Abstract
The adaptive immune system has evolved distinct responses against different pathogens, but the mechanism(s) by which a particular response is initiated is poorly understood. In this study, we investigated the type of Ag-specific CD4+ Th and CD8+ T cell responses elicited in vivo, in response to soluble OVA, coinjected with LPS from two different pathogens. We used Escherichia coli LPS, which signals through Toll-like receptor 4 (TLR4) and LPS from the oral pathogen Porphyromonas gingivalis, which does not appear to require TLR4 for signaling. Coinjections of E. coli LPS + OVA or P. gingivalis LPS + OVA induced similar clonal expansions of OVA-specific CD4+ and CD8+ T cells, but strikingly different cytokine profiles. E. coli LPS induced a Th1-like response with abundant IFN-γ, but little or no IL-4, IL-13, and IL-5. In contrast, P. gingivalis LPS induced Th and T cell responses characterized by significant levels of IL-13, IL-5, and IL-10, but lower levels of IFN-γ. Consistent with these results, E. coli LPS induced IL-12(p70) in the CD8α+ dendritic cell (DC) subset, while P. gingivalis LPS did not. Both LPS, however, activated the two DC subsets to up-regulate costimulatory molecules and produce IL-6 and TNF-α. Interestingly, these LPS appeared to have differences in their ability to signal through TLR4; proliferation of splenocytes and cytokine secretion by splenocytes or DCs from TLR4-deficient C3H/HeJ mice were greatly impaired in response to E. coli LPS, but not P. gingivalis LPS. Therefore, LPS from different bacteria activate DC subsets to produce different cytokines, and induce distinct types of adaptive immunity in vivo.
The immune system has evolved different types of adaptive immunity, each specialized for the elimination of particular classes of pathogens (1). In response to intracellular microbes, CD4+ Th cells differentiate into Th1 cells, which produce IFN-γ; in contrast, helminths induce the differentiation of Th2 cells, whose cytokines (principally IL-4, IL-13, IL-5, and IL-10) induce IgE- and eosinophil-mediated destruction of the pathogens (2–7). While cytokines produced early in the response are crucial in determining the type of immune response, the mechanism by which a given pathogen induces a particular type of response is unknown.
Recently, it was demonstrated that distinct subsets of dendritic cells (DCs)3 differentially induce Th1 and Th2 responses (8–12). In mice, splenic CD8α+ DCs (8, 9, 13) induce Th1 responses, while the CD8α− myeloid DCs skew toward Th2 responses (10, 11). Therefore, it is possible that a given pathogen may induce a given type of immune response, by selectively activating a particular DC subset. In this study, we investigated this hypothesis using LPS from two different strains of bacteria: 1) E. coli LPS, which signals through the Toll-like receptor 4 (TLR4) complex (14, 15), and induces Th cells that secrete high levels of IFN-γ in vivo (16, 17); 2) LPS from the extracellular, Gram-negative bacterium Porphyromonas gingivalis that is a causative agent of adult periodontitis, a chronic inflammatory disease of the oral mucosa (18–28). P. gingivalis LPS appears less dependent on TLR4 signaling than E. coli LPS (18–25). This property is attributed mainly to the unique lipid A motif of P. gingivalis LPS, which contains unusually branched and relatively long fatty acids (19, 20, 23, 24, 25). Unlike enteric LPS, P. gingivalis LPS has been reported to induce the symptoms of endotoxic shock in C3H/HeJ mice (19, 20, 23, 24), which have a point mutation in the gene that encodes TLR4, and are thus hyporesponsive to E. coli LPS (14, 15). Some clinical studies indicate that during adult periodontitis caused by P. gingivalis infections, there is a preponderance of Th2 cytokines and plasma cell infiltration (26–29). However, the reason for this is unknown. While it is becoming appreciated that different microbial products signal through distinct pattern recognition receptors (30–39), the consequences of such differential signaling on the type of adaptive immune response are not known. The present study was prompted by the possibility that the different LPS molecules may induce distinct patterns of immunities by targeting specific DC subsets via TLRs that are uniquely expressed on the DC subsets. Our data suggest that although the two LPS induce potent clonal expansion of Ag-specific CD4+ and CD8+ T cells in mice, they elicit strikingly different cytokine profiles in the T cells. Furthermore, these two LPS molecules appear to do this by eliciting different cytokines by the CD8α+ and CD8α− DCs.
Materials and Methods
Mice
OT-2 TCR transgenic mice (strain 426-6), generated by W. Heath (Walter & Eliza Hall Institute, Melbourne, Australia) and F. Carbone (Monash University, Melbourne, Australia), were obtained from J. Kapp (Emory University, Atlanta, GA). OT-1 TCR transgenic mice were purchased from The Jackson Laboratory (Bar Harbor, ME). C57BL/6 mice, B6.PL.Thy-1a (B6.PL) mice, and C3H/HeJ mice were purchased from The Jackson Laboratory. C3H/HeN mice were purchased from Harlan Sprague Dawley (Indianapolis, IN). All mice were kept in microisolator cages in a specificpathogen free facility. For adoptive transfers, age-matched, male C57BL/6 or B6.PL.Thy-1a recipients were given 2.5–5 × 106 of either OT-2 cells or OT-1 TCR transgenic T cells i.v.
LPS purification
P. gingivalis strain A7436 and E. coli strain 25922 were cultured under identical conditions and LPS purified, as previously described (40). LPS extraction was achieved by the hot-phenol-water method (41), followed by further purification using isopycnic density gradient centrifugation. Briefly, 10 g (wet weight) bacterial cell pellet was suspended in 35 ml pyrogen-free water, and then 35 ml 90% phenol at 65°C was added dropwise for 20 min and stirred constantly. The aqueous phase was separated by centrifugation at 7000 × g for 20 min and collected. This process was repeated, and the aqueous phase was pooled and dialyzed against deionized water for 3 days. The dialyzed LPS preparation was then subjected to cesium chloride isopycnic density gradient centrifugation (in 0.5837 g CsCl2 4.4 ml of the LPS preparation) at 42,000 rpm for 72 h in a Beckman (Palo Alto, CA) L-60 Ultracentrifuge. The refractive indices of the gradient fractions were determined with a refractometer (Milton Roy, Rochester, NY), and values were converted to density (grams per milliliter). Fractions containing LPS (density fractions between 1.42 and 1.52 g/ml) were pooled, dialyzed against distilled water for 3 days, lyophilized, and stored at room temperature. LPS was analyzed for protein by the Pierce (Rockford, IL) bicinchoninic acid protein assay. LPS samples were also separated by SDS-PAGE and stained for protein with Coomassie blue. Selected samples were also subjected to proteinase K digestion and nuclease treatment and reanalyzed by SDS-PAGE to confirm the purity of the LPS moieties (data not shown).
Endotoxin-free OVA
Chicken OVA (Sigma, St. Louis, MO) was freshly prepared in PBS and depleted of the endotoxin activity (measured by LAL QCL-1000 kit from BioWhittaker, Walkersville, MD), using the Detoxi-Gel Affinity Pack Columns (Pierce). After depletion, the endotoxin level was below the limit of detection of the LAL QCL-1000 kit (<0.1 EU).
