Abstract
Objective
To examine the prevalence and biopsychosocial predictors of sub-optimal virologic response to highly active antiretroviral therapy (HAART) among human immunodeficiency virus (HIV)-infected adolescents.
Design
Population-based cohort study.
Setting
Sixteen academic medical centers across thirteen cities in the United States.
Participants
One hundred and fifty four HIV-infected adolescents who presented for at least two consecutive visits after initiation of HAART.
Main Outcome Measures
Viral load (plasma concentration of HIV RNA), CD4+ T-lymphocyte count.
Results
Of the 154 adolescents enrolled in the study, 50 (32.5%) demonstrated early and sustained virologic suppression while receiving HAART. The remaining 104 adolescents (67.5%) had a poor virologic response. Adequate adherence (>50%) to HAART—reported by 70.8% of respondents—was associated with a 60% reduced odds of suboptimal virologic suppression in a multivariable logistic regression model (adjusted odds ratio = 0.4; 95% confidence interval : 0.2 – 1.0). Exposure to sub-optimal antiretroviral therapy (ART) prior to HAART, on the other hand, was associated with more than a two-fold increased odds of sub-optimal virologic response (adjusted odds ratio = 2.6; 95% confidence interval: 1.1 – 5.7).
Conclusions
Fully two-thirds of HIV-infected adolescents in the current study demonstrated a sub-optimal virologic response to HAART. Non-adherence and prior single or dual ART were associated with subsequent poor virologic responses to HAART. These predictors of HAART failure echo findings in pediatric and adult populations. Given the unique developmental stage of adolescence, age-specific interventions are indicated to address high rates of non-adherence and therapeutic failure.
Keywords: HIV, Adolescent, Antiretroviral Therapy, Highly Active, Adherence, Viral Load, CD4 Lymphocyte Count
INTRODUCTION
In 2006, adolescents aged 13 to 19 years accounted for 4% of newly diagnosed cases of human immunodeficiency virus (HIV) infection in the United States,1 half the percentage reported in 2003.2 Despite a decrease in the relative proportion of adolescents diagnosed with HIV, incidence rates within this age group have been steadily increasing since the late 1990s, nearly doubling between 1998 and 2006.1 However, these data are likely to underestimate the true incidence and proportion of adolescent HIV cases, as overall incidence rate estimates were revised upward in 2008 and HIV testing coverage is much lower among adolescent compared to adult populations.3, 4 Furthermore, the prolonged latent phase of HIV’s natural history suggests a large proportion of individuals diagnosed with HIV in early adulthood were likely infected during adolescence.
Adolescents are less likely to be tested for HIV as they access health care services less frequently and with greater difficulty than their adult counterparts.5–7 Behavioral studies have demonstrated that high-risk behaviors, lack of knowledge about HIV, and logistic impediments to accessing health systems may all contribute to delays in testing adolescents for HIV infection.8, 9 Delays in HIV diagnosis lead to delays in treatment initiation which can result in poorer clinical outcomes. The delays to initiating care are compounded by the fact that adolescents lack adequate health insurance and do not often have access to youth-friendly clinical settings.10, 11 These disadvantages along with complicated therapeutic regimens may predispose adolescents to poor antiretroviral therapy (ART) adherence rates that can translate into viral resistance and inadequate viral suppression.
The relationship between viral suppression and immune reconstitution in the context of highly active antiretroviral therapy (HAART) has been well documented in HIV-infected adult and pediatric populations.12–16 Predictors of poor virologic, immunologic, and clinical outcomes have also been validated in these cohorts.17–20 Adolescents differ in their biological and behavioral characteristics from these age groups, but are still often grouped with adults in epidemiologic studies. Given the paucity of data on HIV-infected youth populations, we sought to identify predictors of elevated viral load and decreased CD4+ cell counts among adolescents on HAART.
METHODS
STUDY DESIGN
The REACH Project (Reaching for Excellence in Adolescent Care and Health) of the Adolescent Medicine HIV/AIDS Research Network is a multicenter, prospective, observational study that longitudinally evaluated prevalent HIV infections and related behavioral and biological factors in adolescents (age 12 – 19 years) for a minimum of one year (mean follow-up 2.4 years) between 1996 and 2000. This time frame allowed the assessment of the transition from mono- and dual ART to HAART. The study cohort was restricted to participants who acquired HIV via sexual transmission or injection drug use and excluded youth infected through vertical transmission, contaminated blood products, or early childhood sexual abuse. A detailed description of the REACH study design, objectives, and procedures have been published elsewhere.21, 22
Participants included in the current analysis were required to meet five inclusion criteria: 1) HIV seropositive at baseline; 2) HAART naïve at baseline; 3) on HAART for at least two consecutive visits following HAART initiation; 4) documented baseline viral load; and 5) viral load available before and after HAART initiation. Approvals for the original study and subsequent analyses were obtained from all participating universities’ institutional review boards. Informed consent was obtained from all participants, parents or guardians as deemed appropriate by local laws and/or university regulations.
