Abstract
The aim of this study was to determine the impact that age and comorbidity status have on both overall and bladder cancer-specific survival of bladder cancer patients. We obtained medical information pertaining to a population of 528 patients with newly diagnosed bladder cancer from Chung-Ang University Hospital cancer registry. The Adult Comorbidity Evaluation-27 (ACE-27) test, which has been previously validated in adult cancer patients, was used to assess comorbidity. We evaluated differences in the demographic and clinical characteristics of included patients, as well as differences in the treatments they received after categorizing them by age. The median age at the time of bladder cancer diagnosis of the entire cohort was 63 years, and the median follow-up time was 97 months. Of the 528 patients who were included in our study, 303 had at least one comorbid condition and 249 died during the follow-up period. When patients were stratified by age, we found that older patients had a higher proportion of severe comorbidities (P < 0.01) than younger patients, and that a lower proportion of them underwent radical cystectomy for invasive bladder cancer (IBC) (P < 0.01). By multivariate analysis, we found that older age was predictive of lower overall survival (OS) and bladder cancer-specific survival (BCSS) rates among patients with superficial bladder cancer (SBC) and of lower OS rates among patients with IBC. We also found that moderate–severe comorbidity status and treatment through a bladder-conserving approach were predictive of lower OS and cancer-specific survival rates among patients with IBC. The disparity between overall deaths and bladder cancer deaths was shown in SBC and increased along with age and higher comorbidity. Age and comorbidity were found to be independent predictive factors of OS and BCSS among bladder cancer patients, and explained the disparity that we observed between overall bladder cancer-specific mortality rates.
Keywords: age, comorbidity, neoplasm, prognostic indicator, urinary bladder
Introduction
Age and comorbidity have been shown to be associated with overall and disease-specific outcomes in various types of cancer. Although previous studies have tended to focus on gross and microscopic tumour characteristics for predicting the prognosis of bladder cancer patients, patients' overall health status often impacts survival as well 1, 2. Like many other neoplasms, bladder cancer is a disease that increases in incidence with age. The National Cancer Institute Surveillance, Epidemiology, and End Results (SEER) programme estimates that 89% of bladder carcinoma patients are 55 years of age or older at the time of diagnosis and that the median age at the time of diagnosis for both men and women is 73 years 3, 4. This older patient population is more likely to have preexisting diseases because of their age. Moreover, bladder cancer is strongly associated with smoking and increased dietary fat intake, both of which are associated with multiple types of medical comorbidity 5, 6. Therefore, it is not surprising that patients with bladder cancer often have significant comorbidities, such as cardiovascular, cerebrovascular and/or pulmonary disease 7.
Although these associations have yet to be fully explained, in previous studies, the statistical methods that were used to calculate bladder cancer-specific survival (BCSS) did not adjust for competing risks, that is, death unrelated to bladder cancer without previous evidence of disease progression 1, 2. As a result, the calculated BCSS rate differed from the overall survival (OS) rate, which poses a problem when there is a large amount of censoring.
In this study, we sought to determine the association between two demographic characteristics (age and comorbidity), OS and BCSS using competing risk analysis. The goal of this study was to determine whether or not age and comorbidity status provided important independent prognostic information for patients with bladder cancer after controlling for morphological (stage and grade of tumour) and demographic (gender) variables. Our primary end points were OS and BCSS. We investigated these end points in the entire cohort, as well as in two subgroups, namely, patients with superficial bladder cancer (SBC) and those with invasive bladder cancer (IBC). Our overall goal was to determine the association that age and comorbidity had with OS and BCSS using competing risk analysis. In addition, we sought to investigate the differences in OS and BCSS rates among patients with SBC and IBC after stratifying by age and comorbidity status.
Materials and methods
Chung-Ang Hospital Urologic Oncologic Data Services
After obtaining institutional review board approval, we reviewed the clinical and pathological data of 709 patients who were seen at Chung-Ang University Hospital between 1981 and 2007 for newly diagnosed bladder cancer. The Chung-Ang University Hospital Urologic Oncology Data Service is an electronic database that includes data on patients who were diagnosed or treated at our institution for a urological malignancy. From the 709 patients who were identified from the database, we excluded 43 patients who did not have comorbidity information, 13 patients who did not have pathological or clinical tumour stage information available, 47 patients who did not have a history of transitional cell carcinoma (specifically, we excluded patients with sarcomas, adenocarcinomas and squamous cell carcinomas), 53 patients who did not have follow-up data available and 25 patients who had been diagnosed with carcinoma in situ. This resulted in a final study population of 528 patients.
