Abstract
In this retrospective study, we evaluated and compared the efficacy and toxicities of maximal androgen blockade (MAB) versus castration alone in Chinese patients with advanced prostate cancer. From 1996 to 2004, 608 patients with advanced prostate cancer were included in the study. Patients were retrospectively divided into two groups according to different therapeutic regimens. Of the 608 patients, 300 patients were treated with MAB (castration plus nonsteroidal antiandrogens) and the remaining 308 were treated with castration alone. The 2- and 5-year overall survival rates of these patients were 73.7% and 56%, respectively. Multivariate analysis showed that, in patients with metastatic prostate cancer, MAB was associated with not only the improvement of progression-free survival (PFS) (increased by 10 months) but also a 20.6% reduction in mortality risk compared with castration alone. In contrast, the efficacy of MAB was not superior to castration alone for patients with nonmetastatic prostate cancer. Interestingly, among patients with MAB, those using bicalutamide had a longer PFS than those using flutamide; this was especially so in patients with metastatic prostate cancer. Almost all of the toxicities due to the hormone therapy were mild to moderate and manageable. To conclude, in China, hormone therapies, including MAB and castration alone, have been standard treatments for advanced prostate cancer. For patients with nonmetastatic prostate cancer, castration alone might be adequately practical and efficient. In patients with metastatic prostate cancer, however, MAB has superior efficacy over castration alone. It is clear that MAB should be considered the first-line standard treatment for patients with metastatic prostate cancer.
Keywords: bicalutamide, castration alone, maximal androgen blockade, prostate cancer
Introduction
Prostate cancer is a significant burden on the health of elder men. It is most frequently diagnosed in Western countries, and it is one of the first two leading causes of cancer-related mortality for Westerners 1. Owing to the increased application of prostate-specific antigen (PSA) screening, changes in dietary habits and aging of the population, the incidence of prostate cancer has increased sharply in China; however, the geographical distribution and prevalence of the disease is imbalanced. Compared with eastern regions of China, many more patients with advanced prostate cancer have been diagnosed in western regions in the past several decades. As Huggins et al. 2 showed that castration resulted in the regression of prostate cancer, androgen deprivation therapy, including castration alone and maximal androgen blockade (MAB), has been the mainstay of the treatment for advanced prostate cancer when radical therapy is impossible. However, whereas the treatment usually attains an initial regression in many patients with advanced prostate cancer, the duration of this regression is limited to only 18–36 months 3. After this period, most of the tumors progress to become androgen refractory, leading to death in 9–12 months 4. MAB, which was first described early in 1982 5, is composed of a nonsteroidal or steroidal anti-androgen and medical or surgical castration. To date, the use of MAB over castration alone has been widely debated because of conflicting efficacy data from individual clinical trials, as well as tolerability and cost issues 6, 7, 8, 9. The effect of androgen deprivation therapy on advanced prostate cancer has been observed; however, the exact efficacy of either MAB or castration alone in the treatment of Chinese patients has not been summarized systemically.
In the present retrospective study, clinical data from patients in West China Hospital who had advanced prostate cancer and received either MAB or castration alone were collected and analysed. We evaluated the efficacy of androgen deprivation therapy in the treatment of advanced prostate cancer in China and compared the efficacy and toxicity of MAB and castration alone.
Materials and methods
Patients and follow-up
Between January 1996 and December 2004, 722 patients treated at the West China Hospital were considered for evaluation. All the patients were histologically documented, previously untreated and had advanced (clinical staging 3 or 4) prostate cancer with different levels of PSA. Patients were retrospectively classified according to therapeutic regimen, that is, into the MAB group or the castration alone group. Patients in the MAB group received castration plus nonsteroidal anti-androgens (NSAAs) until the disease progressed.
Detailed follow-up data were available for 608 prostate cancer patients, including 300 patients treated with MAB and 308 patients treated with castration alone. At the final cutoff day for analysis, the median duration of follow-up was 40 months (ranging from 2 to 99 months, mean 40.8 months). The clinical history of cancer, biopsy data, Gleason score and clinical staging at diagnosis had been recorded. Serum PSA had been measured at the beginning of hormonal therapy and monitored regularly during treatment (Table 1).
Table 1. Initial characteristics of patients treated with endocrine therapy.
| Characteristics | Patients, n (%) |
||
|---|---|---|---|
| All patients (n = 608) | MAB group (n = 300) | Castration alone group (n = 308) | |
| Age (years) | |||
| ≤ 70 | 220 (36.2) | 106 (35.3) | 114 (37.0) |
| > 70 | 388 (63.8) | 194 (64.7) | 194 (63.0) |
| PSA at initial (ng mL−1) | |||
| ≤ 50 | 284 (46.7) | 130 (43.3) | 154 (50.0) |
| > 50 | 324 (53.3) | 170 (56.7) | 154 (50.0) |
| Gleason score | |||
| ≤ 6 | 40 (6.6) | 6 (2.0) | 34 (11.0) |
| 7 | 188 (30.9) | 82 (27.3) | 106 (34.4) |
| 8–10 | 380 (62.5) | 212 (70.7) | 168 (54.6) |
| Metastastic status | |||
| M0 | 360 (59.2) | 176 (58.7) | 184 (59.7) |
| M1 | 248 (40.8) | 124 (41.3) | 124 (40.3) |
| T-staging | |||
| ≤ T3 | 228 (37.5) | 94 (31.3) | 134 (43.5) |
| T4 | 380 (62.5) | 206 (68.7) | 174 (56.5) |
| ECOG score | |||
| 0–1 | 566 (93.1) | 276 (92.0) | 290 (94.2) |
| ≥ 2 | 42 (6.9) | 24 (8.0) | 18 (5.8) |
Abbreviations: ECOG, eastern cooperative oncology group; M0, nonmetastatic prostate cancer group; M1, metastatic prostate cancer group; MAB, maximal androgen blockade; PSA, prostate specific antigen.
