Abstract
The prostate secretory protein of 94 amino acids (PSP94) has been shown to interact with cysteine-rich secretory protein 3 (CRISP-3) in human seminal plasma. Interestingly, PSP94 expression is reduced or lost in the majority of the prostate tumours, whereas CRISP-3 expression is upregulated in prostate cancer compared with normal prostate tissue. To obtain a better understanding of the individual roles these proteins have in prostate tumourigenesis and the functional relevance of their interaction, we ectopically expressed either PSP94 or CRISP-3 alone or PSP94 along with CRISP-3 in three prostate cell lines (PC3, WPE1-NB26 and LNCaP) and performed growth inhibition assays. Reverse transcription-polymerase chain reaction and Western blot analysis were used to screen prostate cell lines for PSP94 and CRISP-3 expression. Mammalian expression constructs for human PSP94 and CRISP-3 were also generated and the expression, localization and secretion of recombinant protein were assayed by transfection followed by Western blot analysis and immunofluorescence assay. The effect that ectopic expression of PSP94 or CRISP-3 had on cell growth was studied by clonogenic survival assay following transfection. To evaluate the effects of co-expression of the two proteins, stable clones of PC3 that expressed PSP94 were generated. They were subsequently transfected with a CRISP-3 expression construct and subjected to clonogenic survival assay. Our results showed that PSP94 and CRISP-3 could each induce growth inhibition in a cell line specific manner. Although the growth of CRISP-3-positive cell lines was inhibited by PSP94, growth inhibition mediated by CRISP-3 was not affected by the presence or absence of PSP94. This suggests that CRISP-3 may participate in PSP94-independent activities during prostate tumourigenesis.
Keywords: β-microseminoprotein, CRISP-3, clonogenic survival assay, LNCaP, PC3, WPE1-NB26
Introduction
Prostate secretory protein of 94 amino acids (PSP94, also known as β-microseminoprotein) is a small, cysteine-rich, non-glycosylated protein that is secreted by the prostate 1, 2. It is shown to be uniformly present in the glandular epithelium of the normal prostate but not in the prostatic stroma 1. It has been examined extensively in studies aimed at assessing its utility as a diagnostic and/or prognostic marker for prostate cancer. Its therapeutic potential has also been evaluated, and a synthetic peptide derived from PSP94 has been tested as a potential treatment for prostate cancer 3. The native PSP94 protein has been shown to suppress the growth of an androgen-independent prostate cancer cell line (PC3) and xenografts by inducing apoptosis 4. Rat PSP94 has also been reported to inhibit the growth of rat prostate cancer cell lines when added exogenously and when expressed ectopically in the cells following transfection 5. This hypothesized protective role of PSP94 is supported by the reports linking low levels of PSP94 either in the serum or tumours of patients with high-grade disease or a high probability of recurrence, respectively 6, 7. Genome-wide association studies that have been recently conducted have identified a polymorphism linked with prostate cancer susceptibility in the promoter of MSMB gene, which codes for PSP94 8, 9. This polymorphism has been shown to affect the CREB binding site, which in turn causes reduced levels of PSP94 transcription 10, 11. However, the exact role that PSP94 plays in the prostate and how its loss affects the process of tumourigenesis is not clear.
Human PSP94 has been shown to form high-affinity complexes with cysteine-rich secretory protein 3 (CRISP-3, also known as SGP28), which is present in human seminal plasma 12. CRISP-3 belongs to the CRISP family of proteins that are found in mammals and reptiles. A typical CRISP is a two-domain protein consisting of a larger N-terminal SCP domain, which is also referred to as a CAP (cysteine-rich secretory proteins, antigen 5, and pathogenesis-related 1 proteins) domain, and a cysteine-rich C-terminal domain, which possesses ion channel-regulating activity 13. In addition to PSP94, CRISP-3 also binds to serum α1B-glycoprotein in multiple species 14, 15. It has been speculated that the interaction between CRISP-3 and PSP94 or α1B-glycoprotein may inhibit its activity 12, 14, 15.
In the male reproductive tract, CRISP-3 transcripts are shown to be present predominantly in the prostate and to a lesser extent in the epididymis 16. Its expression is low in benign prostatic epithelium but is highly upregulated in the majority of high-grade prostatic-intraepithelial neoplasia lesions and in most primary prostate tumours and metastases 17. Two independent studies have shown that CRISP-3 is one of the genes that is most often upregulated in prostate cancer 18, 19. A negative association between upregulated CRISP-3 and recurrence-free survival probability in prostate cancer patients has also been demonstrated 7. However, PSP94 exhibits lower levels of expression in prostate cancer tissue compared with benign prostate tissue 20. The physiological significance of the inverse expression patterns of PSP94 and CRISP-3 in prostate cancer tissue needs to be further explored. The role of CRISP-3 in prostate tumourigenesis also needs to be investigated.
To this end, we screened a panel of human prostate cell lines for their PSP94 and CRISP-3 expression status. Based on the results of this screening, we selected high and low CRISP-3-expressing cell lines for ectopic expression of PSP94. Given the proposed growth-regulatory effect that PSP94 has on prostate cancer cells, it is important to determine whether this function of PSP94 is positively or negatively affected by the presence of CRISP-3. Similarly, examining the effect of ectopic CRISP-3 expression in PSP94-positive and PSP94-negative cell lines would provide us with more information regarding the mechanism of action of CRISP-3. We performed transfection followed by clonogenic survival assay to assess the effect of ectopic expression of either PSP94 or CRISP-3 on cell growth.
Materials and methods
Cell culture and antibodies
Human PC3, DU145 and LNCaP were obtained from the National Centre for Cell Sciences, Pune, India. RWPE-1 and WPE1-NB26 cells were obtained from the American Type Culture Collection (ATCC, Manassas, VA, USA). PC3 and LNCaP cells were maintained in RPMI-1640 containing 10% foetal calf serum (FCS). DU145 cells were maintained in DMEM/F12 containing 10% FCS. Both types of media contained penicillin (100 U mL−1) and streptomycin (100 μg mL−1). RWPE-1 and WPE1-NB26 cells were maintained on keratinocyte serum-free medium supplemented with bovine pituitary extract (0.05 mg mL−1), epidermal growth factor (5 ng mL−1), and gentamycin (0.4 mg mL−1) as recommended by ATCC. All cell culture reagents were purchased from Invitrogen (Gibco; Invitrogen, Carlsbad, CA, USA). Cells were grown at 37°C in a humidified environment containing 5% CO2.
The antibodies used for this study included anti-HA rabbit polyclonal antibody (SC-805; Santa Cruz Biotech Inc., CA, USA), anti-β-actin mouse monoclonal antibody (SC-47778; Santa Cruz Biotech Inc.) and anti-hCRISP-3 goat polyclonal antibody (AF2397, R&D Systems Inc., Minneapolis, MN, USA). The anti-PSP94 rabbit polyclonal antiserum was raised against native PSP94 protein purified from human seminal plasma in our laboratory. The anti-HA antibody and the anti-hCRISP-3 antibody were used at dilutions of 1:1 000 and 1:2 000, respectively, for Western blot analysis. The horseradish peroxidase-conjugated anti-rabbit, anti-mouse and anti-goat secondary antibodies (Santa Cruz Biotech Inc.) were used at dilutions of 1:2 000.