Injections
Reconstituted mice (three to five per group) were injected either i.p. or in the footpad, with either 2 mg OVA in saline (Baylor Hospital, Dallas, TX), or 2 mg OVA + 25 µg E. coli LPS, or 2 mg OVA + 25 µg P. gingivalis LPS. Endotoxin activity in saline was measured by LAL QCL-1000 kit, and observed to be below the detection limit. Before mixing with OVA, LPS was sonicated extensively, to ensure uniform mixing of micelles. Footpad injections were given in a volume of 25 µd. i.p. injections were given in a volume of 100 µd.
Flow cytometry
For analyses of OT-2 cells, cell suspensions were prepared from the draining popliteal lymph nodes or spleens, and incubated on ice with PE-labeled anti-Thy-1.2 (BD PharMingen, San Diego, CA), FITC-labeled Vα2 (BD PharMingen), CyChrome-labeled CD4 (BD PharMingen), and biotin-la-beled Vβ5 (BD PharMingen), followed by streptavidin allophycocyanin (ALPC; BD PharMingen). In some of the experiments, we simply used Thy-1.2 vs CD4. For analyses of OT-1 cells, cell suspensions of draining popliteal lymph nodes or spleens were stained with PE-labeled anti-Thy-1.2 (BD PharMingen), FITC-labeled Vα2 (BD PharMingen), and biotin-labeled CD8 (BD PharMingen), followed by streptavidin ALPC (BD PharMingen). In some of the experiments, we simply used Vα2 vs CD8. DCs were stained with FITC-labeled CD11c (BD PharMingen), in combination with PE-labeled CD11b (BD PharMingen), or biotin-labeled CD8α (BD PharMingen), followed by streptavidin ALPC (BD PharMingen).
In vitro cultures
Four days after priming with OVA or OVA + LPS, 2.5–5 × 105 popliteal lymph node cells (footpad injections) or splenocytes (i.p. injections) were plated in triplicate in 96-well round-bottom plates (Costar, Cambridge, MA) in 200 µd RPMI complete medium supplemented with 5% FBS, together with different concentrations of OVA, or OVA peptide (SIINFEKL). Proliferative responses were assessed after 72 h of culture in a humidified atmosphere of 5% CO2 in air. Cultures were pulsed with 1 µCi [3H]thy-midine for 12 h, and incorporation of the radionucleotide was measured by β-scintillation spectroscopy. For cytokine assays, aliquots of culture supernatants were removed after 72 h, pooled, and assayed for the presence of IFN-γ, IL-2, IL-4, IL-5, and IL-10 by ELISA.
Cytokine ELISAs
IFN-γ, IL-2, IL-10, IL-4, IL-5, IL-6, IL-12(p70), and TNF-α were quantified by ELISA kits from BD PharMingen, and IL-13 was measured by an ELISA kit from R&D Systems (Minneapolis, MN).
Purification of DCs
CD11c+CD8α+ and CD11c+CD8α− DC subsets were purified from spleens, as follows. Spleens of C57BL/6 mice were dissected, cut into small fragments, and then digested with collegenase D (0.5 mg/ml; Boehringer Mannheim, Mannheim, Germany) and DNase I (40 mg/ml; Boehringer Mannheim) in RPMI 1640 medium supplemented with 5% FCS for 10 min at 37°C Digested fragments were washed twice in PBS/5% FCS. Then, the CD11c+ DCs were enriched using the CD11c+ microbeads from Miltenyi Biotech (San Diego, CA). This enrichment process was repeated twice, and the resulting purity of CD11c+ cells after two rounds was >90%. The enriched DCs were stained with FITC-conjugated CD11c (BD PharMingen) and PE-conjugated CD8α+ (BD PharMingen) and sorted into the CD11c+CD8α+ and CD11c+CD8α− subsets, using a FACSVantage flow cytometer (BD Biosciences, San Jose, CA), equipped with Enterprise 11 laser (Coherent Radiatin, Palo Alto, CA). In some experiments, CD11c+ DCs were enriched from the spleens of C3H/HeN and C3H/HeJ mice using the CD11c+ microbeads; the purity of such enriched DCs was ~90%.
Induction of cytokines from DC subsets
DCs were cultured in RPMIc + 5% FBS with GM-CSF (20 ng/ml) + IFN-γ (20 ng/ml), in the presence of either E. coli LPS or P. gingivalis LPS for 24 or 48 h, and the cytokines were secreted in the supernatants assayed by ELISA.
Results
E. coli LPS and P. gingivalis LPS enhance Ag-specific Th responses in vivo
We examined whether LPS from E. coli and P. gingivalis could enhance Ag-specific Th responses against a soluble protein, such as OVA. To investigate this, we used OVA-specific, MHC class II-restricted (I-Ab), αβ TCR transgenic mice (OT-2 mice) (42). In these mice, the CD4+ OVA-specific T cells express Vα2 and Vβ5. TCR transgenic T cells were adoptively transferred into Thy-1 congenic B6.PL.Thy-1a (B6.PL) mice, such that they constituted a small, but detectable proportion of all T cells (43). In this system, the fate of OVA-specific, transgenic T cells was followed using the Thy-1.2 Ab, which stains the transferred cells, but not the host cells. Cells with the phenotype Thy-1.2+ CD4+ Vα2+ Vβ5+ are considered OVA-specific CD4+ T cells. In some of the experiments, we simply used Thy-1.2 in combination with CD4, to detect the OVA-specific T cells.