Treatments
Castration alone was achieved through medical or surgical techniques, by the use of a Luteinizing-hormone-releasing hormone (LHRH) agonist (Goserelin acetate; 3.6 mg subcutaneous injection every 28 days) or bilateral orchiectomy. When patients were treated with medical castration alone, oral NSAAs (bicalutamide or flutamide) were simultaneously given for 4 weeks to prevent a probable flare reaction. MAB was defined as castration plus NSAAs (bicalutamide [50 mg; once daily] or flutamide [250 mg; thrice daily]. If the duration of castration combined with NSAAs was less than 3 months, the regimen was classified as castration alone.
Assessment of efficacy and toxicity
Two end points were evaluated: progression-free survival (PFS) time and overall survival (OS) time. Toxicities were also evaluated. Disease progression was defined as any of the following during the initial hormone therapy: the appearance of one or more new bone metastases on bone scan, attributable to metastatic disease; evidence of worsening of any existing bone metastasis on bone scan, attributable to metastatic disease; the appearance of one or more new extra-skeletal metastases or an increase by 20% or more (compared with maximal diameter recorded before treatment) of any existing extra-skeletal metastasis; the elevation of serum PSA level (three consecutive tests of rising serum PSA or post-treatment PSA level over 4 ng mL−1); and death from disease. The end point for analysing patients in the study was 31 December 2008. During the follow-up time, disease progression occurred in 348 patients and 256 patients died. Toxicities were also evaluated according to the common toxicity criteria of the National Cancer Institute, China.
Statistical analysis
Treatment outcome was assessed as OS and PFS rates using the Kaplan–Meier method, and it was correlated with age, clinical staging, Gleason score, basal level of serum PSA, PSA variation after treatment, therapeutic regimen and metastatic status. The Mann–Whitney U-test, χ2-test, Spearman's correlation test and log-rank test were also used for analysis of relationship and difference among variables, as appropriate, with P < 0.05 considered to indicate statistical significance in all tests. Cox's proportional hazard regression model was used for the identification of disease and patient variables that were most correlated to the end points. All of the analyses were performed with SPSS v.13.0 (SPSS Inc., Chicago, IL, USA).
Results
Patient characteristics and disease status before therapy
Of the 300 patients in the MAB group, over 80% (242/300) of the patients had castration by bilateral orchiectomy, 58 patients were given medical castration. In total, 144 patients were treated with castration plus flutamide and the remaining 156 patients were treated with castration plus bicalutamide. In the castration alone group, 261/308 patients (84.7%) had an orchiectomy and the left 47 were given medical castration. Of the 261 patients who underwent surgical castration, 102 were given NSAAs simultaneously for less than 3 months. Details of the pre-treatment demographic characteristics are shown in Table 1. All baseline characteristics between the two groups were similar.
Progression-free survival
PFS in patients with advanced prostate cancer
Overall, disease progression occurred in 50% of patients in the MAB group (150/300) and in 64.3% of patients in the castration alone group (198/308) (λ2 = 12.67, P < 0.001). Univariate analysis showed that patients in the MAB group benefited from a longer PFS time than those in the castration alone group (49.39 ± 14.88 vs. 44.30 ± 13.41 months; P = 0.037). Compared with the castration alone group, the median PFS time of the MAB group had an absolute increase of 6 months from 45.5 to 51.5 months (Figure 1E).
Figure 1.
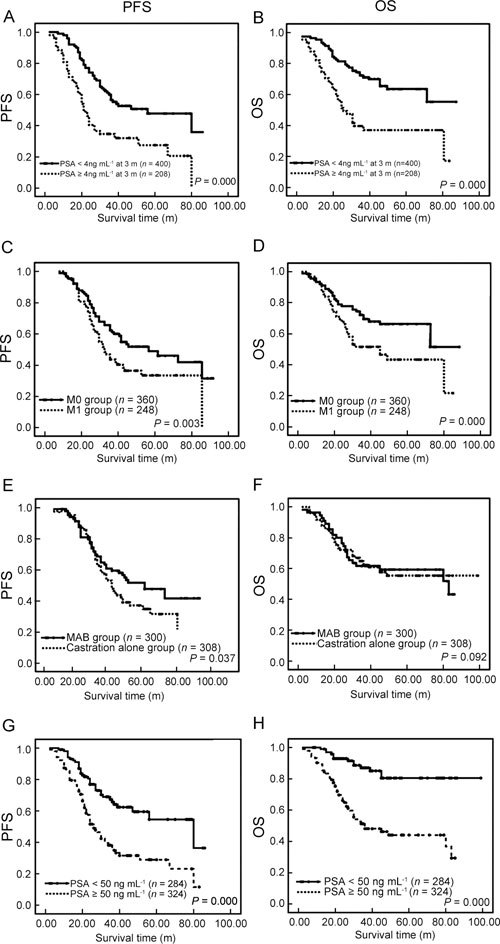
Association of clinical and pathological variables with survival in patients with advanced prostate cancer. Kaplan–Meier survival analysis of progression-free survival (PFS, left array) and overall survival (OS, right array) for PSA variation within 3 months after treatment (A and B), metastatic status (C and D), regimens of hormone therapy (E and F) and basal level of serum PSA (G and H). Log-rank test P-values are listed for each parameter.