Reverse transcription-polymerase chain reaction (RT-PCR)
Each cell line was grown in 60-mm dishes and RNA was extracted using Trizol (Invitrogen) according to the manufacturer's instructions. Reverse transcription was performed using a DNase I-treated RNA template, 200 units of Superscript II reverse transcriptase (Invitrogen), the companion reagents and 25 ng μL−1 of oligo-dT primers according to the manufacturer's instructions. PCR reactions were performed using templates from the reverse transcription reaction (1 μL), 2.5 units of Taq polymerase (Invitrogen), 1 μmol L−1 of gene-specific primers and the companion reagents. The primers for GAPDH and CRISP-3 and the PCR conditions were as published by Kosari et al. 21. The sequence for PSP94 forward primer was 5′-ggagattcaaccaggaaatg-3′ and the PSP94 reverse primer was 5′-ttatccattcactgacag-3′. The PCR for PSP94 amplification included an initial denaturation step (2 min 94°C), 35 rounds of amplification (30 s at 94°C, 1 min at 52°C and 1 min at 72°C) and a final extension period (5 min at 72°C).
PSP94 protein expression, detection and selection of stable clones of PC3
A human PSP94 expression construct, pcDNA3.1PSP94HA, was created by cloning full-length human PSP94 cDNA with a HA tag at the 3′ end into pcDNA3.1+ (Invitrogen) at HindIII and EcoRI sites. The complete coding sequence was then verified. The construct was used for transient transfection of PC3 cells. Transfection was carried out using Lipofectamine and Plus Reagent (Invitrogen) and 1 μg of DNA (either the PSP94 expression construct or the empty vector) according to the manufacturer's protocol. Next, 24 h post-transfection, serum-free medium was added to the cells. Following incubation for 36 h, serum-free medium was harvested and the secreted proteins in the conditioned medium were precipitated using trichloroacetic acid (TCA). The cells were rinsed once with PBS and then lysed in lysis buffer (50 mmol L−1 Tris-HCl, pH 7.4, 2 mmol L−1 EDTA, 100 mmol L−1 NaCl, 1% NP40, 0.1% SDS and protease inhibitors) to obtain total cell extract. Precipitation of the secreted proteins was carried out via TCA precipitation. Proteins were separated on a 15% SDS-PAGE gel. Western blot analysis was carried out using anti-HA antibody for the detection of PSP94HA. Anti-β-actin was used as a loading control.
Stable clones were selected following incubation for 2 weeks in medium containing 500 μg mL−1 of G418 (Gibco; Invitrogen). The medium was changed every third day. The stable clones were screened for the expression of PSP94HA by Western blot analysis.
Immunofluorescence
The sub-cellular localization of recombinant PSP94 was assessed using PC3 and LNCaP cells that had been transiently transfected with a PSP94 expression construct. The same cell lines that had been transfected with empty vector served as controls. Cells were fixed in cold methanol for 10 min and then in cold acetone for 2 min. Cells were then permeabilized in 0.5% Triton X-100 and blocked with 2% BSA in PBS. Staining was performed using 1:100 anti-HA antibody followed by 1:150 FITC-conjugated anti-rabbit antibody (Bangalore Genei, India). Cells were visualized under a fluorescent microscope.
Co-immunoprecipitation
WPE1-NB26 cells were transfected with the PSP94 expression construct and placed in serum-free medium on the following day. After 48 h, conditioned medium was collected and subjected to immunoprecipitation using anti-HA agarose beads (Thermo Fisher Scientific, Pierce Protein Research Products, Rockford, IL, USA) according to the manufacturer's instructions. Co-immunoprecipitated proteins were separated on 12% SDS-PAGE gel, subjected to Western blot analysis and detected using anti-CRISP-3 and anti-HA antibodies.
CRISP-3 protein expression and detection
A hCRISP-3 clone (Human MGC-verified FL cDNA clone ID 8069045) was purchased from Saf labs (Navi Mumbai, India). It was used as a template in a PCR reaction along with CRISP-3-specific primers to add an EcoRI site at the 5′ end and a His tag and an XbaI site at 3′ end of the amplified hCRISP-3 fragment. This fragment was cloned in pEFIRES-P (a kind gift from Dr Steve Hobbs, Institute of Cancer Research, Sutton, UK). The resulting CRISP-3 expression construct was sequence verified. To check the expression of recombinant CRISP-3, this construct was used to transfect PC3 cells and the presence of CRISP-3 in the conditioned medium was detected by Western blotting.
Clonogenic survival assay
As mentioned above, the transfections were carried out using 1 μg of DNA as well as the Lipofectamine and Plus Reagent in a six-well plate. Each cell line was plated in two wells. One was used for transfection with empty vector which served as a control, and the other well was used for transfection with a PSP94 or CRISP-3 expression construct. At 24 h post-transfection, cells in each well were trypsinized and split (in triplicate) in a ratio that was standardized for each cell line depending on the transfection efficiency. Next, 24 h after the cells were split, a PSP94 or CRISP-3 selective agent (G418 for PSP94 expression construct or puromycin for CRISP-3 construct) was added and the selection was continued for 2 weeks. The optimal G418 concentration for the selection of clones for the different cell lines was determined by the kill curve. The final G418 concentrations that were used were as follows: 500 μg mL−1 for PC3 cells; 250 μg mL−1 for WPE1-NB26 cells; and 800 μg mL−1 for LNCaP cells. For puromycin, a concentration of 0.4 μg mL−1 was used for PC3 and WPE1-NB26 cells and a concentration of 0.7 μg mL−1 was used for LNCaP cells. Clones obtained after 2 weeks were fixed with 1% glutaraldehyde and stained with 0.1% crystal violet. These clones were counted manually by two different individuals independently and confirmed by examining the plates under a microscope. The presence of at least 64 cells was considered the cut-off for designating the colonies as clones. When plotting the graph, the number of clones that were obtained following empty vector transfection was used as a measure of transfection efficiency of that cell line. This number was taken as 100% survival for that cell line. The clones that were obtained following PSP94 or CRISP-3 transfection were counted and the percentage of clones that survived was calculated. In order to get a quantitative measure of the survival, cell-associated dye from the crystal violet-stained clones was extracted with 10% acetic acid and the optical density (OD) at 590 nm was determined 22. OD values of the vector-transfected cells were compared with those of the PSP94 or CRISP-3 expression construct-transfected cells.
Statistical analysis
Clonogenic survival data of the three cell lines are presented as the mean ± SE. Statistical analysis of the results was performed by one-way analysis of variance (ANOVA). Multiple comparisons between the cell lines were carried out by Post Hoc Tests (LSD). All the statistical analysis was done using SPSS software (SPSS Inc., Chicago, IL, USA). A conventional value of P < 0.05 was used to represent statistical significance.