Patient characteristics and comorbidities
The data collected were standard tumour registry data points such as the following: (1) date of diagnosis; (2) demographic information; (3) tumour morphology; and (4) nodal spread at the time of presentation or initial therapy. The Adult Comorbidity Evaluation-27 (ACE-27; available online at http://oto.wustl.edu/clinepi/comorbid.html) is a 27-item comorbidity instrument that has been validated for use in adult oncology patients 8. Comorbidities were defined as preexisting medical conditions that were present at the time of cancer diagnosis, including previous or synchronous cancers. The ACE-27 was used to grade the severity of specific diseases and conditions on a scale of 1–3 (grade 1, mild; grade 2, moderate; and grade 3, severe). The grading is based on the severity of organ decompensation and the prognostic impact that comorbid condition had.
Once the patient's individual diseases and comorbid conditions had been classified, an overall comorbidity score (none, mild, moderate or severe) was assigned based on the highest-ranked single ailment. In cases in which two or more moderate ailments occurred in different organ systems or disease groupings, the overall comorbidity score was designated as severe.
Surgical and pathological parameters
Surgical and pathological parameters were obtained from patients' medical records. The surgical parameters that were recorded included the type of surgical procedure that was performed. Pathological parameters were recorded according to the 2004 American Joint Committee on Cancer (AJCC)/Union International Contre le Cancer Tumor–Node–Metastasis (TNM) Pathology Reporting Protocol (6th edition), which were reviewed by a pathologist and included both TNM and AJCC stage. Grading was performed at the time of surgery on the basis of either the World Health Organization/International Society of Urological Pathology consensus classification or the 1965 classification system 9, 10. Given that different grading systems were used during our study period, we standardized the grade by classifying it into high- and low-grade for analysis. Follow-up was obtained for all patients in the database by medical record review, sending letters of enquiry to patients and querying the Social Security Death Index.
Statistical analysis
We used the SPSS software package version 14.0 (SPSS Inc., Chicago, IL, USA) for our statistical analyses. The patients were stratified according to age at diagnosis into the following three groups: (1) < 60 years old; (2) 60–69 years old; and (3) > 69 years old. χ2 analysis was used to evaluate differences in the distribution of demographic, clinical and treatment characteristics across the patient age categories. The cumulative incidence functions (CIFs) or the probability of experiencing an event by the time of death from any cause were estimated using the Kaplan–Meier method. The CIFs for both bladder and nonbladder cancer deaths were estimated using the competing risks method. The CIF for overall death in each group is the sum of the corresponding CIFs of bladder cancer and nonbladder cancer deaths. The log-rank test was used to compare the distribution of the CIFs for all-cause mortality and bladder cancer-specific mortality across patient groups. For each cohort that was studied, all predictor variables were entered into a single multivariate model (Cox proportional hazard analysis). All P-values < 0.05 were considered statistically significant.
Results
Distribution of demographic, clinical and treatment characteristics according to patient age
The median age at the time of diagnosis for the entire cohort was 63 years and the median follow-up time was 97 months. Of the 528 included patients, 303 had at least one comorbid condition and 249 died during the follow-up period. When these patients were stratified by age, we found that in the oldest group of patients (> 69 years), the proportions of female patients (P < 0.01) and patients with severity comorbidities (P < 0.01) were higher than the other study population, but that the incidence of radical cystectomy for the treatment of IBC (P < 0.01) was lower than that of the other study populations (Table 1). However, the distribution of pathological stage and histological grade did not differ by age group (Table 1).
Table 1. The demographic and clinical characteristics of included patients and the treatments they received, stratified by age groups.