Abbreviations: m, month; M0, nonmetastatic prostate cancer group; M1, metastatic prostate cancer group.
The association of PFS with other clinical variables of patients, including age, clinical staging, Gleason score, metastatic status, pre-treatment serum PSA level and PSA variation after treatment, was also investigated (Table 2 and Figures 1A, C, G). Age, clinical staging and Gleason scores were not associated with disease progression. A higher basal level of serum PSA at the initial time of diagnosis (PSA ≥ 50 ng mL−1) was strongly associated with poorer PFS time in all patients. Obviously, patients with metastatic prostate cancer (M1) had a shorter PFS time than those with nonmetastatic prostate cancer (M0) (41.07 ± 15.47 vs. 51.67 ± 17.99 months; P = 0.003, Table 2 and Figure 1C). It was highlighted that PSA normalization at 3 months after endocrine treatment (defined as patients with PSA < 4 ng mL−1) was associated with a much longer PFS time (54.39 ± 18.91 vs. 34.73 ± 16.66 months, respectively; P = 0.000, Table 2 and Figure 1A).
Table 2. Univariate analysis of survival in patients with advanced prostate cancer.
| Grouping | Cutoff value | n | OS (months) (mean ± SD) | P (log-rank test) | PFS (months) (mean ± SD) | P (log-rank test) |
|---|---|---|---|---|---|---|
| Age (years) | < 70 | 220 | 59.12 ± 14.07 | 0.908 | 47.19 ± 15.71 | 0.796 |
| ≥ 70 | 388 | 65.37 ± 16.95 | 47.70 ± 15.08 | |||
| Basal PSA level (ng mL−1) | < 50 | 284 | 83.42 ± 19.71 | 0.000 | 60.39 ± 21.14 | 0.000 |
| ≥ 50 | 324 | 45.56 ± 14.67 | 35.90 ± 12.45 | |||
| Clinical staging | ≤ 3 | 228 | 66.47 ± 16.37 | 0.980 | 48.77 ± 16.76 | 0.346 |
| = 4 | 380 | 63.98 ± 15.28 | 46.67 ± 14.13 | |||
| Gleason score | < 8 | 228 | 59.87 ± 17.01 | 0.322 | 51.61 ± 18.77 | 0.071 |
| ≥ 8 | 380 | 62.41 ± 15.39 | 44.17 ± 15.46 | |||
| Metastasis | M0 | 360 | 71.69 ± 18.58 | 0.000 | 51.67 ± 17.99 | 0.003 |
| M1 | 248 | 49.10 ± 13.82 | 41.07 ± 15.47 | |||
| Therapeutic regimens | Castration alone | 308 | 61.23 ± 16.55 | 0.092 | 44.30 ± 13.41 | 0.037 |
| MAB | 300 | 61.45 ± 14.08 | 49.39 ± 14.88 | |||
| PSA after 3 months (ng mL−1) | < 4 | 400 | 71.34 ± 18.36 | 0.000 | 54.39 ± 18.91 | 0.000 |
| ≥ 4 | 208 | 43.17 ± 14.32 | 34.73 ± 16.66 |
Abbreviations: M0, nonmetastatic prostate cancer group; M1, metastatic prostate cancer group; MAB, maximal androgen blockade; OS, overall survival; PFS, progression-free survival; PSA, prostate specific antigen.
PFS in patients with metastatic prostate cancer
On the basis of the status of metastasis, patients were further classified into metastatic prostate cancer group (M1) (n = 248) and nonmetastatic prostate cancer group (M0) (n = 360). In the M1 group, basal PSA level, PSA normalization 3 months after treatment and selection of MAB were correlated with favourable progression of disease (Table 3). The PFS time of patients with MAB in the M1 group was almost 10 months longer than of those with castration alone (P = 0.014) (Figure 2A). Further analysis revealed that, within the M1 group, there were 156/248 patients with a basal level of serum PSA over 50 ng mL−1 at initial diagnosis; they could much more predominantly benefit from treatment with MAB than castration alone (Data not shown). Otherwise, in the M0 group, compared with castration alone, MAB could not statistically improve PFS (P = 0.096) (Figure 2C).