Behavioral data were collected via face-to-face interviews, audio computer-assisted self-administered interviews, and medical record reviews. Study participants defined their race/ethnicity during direct interviews by self-identification. Interviewers measured and rated depressive signs and symptoms on the Center for Epidemiology Study Depression scale (CES-D).23 Details of adherence assessments have been published elsewhere.24 For the purpose of this analysis, a participant who took his/her triple antiretroviral medications as prescribed more than 50% of the time prior to a particular visit was deemed to be adequately adherent at that specific visit. If a participant then maintained adequate adherence at more than 50% of his/her visits then he/she was defined as having overall adequate adherence for the duration of the study. This definition of ART adherence deviates from standard criteria,25 but was crafted to be more sensitive at the cost of being less specific in order to capture an adequate sample size in a population that has a high risk of non-adherence.
Host genetic factors that are collectively predictive of better immunologic control of HIV replication were captured by an algorithm that has been described previously.26 In brief, HLA class I and CCR2/CCR5 genotypes that have a favorable impact on controlling HIV replication and/or delaying disease progression were assigned a score of +1, while unfavorable genotypes received a score of −1. At the individual level, a genetic score was calculated by summing the assigned values for all contributing HLA and CCR2/CCR5 variants within the individual. Within the study population, genetic scores ranged from −3 to +1, although individuals with extremely low scores were rare. Therefore, we collapsed -3,-2, and -1 scores into a single category of -1 for the purpose of analysis. Virologic and immunologic measures were obtained according to previously defined protocols.21, 22
OUTCOMES
Participants were categorized according to the magnitude and sustainability of viral load reductions while on HAART. We defined early viral responders as those who had >1.0 log10 viral load reduction between pre- and initial two post-HAART visits. We identified individuals as demonstrating a sustained viral response if their mean viral load from the remaining post-HAART visits was at least 0.5 log10 lower than their pre-HAART value. Participants were ultimately classified into two categories: 1) those who had both early and sustained virologic suppression were termed “responders”; 2) those who either demonstrated early but unsustained virologic suppression or showed no early response at all were classified as “sub-optimal responders”. The latter group is a composite of two types of responders because few participants (N = 15) showed an early but unsustained response. The two groups that comprised the suboptimal response categories were immunologically equivalent as measured by CD4+ cell count over the study’s time course (data not shown). Analyses were performed using both categorization schemes. Results differed marginally between the two analyses. Hence, we present the findings of the dichotomous categorization only.
STATISTICAL ANALYSIS
All analyses were performed using SAS Version 9.1 (SAS Institute Inc., Cary, NC). Differences in proportions of participant characteristics between the two viral response groups were assessed by chi-square test. Cochran-Mantel-Haenszel chi-square was used for ordinal categories. After determining normality assumptions to be robust, mean group differences of baseline CD4+ cell count and logarithmically-transformed viral load were compared using Wilcoxon rank sum test and Student’s t-test, respectively. Multivariable logistic regression models were employed to examine the relationship between viral response and baseline age, race/ethnicity, and all dichotomous variables. Magnitudes of association are reported as adjusted odds ratios (OR) and 95% confidence interval (CI) for each covariate in the model.
Mean log10 viral load and CD4+ cell counts were calculated for pre- and post-HAART visits across the two responder groups. The pre-post HAART differences between the two groups were compared using Student’s t-test. Changes in viral load across all time points were examined using longitudinal data analytic methods. Specifically, we estimated changes in log10 viral load within participants and between responder groups using a mixed linear model for repeated measures. Model-selection strategies were used and baseline age, sex and race/ethnicity were adjusted for potential confounding. Variance-covariance structure and model fit diagnostics were assessed for each model.
RESULTS
Of the 154 adolescents included in the study, most were black (72.7%), female (73.4%), and between the ages of 17 and 19 years (70.8%) at enrollment (Table 1). Nearly half (49.4%) of the participants were depressed as assessed by the CES-D scale. More than two-thirds (68.4%) of adolescents reported ever having used alcohol. Of those, 83 (79.8%) reported alcohol use in the three months prior to interview. Illicit drug use was even more prevalent (83.6%). Seventy-three (47.4%) adolescents had a history of using mono- or dual ART before commencing HAART. More than one hundred participants (70.8%) met our definition of medication adherence during the course of follow-up.
Table 1.