| Variable | Category | No. (%) of patients | No. (%) of patients grouping by age (years) |
P-value | ||
|---|---|---|---|---|---|---|
| < 60 (n = 198) | 60–69 (n = 172) | > 69 (n = 158) | ||||
| Sex | Male | 479 (90.7) | 188 (94.9) | 157 (91.3) | 134 (84.8) | < 0.01 |
| Female | 49 (9.3) | 10 (5.1) | 15 (8.7) | 24 (15.2) | ||
| Stage | 0 | 155 (29.4) | 70 (35.4) | 37 (21.5) | 48 (30.4) | 0.141 |
| I | 223 (42.2) | 72 (36.4) | 85 (49.4) | 66 (41.8) | ||
| II | 58 (11.0) | 22 (11.1) | 22 (12.8) | 14 (8.9) | ||
| III | 24 (4.5) | 10 (5.1) | 8 (4.7) | 6 (3.8) | ||
| IV | 68 (12.9) | 24 (12.1) | 20 (11.6) | 24 (15.2) | ||
| Comorbidity | 0 | 225 (42.6) | 96 (48.5) | 63 (36.6) | 66 (41.8) | < 0.01 |
| 1 | 132 (25.0) | 56 (28.3) | 48 (27.9) | 28 (17.7) | ||
| 2 | 123 (23.3) | 36 (18.2) | 48 (27.9) | 39 (24.7) | ||
| 3 | 48 (9.1) | 10 (5.1) | 13 (7.6) | 25 (15.8) | ||
| Grade | Low (I, II) | 263 (49.8) | 107 (54.0) | 85 (49.4) | 71 (44.9) | 0.231 |
| High (III, IV) | 265 (50.2) | 91 (46.0) | 87 (50.6) | 87 (55.1) | ||
| Therapy Superficial | TURB | 358 (67.8) | 136 (95.8) | 113 (92.6) | 109 (95.6) | 0.205 |
| Partial/radical cystectomy | 20 (3.8) | 6 (4.2) | 9 (7.4) | 5 (4.4) | ||
| Muscle invasive | TURB/partial cystectomy | 44 (8.3) | 11 (26.2) | 16 (44.4) | 17 (51.5) | < 0.01 |
| Radical cystectomy | 58 (11.0) | 31 (73.8) | 20 (55.6) | 7 (21.2) | ||
| Metastatic | TURB/partial cystectomy | 46 (8.7) | 13 (92.9) | 13 (92.9) | 20 (100.0) | 0.141 |
| Radical cystectomy | 2 (0.4) | 1 (7.1) | 1 (7.1) | 0 | ||
Abbreviation: TURB, transurethral resection of the bladder.
Table 2 shows the incidence of comorbid conditions in the study population. The most common conditions were cardiovascular disease (42.0%), endocrine disease (38.1%) and respiratory disease (12.9%). Although the incidence of comorbidity was similar between age groups, the severity of comorbidity was higher (P < 0.01) in older patients than in younger ones (Table 1).
Table 2. The distribution of medical comorbidities among the 528 included patients, stratified by age groups.
| Comorbidity | No. (%) of patients grouping by age (years) |
No. of patients (%) | ||
|---|---|---|---|---|
| < 60 (n = 198) | 60–69 (n = 172) | > 69 (n = 158) | ||
| All | 303 (57.4) | |||
| Cardiovascular | 69 (34.8) | 81 (47.1) | 72 (45.6) | 222 (42.0) |
| Respiratory | 22 (11.1) | 25 (14.5) | 21 (13.3) | 68 (12.9) |
| Gastrointestinal | 18 (9.1) | 14 (8.1) | 17 (10.8) | 49 (9.3) |
| Renal | 7 (3.5) | 8 (4.7) | 13 (8.2) | 28 (5.3) |
| Endocrine | 64 (32.3) | 76 (44.2) | 61 (38.6) | 201 (38.1) |
| Neurological | 1 (0.5) | 1 (0.6) | 5 (3.2) | 7 (1.3) |
| Psychiatric | 0 | 0 | 0 | 0 |
| Rheumatologic | 0 | 0 | 0 | 0 |
| Immunological | 0 | 0 | 0 | 0 |
| Malignancy | 0 | 2 (1.2) | 10 (6.3) | 12 (2.3) |
| Substance abuse | 0 | 0 | 0 | 0 |
| Obesity | 0 | 0 | 0 | 0 |
Univariate analysis examining the association between multiple variables and OS and CSS
Table 3 shows the results of the univariate Cox proportional hazard regression analysis that we performed to examine the association that age, comorbidity and other variables had with OS and BCSS in the entire cohort, patients with SBC and in patients with IBC. Compared with patients < 60 years of age, older age was associated with a decreased OS rate (entire cohort: 60–69 years old, P < 0.001 and > 69 years old, P < 0.001; SBC: 60–69 years old, P < 0.001 and > 69 years old, P < 0.001; and IBC: 60–69 years old, P = 0.088 and > 69 years old, P < 0.05). In addition, patients > 69 years of age had a decreased BCSS as compared with patients < 60 years of age (entire cohort: P < 0.01; SBC: P < 0.01; and IBC: P < 0.05). Compared with patients with no comorbid conditions, moderate–severe comorbidity was associated with decreased OS (entire cohort: P < 0.001; SBC: P < 0.01; and IBC: P < 0.05) and BCSS (entire cohort: P < 0.001; IBC: P < 0.05). In addition, adverse pathological stage and tumour grade were associated with decreased OS and BCSS in all three patient groups (that is, the entire cohort, patients with SBC and patients with IBC).