Table 3. Univariate analysis of survival in patients with metastatic prostate cancer (M1).
| Grouping | Cutoff value | n | OS (months) (mean ± SD) | P (log-rank test) | PFS (months) (mean ± SD) | P (log-rank test) |
|---|---|---|---|---|---|---|
| Age (years) | < 70 | 112 | 50.01 ± 15.94 | 0.163 | 41.94 ± 14.54 | 0.899 |
| ≥ 70 | 136 | 47.13 ± 13.12 | 37.17 ± 16.06 | |||
| Basal PSA level (ng mL−1) | < 50 | 92 | 63.01 ± 17.44 | 0.000 | 53.48 ± 17.26 | 0.000 |
| ≥ 50 | 156 | 39.93 ± 14.52 | 32.23 ± 13.77 | |||
| T-staging | ≤ T3 | 98 | 54.22 ± 16.25 | 0.126 | 38.82 ± 14.51 | 0.203 |
| = T4 | 150 | 46.18 ± 14.77 | 39.12 ± 13.92 | |||
| Gleason score | < 8 | 100 | 51.22 ± 14.01 | 0.115 | 38.14 ± 15.12 | 0.324 |
| ≥ 8 | 148 | 48.27 ± 13.11 | 39.44 ± 14.71 | |||
| Therapeutic regimens | Castration alone | 124 | 45.26 ± 17.15 | 0.006 | 34.48 ± 14.95 | 0.014 |
| MAB | 124 | 51.49 ± 16.83 | 44.49 ± 15.44 | |||
| PSA after 3 months (ng mL−1) | < 4 | 148 | 56.17 ± 17.44 | 0.000 | 42.41 ± 17.15 | 0.000 |
| ≥ 4 | 100 | 40.02 ± 13.97 | 33.39 ± 15.45 |
Abbreviations: MAB, maximal androgen blockade; PSA, prostate specific antigen; OS, overall survival; PFS, progression-free survival.
Figure 2.
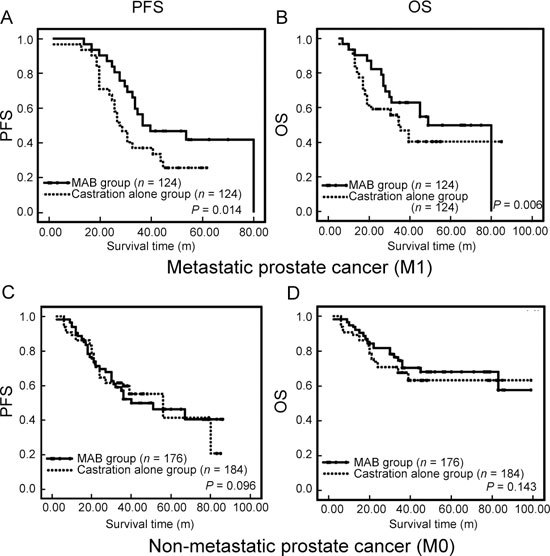
Comparison of two different hormone therapeutic regimens (castration alone versus MAB) in the treatment of patients with metastatic or nonmetastatic prostate cancer. (A), (B): the association of different regimens with PFS and OS in patients with metastatic prostate cancer, respectively. (C), (D): represent the association of different regimens with progression-free survival and overall survival in patients with nonmetastatic prostate cancer, respectively. Abbreviations: m, month; OS, overall survival; PFS, progression-free survival.
Overall survival
The 2-year OS rate of the 608 patients was 73.7% and the 5-year rate decreased to 56.0%. Mean PFS and OS time periods were 47.43 ± 14.47 and 64.38 ± 16.22 months, respectively. Subanalysis showed that the 2-year OS rate of patients with metastatic prostate cancer was lower than that of patients with nonmetastatic prostate cancer (69.8% vs. 77.1%, respectively); the 5-year survival rate of the metastatic prostate cancer group decreased to only 41.4% (compared with 66.3% in nonmetastatic prostate cancer group).
OS in patients with advanced prostate cancer
The mortality of patients treated with MAB was 38.0% (114/300), compared with 46.1% (142/308) patients in the castration alone group (λ2 = 4.694, P = 0.049). Although MAB was associated with favourable PFS time, OS findings were similar between the two groups (61.23 ± 16.55 vs. 61.45 ± 14.08 months, respectively; hazards ratio [HR] 0.957; 95% confidence interval [CI] 0.719–1.131; P = 0.092) (Figure 1F).
The univariate analysis showed that OS was obviously associated with the metastatic status and basal PSA level of patients with prostate cancer (Figures 1D and H). We should pay close attention to the fact that the presence or absence of PSA normalization (< 4 ng mL−1) within 3 months after treatment could also intensively predict the outcome of disease (71.34 ± 18.36 vs. 43.17 ± 14.32 months, respectively; P < 0.001) (Table 2 and Figure 1B). Subgroup analysis suggested that if the PSA level did not decrease to 4 ng mL−1 within 3 months of treatment, then even MAB was not superior to castration alone in prolonging OS time (45.31 ± 16.28 vs. 41.91 ± 13.13 months; P = 0.100). In addition, age, clinical staging and Gleason scores were not considered prognostic factors for advanced prostate cancer.