Baseline Characteristics of 154 HIV-Infected Adolescents According to Virologic Response to HAART
| Respondersb (N = 50) (%) |
Sub-Optimal Responders (N = 104) (%) |
P | |
|---|---|---|---|
| Age, years | |||
| 12 – 16 | 28.0 | 29.8 | .80 |
| 17 – 19 | 72.0 | 70.2 | |
| Sociodemographic characteristics | |||
| Sex, female | 68.0 | 76.0 | .30 |
| Race, black | 64.0 | 76.9 | .09 |
| School drop-out | 20.0 | 26.9 | .40 |
| Ever used alcohol | 80.0 | 61.5 | .03 |
| Used alcohol in last 3 months | 62.0 | 50.0 | .60 |
| Ever used illicit drugs | 84.0 | 67.3 | .40 |
| Used illicit drugs in last 3 months | 68.0 | 55.7 | .70 |
| Depressedc | 52.0 | 48.1 | .60 |
| Clinical characteristics | |||
| Prior history of ART use | 30.0 | 55.8 | .003 |
| Adequate HAART Adherence | 82.0 | 65.3 | .03 |
| HIV clinical staged | |||
| Early | 44.0 | 34.6 | .50 |
| Intermediate | 38.0 | 44.2 | |
| Late | 18.0 | 21.0 | |
| Baseline laboratory measures, mean (SD) | |||
| CD4+ T cell count per µl | 477 (219) | 504 (262) | .54 |
| Log10 HIV RNA copies/mL | 4.05 (0.73) | 3.87 (0.94) | .20 |
| Immunogenetic scoree | |||
| ≤ −1 | 32.0 | 36.5 | .76 |
| 0 | 60.0 | 52.8 | |
| 1 | 6.0 | 7.7 |
Abbreviations: HIV, human immunodeficiency virus; HAART, highly active antiretroviral therapy; ART, antiretroviral therapy; SD, standard deviation
P values were calculated by Pearson chi-square test and Fisher’s exact test for categorical variables and Student’s or Wilcoxon rank sum t-test for continuous variables
Responders had a >1.0 log10 plasma HIV RNA copies/mL reduction between pre-HAART and the initial two post-HAART visits as well as >0.5 log10 reduction between pre-HAART and the mean of all post-HAART visits.
Measured according to the Center for Epidemiology Study Depression scale.23
Stage of disease progression is defined according to the revised Centers for Disease Control and Prevention classification scheme.27
Immunogenetic score was calculated according to an algorithm describe previously.26
According to the Centers for Disease Control and Prevention HIV staging system,27 58 (37.7%) adolescents met clinical criteria for early HIV disease, 65 (42.2%) were at the intermediate stage of infection, and 31 (20.1%) were at the late stage of their disease course. The mean baseline viral load of the study population was 3.93 log10 copies/mL (standard deviation (SD), 0.88) while the mean CD4+ cell count was 495 cells/µL (SD, 248). Fifty (32.5%) adolescents demonstrated early and sustained viral suppression (“responders”) while 15 (9.7%) had an early but unsustained viral load reduction and 89 (57.8%) showed little or no viral response at all while on HAART. The latter two groups comprised the “sub-optimal responders”.
When we compared individuals according to virologic response to HAART, we found no differences in age, sex, race, education, evidence of depression, or history of drug use. Clinical stage of disease progression, immunogenetic profile, baseline CD4+ cell count, and viral load values were also similar across groups. However, responders had a higher proportion of individuals who reported ever using alcohol than sub-optimal responders (80.0% vs. 61.5%, P = .03). Adherence rates and prior use of mono- or dual ART also differed by virologic response. Responders were less likely to have had a prior history of ART use than sub-optimal responders (30.0% vs. 55.8%, P = .003). Also, responders had been significantly more adherent to their HAART regimen when compared to sub-optimal responders (82.0% vs. 65.3%, P = .03).
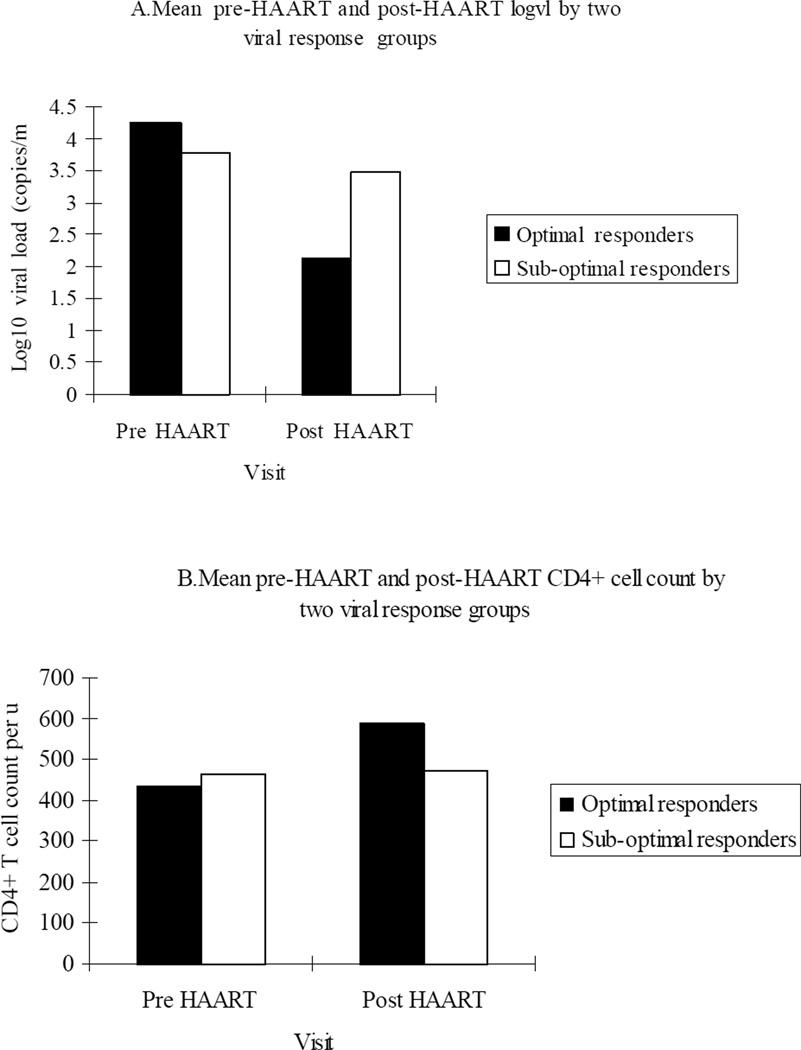