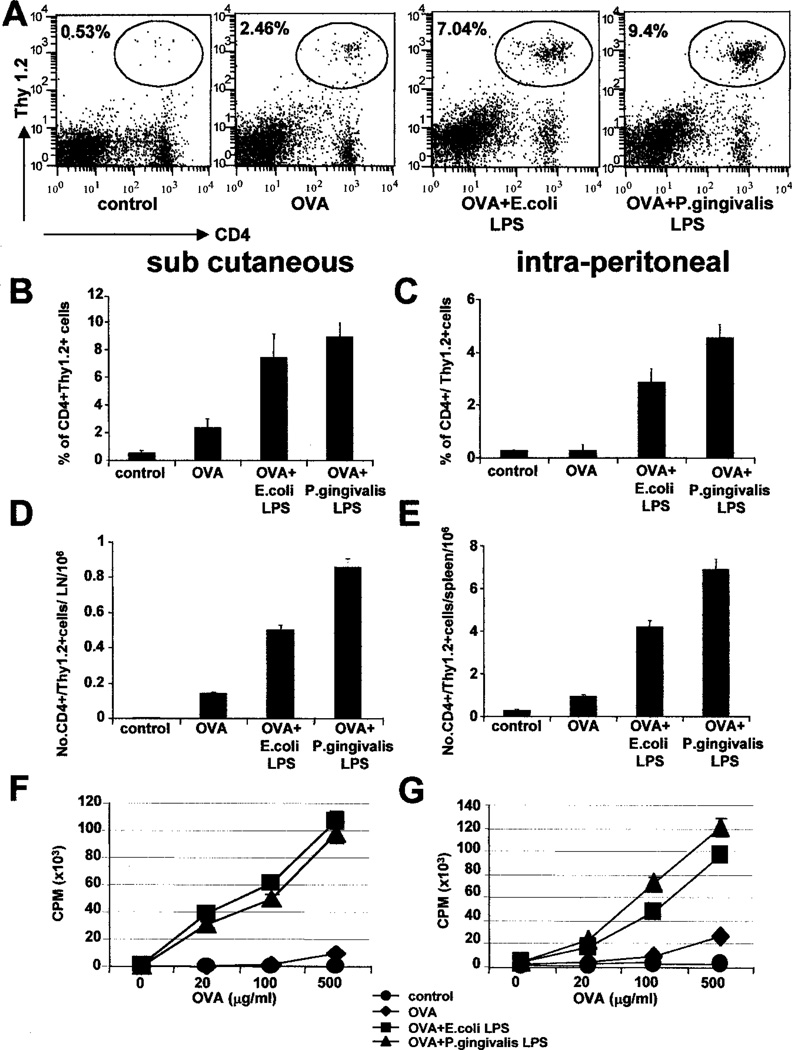
The reconstituted mice were injected with 2 mg soluble OVA alone, or OVA + E. coli LPS, or OVA + P. gingivalis LPS, either i.p. or in the footpads (Fig. 1). Before the experiment, OVA was depleted of endotoxin contamination, using the Detoxi Columns, and tested for endotoxin using the QCL-1000 kit. The CD4+ OVA-specific T cell response either in the draining lymph nodes or in the spleen was monitored by flow cytometry (Fig. 2A). Injection of OVA elicited a significant clonal expansion of the Thy-1.2+ CD4+ T cells in the draining lymph nodes of mice, which received footpad injections (Fig. 2, A, B, and D). Similar expansions were observed with the i.p. route of injection (Fig. 2, C and E). Both E. coli LPS and P. gingivalis LPS could significantly enhance the percentage and absolute numbers of Thy-1.2+ CD4+ T cells, regardless of the route of injection (Fig. 2). However, P. gingivalis LPS was marginally better thanE. coli LPS in enhancing the clonal expansion (7% OVA + E. coli LPS vs 9.4% OVA + P. gingivalis LPS; Fig. 2). Thus, both types of LPS, injected with OVA, enhanced the clonal expansion of OVA-specific T cells. No detectable clonal expansion was observed, when either type of LPS was injected alone (data not shown).
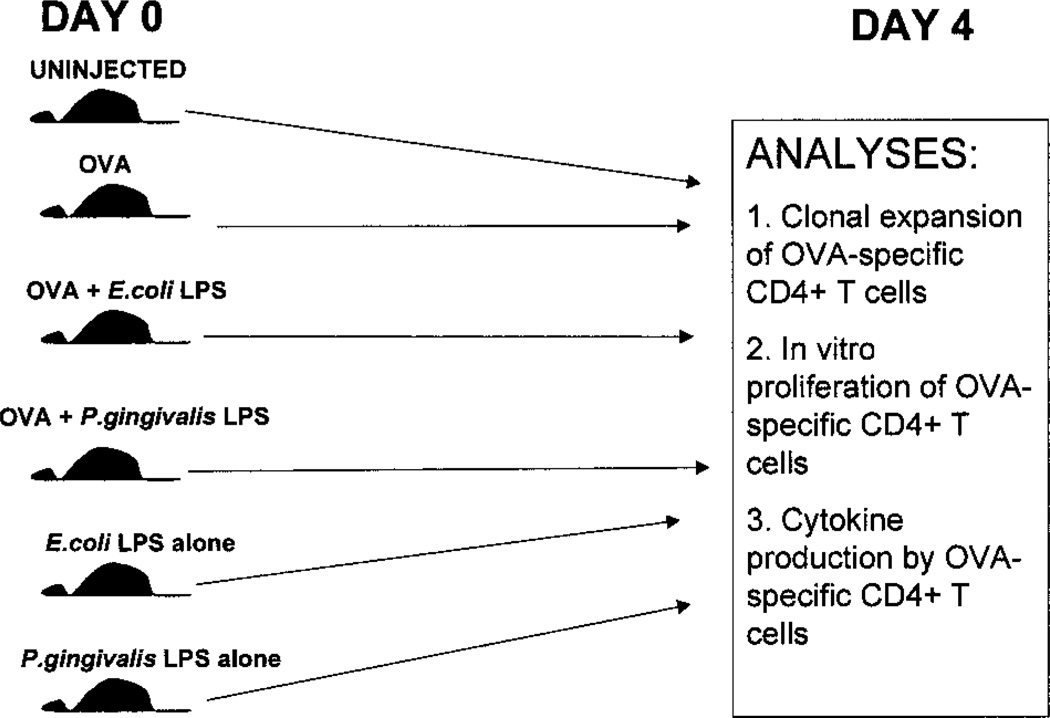
FIGURE 1.
Experimental design. B6.PL.Thy-1a (B6.PL) mice, or C57BL/6 mice that were reconstituted with OT-2 cells were injected with either soluble OVA, soluble OVA + E. coli LPS, soluble OVA + P. gingivalis LPS, E. coli LPS alone, or P. gingivalis LPS alone i.p. or in the footpad. Four days later, the spleens or draining lymph nodes were removed for phenotypic and functional analyses, including clonal expansion of OVA-specific CD4+ T cells, in vitro proliferation of OVA-specific CD4+ T cells, and cytokine production by the OVA-specific CD4+ T cells.
FIGURE 2.
E. coli LPS and P. gingivalis LPS enhance Ag-specific Th responses in vivo. B6.PL.Thy-1a (B6.PL) mice reconstituted with OT-2 transgenic T cells were immunized with soluble OVA, OVA + E. coli LPS, or OVA + P. gingivalis LPS, either in the footpad (s.c; B, D, and F), or i.p. (C,E, and G). Four days later, the draining popliteal lymph nodes (footpad injections) or spleens (i.p. route) were removed, and the clonal expansion of OVA-specific CD4+ T cells was assessed by flow cytometry, by staining with Thy-1.2 vs CD4. A, Flow cytometry profiles from day 4 of the response in the popliteal lymph nodes, from a representative experiment. B and C, The percentage expansion of OVA-specific CD4+ T cells (Thy-1.2+, CD4+) in the draining lymph nodes (B) and the spleens (C) at day 4. Both E. coli LPS and P. gingivalis LPS significantly enhance the clonal expansion, regardless of the route of injection. Data represent the means of four mice per group from one representative experiment. SDs are indicated, and differences between OVA group and the OVA + LPS groups are highly significant (p < 0.01). D andE, The absolute numbers of Thy-1.2+ CD4+ cells per popliteal lymph node (D) or per spleen (E) at day 4. Data represent the means of four mice per group from one representative experiment. SDs are indicated, and differences between OVA group and the OVA + LPS groups are highly significant (p< 0.01). F and G, In vitro restimulation of OVA-specific T cells expanded in vivo, by footpad (F), or i.p. (G) injections. Four days after priming, single cell suspensions from the draining popliteal lymph nodes (F) or spleens (G) were restimulated with varying concentrations of OVA for 72 h, and pulsed with 3H for 12 h. Note that injections of E. coli LPS alone or P. gingivalis LPS alone did not result in significant clonal expansion or in vitro proliferation. A–G, Representative of 10 independent experiments.