OS in patients with metastatic prostate cancer
In the present study, 120/360 patients (33.3%) with nonmetastatic prostate cancer (M0) died, whereas 136/248 patients (54.8%) with metastatic prostate cancer (M1) died. Compared with castration alone, MAB could remarkably improve the OS among patients with metastatic prostate cancer (M1) (51.49 ± 16.83 vs. 45.26 ± 17.15 months, respectively; HR 0.794; 95% CI 0.627–0.954; P = 0.006) (Table 3 and Figure 2B). In contrast, patients with nonmetastatic prostate cancer could not benefit from MAB (HR 1.373, 95% CI 1.053–1.651, P = 0.143) (Figure 2D).
Subgroup analysis confirmed that, among patients with metastatic prostate cancer, 87/124 patients (70.2%) receiving MAB achieved PSA normalization within 3 months, while only 62/124 (50%) patients receiving castration alone gained normal PSA level within 3 months. Furthermore, analysis of combined PSA data from the group of metastatic prostate cancer patients showed longer median OS time among patients with a higher level of basal PSA (higher than 50 ng mL−1) compared with patients with lower PSA level (41 vs. 33 months, respectively; P = 0.010).
Results of multivariate survival analysis
Multivariate analysis by Cox proportional hazard regression showed that in patients with advanced prostate cancer, PSA normalization within 3 months of treatment was an independent prognostic factor, not only for PFS (RR = 2.379, 95% CI = 1.614–3.507, P = 0.009) but also for OS (RR = 2.699, 95% CI = 1.731–4.206, P = 0.004). It is worthy of noting that, except for PSA normalization, MAB was another independent prognostic factor for PFS in patients with metastatic prostate cancer (RR = 1.617, 95% CI = 1.131–2.311, P = 0.048).
Efficacy of different MAB combinations in the treatment of advanced prostate cancer
Although the difference was not statistically significant, patients treated with castration plus bicalutamide (n = 156) had a longer PFS than those using flutamide (n = 144) (47.55 ± 16.74 vs. 41.22 ± 14.75 months, respectively; P = 0.054). However, the OS between these two subgroups was similar (62.23 ± 17.31 vs. 60.77 ± 14.28 months, respectively; P = 0.098). Remarkably, if patients with nonmetastatic prostate cancer were excluded from MAB group, the difference in PFS between patients with castration plus bicalutamide (n = 88) and castration plus flutamide (n = 56) were statistically significant (45.24 ± 15.69 vs. 38.85 ± 15.21 months; HR 0.873; 95% CI 0.656–1.234; P = 0.045). Furthermore, although without statistical significance, patients with metastatic prostate cancer treated with castration plus bicalutamide indeed had a longer OS time than those with flutamide (54.64 ± 17.05 vs. 47.80 ± 16.44 months; HR 0.898; 95% CI 0.546–1.475; P = 0.103). The analysis showed that there was no difference between medical castration and surgical castration in the aspect of efficacy.
Tolerability and toxicity
Overall, adverse events associated with treatment, either MAB or castration alone, were well tolerated and reversible. No treatment-related death was recorded and severe adverse events (grade 3 or 4) seldom occurred. The common, frequently reported adverse events in both groups were hot flushes (41.3% and 42.8%) and fatigue (29.0% and 27.3%). The incidence of diarrhoea, constipation, nausea or vomiting, haematuria, visual disorders and hepatic dysfunction was higher in the MAB group than in the castration alone group (Table 4). When the MAB group was classified into subgroups, castration plus bicalutamide and castration plus flutamide, the incidence of adverse events between subgroups was quite different. The incidence of gastrointestinal symptoms, including diarrhoea, nausea, vomiting and increased alanine transaminase/aspartate aminotransferase, was predominantly higher in the castration plus flutamide subgroup, whereas haematuria and visual disorders were more common in the bicalutamide subgroup (Figure 3).
Table 4. Incidence of common adverse events among patients with advanced prostate cancer receiving either MAB or castration alone.
| All grades in MAB group (%)/grade ≥ 3 |
All grades in castration alone group (%)/grade ≥ 3 (n = 308) | |||
|---|---|---|---|---|
| Bicalutamide subgroup (n = 156) | Flutamide subgroup (n = 144) | Total (n = 300) | ||
| Hot flushes | 63 (40.4)/10 | 61 (42.7)/7 | 124 (41.3)/17 | 132 (42.8)/13 |
| Fatigue | 47 (30.1)/4 | 40 (27.7)/5 | 87 (29.0)/9 | 84 (27.3)/7 |
| Diarrhoea | 17 (10.9)/6 | 37 (25.7)/4 | 54 (18.0)/10 | 17 (5.5)/2 |
| Increased ALT/AST | 16 (10.3)/0 | 39 (27.1)/2 | 54 (18.0)/2 | 24 (7.8)/0 |
| Back pain | 25 (16.0)/5 | 22 (15.3)/6 | 47 (15.6)/11 | 37 (12)/6 |
| Nausea/vomiting | 17 (10.9)/3 | 28 (19.4)/6 | 45 (15.0)/9 | 25 (8.1)/1 |
| Constipation | 25 (16.0)/3 | 15 (10.4)/1 | 40 (13.3)/4 | 23 (7.5)/1 |
| Dyspnea | 21 (13.5)/0 | 19 (13.2)/0 | 40 (13.3)/0 | 35 (11.4)/0 |
| Abdominal pain | 13 (8.3)/0 | 20 (13.9)/0 | 33 (11.0)/0 | 19 (6.2)/0 |
| Anaemia | 14 (9.0)/0 | 11 (7.6)/0 | 25 (8.3)/0 | 25 (8.1)/0 |
| Haematuria | 12 (7.7)/1 | 2 (1.4)/0 | 14 (4.7)/1 | 4 (1.3)/1 |
| Visual disorder | 8 (5.1)/1 | 2 (1.4)/0 | 10 (3.3)/0 | 0 (0.0)/0 |
Abbreviations: ALT, alanine aminotransferase; AST, aspartate aminotransferase; MAB, maximal androgen blockade.