Despite statistically significant differences between groups on three separate parameters, only prior ART use was still significantly associated with sub-optimal virologic response on multivariable logistic regression modeling (adjusted OR, 2.6; 95% CI, 1.1– 5.7) (Table 2). There was also a strong trend toward protection from sub-optimal virologic response with adequate HAART adherence (OR, 0.4; 95% CI, 0.2–1.0). Comparisons between HAART response groups revealed that mean pre-HAART plasma concentration of HIV RNA was 0.36 log10 copies/mL higher in the responders (4.13 log10 copies/mL; SD, 0.76) compared to sub-optimal responders (3.77 log10 copies/mL; SD, 0.90) (P < .001) (Table 3). At post HAART visits, viral responders had a 7-fold log10 copies/mL viral load decrement compared to sub-optimal responders (−2.11 log10 copies/mL; standard deviation (SD), 0.68 vs.−0.29 log10 copies/mL; SD, 0.93; .P < .001) (Figure 1). And the responders maintained at least 1.0 log10 copies/mL lower concentration of plasma HIV RNA than sub-optimal responders on all subsequent post-HAART initiation visits except the last (P < .001). After the baseline visit at which the responders had a lower CD4+ cell count than the sub-optimal response group (458 cells/µL vs 503 cells/µL, P .03), the responders consistently exhibited significantly higher CD4+ cell counts across all remaining visits (P < .001).
Table 2.
Multivariable Adjusted Likelihood of Sub-Optimal Virologic Response to HAART in Association with Sociodemographic Characteristics Among 154 HIV-Infected Adolescents.a,b
| Unadjusted OR | Adjusted OR (95% CI) | |
|---|---|---|
| Age, 17 – 19 years | 0.9 | 0.9 (0.4–2.2) |
| Sex, male | 0.7 | 1.0 (0.4–2.3) |
| Race, black | 1.9 | 1.8 (0.8–4.2) |
| Depressed c | 0.9 | 1.0 (0.5–2.2) |
| Ever used alcohol | 0.4 | 0.4 (0.1–2.3) |
| Ever used illicit drugs | 0.6 | 1.4 (0.2–10.1) |
| Prior history of ART use | 2.9 | 2.6 (1.1–5.7) |
| Adequate HAART Adherence | 0.4 | 0.4 (0.2–1.0) |
| Immunogenetic score, 0 or 1 d | 0.9 | 0.8 (0.4–1.8) |
Abbreviations: HIV, human immunodeficiency virus; HAART, highly active antiretroviral therapy; ART, antiretroviral therapy; OR, odds ratio; CI, confidence interval
Responders had a >1.0 log10 plasma HIV RNA copies/mL reduction between pre-HAART and the initial two post-HAART visits as well as >0.5 log10 reduction between pre-HAART and the mean of all post-HAART visits.
Variables with univariate P values ≤ .2 were included in the multivariable logistic regression model. ORs and 95% CIs were calculated before removal from a backward elimination model.
Measured according to the Center for Epidemiology Study Depression scale.23
Immunogenetic score was calculated according to an algorithm describe previously.26
Table 3.
Mixed Model of Plasma Concentrations of HIV RNA and CD4+ Cell Counts Among Adolescent Responders and Sub-Optimal Responders to HAART Before and After HAART initiation. a, b
| Pre-HAART | Post-HAART | |||||||
|---|---|---|---|---|---|---|---|---|
| Visit 0 | Visit 1 | Visit 2 | Visit 3 | Visit 4 | Visit 5 | Visit 6 | P | |
| Log10 HIV RNA c | ||||||||
| Response | 4.13 (0.76) | 2.14 (0.69) | 2.28 (1.04) | 2.44 (1.02) | 2.54 (1.05) | 2.73 (1.06) | 2.85 (1.05) | <.001 |
| Sub-optimal response | 3.77 (0.90) | 3.49 (1.14) | 3.79 (1.05) | 3.65 (1.16) | 3.71 (1.13) | 3.86 (1.16) | 3.59 (1.22) | |
| CD4+ cell count c | ||||||||
| Response | 458 (224) | 586 (263) | 577 (254) | 600 (272) | 624 (272) | 626 (284) | 628 (331) | <.001 |
| Sub-optimal response | 503 (218) | 464 (253) | 483 (257) | 505 (315) | 465 (259) | 461 (276) | 466 (260) | |
| HAART adherence d | ||||||||
| Response | ----------- | 40 (80.0) | 31 (62.0) | 37 (74.0) | 30 (62.5) | 27 (65.9) | 22 (57.9) | <.001 |
| Sub-optimal response | ----------- | 62 (59.6) | 55 (52.9) | 53 (54.1) | 43 (45.7) | 40 (45.5) | 45 (53.6) | |
Abbreviations: HIV, human immunodeficiency virus; HAART, highly active antiretroviral therapy.