Previous work has shown that productive T cell immunity is elicited only when the Ag is injected with an adjuvant, and that injections of soluble Ags only result in a transient and abortive clonal expansion, in which Ag-specific T cells cannot be efficiently restimulated in vitro with protein or peptide (16, 43, 44). We thus examined the in vitro proliferative capacity of the OVA-specific T cells from the various cohorts of mice, by culturing single cell suspensions of the draining lymph nodes with varying concentrations of OVA. As shown in Fig. 2, F and G, mice that received an injection of OVA + E. coli LPS, or OVA + P. gingivalis LPS, had greatly enhanced responses compared with those that received OVA alone (Fig. 2, F and G). Either type of LPS alone did not result in any significant proliferation (data not shown).
E. coli LPS and P. gingivalis LPS induce distinct types of Ag-specific Th responses in vivo
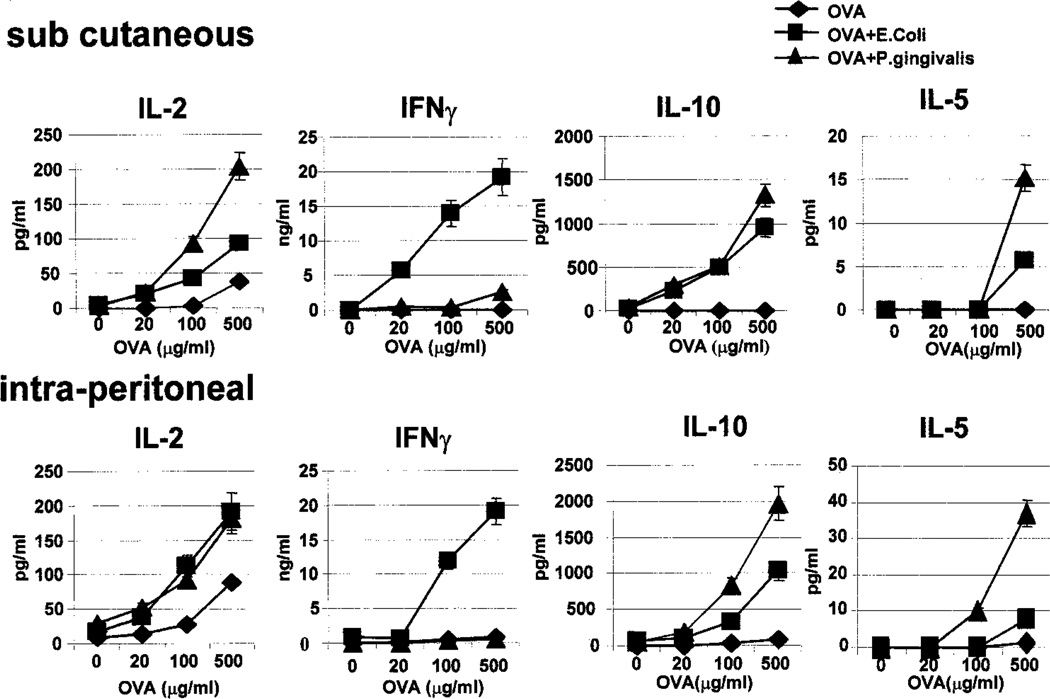
Cytokine production by Ag-specific T cells was measured by assaying the culture supernatants from the cultures described above for IL-2, IFN-γ, IL-4, IL-10, and IL-5. Assessment of cytokine production in these cultures revealed significant differences between mice injected with OVA, OVA + E. coli LPS, and OVA + P. gingivalis LPS (Fig. 3). In cultures from mice injected with OVA alone, there was little, if any, IL-2, IFN-γ, IL-10, IL-4, or IL-5 produced. In contrast, in cultures from mice injected with OVA + E. coli LPS, there was significant IL-2, IL-10, and very high levels of IFN-γ produced by the Ag-specific T cells. Neither IL-4 nor IL-5 could be detected. However, in cultures from mice injected with OVA + P. gingivalis LPS, there was a striking diminution of IFN-γ production, despite significant production of IL-2 and the Th2 cytokines IL-10 and IL-5 (Fig. 3). In fact, the level of IFN-γ was as low as that observed with OVA alone. Therefore, while both types of LPS elicit potent clonal expansion of Ag-specific CD4+ T cells in vivo, E. coli LPS + OVA induces a Th1-like response, characterized by high levels of IFN-γ. In contrast, P. gingivalis LPS + OVA induces a response that is essentially devoid of IFN-γ, and characterized by significant levels of IL-10 and IL-5, regardless of the route of injection (Fig. 3). Either type of LPS alone did not result in any cytokine production in these cultures (data not shown). No significant levels of IL-4 could be detected in any of the conditions, and this may reflect the Th1 bias of the C57BL/6 strain studied. Failure to detect IL-4 was not a peculiarity of the transgenic system because similar cytokine profiles were observed in experiments using nontransgenic mice (data not shown).
FIGURE 3.
E. coli LPS and P. gingivalis LPS induce distinct types of Ag-specific Th responses in vivo. Culture supernatants from the cultures described in Fig. 2, F and G, were assayed for IL-2, IFN-γ, IL-10, IL-5, and IL-5 with ELISA. Each data point represents mean ± SD of triplicate wells, from a typical experiment. Differences in IL-2 production between the E. coli LPS and the P. gingivalis LPS groups are not significant; IL-5 production in the P. gingivalis LPS group at 500 µg/ml OVA is significant (p< 0.05; detection limit of ELISA assay is 6 pg/ml). Note that injections of E. coli LPS alone or P. gingivalis LPS alone did not result in significant cytokine production. Representative of 10 independent experiments.
Another cytokine that is a strong hallmark of Th2 responses is IL-13 (4–7). We wondered whether P. gingivalis LPS induced IL-13 from Ag-specific Th cells in vivo. In the cultures described above, P. gingivalis LPS induced much higher levels of IL-13 than E. coli LPS (Fig. 4). Interestingly, although there is a titration of IL-13 production with the dose of OVA, even when no OVA was used there is some basal level of IL-13 production. Whether this is due to IL-13 production from other cell types (e.g., NK T cells) is presently under investigation. Therefore, in this system, P. gingivalis LPS, while shifting the balance toward the Th2 pathway, does not appear to elicit a classic Th2 response that is characterized by IL-4 production.
FIGURE 4.
P. gingivalis LPS induces much higher levels of IL-13 than E. coli LPS. B6.PL.Thy-1a (B6.PL) mice reconstituted with OT-2 transgenic T cells were immunized with soluble OVA, OVA + E. coli LPS, or OVA + P. gingivalis LPS. Four days after priming, single cell suspensions from the draining popliteal lymph nodes or spleens were restimulated with varying concentrations of OVA for 72 h, and assayed for IL-13 production. Each data point represents mean ± SD of triplicate wells, from a typical experiment. Differences between the E. coli LPS and the P. gingivalis LPS groups are highly significant (p < 0.01). Representative of three independent experiments.