Table 3. Overall and bladder cancer-specific survival as a function of baseline demographic, clinical and pathological features in the entire cohort, as well as stratified by disease invasiveness.
| Variable | Category | No. (%) of patients | Overall survival |
Cancer-specific survival |
||
|---|---|---|---|---|---|---|
| P-value | Unadjusted HR (95% CI) | P-value | Unadjusted HR (95% CI) | |||
| Entire cohort (n = 528) | ||||||
| Gender | Male | 479 (90.7) | ||||
| Female | 49 (9.3) | 0.257 | 0.78 (0.51–1.19) | 0.197 | 1.42 (0.83–2.44) | |
| Age (years) | < 60 | 198 (37.5) | ||||
| 60–69 | 172 (32.6) | < 0.001 | 1.92 (1.38–2.68) | 0.128 | 1.39 (0.91–2.12) | |
| > 69 | 158 (29.9) | < 0.001 | 3.83 (2.77–5.30) | < 0.01 | 1.95 (1.28–2.96) | |
| Stage | 0 | 155 (29.4) | ||||
| I | 223 (42.2) | < 0.001 | 2.00 (1.37–2.92) | < 0.05 | 3.34 (1.27–8.79) | |
| II | 58 (11.0) | < 0.001 | 2.71 (1.67–4.39) | < 0.001 | 15.61 (5.96–40.93) | |
| III | 24 (4.5) | < 0.001 | 9.73 (5.69–16.63) | < 0.001 | 48.53 (18.13–129.92) | |
| IV | 68 (12.9) | < 0.001 | 18.81 (12.33–28.69) | < 0.001 | 97.25 (38.72–244.24) | |
| Tumour grade | Low (I, II) | 263 (49.8) | ||||
| High (III, IV) | 265 (50.2) | < 0.001 | 3.40 (2.60–4.45) | < 0.001 | 12.23 (7.02–21.30) | |
| Comorbidity | 0 | 225 (42.6) | ||||
| 1 | 132 (25.0) | 0.202 | 1.23 (0.89–1.70) | 0.309 | 1.26 (0.81–1.94) | |
| 2, 3 | 171 (32.4) | < 0.001 | 1.87 (1.40–2.51) | < 0.01 | 1.70 (1.15–2.53) | |
| Superficial bladder cancer (n = 378) | ||||||
| Age (years) | < 60 | 142 (37.6) | ||||
| 60–69 | 122 (32.3) | <0.001 | 2.90 (1.73–4.85) | 0.308 | 1.67 (0.62–4.50) | |
| > 69 | 114 (30.2) | < 0.001 | 7.99 (4.89–13.07) | < 0.01 | 3.83 (1.49–9.82) | |
| Comorbidity | 0 | 166 (43.9) | ||||
| 1 | 96 (25.4) | 0.400 | 1.20 (0.78–1.85) | 0.563 | 1.28 (0.55–2.97) | |
| 2, 3 | 116 (30.7) | < 0.01 | 1.76 (1.18–2.64) | 0.556 | 0.74 (0.28–1.98) | |
| Invasive bladder cancer (n = 102) | ||||||
| Age (years) | < 60 | 42 (41.2) | ||||
| 60–69 | 36 (35.3) | 0.088 | 1.63 (0.93–2.86) | 0.071 | 1.74 (0.95–3.17) | |
| > 69 | 24 (23.5) | < 0.05 | 2.24 (1.21–4.13) | < 0.05 | 2.07 (1.08–3.96) | |
| Comorbidity | 0 | 44 (43.1) | ||||
| 1 | 25 (24.5) | 0.691 | 1.13 (0.61–2.09) | 0.741 | 1.12 (0.57–2.19) | |
| 2, 3 | 33 (32.4) | < 0.05 | 1.78 (1.02–3.11) | < 0.05 | 1.90 (1.07–3.40) | |
| Radical cystectomy | Yes | 58 (56.9) | ||||
| No | 44 (43.1) | 0.086 | 1.52 (0.94–2.44) | 0.206 | 1.39 (0.84–2.31) | |
Abbreviations: HR, hazard ratio; CI, confidence interval.
Multivariate analysis of variables associated with OS and CSS in SBC and IBC
Table 4 shows the results of the multivariate Cox proportional hazard regression analysis that we performed to examine the association of age, comorbidity and other variables with worse OS and BCSS among patients with SBC and IBC. In the multivariate model, older age was predictive of worse OS and worse BCSS among patients with SBC. It was also associated with worse OS among patients with IBC. In addition, we found that moderate–severe comorbidity status and a bladder-preserving treatment approach were both predictive of worse OS (HR 2.06, 95% CI 1.15–3.71 and HR 2.08, 95% CI 1.17–3.70, respectively) and worse BCSS (HR 2.03, 95% CI 1.11–3.73 and HR 1.92, 95% CI 1.03–3.58, respectively) among patients with IBC.