The time between each visit was 3 months.
Responders had a >1.0 log10 plasma HIV RNA copies/mL reduction between pre-HAART and the initial two post-HAART visits as well as >0.5 log10 reduction between pre-HAART and the mean of all post-HAART visits.
Data are mean (standard deviation), unless otherwise indicated.
Log10 HIV RNA - copies/mL; CD4+ cell count - cells/µL.
Participants were adherent to HAART at each visit if they reported taking there making medications 50% of the time.
Figure 1.
Pre-Post HAART Viral Load and CD4+ Cell Counts According to Virologic Response
COMMENT
Data on the predictors of clinical outcomes in HIV-infected adolescents are sparse and primarily limited to long-term survivors of perinatal transmission.28, 29 Since adolescents with HIV infection may not know their status and/or may not be in clinical care, it is hard for any single site outside of Africa to have a large enough number of HIV-infected adolescents to tease out the factors salient to clinical failure. The REACH project made it possible for us to overcome these limitations by combining 16 sites from 13 diverse locales in US cities for a prospective care-based epidemiologic study.
We found that adolescents with a prior history of ART use were more than twice as likely to have a sub-optimal virologic response to HAART than if they had been completely ART naïve. These outcomes are consistent with previous studies in both adults and children. Use of mono- or dual therapy is inherently sub-optimal and precipitates the emergence of resistant HIV strains. We acknowledge the possibility that adolescents on prior ART may have been non-adherent with their medication regimen as well. We found, however, that adolescents with at least 50% adherence to their HAART regimen through at least 50% of their study visits were 60% less likely to exhibit a poor virologic response. That even modest ART adherence should have had such a large protective effect is encouraging. Still, moderate adherence poses a high risk for emergence of viral resistance as it is well established that the lower the proportion of adherent participants, the higher the mean viral load.30, 31 Most studies have indicated that exceptionally high adherence (>95%) is needed in order to maintain adequate virologic suppression.32–34 These results are, therefore, consistent with findings from studies in both adults and children that show HAART adherence is essential to achieve sustained suppression of HIV replication and immunologic reconstitution, although an elevated risk of developing resistant viral strains remains.35–37
Despite the consistency of our results with prior studies in adult and pediatric populations, our study had several limitations. Adherence data that are based on self-report are subject to imprecision and recall bias. To reduce such bias, REACH study interviews were conducted in a uniform fashion, making a differential bias between the two outcome groups less likely. We also used less rigorous criteria for the definition of adherence. That better adherence should have been highly protective against both viral load increments and CD4+ cell count decrements suggests that a stricter definition of adherence would not necessarily have changed our findings (though it would have reduced our statistical power as excellent adherence was rare in this population). Also, we only assessed adherence to HAART; adherence to any prior antiretroviral use was not considered. Compared to other adult cohorts, our study’s sample size was much smaller. However, the current study is one of the largest analyses of an HIV-infected adolescent population, drawing upon a geographically diverse set of sites across the US. Finally, due to cost restraints, we were not able to perform resistance genotyping or phenotyping on study participants
HIV-infected youth have many special needs in coping with their disease.38 While we found that predictors of virologic failure in adolescents are similar to those in children and adults, adolescent psychosocial issues must be addressed to ensure clinical success. Many adolescents live at home and may not reveal their status to parents and guardians who are then unable to provide assistance in adhering to therapy.39, 40 Other youth not living with parents or guardians may have transient housing arrangements or may be living on the street that complicate adequate adherence as housing, food, and shelter often take precedence over medication adherence.41, 42 Furthermore, even when treatment is sought, additional barriers related to availability, acceptability, and equity of services make adherence difficult.
Adherence to HAART will require youth friendly clinical settings and social support through community outreach efforts.9, 43 The adolescent population is in a unique transition state from child to adulthood; this requires tailored services to ensure proper adherence. Our data add support for increased focus on adherence support services for HIV-infected adolescents, particularly as fully two-thirds of youth failed to have an adequate virologic response to HAART. The strong association observed in this study between better adherence and both early and sustained virologic responses among adolescents suggests an urgent need to maximize supportive adherence programs in the context of the broader social needs of HIV-infected youth.
Acknowledgements
The authors acknowledge Katherine L. Allen, MSc for assistance with manuscript development, the contributions of investigators and staff of the Adolescent Medicine HIV/AIDS Research Network (1994–2001, then superseded by the Adolescent Trials Network), and the youth who participated in the research. Participating investigators and staff are listed in J Adolesc Health 2001; 29 (suppl):5–6
Financial Disclosure: The REACH study was funded by grant U01-HD32842 from the National Institute of Child Health and Human Development and the National Institute on Drug Abuse, National Institute of Allergy and Infectious Diseases, and National Institute of Mental Health. Support for the analytic work was received from the Vanderbilt-Meharry Center for AIDS Research, grant P30-AI054999 from the National Institute of Allergy and Infectious Diseases.