E. coli LPS and P. gingivalis LPS enhance Ag-specific CD8+ T cell responses in vivo
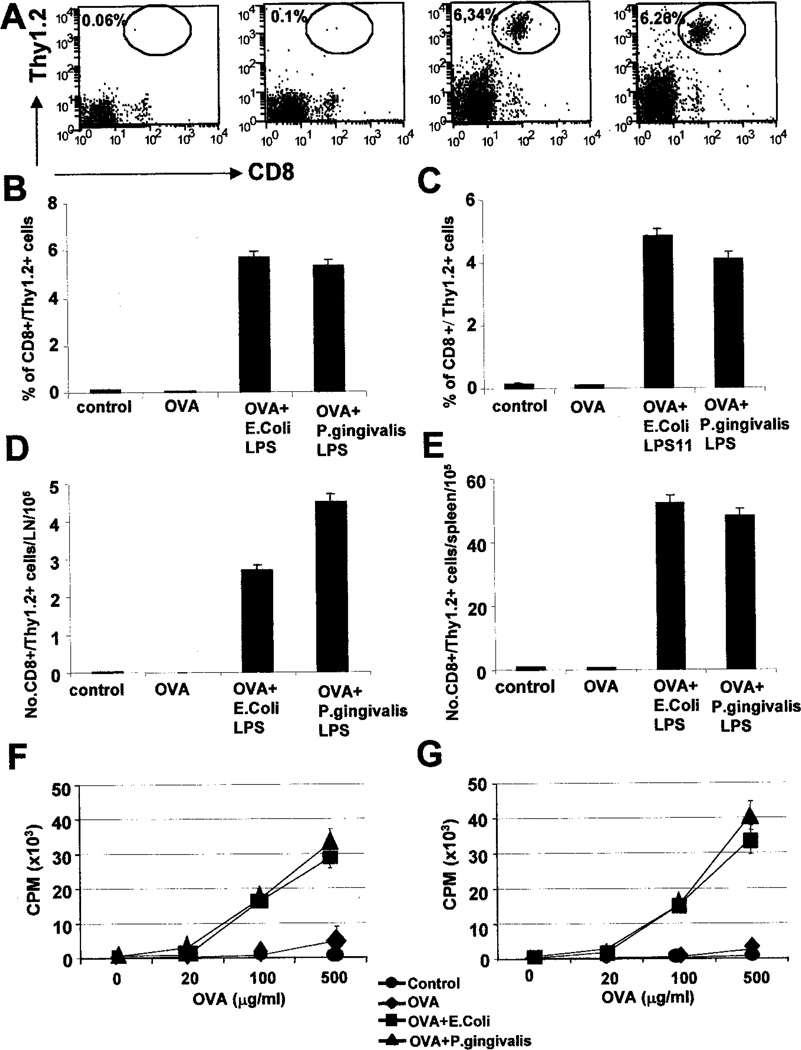
The strikingly different Th responses induced by E. coli LPS and P. gingivalis LPS suggested that there may be differences in Ag-specific CD8+ T cell responses. We investigated this using OT-1 mice (H-2Kb-restricted, OVA-specific TCR transgenic mice) (45, 46). A total of 2.5 × 106 or 5 × 106 spleen cells from OT-1 mice (B6.PL, Thy-1.2) was adoptively transferred into B6.PL (Thy-1.1) hosts. Cohorts of host mice were injected with either OVA, OVA + E. coli LPS, or OVA + P. gingivalis LPS. Clonal expansion of OVA-specific CD8+ T cells (CD8+Thy-1.2+) was assessed by flow cytometry (Fig. 5, A–E). Both E. coli LPS + OVA and P. gingivalis LPS + OVA enhanced the clonal expansion of OVA-specific CD8+ T cells, >60-fold (Fig. 5, A–E). LPS alone had no detectable effect (data not shown).
FIGURE 5.
E. coli LPS and P. gingivalis LPS enhance Ag-specific CD8+ T cell responses in vivo. C57BL/6 mice or B6.PL.Thy-1a (B6.PL) mice reconstituted with OT-1 transgenic T cells were immunized with soluble OVA, OVA + E. coli LPS, or OVA + P. gingivalis LPS, either in the footpad (s.c; B, D, and F) or i.p. (C,E, and G). Four days later, the draining popliteal lymph nodes (footpad injections) or spleens (i.p. route) were removed, and the clonal expansion of OVA-specific CD8+ T cells was assessed by flow cytometry, by staining with CD8 vs Vα2, or CD8 vs Thy-1.2 vs Vα2 (A). B and C, The percentage expansion of OVA-specific CD8+ T cells (CD8+ Thy-1.2+) in the draining lymph nodes (B) and the spleens (C) at day 4. Both E. coli LPS and P. gingivalis LPS significantly enhance the clonal expansion, regardless of the route of injection. Data represent the means of four mice per group from one representative experiment. SDs are indicated, and differences between OVA group and the OVA + LPS groups are highly significant (p < 0.001). D and E, The absolute numbers of OVA-specific CD8+ T cells per popliteal lymph node (D) or per spleen (E) at day 4. Data represent the means of four mice per group from one representative experiment. SDs are indicated, and differences between OVA group and the OVA + LPS groups are highly significant (p < 0.001). F and G, In vitro restimulation of OVA-specific CD8+ T cells expanded in vivo, by footpad (F), or i.p. (G) injections. Four days after priming, single cell suspensions from the draining popliteal lymph nodes (F) or spleens (G) were restimulated with varying concentrations of OVA for 72 h, and pulsed with 3H for 12 h. Note that injections ofE. coli LPS alone or P. gingivalis LPS alone did not result in significant clonal expansion, or in vitro proliferation. Representative of three independent experiments.
We then examined the in vitro proliferative capacity of the OVA-specific CD8+ T cells from the various cohorts of mice, by culturing single cell suspensions of the draining lymph nodes (s.c. route) or spleen (i.p.) with varying concentrations of OVA. As shown in Fig. 5, F and G, mice that received an injection of either E. coli LPS + OVA or P. gingivalis LPS + OVA had greatly enhanced responses, compared with those that received OVA alone. Identical results were obtained when class I-restricted OVA peptide (SIINFEKL) was used to restimulate the cells in vitro, suggesting that it was indeed the CD8+ OVA-specific T cells that proliferated in culture (data not shown). LPS alone did not result in any significant proliferation (data not shown).
E. coli LPS and P. gingivalis LPS induce distinct types of Ag-specific CD8+ T cell responses in vivo
We next examined the cytokines produced in these cultures by ELISA. As observed with the CD4+ OT-2 cells, CD8+ OT-1 cells stimulated with OVA alone did not secrete significant levels of IL-2, IFN-γ, IL-10, or IL-5 (Fig. 6). Cells from mice injected with E. coli LPS + OVA produced very high levels of IFN-γ and significant IL-10, but no IL-5 (Fig. 6). In contrast, cells from mice injected with P. gingivalis LPS + OVA produced strikingly lower levels of IFN-γ, and significant levels of IL-10 and IL-5 (Fig. 6), consistent with the cytokine patterns observed with OT-2 cells (Fig. 3). Identical results were obtained when SIINFEKL peptide was used to restimulate the cells in vitro (data not shown). LPS alone did not result in any significant cytokine production (data not shown). No significant levels of IL-4 were detected, and this may reflect the Th1 bias of the C57BL/6 strain.
FIGURE 6.
E. coli LPS and P. gingivalis LPS induce distinct types of Ag-specific CD8+ T cell responses in vivo. Culture supernatants from the cultures described in Fig. 5, E and F, were assayed for IL-2, IFN-γ, IL-10, IL-5, and IL-5 with ELISA. Note that injections of E. coli LPS alone or P. gingivalis LPS alone did not result in significant cytokine production. Each data point represents mean ± SD of triplicate wells, from a typical experiment. Differences in IL-2 production between the E. coli LPS and the P. gingivalis LPS groups are not significant; IL-5 production in the P. gingivalis LPS group at 500 µg/ml OVA is significant (p < 0.05; detection limit of ELISA assay is 6 pg/ ml). Note that injections of E. coli LPS alone or P. gingivalis LPS alone did not result in significant cytokine production. Representative of three independent experiments.