Results
Screening of prostate cell lines based on their endogenous PSP94 and CRISP-3 expression
We screened five prostate cell lines (PC3, DU145, LNCaP, RWPE-1 and WPE1-NB26) for expression of PSP94 and CRISP-3 by RT-PCR. The PC3, DU145 and LNCaP cell lines are derived from metastatic prostate cancers, whereas the RWPE-1 is a cell line that is derived from normal human prostate cells that were immortalized by transfection with HPV-18 DNA. WPE1-NB26 was derived from RWPE-1 cells after treatment with N-methyl-N-nitrosourea 23. Except for RWPE-1, all of the cell lines we tested are tumourigenic in nude mice. As shown in Figure 1A, PSP94 mRNA was not detected in PC3, DU145, RWPE-1 or WPE1-NB26 cells, but a weak signal was observed in LNCaP cells. Expression of CRISP3 was low in PC3, DU145 and RWPE-1 cells, but it was upregulated in WPE1-NB26 and LNCaP cells. LNCaP was the only cell line that expressed PSP94 and exhibited upregulation of CRISP-3. We therefore categorized these cell lines into three groups: group 1: PSP94-negative with low-level CRISP-3 expression; group 2: PSP94-negative with upregulated CRISP-3 expression; and group 3: PSP94-positive with upregulated CRISP-3 expression. Using this grouping strategy, PC3, DU145 and RWPE-1 were allocated to group 1, WPE1-NB26 was allocated to group 2 and LNCaP was allocated to group 3. We chose three cell lines (PC3, WPE1-NB26 and LNCaP) that were representative of each of the three groups and carried out Western blot analysis for the detection of CRISP-3 and PSP94 protein. As both CRISP-3 and PSP94 are secretory proteins, we used the proteins that were precipitated by TCA from the conditioned medium of these three cell lines. WPE1-NB26 and LNCaP cells showed evidence of two bands of roughly 30 and 28 kDa that corresponded to glycosylated and non-glycosylated CRISP-3 (Figure 1B). For semi-quantitative Western blot analysis, the amount of protein that was present was adjusted for cell number. As shown in Figure 1B, the amount of CRISP-3 secreted by an equivalent number of WPE1-NB26 and LNCaP cells (500 000) at 48 and 72 h was similar, whereas PC3 cells did not express CRISP-3 protein. For PSP94, we were unable to detect any specific signal in LNCaP cells using the polyclonal anti-PSP94 antibody raised in our laboratory (data not shown). This antibody detects native PSP94 purified from the seminal plasma, but the amount of PSP94 protein expressed by LNCaP cells may have been too low to be detected.
Figure 1.
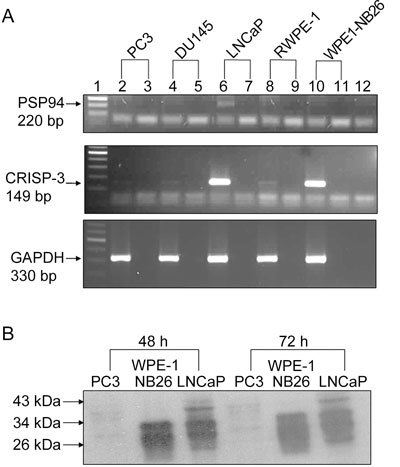
Pattern of endogenous PSP94 and CRISP-3 expression in different prostate cell lines. (A): Reverse transcription-polymerase chain reaction (RT-PCR) results of the total RNA content that was extracted from the five tested cell lines. The panels from the top to the bottom show amplification of PSP94, CRISP-3 and GAPDH, respectively. Lane 1 is a 100-bp ladder. Lanes 2, 4, 6, 8 and 10 are RT-PCR products for PC3, DU145, LNCaP, RWPE-1 and WPE1-NB26, respectively. Lanes 3, 5, 7, 9 and 11 are PCR reactions for no RT enzyme controls for the same samples. Lane 12 is a water control. (B): Semi-quantitative Western blot analysis for detection of CRISP-3 protein. TCA-precipitated proteins from conditioned medium that was harvested at 48 h (left side) and 72 h (right side) from 5 × 105 PC3 cells, WPE1-NB26 cells and LNCaP cells were subjected to Western blot analysis using an anti-CRISP-3 polyclonal antibody. The presence of two bands at around 30 and 28 kDa in the WPE1-NB26 and LNCaP cells represent glycosylated and unglycosylated CRISP-3 protein, respectively.
Expression of recombinant PSP94 and its interaction with endogenous CRISP-3
We cloned human PSP94 with an HA tag in a mammalian expression vector pcDNA3.1+ (Invitrogen). To confirm the presence of PSP94 expression from this construct, PC3 cells were transiently transfected with this construct or an empty vector. The total protein extract and the TCA-precipitated proteins that were secreted from transfected cells were analysed by Western blot analysis using an anti-HA antibody. A specific signal was obtained at approximately 18–20 kDa in the cell lysate and the conditioned medium of the PC3 cells that had been transfected with the PSP94 expression construct (Figure 2A). Although the calculated mass of PSP94 is 10.7 kDa, it has been shown to run at a higher molecular mass on SDS gels 12, 24. The localization of PSP94 in transiently transfected PC3 and LNCaP cells was examined by immunofluorescence (Figure 2B). A strong staining was observed in the cytoplasm of cells transfected with the PSP94 expression construct.
Figure 2.
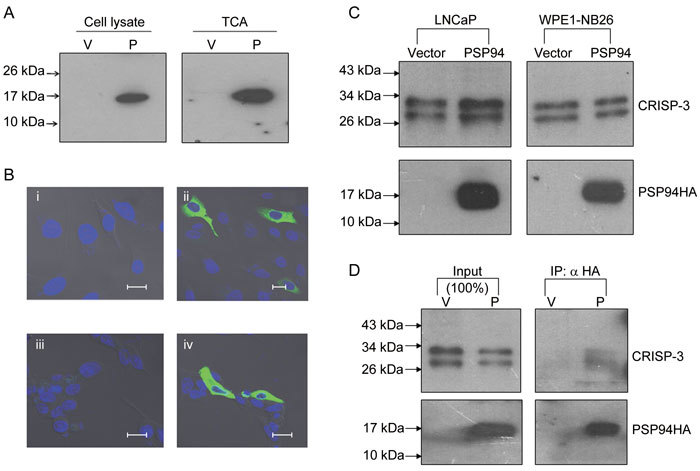
Secreted recombinant PSP94 interacts with endogenous CRISP-3. (A): Western blot analysis for detection of PSP94HA. Cell lysate and TCA-precipitated proteins derived from the conditioned medium from PC3 cells that were transiently transfected with either empty vector (V) or a PSP94 expression construct (P) were probed with anti-HA antibody. (B): Localization of PSP94HA by immunofluorescence. PC3 cells that were transiently transfected with empty vector (i) and a PSP94 expression construct (ii) as well as LNCaP cells transiently transfected with empty vector (iii) and a PSP94 expression construct (iv) were first probed with polyclonal anti-HA antibody and then with FITC-labelled secondary antibody. Bars = 20 μm. (C): Effect of ectopically expressed PSP94 on endogenous CRISP-3. TCA-precipitated proteins from vector-transfected and PSP94 expression construct-transfected WPE1-NB26 and LNCaP cells were subjected to Western blot analysis 48 h after transfection. The upper panel was probed with anti-CRISP-3 antibody and the lower panel was probed with anti-HA antibody. (D): Co-immunoprecipitation of PSP94 and CRISP-3 from the conditioned medium of vector-transfected and PSP94 expression construct-transfected WPE1-NB26 cells. PSP94HA was immunoprecipitated using anti-HA agarose-conjugated beads. The upper portion of the Western blot was probed with anti-CRISP-3 antibody and the lower portion was probed with anti-HA antibody. The lanes marked with input show the total amount of CRISP-3 and PSP94HA that was immunoprecipitated from the conditioned medium.