Participating Institutions of the Reaching for Excellence in Adolescent Care and Health (REACH) project of the Adolescent Medicine HIV/AIDS Research Network are: University of Miami, Miami, FL; Children’s Hospital of Philadelphia, Philadelphia, PA; Tulane Medical Center, New Orleans, LA; Children’s National Medical Center, Washington, DC; Montefiore Medical Center, Bronx, NY; University of Maryland, Baltimore, MD; Children’s Hospital of Los Angeles, Los Angeles, CA; Cook County Hospital/University of Chicago, Chicago, IL; Mt. Sinai Medical Center, New York, NY; Alabama Children’s Hospital, Birmingham, AL; Emory University, Atlanta, GA; St. Jude Children’s Research Hospital, Memphis, TN; SUNY Health Science Center at Brooklyn, Brooklyn, NY; Children’s Diagnostic and Treatment Center, University of Puerto Rico, San Juan, PR; University of Medicine and Dentistry of New Jersey, Newark, NJ.
Footnotes
Author Contributions: Dr Ding had full access to all of the data in the study and takes responsibility for the integrity of the data and the accuracy of the data analysis.
Study concept and design: Ding, Wilson, and Vermund.
Analysis and interpretation of data: Ding, Wilson, Modjarrad, McGwin, Tang, and Vermund.
Drafting of the manuscript: Ding, Wilson, Modjarrad, McGwin, Tang, and Vermund.
Critical revision of the manuscript for important intellectual content: Ding, Wilson, Modjarrad, McGwin, Tang and Vermund.
Statistical analysis: Ding, Wilson, Modjarrad, McGwin, Tang, and Vermund.
Obtained funding: Vermund.
Administrative, technical, and material support: Ding and Modjarrad.
Study supervision: Wilson and Vermund.
REFERENCES
- 1.Prevention CfDCa. HIV/AIDS Surveillance Report, 2006. Atlanta: U.S. Department of Health and Human Services, Centers for Disease Control and Prevention; 2008. [Google Scholar]
- 2.Rosenberg PS, Biggar RJ. Trends in HIV incidence among young adults in the United States. JAMA. 1998 Jun 17;279(23):1894–1899. doi: 10.1001/jama.279.23.1894. [DOI] [PubMed] [Google Scholar]
- 3.Hall HI, Song R, Rhodes P, et al. Estimation of HIV incidence in the United States. JAMA. 2008 Aug 6;300(5):520–529. doi: 10.1001/jama.300.5.520. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Rangel MC, Gavin L, Reed C, Fowler MG, Lee LM. Epidemiology of HIV and AIDS among adolescents and young adults in the United States. J Adolesc Health. 2006 Aug;39(2):156–163. doi: 10.1016/j.jadohealth.2006.02.011. [DOI] [PubMed] [Google Scholar]
- 5.Murphy DA, Mitchell R, Vermund SH, Futterman D. Factors associated with HIV testing among HIV-positive and HIV-negative high-risk adolescents: the REACH Study. Reaching for Excellence in Adolescent Care and Health. Pediatrics. 2002 Sep;110(3):e36. doi: 10.1542/peds.110.3.e36. [DOI] [PubMed] [Google Scholar]
- 6.Obermeyer CM, Osborn M. The utilization of testing and counseling for HIV: a review of the social and behavioral evidence. Am J Public Health. 2007 Oct;97(10):1762–1774. doi: 10.2105/AJPH.2006.096263. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Vermund SH, Wilson CM. Barriers to HIV testing--where next? Lancet. 2002 Oct 19;360(9341):1186–1187. doi: 10.1016/S0140-6736(02)11291-8. [DOI] [PubMed] [Google Scholar]
- 8.Johnson RL, Martinez J, Botwinick G, et al. Introduction: what youth need--adapting HIV care models to meet the lifestyles and special needs of adolescents and young adults. J Adolesc Health. 2003 Aug;33(2 Suppl):4–9. doi: 10.1016/s1054-139x(03)00161-7. [DOI] [PubMed] [Google Scholar]
- 9.Tylee A, Haller DM, Graham T, Churchill R, Sanci LA. Youth-friendly primary-care services: how are we doing and what more needs to be done? Lancet. 2007 May 5;369(9572):1565–1573. doi: 10.1016/S0140-6736(07)60371-7. [DOI] [PubMed] [Google Scholar]
- 10.Johnson RL, Botwinick G, Sell RL, et al. The utilization of treatment and case management services by HIV-infected youth. J Adolesc Health. 2003 Aug;33(2 Suppl):31–38. doi: 10.1016/s1054-139x(03)00158-7. [DOI] [PubMed] [Google Scholar]
- 11.Murphy DA, Sarr M, Durako SJ, Moscicki AB, Wilson CM, Muenz LR. Barriers to HAART adherence among human immunodeficiency virus-infected adolescents. Arch Pediatr Adolesc Med. 2003 Mar;157(3):249–255. doi: 10.1001/archpedi.157.3.249. [DOI] [PubMed] [Google Scholar]