Both E. coli LPS and P. gingivalis LPS activate CD8α+ and CD8α− DC subsets in vivo
Adjuvants and certain microbial products, such as LPS, are known to activate DCs, thereby enhancing T cell immunity (47–51). In this study, we investigated whether both types of LPS were capable of activating DC subsets in vivo. C57BL/6 mice were injected with 25 µg E. coli LPS, or P. gingivalis LPS, either s.c, i.p., or i.v., and sacrificed 6 h later. Spleens and lymph nodes were collected, and the expression of activation markers (CD80, CD86, and CD40) on DCs was determined. CD8α+ and CD8α− DC subsets are well characterized (8–13, 47, 50–53), and may derive from different lineages or may simply reflect different developmental stages of the same lineage (8, 9, 13). CD8α+ and CD8α− DCs from the spleens of PBS-treated, control mice express significant levels of CD80, CD86, and CD40, as reported previously (50). However, upon injection of either type of LPS, there was a significant up-regulation of CD80, CD86, and CD40 on both DC subsets (Fig. 7). Therefore, both types of LPS appear to activate the CD8α+ and CD8α− DC subsets in vivo. Similar results were obtained with the DC subsets in the lymph nodes (data not shown).
FIGURE 7.
BothE. coli LPS and P. gingivalis LPS activate CD8α+ and CD8α− DC subsets in vivo. C57BL/6 mice were injected with either PBS (open red histograms) E. coli LPS, or P. gingivalis LPS (open green histograms), either i.v. or i.p., and 6 h later, the expression of CD80, CD86, and CD40 was assessed on gated, splenic CD11c+CD8α+ and CD11c+CD8α− DC subsets by flow cytometry. Isotype controls are filled purple histograms. Data are representative of three experiments.
E. coli LPS, but not P. gingivalis LPS, induces IL-12(p70) in CD8α+ DCs
It has been previously shown that splenic CD8α+ DCs can be induced to secrete IL-12 by various microbial products (8–10, 50–52), and that this IL-12 is influential in the elicitation of Th1 responses by the CD8α+ DC subset (10). In this study, we wished to determine whether both types of LPS could induce biologically active IL-12(p70) in CD8α+ DCs. Splenic CD11c+CD8α+ and CD11c+CD8α− DC subsets were isolated by flow cytometry, and cultured for 48 h with either E. coli LPS, P. gingivalis LPS, or alone. Then the supernatants were assayed for IL-12, IL-6, and TNF-α by ELISA. Both types of LPS induced IL-6 and TNF-α in both DC subsets (Fig. 8). However, only E. coli LPS induced IL-12 in the CD8α+ DC subset (Fig. 8). Therefore, while both types of LPS could activate both DC subsets, only the E. coli LPS could elicit the Th-1-inducing cytokine IL-12, this being consistent with the strikingly different Th responses induced by E. coli LPS and P. gingivalis LPS in vivo. Whether P. gingivalis LPS induces Th2 responses by simply failing to elicit IL-12 in DCs, or whether it actually stimulates the production of a Th2-inducing cytokine is not known. Good candidates for Th2-inducing cytokines are IL-10 and IL-4. However, significant levels of IL-10 or IL-4 could not be consistently detected in these cultures (data not shown).
FIGURE 8.
E. coli LPS, but not P. gingivalis LPS, induces IL-12 in CD8α+ DCs. Splenic CD11c+CD8α+ and CD11c+CD8α− DCs were isolated from C57BL/6 mice by microbead enrichment, followed by flow cytometry, and stimulated in vitro with 10 µg/ml E. coli LPS or P. gingivalis LPS. Culture supernatants were assayed for IL-12, IL-6, or TNF-α− 24 h later. Representative of 10 independent experiments.
P. gingivalis LPS is less dependent on TLR4 signaling than E. coli LPS
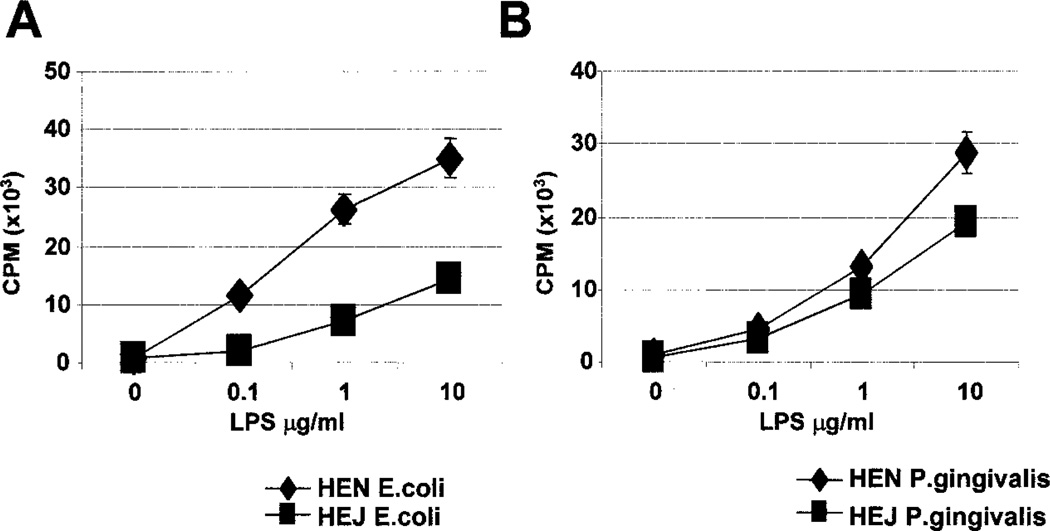
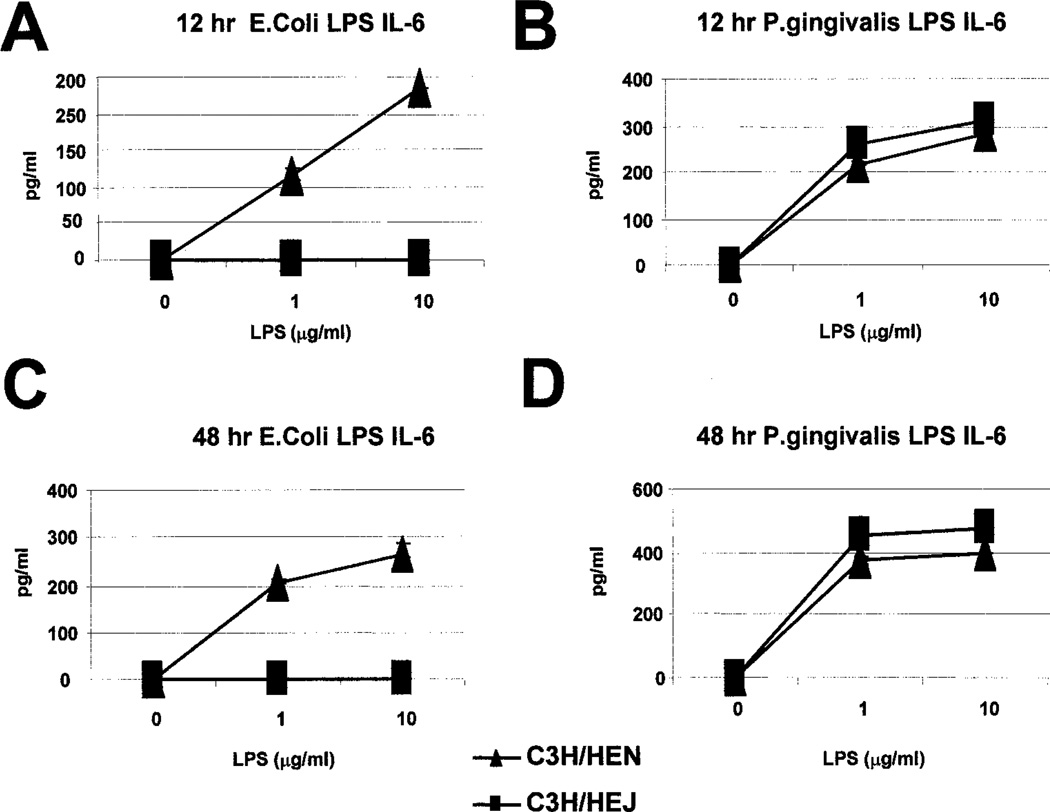
While E. coli LPS mediates its effects by signaling though TLR4 (14, 15), P. gingivalis LPS is reported signal through a TLR4- independent mechanism (18–25). Thus, we examined the effect(s) of either type of LPS on proliferation of splenocytes from C3H/ HeJ mice, which have a point mutation in the TLR4 gene, and wild-type (C3H/HeN) mice. C3H/HeJ splenocytes cultured with E. coli LPS were greatly impaired in their proliferative capacity, compared with the C3H/HeN controls (Fig. 9A). In contrast, C3H/HeJ splenocytes cultured with P. gingivalis LPS were only modestly impaired in their proliferative capacity, compared with the C3H/ HeN controls (Fig. 9B). Consistent with this, production of IL-6 induced by E. coli LPS was greatly impaired in C3H/HeJ splenocytes, compared with C3H/HeN splenocytes (Fig. 10). However, production of IL-6 induced by P. gingivalis LPS was not impaired in C3H/HeJ mice (Fig. 10). Furthermore, induction of IL-12 and other cytokines, by E. coli LPS, from enriched CD11c+ splenic DCs was severely impaired in C3H/HeJ mice; in contrast, cytokine production induced by P. gingivalis LPS was only moderately affected (Fig. 11). Therefore, while E. coli LPS signaling is largely dependent on TLR4, P. gingivalis LPS appears less dependent on this pathway.
FIGURE 9.
P. gingivalis LPS is less dependent on TLR4 signaling than E. coli LPS. Splenocytes from C3H/HeJ mice and C3H/HeN mice were cultured with varying concentrations of E. coli LPS or P. gingivalis LPS for 72 h. The cultures were pulsed with 3H during the last 12 h of culture. Representative of three independent experiments.
FIGURE 10.