Figure 3.
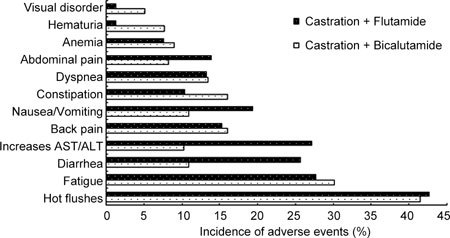
Comparison of the incidence of common adverse events between patients with advanced prostate cancer treated with castration plus flutamide and castration plus bicalutamide. ALT, alanine aminotransferase; AST, aspartate aminotransferase.
Discussion
From the early 1980s, over 30 randomized clinical trials have been performed to evaluate the efficacy of MAB 10. These trials used flutamide, nilutamide or cyproterone acetate as the anti-androgen combined with surgical or medical castration. Among these trials, three large randomized controlled trials (RCT) showed the significant survival advantage of MAB over castration alone in the treatment of metastatic prostate cancer 11, 12, 13. However, other trials did not find a superior advantage of MAB. To evaluate the role of MAB on advanced prostate cancer deeply and thoroughly, further systematic review and meta-analysis of data pooled from the RCTs compared the difference between MAB and castration alone in the treatment of advanced prostate cancer 6, 8, 9, 14. Most of the meta-analyses revealed a small but statistically significant survival difference among patients with MAB compared with castration alone, which confirmed the survival benefit of this kind of regimen; however, combination therapy led to higher medical expense and, inevitably, more adverse effects.
In the present retrospective study, the 5-year OS was 56%, and subgroup analysis for patients with metastatic prostate cancer showed that the 5-year OS decreased to 41.4%. Nevertheless, compared with data from PCTCG 6, OS in our group with metastatic prostate cancer was still much higher (41.4% vs. 27.6%). Hazard model analysis showed that patients with metastatic prostate cancer could benefit significantly more from MAB than patients with nonmetastatic prostate cancer. MAB treatment of metastatic prostate cancer was associated with a 20.6% reduction in mortality risk compared with castration alone. A recent clinical trial from Japan has reported the superior effect of MAB in Japanese patients with prostate cancer 15. In the present study, Chinese patients seemed to have similar results to Japanese patients. The possible reasons for our superior results are explored and discussed. First, although there is a lack of evidence in the current literature, we hypothesized that Asian men with metastatic prostate cancer might be more sensitive to androgen deprivation therapy than Western men. Second, in our study, most of the patients were given a bilateral orchiectomy rather than medical castration. Whether surgical castration has a more persistent and efficient androgen deprivation effect should be studied. Our data strongly support the recommendation of MAB for patients with metastatic prostate cancer, whereas for patients with nonmetastatic prostate cancer, the efficacy of MAB was not superior to that of castration alone.
In the present study, the relationship between several clinical and pathological variables and outcomes was analyzed. Univariate and multivariate analyses showed that, among these variables, metastatic status, basal level of serum PSA and speed of PSA normalization within 3 months of treatment were strongly associated with prognosis of the disease. Adequate understanding of these predictive factors could undoubtedly be applied to early intervention and timely evaluation for suitable patients. These factors could also help clinicians determine the most efficacious therapeutic regimens.
It is worth noting that because most studies of combined therapy versus castration alone were conducted before the availability of bicalutamide, few meta-analyses or randomized clinical trials have evaluated the role of bicalutamide. To estimate the benefit of bicalutamide in combined therapy, Klotz et al. 16 used data from the PCTCG meta-analysis for flutamide plus castration versus castration alone (HR 0.92; 95% CI 0.86–0.98) to calculate an estimate of the likely benefit of bicalutamide combined therapy versus castration alone (HR 0.80; 95% CI 0.66–0.98). The estimate suggested the probable beneficial advantage of MAB using bicalutamide over MAB using flutamide. Recently, a randomized clinical trial from Japan updated its previous study results: MAB using bicalutamide offered a significant OS benefit compared with LHRH agonist alone in patients with metastatic prostate cancer, and even in locally advanced prostate cancer 17. In the present study, patients within the MAB group were treated either with castration plus bicalutamide (n = 156) or with castration plus flutamide (n = 144). To further evaluate the efficacy of these two regimens of MAB, we attempted to compare PFS and OS time between patients with MAB using flutamide and patients with MAB using bicalutamide. Although the total number of our patients was relatively small, patients with MAB using bicalutamide had a much longer PFS than those using flutamide (45.24 ± 15.69 vs. 38.85 ± 15.21 months). Although it was not statistically significant, the mean OS time of patients with MAB using bicalutamide was increased by almost half a year. Again, this result revealed the superior efficacy of bicalutamide in combined hormone therapy.