To determine whether ectopic expression of PSP94 affected endogenous CRISP-3 expression, LNCaP and WPE1-NB26 cells were transfected with the PSP94 expression construct and their CRISP-3 protein levels were evaluated by Western blot analysis 48 h post-transfection (Figure 2C). The amount of CRISP-3 protein produced by PSP94-transfected cells was similar to that produced by vector-transfected cells for both cell lines. Next, to attempt to characterize the interaction between ectopically expressed PSP94 and endogenous CRISP-3, co-immunoprecipitation was carried out (Figure 2D). We found that endogenous CRISP-3 protein secreted by WPE1-NB26 cells interacted with the ectopically expressed PSP94HA and was co-precipitated along with it.
Different cell lines respond differently to the ectopic expression of PSP94 as assessed by clonogenic survival assay
The three representative prostate cancer cell lines were subjected to transfection with a PSP94 expression construct followed by clonogenic survival assay. PC3 (which lacks PSP94 and CRISP-3 expression), WPE1-NB26 (which lacks PSP94 expression but has upregulated CRISP-3 expression) and LNCaP (which has low levels of PSP94 transcription and upregulated CRISP-3 expression) cells were used for this assay. Clonogenic survival was calculated by comparing the number of clones that were obtained after empty vector transfection with those obtained after PSP94 transfection. Figure 3A shows a representative picture of the crystal violet staining of the stable clones that were obtained from PC3, WPE1-NB26 and LNCaP cells. WPE1-NB26 cells showed more than 90% reduction in clonogenic survival and LNCaP cells showed approximately 50% reduction in clonogenic survival. In contrast, there was less than 10% reduction in clonogenic survival of PC3 cells upon transfection with the PSP94 expression construct (Figure 3B). In addition to manual counting of the clones, colorimetric evaluation of the cell-associated crystal violet dye was carried out as described in methodology and the results of the clonogenic survival assay were re-confirmed (Supplementary Figure 1).
Figure 3.
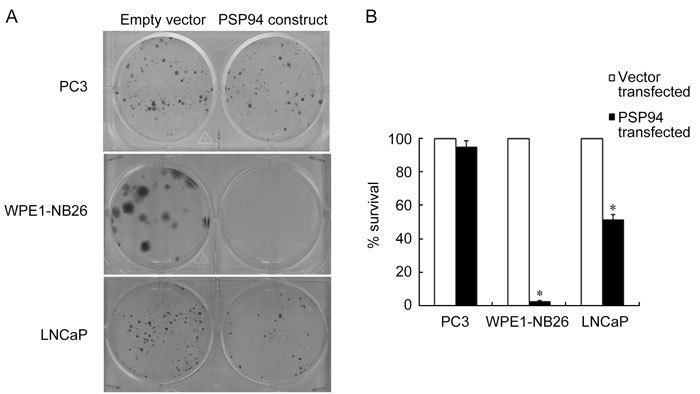
Ectopic expression of PSP94 has an inhibitory effect on cell growth in a cell line specific manner. (A): A representative composite picture of the crystal violet staining from the clonogenic survival assay. A single well (of the six-well plate) is shown for each cell line (PC3, WPE1-NB26 and LNCaP) following transfection with empty vector, pcDNA3.1+ (left wells) or PSP94 expression construct (right wells). At 24 h after transfection, the cells were split and stable clones were selected on G418. Clones obtained after two weeks of selection were stained and counted. (B): Effect of the over-expression of PSP94 on the clonogenic survival of PC3, WPE1-NB26 and LNCaP cells. For each cell line, the number of clones obtained in the vector-transfected wells was considered to represent 100% survival. The number of clones obtained in the PSP94 expression construct-transfected wells was used to calculate the percentage of surviving cells with respect to the empty vector-transfected cells. The assay was repeated at least three times for each cell line. The graph is the average of three independent observations and is plotted as the mean percentage survival ± SE. The observed reduction in the percentage survival of WPE1-NB26 and LNCaP cells was statistically significant when compared with PC3 cells (*P < 0.05, compared with PC3 cells; one-way ANOVA).
Isolation and characterization of stable clones of PC3 expressing PSP94
Our observation that PSP94 expression had no effect on PC3 cell growth led us to attempt to isolate stable clones of PC3 that expressed PSP94 that could be used to further study the effect of the co-expression of PSP94 and CRISP-3. A total of 12 stable clones were randomly selected, expanded and screened for the expression of PSP94 by Western blot analysis. Five clones showed the presence of PSP94 protein. A representative Western blot shown in Figure 4A demonstrates that two of the five clones (Lanes 4 and 5) expressed PSP94HA. Lane 6 shows a clone carrying empty vector (PC3-A1v). A clone that has high levels of PSP94 expression (PC3-D4) and one with moderate PSP94 expression (PC3-A5) were subsequently maintained in culture for more than 30 passages. These two clones retained the expression and secretion of PSP94 (Figure 4B). These clones along with the three cell lines used in our experiments were transfected with a CRISP-3 expression construct to study the effect that CRISP-3 had on cell growth.
Figure 4.
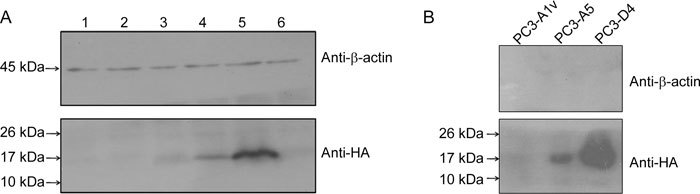
Screening of stable clones expressing PSP94 by Western blot analysis. (A): Lanes 1–5 represent cell lysates from five different clones following PSP94 expression construct transfection of PC3 cells. Lane 6 is a cell lysate from one of the clones that was obtained following transfection with empty vector (pcDNA3.1+). The expression of recombinant PSP94HA was confirmed by probing the blot with anti-HA antibody. β-Actin was used as a loading control. (B): Western blot analysis of the TCA-precipitated proteins from the culture supernatants of stable clones PC3-A1v (which carried empty vector), PC3-A5 (which expressed PSP94HA) and PC3-D4 (which expressed PSP94HA) after roughly 30 passages in culture. Clones PC3-A5 and PC3-D4 stably expressed and secreted PSP94HA. The blot was probed with anti-HA antibody to detect PSP94HA, and with anti-β-actin antibody to show that the conditioned media were not contaminated with cellular contents.
Ectopic expression of CRISP-3 alone exhibits growth-inhibitory activity that is independent of the presence of PSP94
We first cloned the human CRISP-3 cDNA in a mammalian expression vector, pEFIRES-P, which carries a puromycin resistance gene. An advantage of using this vector was that it has an internal ribosome entry site. This means that the gene of interest and the puromycin resistance gene are expressed from the same transcript, thereby ensuring that almost all of the stable clones express the gene of interest 25.