Table 4. Adjusted hazard ratios for overall survival and bladder cancer-specific survival as a function of age and comorbidity severity*.
| Variable | Category | Superficial bladder cancer |
Invasive bladder cancer |
||
|---|---|---|---|---|---|
| P-value | HR (95% CI) | P-value | HR (95% CI) | ||
| Overall survival | |||||
| Age | < 60 | ||||
| 60–69 | < 0.01 | 2.52 (1.49–4.25) | < 0.05 | 1.79 (1.01–3.18) | |
| > 69 | < 0.001 | 7.37 (4.47–12.16) | < 0.05 | 2.08 (1.01–4.27) | |
| Comorbidity | 0 | ||||
| 1 | 0.544 | 1.14 (0.74–1.77) | 0.423 | 1.31 (0.67–2.56) | |
| 2, 3 | 0.220 | 1.29 (0.86–1.95) | < 0.05 | 2.06 (1.15–3.71) | |
| No radical cystectomy | < 0.05 | 2.08 (1.17–3.70) | |||
| Cancer-specific survival | |||||
| Age | < 60 | ||||
| 60–69 | 0.489 | 1.43 (0.52–3.90) | 0.056 | 1.82 (0.98–3.35) | |
| > 69 | < 0.05 | 3.28 (1.26–8.55) | 0.082 | 1.96 (0.92–4.17) | |
| Comorbidity | 0 | ||||
| 1 | 0.568 | 1.28 (0.55–2.99) | 0.432 | 1.33 (0.65–2.69) | |
| 2, 3 | 0.262 | 0.57 (0.21–1.53) | < 0.05 | 2.03 (1.11–3.73) | |
| No radical cystectomy | < 0.05 | 1.92 (1.03–3.58) | |||
Abbreviations: HR, hazard ratio; CI, confidence interval.
*HRs were adjusted for stage, grade and gender.
The disparity in CIF between overall deaths and bladder cancer deaths
Figure 1 shows the CIFs for all-cause and bladder cancer-specific deaths stratified by age among patients with SBC and IBC. Log-rank analysis revealed a disparity in the CIF between all-cause and bladder cancer-specific deaths for all age groups. This disparity increased as age increased among patients with SBC, but there was no disparity between age groups among patients with IBC.
Figure 1.
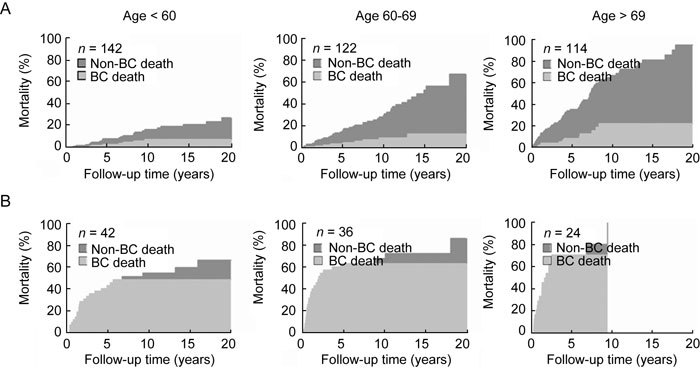
Cumulative incidence of death due to bladder cancer (BC), stratified by age of patients with (A) superficial bladder cancer and (B) invasive bladder cancer.
Figure 2 shows the CIFs for all-cause and bladder cancer-specific deaths stratified by comorbidity status among patients with SBC and IBC. Log-rank analysis revealed a disparity in the CIF between all-cause and bladder cancer-specific deaths among patients in all comorbidity groups. This disparity increased with comorbidity severity among patients with SBC, but there was no disparity between comorbidity groups among patients with IBC.
Figure 2.
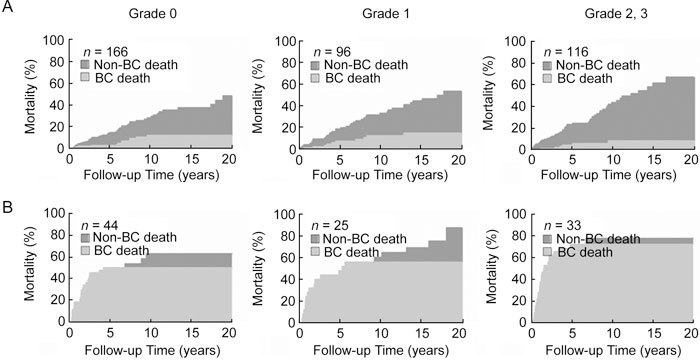
Cumulative incidence of death due to bladder cancer (BC), stratified by comorbidity status in patients with (A) superficial bladder cancer and (B) invasive bladder cancer.