In fact, as bicalutamide was approved by the Food and Drug Administration as one of the NSAAs in the treatment of prostate cancer, several relative studies have elucidated the fact that it has more potential advantages than other nonsteroidal anti-androgens, such as flutamide and nilutamide. First, pharmacokinetic studies have illuminated that bicalutamide has a fourfold greater affinity for the androgen receptor than flutamide and nilutamide 18. Second, basic studies have already confirmed that the androgen receptor signalling pathway is not exclusively activated by androgen ligands, but can also be activated by nonsteroidal growth factors, cytokines and other non-ligand-dependent molecules 19. These include interleukin-6 and interleukin-10, insulin-like growth factor, epidermal growth factor and signal transduction factors such as protein kinase A 20. Preclinical data have shown that, among nonsteroidal anti-androgens, bicalutamide is a better candidate potential inhibitor for these androgen-independent factors. Furthermore, bicalutamide was found to interact more avidly with AR nuclear coactivators, cosuppressors and androgen-regulated genes, which have an important role in the activation of the androgen signaling pathway 21, 22.
In regard to toxicity, adverse events between bicalutamide and flutamide were also evaluated in the present study. Overall, compared with that in patients with castration alone, the incidence of adverse events was higher in patients with MAB. When the incidence of adverse events was compared for patients treated with castration plus bicalutamide and for those treated with castration plus flutamide, the incidence of adverse events of the bicalutamide group was slightly lower than that of the flutamide group. Gastrointestinal symptoms, including diarrhoea, nausea, vomiting and hepatic dysfunction, commonly occurred in patients with flutamide. Although incidences of haematuria and visual disorders were relatively low, they almost exclusively occurred in patients with bicalutamide. Generally, most of the adverse events induced by androgen deprivation were mild to moderate, tolerated, reversible and manageable.
Conclusions
In summary, this is the first time that the efficacy of hormone therapy for advanced prostate cancer has been reported on a large-scale retrospective study in China. Patients with advanced prostate cancer in this study seemed to be more sensitive to hormone therapies than Western patients. For patients with metastatic prostate cancer, first-line treatment with a combination of NSAAs (including flutamide and bicalutamide) and surgical or medical castration could provide superior efficacy over castration alone in terms of achieving PSA normalization within 3 months, PFS and OS. On the other hand, for patients with nonmetastatic prostate cancer, if the cost–efficacy ratio is considered, castration alone might be adequately practical and efficient until the disease progresses to metastasis.
Although this is a retrospective study, these results should contribute to the choice of treatment by Chinese clinicians. A larger sample and a longer time of follow-up would be expected to disclose the real status of hormone therapy for advanced prostate cancer in Chinese patients.
Acknowledgments
We thank Professor Qiao Zhou from the Department of Pathology, West China Hospital, Dr Jing Gong from the Laboratory of Pathology, the State Key Laboratory of Biotherapy, and many other clinicians from the Department of Urology, West China hospital for their kind assistance. This work was supported by the National Natural Science Foundation of China (No. NSFC30700977, No. NSFC30800637 and No. NSFC30871383).
References
- Jemal A, Siegel R, Ward E, Hao Y, Xu J, et al. Cancer statistics, 2009. CA Cancer J Clin. 2009;59:225–49. doi: 10.3322/caac.20006. [DOI] [PubMed] [Google Scholar]
- Huggins C, Hodges CV. Studies on prostatic cancer: The effect of castration, of estrogen and of androgen injection on serum phosphatases in metastatic carcinoma of the prostate. Cancer Res. 1941;1:293–7. [Google Scholar]
- Sharifi N, Dahut WL, Steinberg SM, Figg WD, Tarassoff C, et al. A retrospective study of the time to clinical endpoints for advanced prostate cancer. BJU Int. 2005;96:985–9. doi: 10.1111/j.1464-410X.2005.05798.x. [DOI] [PubMed] [Google Scholar]
- Heidenreich A, von Knobloch R, Hofmann R. Current status of cytotoxic chemotherapy in hormone refractory prostate cancer. Eur Urol. 2001;39:121–30. doi: 10.1159/000052426. [DOI] [PubMed] [Google Scholar]
- Labrie F, Dupont A, Belanger A, Cusan L, Lacourciere Y, et al. New hormonal therapy in prostatic carcinoma: combined treatment with an LHRH agonist and an antiandrogen. Clin Invest Med. 1982;5:267–75. [PubMed] [Google Scholar]