The expression of recombinant CRISP-3 from this construct was verified following transfection (Figure 5A). Western blot analysis demonstrated the presence of recombinant CRISP-3 as two bands migrating at ∼30 and 28 kDa in the CRISP-3-transfected PC3 cells (Lane 1) but not in the empty vector-transfected cells (Lane 2). The WPE1-NB26 served as a positive control (Lane 3) and RWPE-1 served as a negative control (Lane 4). The CRISP-3 expression construct and the empty vector (pEFIRES-P) were then used for clonogenic survival assays in PC3, WPE1-NB26 and LNCaP cells. Ectopic expression of CRISP-3 caused more than 90% reduction in the clonogenic survival of PC3 and WPE1-NB26 cells, whereas ectopic CRISP-3 expression by LNCaP cells did not have a marked effect on the clonogenic survival of this cell line (Figures 5B and C).
Figure 5.
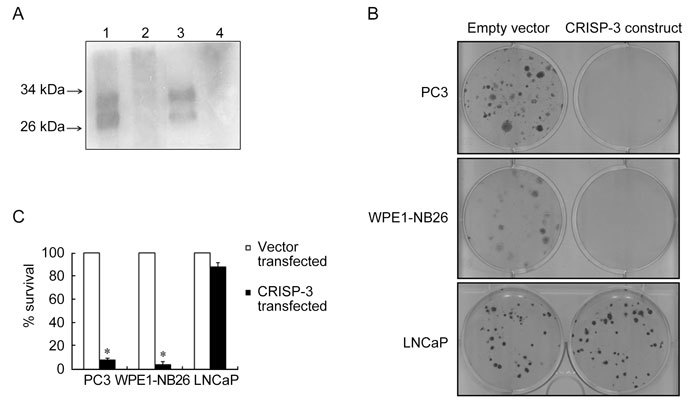
Cell line specific growth-inhibitory effect caused by CRISP-3 over-expression. (A): Expression of recombinant CRISP-3 was detected by Western blot analysis using anti-CRISP-3 antibody. TCA-precipitated proteins from the conditioned medium of PC3 cells that were transiently transfected with CRISP-3 expression construct (Lane 1) showed the presence of glycosylated and unglycosylated CRISP-3 similar to the positive control (Lane 3) that contained TCA-precipitated proteins from the conditioned medium of WPE1-NB26 cells. TCA-precipitated proteins from the conditioned medium of PC3 cells transfected with empty vector (Lane 2) as well as untransfected RWPE-1 (Lane 4) served as a negative control. (B): A representative composite picture of the crystal violet staining from the clonogenic survival assay. A single well (of the six-well plate) is shown for each cell line (PC3, WPE1-NB26 and LNCaP) that was transfected with either the empty vector, pEFIRES-P (left wells), or the CRISP-3 expression construct (right wells). At 24 h after transfection, the cells were split and stable clones were selected on puromycin. Clones obtained after 2 weeks of selection were stained and counted. (C): Effect of over-expression of CRISP-3 on the clonogenic survival of PC3, WPE1-NB26 and LNCaP cells. The percentage survival with respect to empty vector was calculated as described above. The assay was repeated at least three times for each cell line. The graph is the average of three independent observations and is plotted as mean percentage survival ± SE. The reduction in percentage survival of PC3 and WPE1-NB26 cells was statistically significant when compared with LNCaP cells (*P < 0.05, compared with LNCaP; one-way ANOVA).
Two stable clones of PC3 that expressed PSP94 (PC3-D4 and PC3-A5) and a stable clone carrying the empty vector (PC3-A1v) were then used for ectopic expression of CRISP-3 followed by clonogenic survival assay to assess the effect of CRISP-3 in the presence of PSP94. All three clones exhibited growth inhibition upon induction of CRISP-3 expression (Figure 6) in a manner that was similar to the parent cell line, indicating that the presence or absence of PSP94 did not affect CRISP-3-mediated growth inhibition of PC3 cells. Clonogenic survival data were re-confirmed by colorimetric assay (Supplementary Figure 1). The results of the clonogenic survival assays that examined the effects of PSP94 and CRISP-3 over-expression are summarized in Table 1.
Figure 6.
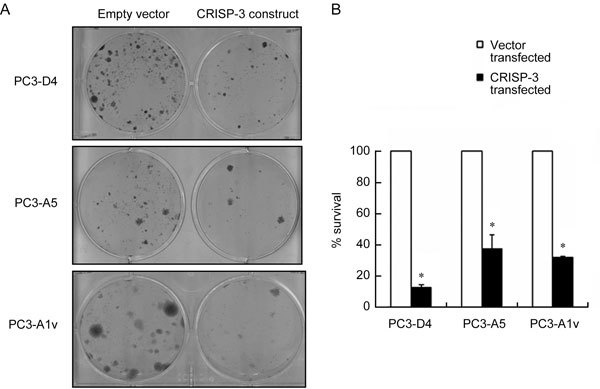
The growth-inhibitory effect of CRISP-3 is not modulated by PSP94. (A): A representative composite picture of the crystal violet staining from the clonogenic survival assay for the stable clones PC3-D4, PC3-A5 (PSP94-expressing clones) and PC3-A1v (empty vector-transfected clone). A single well (of the six-well plate) is shown for each clone that was transfected with either empty vector, pEFIRES-P (left wells), or the CRISP-3 expression construct (right wells). At 24 h after transfection, cells were split and selected on puromycin. Clones obtained after 2 weeks of selection were stained and counted. (B): The effect of over-expression of CRISP-3 on the clonogenic survival of PC3-D4, PC3-A5 and PC3-A1v. The percentage survival with respect to empty vector was calculated as described above. The assay was repeated at least three times for each cell line. The graph is the average of three independent observations and is plotted as the mean percentage survival ± SE. The reduction in percentage survival of all three clones was statistically significant when compared with LNCaP (*P < 0.05, compared with LNCaP shown in Figure 5B; one-way ANOVA).
Table 1. Cell line specific effect of the ectopic expression of PSP94 or CRISP-3.
| Cell line | Endogenous PSP94 |
Endogenous CRISP-3 |
Clonogenic survival |
|||
|---|---|---|---|---|---|---|
| Transcript | Protein | Transcript | Protein | Upon PSP94 over-expression | Upon CRISP-3 over-expression | |
| PC3 | Absent | Absent | Low | Absent | No effect | Reduced |
| WPE1-NB26 | Absent | Absent | High | Present | Reduced | Reduced |
| LNCaP | Present | Not detected | High | Present | Moderately reduced | No effect |
| PC3 clones expressing PSP94 | Present | Present | Low | Absent | NAa | Reduced |
aNot applicable.