Discussion
The management of any disease is guided by many factors, including the risk of morbidity and mortality from the disease, as well as the likely success of and anticipated morbidity and mortality of the therapy. Age and comorbidity status are important factors to consider in almost all patients with cancer, as they both clearly have an impact on the diagnosis, treatment and consequently, on the outcome of these patients. In oncology, it is well recognized that age and comorbidity are inversely related to the treatment intensity that is delivered 11. However, this is based on the assumption that the assessment of comorbidity and age that clinicians use is accurate, and that both these factors are independently related to long-term patient outcome (that is, survival) and have a direct bearing on management decisions that must be made for the patient's specific type of cancer. Age and comorbidity can impact both OS and BCSS among bladder cancer patients either directly (that is, by causing death of the patient) or indirectly (that is, by limiting treatment options). Increased perioperative complications and less aggressive treatment have both often been considered to be possible links between comorbidity status and patient outcome 12.
In the current study, we sought to determine the impact of age and comorbidity on the survival rates of bladder cancer patients. By multivariate analysis, we found that older age is a predictive factor of worse OS and BCSS among patients with SBC and worse OS among patients with IBC.
The higher mortality rates that we observed among older patients can be explained by several factors. In bladder cancer patients < 40 years of age, tumours tend to have a well-differentiated histology and behave in a more indolent fashion. In addition, in contrast to bladder tumours that occur in older individuals, they tend not to recur 13, 14, 15. Furthermore, the molecular and genetic aberrations that are present in the bladder tumours of younger patients do not share the fairly close correlation with histological grade and clinical behaviour that is seen in the urothelial tumours of middle-aged and elderly patients 15. This may indicate that bladder cancer usually occurs from a series of molecular aberrations, many of which are present as part of a background of genetic instability that occurs with aging, on which the signature genetic abnormalities that have been well described (for example, mutations inactivating p53) arise over time. Thus, by the time patients have reached older age, these signature genetic events would have occurred, leading to more aggressive tumours. In addition, elderly patients have more unfavourable survival within a given disease stage. This may ultimately be a reflection of decreased host immunological and defence factors, more aggressive tumour patterns and greater treatment resistance (for example, the worse tumour response that is seen to intravesical bacillus Calmette-Guérin [BCG] therapy among older patients). Several studies have reported that aging seems to be associated with a decreased response to intravesical immunotherapy, which is particularly marked in patients > 80 years 16, 17.
Another explanation for the higher mortality rates that are seen in older patients is that the diagnosis of bladder cancer is likely to be delayed more often among these patients than it is among younger patients. This factor is probably both physician- and patient-related 18. The elderly also often have multiple medical comorbidities that may influence the treatments that are offered (by medical care providers) or that are eventually chosen (by patients), in that more effective yet often more aggressive treatment options tend not to be chosen 18, 19. In our study, when the group of patients was stratified by age, the proportion of severe comorbidities increased and the incidence of radical cystectomy for the treatment of IBC decreased among older patients. This finding is particularly interesting because when we examined the distribution of pathological stage, we found that it had no difference between the three age groups. Several studies have shown that radical cystectomy can be performed safely in selected older patients with minimal impact on their functional status or morbidity and mortality rates 20, 21. However, age and comorbidity are clearly associated with treatment selection for patients with bladder cancer. Prout et al. 11 evaluated treatment patterns among patients with bladder cancer in the SEER database and found that older patients and patients with a higher comorbidity status were less likely to undergo radical cystectomy than younger, healthier patients. This finding held true even among patients with invasive disease. There were no significant treatment differences noted with regard to age among patients with SBC, but among patients with muscle-invasive disease, individuals who were > 75 years of age were less likely to undergo radical cystectomy (14%) than patients who were 55–64 years of age (48%) or 65–74 years of age (43%) 11. We found similar results in our study. Moreover, in our study, the use of a bladder-preserving approach was predictive of worse OS and BCSS in patients with IBC on multivariate analysis. In addition, the disparity in the CIFs between all-cause deaths and bladder cancer deaths was evident among patients with SBC, and increased with older age and higher comorbidity status. Interestingly, this finding was not present in patients with IBC. We believe that among patients with IBC, radical cystectomy may have a greater effect on patient survival than age and comorbidity, because most of the patients with IBC in our study died of bladder cancer.