- 12.De Rossi A. Primary HIV infection in infants: impact of highly active antiretroviral therapy on the natural course. J Biol Regul Homeost Agents. 2002 Jan-Mar;16(1):53–57. [PubMed] [Google Scholar]
- 13.Detels R, Munoz A, McFarlane G, et al. Effectiveness of potent antiretroviral therapy on time to AIDS and death in men with known HIV infection duration. Multicenter AIDS Cohort Study Investigators. JAMA. 1998 Nov 4;280(17):1497–1503. doi: 10.1001/jama.280.17.1497. [DOI] [PubMed] [Google Scholar]
- 14.Erb P, Battegay M, Zimmerli W, Rickenbach M, Egger M. Effect of antiretroviral therapy on viral load, CD4 cell count, and progression to acquired immunodeficiency syndrome in a community human immunodeficiency virus-infected cohort. Swiss HIV Cohort Study. Arch Intern Med. 2000 Apr 24;160(8):1134–1140. doi: 10.1001/archinte.160.8.1134. [DOI] [PubMed] [Google Scholar]
- 15.Hunt PW, Deeks SG, Rodriguez B, et al. Continued CD4 cell count increases in HIV-infected adults experiencing 4 years of viral suppression on antiretroviral therapy. AIDS. 2003 Sep 5;17(13):1907–1915. doi: 10.1097/00002030-200309050-00009. [DOI] [PubMed] [Google Scholar]
- 16.Press N, Tyndall MW, Wood E, Hogg RS, Montaner JS. Virologic and immunologic response, clinical progression, and highly active antiretroviral therapy adherence. J Acquir Immune Defic Syndr. 2002 Dec 15;31(Suppl 3):S112–S117. doi: 10.1097/00126334-200212153-00005. [DOI] [PubMed] [Google Scholar]
- 17.Barreiro PM, Dona MC, Castilla J, Soriano V. Patterns of response (CD4 cell count and viral load) at 6 months in HIV-infected patients on highly active antiretroviral therapy. AIDS. 1999 Mar 11;13(4):525–526. doi: 10.1097/00002030-199903110-00014. [DOI] [PubMed] [Google Scholar]
- 18.Florence E, Lundgren J, Dreezen C, et al. Factors associated with a reduced CD4 lymphocyte count response to HAART despite full viral suppression in the EuroSIDA study. HIV Med. 2003 Jul;4(3):255–262. doi: 10.1046/j.1468-1293.2003.00156.x. [DOI] [PubMed] [Google Scholar]
- 19.Jevtovic D, Salemovic D, Ranin J, Pesic I, Zerjav S, Djurkovic-Djakovic O. The dissociation between virological and immunological responses to HAART. Biomed Pharmacother. 2005 Sep;59(8):446–451. doi: 10.1016/j.biopha.2005.07.006. [DOI] [PubMed] [Google Scholar]
- 20.Le Moing V, Thiebaut R, Chene G, et al. Long-term evolution of CD4 count in patients with a plasma HIV RNA persistently <500 copies/mL during treatment with antiretroviral drugs. HIV Med. 2007 Apr;8(3):156–163. doi: 10.1111/j.1468-1293.2007.00446.x. [DOI] [PubMed] [Google Scholar]
- 21.Rogers AS, Futterman DK, Moscicki AB, Wilson CM, Ellenberg J, Vermund SH. The REACH Project of the Adolescent Medicine HIV/AIDS Research Network: design, methods, and selected characteristics of participants. J Adolesc Health. 1998 Apr;22(4):300–311. doi: 10.1016/s1054-139x(97)00279-6. [DOI] [PubMed] [Google Scholar]
- 22.Wilson CM, Houser J, Partlow C, Rudy BJ, Futterman DC, Friedman LB. The REACH (Reaching for Excellence in Adolescent Care and Health) project: study design, methods, and population profile. J Adolesc Health. 2001 Sep;29(3 Suppl):8–18. doi: 10.1016/s1054-139x(01)00291-9. [DOI] [PubMed] [Google Scholar]
- 23.Radloff LS. The CES-D Scale: A Self-Report Depression Scale for Research in the General Population. Applied Psychological Measurement. 1977;1(3):385–401. [Google Scholar]
- 24.Murphy DA, Wilson CM, Durako SJ, Muenz LR, Belzer M. Antiretroviral medication adherence among the REACH HIV-infected adolescent cohort in the USA. AIDS Care. 2001 Feb;13(1):27–40. doi: 10.1080/09540120020018161. [DOI] [PubMed] [Google Scholar]
- 25.Reisner SL, Mimiaga MJ, Skeer M, Perkovich B, Johnson CV, Safren SA. A Review of HIV Antiretroviral Adherence and Intervention Studies Among HIV-Infected Youth. Top HIV Med. 2009 Feb-Mar;17(1):14–25. [PMC free article] [PubMed] [Google Scholar]
- 26.Tang J, Wilson CM, Meleth S, et al. Host genetic profiles predict virological and immunological control of HIV-1 infection in adolescents. AIDS. 2002 Nov 22;16(17):2275–2284. doi: 10.1097/00002030-200211220-00007. [DOI] [PubMed] [Google Scholar]
- 27.1993 revised classification system for HIV infection and expanded surveillance case definition for AIDS among adolescents and adults. MMWR Recomm Rep. 1992 Dec 18;41(RR-17):1–19. [PubMed] [Google Scholar]