Discussion
The potential utility of PSP94 as a diagnostic or prognostic marker for prostate cancer has been evaluated by many researchers. Although some controversy exists, the majority of the reports associate low levels of PSP94 with a diagnosis of prostate cancer or a worse prognosis among patients with known prostate cancer 6, 7, 20, 26, 27. The reduction in the PSP94 levels that are seen in prostate cancer has been attributed to the upregulation of a transcriptional repressor, EZH2, which silences the MSMB gene 28. Recently, an association between prostate cancer susceptibility and a polymorphism in the promoter region of PSP94 that causes reduction in its expression has been discovered 8, 9, 10, 11. However, how the loss of PSP94 might promote tumourigenesis remains unclear. Important clues are expected to be found in the results of studies that examine proteins that interact with PSP94. This may aid in the understanding of the intracellular signalling mediated by PSP94 that leads to inhibition of cell growth. CRISP-3 and PSP94-binding protein are the two proteins that have been reported to interact with PSP94 12, 29. In addition to interacting with these two proteins, PSP94 also forms homodimers as shown by the crystal structure reported by our group 30. CRISP-3 has been shown to be upregulated in majority of the prostate tumours and its upregulation is linked to an increased probability of recurrence 7, 17. The inverse pattern of expression of PSP94 and CRISP-3 in prostate tumours is intriguing. In this study, we attempted to understand whether the effect of PSP94 on cell growth was modulated by the presence of its interacting partner, CRISP-3. We also wanted to gain insight into the role of CRISP-3 in prostate cancer and therefore we also evaluated its effect on cell growth in the presence and absence of PSP94.
To do so, we made mammalian expression constructs for PSP94 as well as CRISP-3 and verified the expression of these recombinant proteins by Western blotting and immunofluorescence. These constructs were used to induce the ectopic expression of the respective proteins in prostate cancer cell lines. To assess the effect of PSP94/CRISP-3 on cell growth, we used clonogenic survival assays of the stable clones after transfection. This assay is used to assess the proliferative potential of a cell population following a certain treatment, e.g., exposure to ionizing radiation or treatment with a drug 31. We subjected PC3, WPE1-NB26 and LNCaP to this analysis after transfection with PSP94/CRISP-3 expression construct. We did not observe any significant reduction in clonogenic survival of PC3 following PSP94 transfection. This was unexpected considering the proposed tumour suppressor role of PSP94. Earlier reports used exogenous addition of native PSP94 protein at a concentration of 10 μg mL−1 or higher and demonstrated that this led to 60% inhibition of the growth of PC3 cells and 80% inhibition of the growth of rat PAIII cells 4, 5. In this study, we examined the effect of ectopic expression of PSP94, which may result in a different signalling event than that induced by exogenous addition of the protein. The amount of protein generated after transfection was also much lower than the amount of exogenous protein that was added in earlier reports. It should also be noted that the growth-inhibitory effect of PSP94 protein on PC3 cells shown by Garde et al. 4 was not 100% and that there is a chance that resistant cells may continue to grow when observed for a longer period of time. Similar to our results, Cadieux et al. 5 reported that PSP94-transfected PAIII cells did not exhibit growth inhibition after 24 h and that they were completely insensitive to its effect after a 120 h period as assessed by 3H-thymidine uptake assay. This effect has been attributed to the low levels of protein produced in the transfected cells. In contrast to PC3 cells, we observed a major reduction in the clonogenic survival of WPE1-NB26 cells and a moderate reduction of LNCaP cells in the respective clonogenic survival assays. Whether this growth inhibition is due to apoptosis or senescence-like growth arrest needs to be further evaluated. The endogenous CRISP-3 levels of these two cell lines were not altered by ectopic PSP94 expression. Ectopically expressed PSP94 exhibited interaction with endogenous CRISP-3. As both of the cell lines whose growth was affected by PSP94 expression show upregulated CRISP-3 expression, there is a possibility that CRISP-3 plays a role in PSP94-mediated growth inhibition. To test this possibility, we further evaluated the effect of CRISP-3 alone as well as the effect of CRISP-3 in combination with PSP94 on growth of prostate cancer cell lines. The same three cell lines along with two stable PC3 clones that expressed PSP94 protein were used for CRISP-3 expression and clonogenic survival assays.
Interestingly, the ectopic expression of CRISP-3 led to a strong growth-inhibitory effect in PC3 and WPE1-NB26 cells, whereas LNCaP cells only exhibited a marginal degree of growth inhibition. As CRISP-3 alone causes growth inhibition of PC3 cells, we could not generate CRISP-3-expressing stable PC3 clones. The few clones we identified following CRISP-3 transfection also showed growth arrest upon further passages (data not shown), and a stable line that expressed CRISP-3 could not be established in PC3 cells. Hence, the effect of PSP94 on CRISP-3-positive PC3 cells could not be tested. Further studies aimed at knocking down endogenous CRISP-3 expression in LNCaP and WPE1-NB26 cells to evaluate the effect of PSP94 expression on a CRISP-3-negative background would prove that CRISP-3 is involved in PSP94-mediated growth inhibition. The CRISP-3-negative cell line RWPE-1 showed poor transfection efficiency and therefore we could not evaluate the effect of PSP94 on this cell line.
Certain members of the CRISP family of proteins possess ion channel-blocking activity in their carboxyl terminals 32. No such activity has yet been shown for human CRISP-3, but the interaction of CRISP-3 with PSP94 or with α1-B glycoprotein is thought to act as an inhibitory mechanism for the possible toxic activity of CRISP-3 14, 15. Both PC3 and WPE1-NB26 cells lack endogenous PSP94 expression. LNCaP exhibits weak expression of the PSP94 transcript but does not show detectable amounts of PSP94 protein. To investigate whether the presence of PSP94 affected CRISP-3-mediated growth inhibition, we used stable clones of PC3 that expressed PSP94. These clones also exhibited growth inhibition following induction of ectopic CRISP-3 expression, much like our findings in PC3 cells, indicating that the CRISP-3-mediated growth inhibition of PC3 cells is PSP94-independent. The variation in the percentage of surviving clones can be attributed to the clonal variation seen among different clones during clonal selection.
The growth of one of the two cell lines that expressed CRISP-3 endogenously (WPE1-NB26) was affected by additional expression of this protein. The amounts of CRISP-3 protein secreted by these two cell lines appeared to be similar based on the results of our semi-quantitative Western blot analysis. The reason behind the cell line specific effect of CRISP-3 expression is not clear at present. Specific receptors or binding sites for human CRISP-3 on the cell membrane have not yet been identified. However, for human PSP94, Yang et al. 33 have demonstrated the existence of specific binding proteins in the PC3 and LNCaP cell lines and also in human prostate tissue, but the molecular identity of these proteins is still unknown. Differential expression of these binding proteins between cell lines can cause cell line specific effects, which we observed following the induction of ectopic expression of either CRISP-3 or PSP94.