IL-6 production by splenocytes stimulated with E. coli LPS or P. gingivalis LPS in C3H/HeN and C3H/HeJ mice. Splenocytes from C3H/HeJ mice and C3H/HeN mice were cultured with varying concentrations of E. coli LPS (A and C) or P. gingivalis LPS (B and D) for 12 h (A and B) or 48 h (C and D). IL-6 was measured by cytokine ELISA. Representative of three independent experiments.
FIGURE 11.
Cytokine production by splenic DCs from C3H/HeJ and C3H/HeN mice stimulated in vitro withE. coli LPS or P. gingivalis LPS. CD11c+ DCs, enriched from the spleens of mice, were cultured with either type of LPS for 24 h, and secretion of IL-12, IL-6, and TNF-α was measured by cytokine ELISA. Representative of three separate experiments.
Discussion
Our current study demonstrates that LPS from different bacteria elicit distinct types of adaptive immune responses against an exogenous soluble Ag, such as OVA. E. coli LPS induces OVA-specific T cell responses characterized by very high levels of the Th1 cytokine IFN-γ. In contrast, P. gingivalis LPS induces OVA-specific T cell responses, characterized by significant levels of the Th2 cytokines IL-13, IL-5, and IL-10, but lower levels of IFN-γ. Consistent with these findings, E. coli LPS induces IL-12 production from CD8α+ DCs, while P. gingivalis LPS does not. These observations are consistent with several previous reports implicating a central role for IL-12 in IFN-γ induction by E. coli LPS (54, 55). Despite their strikingly different effects on IL-12 production, both LPS molecules do activate the CD8α+ and CD8α− DC subsets, as judged by the production of IL-6 and TNF-α, and the up-regulation of costimulatory molecules.
Our data also suggest that P. gingivalis LPS is less dependent on TLR4 signaling than E. coli LPS. This is consistent with several previous reports, and is attributed mainly to the unique lipid A motif of P. gingivalis LPS, which contains unusually branched and relatively long fatty acids, compared with lipid A from enteric bacterial LPS (19, 20, 23, 24). Unlike enteric LPS, P. gingivalis LPS has been reported to induce endotoxic shock in C3H/HeJ mice (19, 20, 23, 24). These data are consistent with recent findings from Vogel’s group (25), which suggest that P. gingivalis LPS activates murine peritoneal macrophages through a TLR4-independent mechanism to elicit the production of IL-6 and TNF-α, but not IL-12(p70); in contrast, E. coli LPS does induce IL-12(p70) in macrophages, in addition to IL-6 and TNF-α (25).
There could be several mechanisms by which E. coli LPS and P. gingivalis LPS interact with DCs to elicit distinct adaptive immune responses, but in principle two opposite kinds of mechanisms can be envisaged. First, a single DC subset may interpret distinct microbial or environmental signals differently to yield distinct types of adaptive immune responses (56–58). This instructive model is based on recent reports, which suggest that apparently homogenous DC subsets can differentially transduce signals from distinct microbial products or cytokines, to elicit distinct Th cytokines in vitro (59–62). In this model, a given DC subset can elicit virtually any Th response, depending on the environmental stimulus. In the second selective model, functionally distinct DC subsets may express distinct repertoires of pattern recognition receptors that recognize different classes of microbial products (62). Thus, recognition of a particular microbial product by a given DC subset will result in a given Th response, different from that induced by another microbe that activated a different DC subset. At present, we have limited evidence to discriminate between these scenarios, with respect to the differential effects of E. coli and P. gingivalis LPS. Clearly, both types of LPS activate the CD8α+ and CD8α− DC subsets (Fig. 8), suggesting that the signaling receptors for the two types of LPS are present on both subsets. E. coli LPS activates both DC subsets, most likely through TLR4, and P. gingivalis LPS activates both DC subsets, perhaps through a different TLR, expressed on both DC subsets. In this context, our additional data suggest that mRNA for TLR4 is expressed on both the CD8α+ and the CD8α− DC subsets, as measured by PCR (data not shown). Further studies are however needed to ascertain surface expression of the various TLRs on the different DC subsets. Whatever receptors through which they signal, our data suggest that two distinct LPS can elicit strikingly different patterns of adaptive immunity, most likely by stimulating different cytokines in DC subsets. These observations highlight a central role for distinct DC subsets for differentially interpreting signals from different microbial products and instructing the adaptive immune response to mount distinct patterns of immunity. Although the present study compares the effect of a single molecule (LPS) from different microbes, it is tempting to speculate that this may represent a model for how different microorganisms stimulate distinct types of immunities. Thus, the overall type of response against the whole microorganism may well be an integration of all microbial signals through all receptors and all APC subsets. Whether live pathogens or their extracts do indeed obey the rules observed for individual microbial products remains to be established. Finally, these data also point to the use of P. gingivalis LPS, or its derivatives such as lipid A or synthetic analogues in the elicitation of therapeutic Th2-like responses in clinical settings.