- 28.Flynn PM, Rudy BJ, Lindsey JC, et al. Long-term observation of adolescents initiating HAART therapy: three-year follow-up. AIDS Res Hum Retroviruses. 2007 Oct;23(10):1208–1214. doi: 10.1089/aid.2006.0290. [DOI] [PubMed] [Google Scholar]
- 29.Rudy BJ, Lindsey JC, Flynn PM, et al. Immune reconstitution and predictors of virologic failure in adolescents infected through risk behaviors and initiating HAART: week 60 results from the PACTG 381 cohort. AIDS Res Hum Retroviruses. 2006 Mar;22(3):213–221. doi: 10.1089/aid.2006.22.213. [DOI] [PubMed] [Google Scholar]
- 30.Braithwaite RS, Kozal MJ, Chang CC, et al. Adherence, virological and immunological outcomes for HIV-infected veterans starting combination antiretroviral therapies. AIDS. 2007 Jul 31;21(12):1579–1589. doi: 10.1097/QAD.0b013e3281532b31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Gandhi M, Ameli N, Bacchetti P, et al. Protease inhibitor levels in hair strongly predict virologic response to treatment. AIDS. 2009 Feb 20;23(4):471–478. doi: 10.1097/QAD.0b013e328325a4a9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Boden D, Hurley A, Zhang L, et al. HIV-1 drug resistance in newly infected individuals. JAMA. 1999 Sep 22–29;282(12):1135–1141. doi: 10.1001/jama.282.12.1135. [DOI] [PubMed] [Google Scholar]
- 33.Chesney M. Adherence to HAART regimens. AIDS Patient Care STDS. 2003 Apr;17(4):169–177. doi: 10.1089/108729103321619773. [DOI] [PubMed] [Google Scholar]
- 34.Garcia de Olalla P, Knobel H, Carmona A, Guelar A, Lopez-Colomes JL, Cayla JA. Impact of adherence and highly active antiretroviral therapy on survival in HIV-infected patients. J Acquir Immune Defic Syndr. 2002 May 1;30(1):105–110. doi: 10.1097/00042560-200205010-00014. [DOI] [PubMed] [Google Scholar]
- 35.Gifford AL, Bormann JE, Shively MJ, Wright BC, Richman DD, Bozzette SA. Predictors of self-reported adherence and plasma HIV concentrations in patients on multidrug antiretroviral regimens. J Acquir Immune Defic Syndr. 2000 Apr 15;23(5):386–395. doi: 10.1097/00126334-200004150-00005. [DOI] [PubMed] [Google Scholar]
- 36.Gross R, Bilker WB, Friedman HM, Strom BL. Effect of adherence to newly initiated antiretroviral therapy on plasma viral load. AIDS. 2001 Nov 9;15(16):2109–2117. doi: 10.1097/00002030-200111090-00006. [DOI] [PubMed] [Google Scholar]
- 37.Nachega JB, Hislop M, Dowdy DW, Chaisson RE, Regensberg L, Maartens G. Adherence to nonnucleoside reverse transcriptase inhibitor-based HIV therapy and virologic outcomes. Ann Intern Med. 2007 Apr 17;146(8):564–573. doi: 10.7326/0003-4819-146-8-200704170-00007. [DOI] [PubMed] [Google Scholar]
- 38.Hein K, Futterman D. Guidelines for the care of children and adolescents with HIV infection. Medical management in HIV-infected adolescents. J Pediatr. 1991 Jul;119(1 (Pt 2)):S18–S20. doi: 10.1016/s0022-3476(05)81448-9. [DOI] [PubMed] [Google Scholar]
- 39.D'Angelo LJ, Abdalian SE, Sarr M, Hoffman N, Belzer M. Disclosure of serostatus by HIV infected youth: the experience of the REACH study. Reaching for Excellence in Adolescent Care and Health. J Adolesc Health. 2001 Sep;29(3 Suppl):72–79. doi: 10.1016/s1054-139x(01)00285-3. [DOI] [PubMed] [Google Scholar]
- 40.Tornero C, Santamaria A, Gil E. The presence of a caregiving relative implicated in the administration of antiretroviral drug administration improves virologic response. J Acquir Immune Defic Syndr. 2007 Dec 15;46(5):658. doi: 10.1097/QAI.0b013e3181568b11. [DOI] [PubMed] [Google Scholar]
- 41.Aidala AA, Lee G, Abramson DM, Messeri P, Siegler A. Housing need, housing assistance, and connection to HIV medical care. AIDS Behav. 2007 Nov;11(6 Suppl):101–115. doi: 10.1007/s10461-007-9276-x. [DOI] [PubMed] [Google Scholar]
- 42.Martinez J, Bell D, Dodds S, et al. Transitioning youths into care: linking identified HIV-infected youth at outreach sites in the community to hospital-based clinics and or community-based health centers. J Adolesc Health. 2003 Aug;33(2 Suppl):23–30. doi: 10.1016/s1054-139x(03)00159-9. [DOI] [PubMed] [Google Scholar]
- 43.Futterman DC. HIV and AIDS in adolescents. Adolesc Med Clin. 2004 Jun;15(2):369–391. doi: 10.1016/j.admecli.2004.02.009. [DOI] [PubMed] [Google Scholar]