Although most prostate tumours exhibit upregulated CRISP-3 expression, we have observed a growth-inhibitory effect of CRISP-3 on two prostate cancer cell lines. At present, the factor that triggers the upregulation of CRISP-3 is not known. CRISP-3 protein has been implicated in innate immunity and inflammation as it is expressed in neutrophilic granules, but its exact role in prostate tumourigenesis has not yet been demonstrated 34. Prostate tumours have been reported to show cellular heterogeneity in CRISP-3 expression. In a study by Bjartell et al. 7 that included more than 900 prostate cancer patients, the authors found that CRISP-3 expression in tumour tissue varied greatly. A total of 55% of prostate cancer patients in their study had ≥ 50% of their tumour cells stain positive for CRISP-3, whereas only 18% patients (146 patients out of 806) had ≥ 80% of their tumour cells stain positive for CRISP-3 with a staining intensity of ≥ 1.5 7. The negative association between CRISP-3 positivity and recurrence-free survival probability has been drawn with reference to these 18% prostate cancer patients, whereas the majority of the patients (82%) were considered CRISP-3 negative because less than 80% of their tumour cells were positive for CRISP-3. As the role that CRISP-3 plays in prostate tumours is not known, the fate of individual CRISP-3-positive or CRISP-3-negative cells from the same tumour cannot be predicted. Of the five cell lines that we tested for CRISP-3 expression, two (PC3 and DU145) were isolated from metastatic prostate cancers, and they do not demonstrate CRISP-3 upregulation. These cell lines were either established from the CRISP-3-negative cell population of the tumours from which they were derived or during the establishment of the cell lines from the tumour CRISP-3 expression was switched off. However, based on our results, it can be observed that re-introduction of CRISP-3 in PC3 cells leads to growth inhibition. WPE1-NB26, an MNU-treated tumourigenic cell line derived from RWPE-1 cells, exhibits upregulation of CRISP-3, indicating that carcinogenic exposure leading to neoplastic transformation can also trigger transcriptional activation of CRISP-3. Over-expression of CRISP-3 in this cell line was growth inhibitory, whereas the growth of LNCaP cells was not affected by additional expression of CRISP-3, suggesting that the latter cell line can escape from CRISP-3-mediated growth inhibition.
In conclusion, either PSP94 or CRISP-3 alone can induce growth inhibition in prostate cancer cells in a cell line specific manner. The growth inhibition mediated by CRISP-3 in PSP94-negative PC3 cells could not be rescued by re-expression of PSP94, indicating that there is a distinct signalling pathway in which CRISP-3 is implicated that is not affected by PSP94 expression. This cell line based study that examined the growth-inhibitory action of PSP94 and CRISP-3 also suggests that PSP94 and/or CRISP-3 expression status in prostate tumours might not be the sole determinant of a patient's risk of recurrence. The prognostic as well as therapeutic utility of PSP94 and/or CRISP-3 may depend on the signalling pathways in which they are involved, and therefore, further studies examining this issue should be undertaken.
Acknowledgments
This research (NIRRH/MS/81/09) was supported by grants from the Indian Council of Medical Research. We gratefully acknowledge Dr Steve Hobbs, Institute of Cancer Research, Belmont, Sutton, UK for providing us with the pEFIRES-P vector. We thank Ms Amanda Gomes and Ms Anuradha Mali, who worked as trainees in the department, for their technical assistance. We also thank Dr Nafisa Balasinor and Ms Reshma Gaonkar for their assistance with the confocal microscope. We acknowledge Dr Anurupa Maitra, Mr Chinnaraj Saravanan and Ms Nanda Joshi for their help with DNA sequencing.
Appendix
Figure 1S.
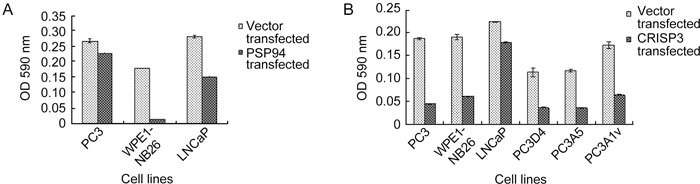
Colorimetric assay for the clonogenic survival after transfection. Absorbance at 590 nm was measured for the cell associated dye from stable clones obtained after transfection with PSP94 expression construct and empty vector (A) and for those obtained after CRISP-3 transfection and empty vector transfection (B). Optical density is plotted as mean ± SE. The experiment was repeated thrice and the graph is for one representative experiment.
References
- Doctor VM, Sheth AR, Simha MM, Arbatti NJ, Aaveri JP, et al. Studies on immunocytochemical localization of inhibin-like material in human prostatic tissue: comparison of its distribution in normal, benign and malignant prostates. Br J Cancer. 1986;53:547–54. doi: 10.1038/bjc.1986.85. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lilja H, Abrahamsson PA. Three predominant proteins secreted by the human prostate gland. Prostate. 1988;12:29–38. doi: 10.1002/pros.2990120105. [DOI] [PubMed] [Google Scholar]
- Shukeir N, Garde S, Wu JJ, Panchal C, Rabbani SA. Prostate secretory protein of 94 amino acids (PSP-94) and its peptide (PCK3145) as potential therapeutic modalities for prostate cancer. Anti-Cancer Drugs. 2005;16:1045–51. doi: 10.1097/00001813-200511000-00002. [DOI] [PubMed] [Google Scholar]
- Garde SV, Basrur VS, Li L, Finkelman MA, Krishan A, et al. Prostate secretory protein (PSP94) suppresses the growth of androgen-independent prostate cancer cell line (PC3) and xenografts by inducing apoptosis. Prostate. 1999;38:118–25. doi: 10.1002/(sici)1097-0045(19990201)38:2<118::aid-pros5>3.0.co;2-g. [DOI] [PubMed] [Google Scholar]
- Cadieux PA, Mikolajczak SA, Reeves J, Strathdee C, Reid G, et al. Rat PSP94 inhibits the growth and viability of the rat adenocarcinoma cell line PAIII in vitro. Cancer Invest. 2006;24:242–51. doi: 10.1080/07357900600629575. [DOI] [PubMed] [Google Scholar]
- Nam RK, Reeves JR, Toi A, Dulude H, Trachtenberg J, et al. A novel serum marker, total prostate secretory protein of 94 amino acids, improves prostate cancer detection and helps identify high grade cancers at diagnosis. J Urol. 2006;175:1291–7. doi: 10.1016/S0022-5347(05)00695-6. [DOI] [PubMed] [Google Scholar]
- Bjartell AS, Al-Ahmadie H, Serio AM, Eastham JA, Eggener SE, et al. Association of cysteine-rich secretory protein 3 and beta-microseminoprotein with outcome after radical prostatectomy. Clin Cancer Res. 2007;13:4130–8. doi: 10.1158/1078-0432.CCR-06-3031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eeles RA, Kote-Jarai Z, Giles GG, Olama AA, Guy M, et al. Multiple newly identified loci associated with prostate cancer susceptibility. Nat Genet. 2008;40:316–21. doi: 10.1038/ng.90. [DOI] [PubMed] [Google Scholar]
- Thomas G, Jacobs KB, Yeager M, Kraft P, Wacholder S, et al. Multiple loci identified in a genome-wide association study of prostate cancer. Nat Genet. 2008;40:310–5. doi: 10.1038/ng.91. [DOI] [PubMed] [Google Scholar]
