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Förtsch D, Röllinghoff M, Stenger S. IL-10 converts human dendritic cells into macrophage-like cells with increased antibacterial activity against virulentMycobacterium tuberculosis. J Immunol. 2000;165:978. doi: 10.4049/jimmunol.165.2.978. [DOI] [PubMed] [Google Scholar]
- 30.Yamamura M, Uyemura K, Deans RJ, Weinberg K, Rea TH, Bloom BR, Modlin RL. Defining protective responses to pathogens: cytokine profiles in leprosy lesions. Science. 1991;254:277. doi: 10.1126/science.254.5029.277. [DOI] [PubMed] [Google Scholar]
- 31.Cutler CW, Shinedling EA, Nunn M, Jotwani R, Kim BO, Nares S, Iacopino AM. Association between periodontitis and hyperlipidemia: cause or effect? J Periodontol. 1999;70:1429. doi: 10.1902/jop.1999.70.12.1429. [DOI] [PubMed] [Google Scholar]
- 32.Cutler CW, Stanford TW, Abraham C, Cederberg RA, Boardman T, Ross C. Clinical benefits of oral irrigation for periodontitis are related to reduction of pro-inflammation cytokine levels and plaque. J Clin Periodontol. 2000;27:134. doi: 10.1034/j.1600-051x.2000.027002134.x. [DOI] [PubMed] [Google Scholar]
- 33.Bell D, Chomarat P, Broyles D, Netto G, Harb GM, Lebecque S, Valladeau J, Davoust J, Palucka KA, Banchereau J. In breast carcinoma tissue, immature dendritic cells reside within the tumor, whereas mature dendritic cells are located in peritumoral areas. J Exp Med. 1999;190:1417. doi: 10.1084/jem.190.10.1417. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Palucka KA, Taquet N, Sanchez-Chapuis F, Gluckman JC. Dendritic cells as the terminal stage of monocyte differentiation. J Immunol. 1998;160:4587. [PubMed] [Google Scholar]
- 35.Cutler CW, Eke PI, Genco CA, Van Dyke TE, Arnold RR. Hemin-induced modifications of the antigenicity and hemin-binding capacity of Porphyromonas gingivalis lipopolysaccharide. Infect Immun. 1996;64:2282. doi: 10.1128/iai.64.6.2282-2287.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Pulendran B, Banchereau J, Burkeholder S, Kraus E, Guinet E, Chalouni C, Caron D, Maliszewski C, Davoust J, Fay J, Palucka KA. Flt3-ligand and granulocyte colony-stimulating factor mobilize distinct human dendritic cell subsets in vivo. J Immunol. 2000;165:566. doi: 10.4049/jimmunol.165.1.566. [DOI] [PubMed] [Google Scholar]
- 37.Gemmell E, McHugh GB, Grieco DA, Seymour GJ. Costimulatory molecules in human periodontal disease tissues. J Periodontol Res. 2001;36:92. doi: 10.1034/j.1600-0765.2001.360205.x. [DOI] [PubMed] [Google Scholar]
- 38.Kaliński P, Schuitmaker JHN, Hilkens CMU, Kapsenberg ML. Prostaglandin E2 induces final maturation of IL-12-deficient DC1a+CD83+ dendritic cell: the levels of IL-12 are determined during the final dendritic cell maturation and are resistant to further modulation. J Immunol. 1998;161:2804. [PubMed] [Google Scholar]
- 39.Newcomb GM, Seymour GJ, Pomell RN. Association between plaque accumulation and Langerhans cell numbers in the oral epithelium of attached gingiva. J Clin Periodontol. 1982;9:297. doi: 10.1111/j.1600-051x.1982.tb02096.x. [DOI] [PubMed] [Google Scholar]