As previously mentioned, a more severe comorbidity status was independently associated with an increased risk of all-cause mortality of bladder cancer after controlling for all known confounding factors in our study. Koppie et al. 22 reported that the older age-adjusted Charlson Comorbidity Index score was associated with OS, but not with progression-free survival, in 1 121 patients with bladder cancer who were treated with radical cystectomy (after adjusting for pathological stage and lymph node status). Similarly, Megwalu et al. 23 found that worse comorbidity (as measured by the ACE-27) was associated with worse OS in 675 bladder carcinoma patients (after controlling for age, AJCC stage, histological grade and race). This finding held true for the subset of patients with non-invasive disease and the subset of patients treated with radical cystectomy. In our study on multivariate analysis, moderate–severe comorbidity was predictive of worse OS and BCSS among patients with IBC. The reasons for these disparate findings are not completely clear, but may be related to differences in study populations, study methodology and/or the comorbidity assessment that was used.
There are several limitations to our study. First, it was a single-institution, retrospective study. Another limitation is that the relationship between treatment and outcome was not explored. However, the primary goal of this study was to examine the effect of comorbidity on all patients with newly diagnosed bladder cancer, not the impact it had on a specific subset of patients. The treatments that are delivered for bladder cancer differ dramatically for patients with different stages of disease. They range from local resection alone in patients with low-grade non-invasive disease to chemotherapy in patients with metastatic disease. Treatment is also influenced by patient and physician preference. Because of the complexity involved in assessing treatment effectiveness based on observational data, we were unable to accurately model treatment in this study.
Despite these limitations, our study has certain strengths. Our study population was diverse, as it encompassed patients with all stages of the disease. We did not limit our analysis to one specific disease stage or treatment group. This study, therefore, showed the global effects that age and comorbidity have on bladder cancer outcomes, while also addressing factors that might influence the outcomes of specific subsets of patients. Age and comorbidity were able to independently predict OS and BCSS in bladder cancer patients, and these factors explained the disparity that existed between all-cause mortality and bladder cancer-specific mortality in this cohort. Therefore, both tumour-specific variables and patient-specific variables (for example, age and comorbidity) should be evaluated in bladder cancer patients when treatment decisions are being made. In addition, both these types of variables are critical to the appropriate design and implementation of clinical trials evaluating bladder cancer treatments.
Acknowledgments
This research was supported by the Chung-Ang University research grants in 2010, Korea.
References
- Millan-Rodriguez F, Chechile-Toniolo G, Salvador-Bayarri J, Palou J, Vicente-Rodriguez J. Multivariate analysis of the prognostic factors of primary superficial bladder cancer. J Urol. 2000;163:73–8. doi: 10.1016/s0022-5347(05)67975-x. [DOI] [PubMed] [Google Scholar]
- Stein JP, Lieskovsky G, Cote R, Groshen S, Feng AC, et al. Radical cystectomy in the treatment of invasive bladder cancer: long-term results in 1,054 patients. J Clin Oncol. 2001;19:666–75. doi: 10.1200/JCO.2001.19.3.666. [DOI] [PubMed] [Google Scholar]
- Surveillance, Epidemiology, and End Results (SEER) Program SEER Stat Database: incidence. SEER 13 Registered Public Use, November 2004 Submission (1973–2000).
- Ries LAG, Eisner MP, Kosary CL.SEER Cancer Statistics Review, 1973–1999Bethesda: National Cancer Institute; 2002
- Steineck G, Hagman U, Gerhardsson M, Norell SE. Vitamin A supplements, fried foods, fat and urothelial cancer. A case-referent study in Stockholm in 1985–87. Int J Cancer. 1990;45:1006–11. doi: 10.1002/ijc.2910450604. [DOI] [PubMed] [Google Scholar]