- Prostate Cancer Trialists' Collaborative Group. Maximum androgen blockade in advanced prostate cancer: an overview of the randomised trials Lancet 20003551491–8. [PubMed] [Google Scholar]
- Bennett CL, Tosteson TD, Schmitt B, Weinberg PD, Ernstoff MS, et al. Maximum androgen-blockade with medical or surgical castration in advanced prostate cancer: a meta-analysis of nine published randomized controlled trials and 4128 patients using flutamide. Prostate Cancer Prostatic Dis. 1999;2:4–8. doi: 10.1038/sj.pcan.4500265. [DOI] [PubMed] [Google Scholar]
- Caubet JF, Tosteson TD, Dong EW, Naylon EM, Whiting GW, et al. Maximum androgen blockade in advanced prostate cancer: a meta-analysis of published randomized controlled trials using nonsteroidal antiandrogens. Urology. 1997;49:71–8. doi: 10.1016/S0090-4295(96)00325-1. [DOI] [PubMed] [Google Scholar]
- Schmitt B, Bennett C, Seidenfeld J, Samson D, Wilt T.Maximal androgen blockade for advanced prostate cancer Cochrane Database Syst Rev 2003. 2000. CD001526. Review. [DOI] [PMC free article] [PubMed]
- Denis L, Murphy GP. Overview of phase III trials on combined androgen treatment in patients with metastatic prostate cancer. Cancer. 1993;7l:3888–95. doi: 10.1002/1097-0142(19931215)72:12+<3888::aid-cncr2820721726>3.0.co;2-b. [DOI] [PubMed] [Google Scholar]
- Crawford ED, Eisenberger MA, McLeod DG, Spaulding JT, Benson R, et al. A controlled trial of leuprolide with and without flutamide in prostatic carcinoma. N Engl J Med. 1989;321:419–24. doi: 10.1056/NEJM198908173210702. [DOI] [PubMed] [Google Scholar]
- Denis LJ, Keuppens F, Smith PH, Whelan P, de Moura JL, et al. EORTC Genito-Urinary Tract Cancer Cooperative Group and the EORTC Data Center. Maximal androgen blockade: final analysis of EORTC phase III trial 30853. Eur Urol. 1998;33:144–51. doi: 10.1159/000019546. [DOI] [PubMed] [Google Scholar]
- Dijkman GA, Janknegt RA, De Reijke TM, Debruyne FM. International Anandron Study Group. Long-term efficacy and safety of nilutamide plus castration in advanced prostate cancer, and the significance of early prostate specific antigen normalization. J Urol. 1997;158:160–3. doi: 10.1097/00005392-199707000-00051. [DOI] [PubMed] [Google Scholar]
- Bertagna C, De Géry A, Hucher M, François JP, Zanirato J. Efficacy of the combination of nilutamide plus orchidectomy in patients with metastatic prostatic cancer. A meta-analysis of seven randomized double-blind trials (1056 patients) Br J Urol. 1994;73:396–402. doi: 10.1111/j.1464-410x.1994.tb07603.x. [DOI] [PubMed] [Google Scholar]
- Usami M, Akaza H, Arai Y, Hirano Y, Kagawa S, et al. Bicalutamide 80 mg combined with a luteinizing hormone-releasing hormone agonist (LHRH-A) versus LHRH-A monotherapy in advanced prostate cancer: findings from a phase III randomized, double-blind, multicenter trial in Japanese patients. Prostate Cancer Prostatic Dis. 2007;10:194–201. doi: 10.1038/sj.pcan.4500934. [DOI] [PubMed] [Google Scholar]
- Klotz L, Schellhammer P, Carroll K. A re-assessment of the role of combined androgen blockade for advanced prostate cancer. BJU Int. 2004;93:1177–82. doi: 10.1111/j.1464-410x.2004.04803.x. [DOI] [PubMed] [Google Scholar]
- Akaza H, Hinotsu S, Usami M, Arai Y, Kanetake H, et al. Combined androgen blockade with bicalutamide for advanced prostate cancer: long-term follow-up of a phase 3, double-blind, randomized study for survival. Cancer. 2009;115:3437–45. doi: 10.1002/cncr.24395. [DOI] [PubMed] [Google Scholar]
- Klotz L. Maximal androgen blockade for advanced prostate cancer. Best Pract Res Clin Endocrinol Metab. 2008;22:331–40. doi: 10.1016/j.beem.2008.01.004. [DOI] [PubMed] [Google Scholar]
- Kuil CW, Berrevoets CA, Mulder E. Ligand-induced conformational alterations of the androgen receptor analyzed by limited trypsinization. Studies on the mechanism of antiandrogen action. J Biol Chem. 1995;270:27569–76. doi: 10.1074/jbc.270.46.27569. [DOI] [PubMed] [Google Scholar]
- Culig Z. Androgen receptor cross-talkwith cell signalling pathways. Growth Factors. 2004;22:179–84. doi: 10.1080/08977190412331279908. [DOI] [PubMed] [Google Scholar]
- Hu X, Lazar MA. Transcriptional repression by nuclear hormone receptors. Trends Endocrinol Metab. 2000;11:6–10. doi: 10.1016/s1043-2760(99)00215-5. [DOI] [PubMed] [Google Scholar]
- Bouchal J, Baumforth KR, Sváchová M, Murray PG, von Angerer E, et al. Microarray analysis of bicalutamide action on telomerase activity, p53 pathway and viability of prostate carcinoma cell lines. J Pharm Pharmacol. 2005;57:83–92. doi: 10.1211/0022357055164. [DOI] [PubMed] [Google Scholar]