- 40.Krisanaprakornkit S, Kimball JR, Weinberg A, Darveau RP, Bainbridge BW, Dale BA. Inducible expression of human β-defensin 2 by Fusobacterium nucleatum in oral epithelial cells: multiple signaling pathways and role of commensal bacteria in innate immunity and the epithelial barrier. Infect Immun. 2000;68:2907. doi: 10.1128/iai.68.5.2907-2915.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Kashihara M, Ueda M, Horiguchi Y, Furukawa F, Hanaoka M, Imamura S. A monoclonal antibody specifically reactive to human Langerhans cells. J Invest Dermatol. 1986;87:602. doi: 10.1111/1523-1747.ep12455849. [DOI] [PubMed] [Google Scholar]
- 42.Van Loon LAJ, Van Elsas PW, Bos JD, Ten Harkel-Hagenaar HC, Krieg SR, Davison CL. T-lymphocyte and Langerhans cell distribution in normal and allergically-induced oral mucosa in contact with nickel-containing dental alloys. J Oral Pathol. 1988;17:129. doi: 10.1111/j.1600-0714.1988.tb01899.x. [DOI] [PubMed] [Google Scholar]
- 43.Valladeau J, Duvert-Frances V, Pin J-J, Dezutter-Dambuyant C, Vincent C, Massacrier C, Vincent J, Yoneda K, Banchereau J, Caux C, et al. The monoclonal antibody DCGM4 recognizes langerin, a protein specific of Langerhans cells, and is rapidly internalized from the cell-surface. Eur J Immunol. 1999;29:2695. doi: 10.1002/(SICI)1521-4141(199909)29:09<2695::AID-IMMU2695>3.0.CO;2-Q. [DOI] [PubMed] [Google Scholar]
- 44.Hasseus B, Jontell M, Bergenholtz G, Elkund C, Dahlgren UI. Langerhans cells from oral epithelial cells are more effective in stimulating allogeneic T cells in vitro than Langerhans cells from skin epithelium. J Dent Res. 1999;78:751. doi: 10.1177/00220345990780030701. [DOI] [PubMed] [Google Scholar]
- 45.Mougal NA, Adonogianaki E, Kinane DF. Langerhans cell dynamics in human gingiva during experimentally induced inflammation. J Biol Buccale. 1992;20:163. [PubMed] [Google Scholar]
- 46.Enk AH. Allergic contact dermatitis: understanding the immune response and potential for targeted therapy using cytokines. Mol Med Today. 1997;3:423. doi: 10.1016/S1357-4310(97)01087-3. [DOI] [PubMed] [Google Scholar]
- 47.Saglie FR, Pertuiset JH, Smith CT, Nestor MG, Carranza FA, Jr, Newman MG, Rezende MT, Nisengard R. The presence of bacteria in the oral epithelium in periodontal disease. III. Correlation with Langerhans cells. J Periodontol. 1987;58:417. doi: 10.1902/jop.1987.58.6.417. [DOI] [PubMed] [Google Scholar]
- 48.Ebersole JL, Taubman MA. The protective nature of host responses in periodontal diseases. Periodontol 2000. 1994;5:112. doi: 10.1111/j.1600-0757.1994.tb00021.x. [DOI] [PubMed] [Google Scholar]
- 49.Rothkotter HJ, Pabst R. Lymphocyte subsets in jejunal and ileal Peyer’s patches of normal and gnotobiotic minipigs. Immunology. 1989;67:103. [PMC free article] [PubMed] [Google Scholar]
- 50.Vertvicka V, Tlaskalova-Hogenova H, Stepankova R. Effects of microflora antigens on lymphocyte migration patterns in germfree and conventional rats. Folia Biologica. 1983;29:412. [PubMed] [Google Scholar]
- 51.Pulendran B, Cutler CW, Kumar P, Mohamadzadeh M, Van Dyke T, Banchereau J. Lipopolysaccharides from distinct pathogens induce different classes of immune responses in vivo. J Immunol. doi: 10.4049/jimmunol.167.9.5067. In press. [DOI] [PMC free article] [PubMed] [Google Scholar]