- Lou H, Yeager M, Li H, Bosquet JG, Hayes RB, et al. Fine mapping and functional analysis of a common variant in MSMB on chromosome 10q11.2 associated with prostate cancer susceptibility. Proc Natl Acad Sci USA. 2009;106:7933–8. doi: 10.1073/pnas.0902104106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chang BL, Cramer SD, Wiklund F, Isaacs SD, Stevens VL, et al. Fine mapping association study and functional analysis implicate a SNP in MSMB at 10q11 as a causal variant for prostate cancer risk. Hum Mol Genet. 2009;18:1368–175. doi: 10.1093/hmg/ddp035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Udby L, Lundwall A, Johnsen AH, Fernlund P, Valtonen-André C, et al. beta-Microseminoprotein binds CRISP-3 in human seminal plasma. Biochem Biophys Res Commun. 2005;333:555–61. doi: 10.1016/j.bbrc.2005.05.139. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gibbs GM, Roelants K, O'Bryan MK. The CAP superfamily: cysteine-rich secretory proteins, antigen 5, and pathogenesis-related 1 proteins—roles in reproduction, cancer, and immune defense. Endocr Rev. 2008;29:865–97. doi: 10.1210/er.2008-0032. [DOI] [PubMed] [Google Scholar]
- Udby L, Sørensen OE, Pass J, Johnsen AH, Behrendt N, et al. Cysteine-rich secretory protein 3 is a ligand of alpha1B-glycoprotein in human plasma. Biochemistry. 2004;43:12877–86. doi: 10.1021/bi048823e. [DOI] [PubMed] [Google Scholar]
- Udby L, Johnsen AH, Borregaard N. Human CRISP-3 binds serum α1B-glycoprotein across species. Biochim Biophys Acta. 2010;1800:481–5. doi: 10.1016/j.bbagen.2010.01.011. [DOI] [PubMed] [Google Scholar]
- Krätzschmar J, Haendler B, Eberspaecher U, Roosterman D, Donner P, et al. The human cysteine-rich secretory protein (CRISP) family. Primary structure and tissue distribution of CRISP-1, CRISP-2 and CRISP-3. Eur J Biochem. 1996;236:827–36. doi: 10.1111/j.1432-1033.1996.t01-1-00827.x. [DOI] [PubMed] [Google Scholar]
- Bjartell A, Johansson R, Björk T, Gadaleanu V, Lundwall A, et al. Immunohistochemical detection of cysteine-rich secretory protein 3 in tissue and in serum from men with cancer or benign enlargement of the prostate gland. Prostate. 2006;66:591–603. doi: 10.1002/pros.20342. [DOI] [PubMed] [Google Scholar]
- Asmann YW, Kosari F, Wang K, Cheville JC, Vasmatzis G. Identification of differentially expressed genes in normal and malignant prostate by electronic profiling of expressed sequence tags. Cancer Res. 2002;62:3308–14. [PubMed] [Google Scholar]
- Ernst T, Hergenhahn M, Kenzelmann M, Cohen CD, Bonrouhi M, et al. Decrease and gain of gene expression are equally discriminatory markers for prostate carcinoma: a gene expression analysis on total and microdissected prostate tissue. Am J Pathol. 2002;160:2169–80. doi: 10.1016/S0002-9440(10)61165-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tsurusaki T, Koji T, Sakai H, Kanetake H, Nakane PK, et al. Cellular expression of beta-microseminoprotein (beta-MSP) mRNA and its protein in untreated prostate cancer. Prostate. 1998;35:109–16. doi: 10.1002/(sici)1097-0045(19980501)35:2<109::aid-pros4>3.0.co;2-e. [DOI] [PubMed] [Google Scholar]
- Kosari F, Asmann YW, Cheville JC, Vasmatzis G. Cysteine-rich secretory protein-3: a potential biomarker for prostate cancer. Cancer Epidemiol Biomarkers Prev. 2002;11:1419–26. [PubMed] [Google Scholar]
- Serrano M, Lin AW, McCurrach ME, Beach D, Lowe SW. Oncogenic ras provokes premature cell senescence associated with accumulation of p53 and p16INK4a. Cell. 1997;88:593–602. doi: 10.1016/s0092-8674(00)81902-9. [DOI] [PubMed] [Google Scholar]
- Webber MM, Quader ST, Kleinman HK, Bello-DeOcampo D, Storto PD, et al. Human cell lines as an in vitro/in vivo model for prostate carcinogenesis and progression. Prostate. 2001;47:1–13. doi: 10.1002/pros.1041. [DOI] [PubMed] [Google Scholar]
- Kumar V, Roske Y, Singh N, Heinemann U, Singh TP, et al. Purification and preliminary X-ray crystallographic studies of beta-microseminoprotein from human seminal plasma. Acta Crystallogr Sect F Struct Biol Cryst Commun. 2009;65:518–21. doi: 10.1107/S1744309109013670. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hobbs S, Jitrapakdee S, Wallace JC. Development of a bicistronic vector driven by the human polypeptide chain elongation factor 1alpha promoter for creation of stable mammalian cell lines that express very high levels of recombinant proteins. Biochem Biophys Res Commun. 1998;252:368–72. doi: 10.1006/bbrc.1998.9646. [DOI] [PubMed] [Google Scholar]
- Imasato Y, Xuan JW, Sakai H, Izawa JI, Saito Y, et al. PSP94 expression after androgen deprivation therapy: a comparative study with prostate specific antigen in benign prostate and prostate cancer. J Urol. 2000;164:1819–24. [PubMed] [Google Scholar]
- Girvan AR, Chang P, van Huizen I, Moussa M, Xuan JW, et al. Increased intratumoral expression of prostate secretory protein of 94 amino acids predicts for worse disease recurrence and progression after radical prostatectomy in patients with prostate cancer. Urology. 2005;65:719–23. doi: 10.1016/j.urology.2004.10.058. [DOI] [PubMed] [Google Scholar]
- Beke L, Nuytten M, Van Eynde A, Beullens M, Bollen M. The gene encoding the prostatic tumor suppressor PSP94 is a target for repression by the Polycomb group protein EZH2. Oncogene. 2007;26:4590–5. doi: 10.1038/sj.onc.1210248. [DOI] [PubMed] [Google Scholar]
- Reeves JR, Xuan JW, Arfanis K, Morin C, Garde SV, et al. Identification, purification and characterization of a novel human blood protein with binding affinity for prostate secretory protein of 94 amino acids. Biochem J. 2005;385:105–14. doi: 10.1042/BJ20040290. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kumar A, Jagtap DD, Mahale SD, Kumar M. Crystal structure of prostate secretory protein PSP94 shows an edge-to-edge association of two monomers to form a homodimer. J Mol Biol. 2010;397:947–56. doi: 10.1016/j.jmb.2010.02.035. [DOI] [PubMed] [Google Scholar]
- Yan SX, Ejima Y, Sasaki R, Zheng SS, Demizu Y, et al. Combination of genistein with ionizing radiation on androgen-independent prostate cancer cells. Asian J Androl. 2004;6:285–90. [PubMed] [Google Scholar]
- Yamazaki Y, Brown RL, Morita T. Purification and cloning of toxins from elapid venoms that target cyclic nucleotide-gated ion channels. Biochemistry. 2002;41:11331–7. doi: 10.1021/bi026132h. [DOI] [PubMed] [Google Scholar]
- Yang JP, Baijal-Gupta M, Garde SV, Fraser JE, Finkelman MA, et al. Identification of binding proteins for PSP94 in human prostate adenocarcinoma cell lines LNCaP and PC-3. Prostate. 1998;35:11–7. doi: 10.1002/(sici)1097-0045(19980401)35:1<11::aid-pros2>3.0.co;2-k. [DOI] [PubMed] [Google Scholar]
- Udby L, Calafat J, Sørensen OE, Borregaard N, Kjeldsen L. Identification of human cysteine-rich secretory protein 3 (CRISP-3) as a matrix protein in a subset of peroxidase-negative granules of neutrophils and in the granules of eosinophils. J Leukoc Biol. 2002;72:462–9. [PubMed] [Google Scholar]