- Wingo PA, Ries LA, Giovino GA, Miller DS, Rosenberg HM, et al. Annual report to the nation on the status of cancer, 1973–1996, with a special section on lung cancer and tobacco smoking. J Natl Cancer Inst. 1999;91:675–90. doi: 10.1093/jnci/91.8.675. [DOI] [PubMed] [Google Scholar]
- Yusuf S, Hawken S, Ounpuu S, Bautista L, Franzosi MG, et al. Obesity and the risk of myocardial infarction in 27,000 participants from 52 countries: a case-control study. Lancet. 2005;366:1640–9. doi: 10.1016/S0140-6736(05)67663-5. [DOI] [PubMed] [Google Scholar]
- Piccirillo JF, Tierney RM, Costas I, Grove L, Spitznagel EL., Jr Prognostic importance of comorbidity in a hospital-based cancer registry. JAMA. 2004;291:2441–7. doi: 10.1001/jama.291.20.2441. [DOI] [PubMed] [Google Scholar]
- Epstein JI, Amin MB, Reuter VR, Mostofi FK. The World Health Organization/International Society of Urological Pathology consensus classification of urothelial (transitional cell) neoplasms of the urinary bladder. Bladder Consensus Conference Committee. Am J Surg Pathol. 1998;22:1435–48. doi: 10.1097/00000478-199812000-00001. [DOI] [PubMed] [Google Scholar]
- Bergkvist A, Ljungqvist A, Moberger G. Classification of bladder tumours based on the cellular pattern. Preliminary report of a clinical-pathological study of 300 cases with a minimum follow-up of eight years. Acta Chir Scand. 1965;130:371–8. [PubMed] [Google Scholar]
- Prout GR, Jr, Wesley MN, Yancik R, Ries LA, Havlik RJ, et al. Age and comorbidity impact surgical therapy in older bladder carcinoma patients: a population-based study. Cancer. 2005;104:1638–47. doi: 10.1002/cncr.21354. [DOI] [PubMed] [Google Scholar]
- Derks W, de Leeuw RJ, Hordijk GJ. Elderly patients with head and neck cancer: the influence of comorbidity on choice of therapy, complication rate, and survival. Curr Opin Otolaryngol. 2005;13:92–6. doi: 10.1097/01.moo.0000156169.63204.39. [DOI] [PubMed] [Google Scholar]
- Benson RC, Jr, Tomera KM, Kelalis PP. Transitional cell carcinoma of the bladder in children and adolescents. J Urol. 1983;130:54–5. doi: 10.1016/s0022-5347(17)50950-7. [DOI] [PubMed] [Google Scholar]
- Fitzpatrick JM, Reda M. Bladder carcinoma in patients 40 years old or less. J Urol. 1986;135:53–4. doi: 10.1016/s0022-5347(17)45513-3. [DOI] [PubMed] [Google Scholar]
- Linn JF, Sesterhenn I, Mostofi FK, Schoenberg M. The molecular characteristics of bladder cancer in young patients. J Urol. 1998;159:1493–6. doi: 10.1097/00005392-199805000-00022. [DOI] [PubMed] [Google Scholar]
- Joudi FN, Smith BJ, O'Donnell MA, Konety BR.The impact of age on the response of patients with superficial bladder cancer to intravesical immunotherapy J Urol 20061751634–9.discussion 9–40. [DOI] [PubMed] [Google Scholar]
- Heiner JG, Terris MK. Effect of advanced age on the development of complications from intravesical bacillus Calmette-Guerin therapy. Urol Oncol. 2008;26:137–40. doi: 10.1016/j.urolonc.2007.04.005. [DOI] [PubMed] [Google Scholar]
- Wymenga AN, Slaets JP, Sleijfer DT. Treatment of cancer in old age, shortcomings and challenges. Neth J Med. 2001;59:259–66. doi: 10.1016/s0300-2977(01)00160-7. [DOI] [PubMed] [Google Scholar]
- Miller DC, Taub DA, Dunn RL, Montie JE, Wei JT. The impact of co-morbid disease on cancer control and survival following radical cystectomy. J Urol. 2003;169:105–9. doi: 10.1016/S0022-5347(05)64046-3. [DOI] [PubMed] [Google Scholar]
- Farnham SB, Cookson MS, Alberts G, Smith JA, Jr, Chang SS. Benefit of radical cystectomy in the elderly patient with significant co-morbidities. Urol Oncol. 2004;22:178–81. doi: 10.1016/j.urolonc.2003.12.005. [DOI] [PubMed] [Google Scholar]
- Hollenbeck BK, Miller DC, Taub D, Dunn RL, Underwood W, III, et al. Aggressive treatment for bladder cancer is associated with improved overall survival among patients 80 years old or older. Urology. 2004;64:292–7. doi: 10.1016/j.urology.2004.03.034. [DOI] [PubMed] [Google Scholar]
- Koppie TM, Serio AM, Vickers AJ, Vora K, Dalbagni G, et al. Age-adjusted Charlson comorbidity score is associated with treatment decisions and clinical outcomes for patients undergoing radical cystectomy for bladder cancer. Cancer. 2008;112:2384–92. doi: 10.1002/cncr.23462. [DOI] [PubMed] [Google Scholar]
- Megwalu II, Vlahiotis A, Radwan M, Piccirillo JF, Kibel AS. Prognostic impact of comorbidity in patients with bladder cancer. Eur Urol. 2008;53:581–9. doi: 10.1016/j.eururo.2007.10.069. [DOI] [PMC free article] [PubMed] [Google Scholar]