Acknowledgments
We thank Bill Heath, Frank Carbone, and Judith Kapp for OT-2 mice, and Chryshanthi Joseph for excellent technical assistance.
Footnotes
This work was supported by grants from National Institutes of Health (DK57665-01, AI48638-01, and 1R21AI/DE48154-01) and the Baylor Health Care System Foundation to B.P. C.W.C. acknowledges the support of the Baylor Oral Health Foundation and the National Institutes of Health (1R21AI/DE48154-01). T.V.D. is funded by National Institutes of Health (DE 13191).
Abbreviations used in this paper: DC, dendritic cell; ALPC, allophycocyanin; TLR, Toll-like receptor.
References
- 1.Abbas AK, Janeway CA., Jr Immunology: improving on nature in the twenty-first century. Cell. 2000;100:129. doi: 10.1016/s0092-8674(00)81689-x. [DOI] [PubMed] [Google Scholar]
- 2.Mosmann TR, Coffman RL. TH1 and TH2 cells: different patterns of lymphokine secretion lead to different functional properties. Annu. Rev. Immunol. 1989;7:145. doi: 10.1146/annurev.iy.07.040189.001045. [DOI] [PubMed] [Google Scholar]
- 3.Seder RA, Paul WE. Acquisition of lymphokine-producing phenotype by CD4+ T cells. Annu. Rev. Immunol. 1994;12:635. doi: 10.1146/annurev.iy.12.040194.003223. [DOI] [PubMed] [Google Scholar]
- 4.Constant SL, Bottomly K. Induction of Th1 and Th2 CD4+ T cell responses: the alternative approaches. Annu. Rev. Immunol. 1997;15:297. doi: 10.1146/annurev.immunol.15.1.297. [DOI] [PubMed] [Google Scholar]
- 5.Carter LL, Swain SL. Single cell analyses of cytokine production. Curr. Opin. Immunol. 1997;9:177. doi: 10.1016/s0952-7915(97)80132-x. [DOI] [PubMed] [Google Scholar]
- 6.O’Garra A. Cytokines induce the development of functionally heterogeneous T helper cell subsets. Immunity. 1998;8:275. doi: 10.1016/s1074-7613(00)80533-6. [DOI] [PubMed] [Google Scholar]
- 7.Murphy KM, Ouyang W, Farrar JD, Yang J, Ranganath S, Asnagli H, Afkarian M, Murphy TL. Signaling and transcription in T helper development. Annu. Rev. Immunol. 2000;18:451. doi: 10.1146/annurev.immunol.18.1.451. [DOI] [PubMed] [Google Scholar]
- 8.Moser M, Murphy KM. Dendritic cell regulation of TH1-TH2 development. Nat. Immunol. 2000;3:199. doi: 10.1038/79734. [DOI] [PubMed] [Google Scholar]
- 9.Pulendran B, Maraskovsky E, Banchereau J, Maliszewski C. Modulating the immune response with dendritic cells and their growth factors. Trends Immunol. 2001;1:41. doi: 10.1016/s1471-4906(00)01794-4. [DOI] [PubMed] [Google Scholar]
- 10.Maldonado-Lopez R, De Smedt T, Michel P, Godfroid J, Pajak B, Heirman C, Thielemans K, Leo O, Urbain J, Moser M. CD8α + and CD8α − subclasses of dendritic cells direct the development of distinct T helper cells in vivo. J. Exp. Med. 1999;189:587. doi: 10.1084/jem.189.3.587. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Pulendran B, Smith JL, Caspary G, Brasel K, Pettit D, Maraskovsky E, Maliszewski CR. Distinct dendritic cell subsets differentially regulate the class of immune response in vivo. Proc. Natl. Acad. Sci. USA. 1999;96:1036. doi: 10.1073/pnas.96.3.1036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Rissoan MC, Soumelis V, Kadowaki N, Grouard G, Briere F, de Waal Malefyt R, Liu YJ. Reciprocal control of T helper cell and dendritic cell differentiation. Science. 1999;283:1183. doi: 10.1126/science.283.5405.1183. [DOI] [PubMed] [Google Scholar]
- 13.Shortman K, Vremec D, Corcoran LM, Georgopoulos K, Lucas K, Wu L. The linkage between T-cell and dendritic cell development in the mouse thymus. Immunol. Rev. 1998;165:39. doi: 10.1111/j.1600-065x.1998.tb01228.x. [DOI] [PubMed] [Google Scholar]
- 14.Poltorak A, He X, Smirnova I, Liu MY, Huffel CV, Du X, Birdwell D, Alejos E, Silva M, Galanos C, et al. Defective LPS signaling in C3H/HeJ and C57BL/10ScCr mice: mutations in Tlr4 gene. Science. 1998;282:2085. doi: 10.1126/science.282.5396.2085. [DOI] [PubMed] [Google Scholar]
- 15.Qureshi ST, Lariviere L, Leveque G, Clermont S, Moore KJ, Gros P, Malo D. Endotoxin-tolerant mice have mutations in Toll-like receptor 4 (Tlr4) J. Exp. Med. 1999;189:615. doi: 10.1084/jem.189.4.615. [Published erratum appears in 1999 J. Exp. Med. 189:1518.] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Pape KA, Khoruts A, Mondino A, Jenkins MK. Inflammatory cytokines enhance the in vivo clonal expansion and differentiation of antigen-activated CD4+ T cells. J. Immunol. 1997;159:591. [PubMed] [Google Scholar]
- 17.Khoruts A, Mondino A, Pape KA, Reiner SL, Jenkins MK. A natural immunological adjuvant enhances T cell clonal expansion through a CD28-dependent, interleukin (IL)-2-independent mechanism. J. Exp. Med. 1998;187:225. doi: 10.1084/jem.187.2.225. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Yamamoto M, Ono K, Mochizuki S, Nishihara Y, Makimura M, Otake S. Mitogenic activity of lipopolysaccharide from Bacteroides intermedius against C3H/HeJ mice spleen cells. Nichidai Koko Kagaku. 1990;16:332. [PubMed] [Google Scholar]
- 19.Ogawa T, Shimauchi H, Uchida H, Mori Y. Stimulation of splenocytes in C3H/HeJ mice with Porphyromonas gingivalis lipid A in comparison with enterobacterial lipid A. Immunobiology. 1996;196:399. doi: 10.1016/S0171-2985(96)80062-3. [DOI] [PubMed] [Google Scholar]
- 20.Ogawa T, Nakazawa M, Masui K. Immunopharmacological activities of the nontoxic monophosphoryl lipid A of Porphyromonas gingivalis . Vaccine. 1996;14:70. doi: 10.1016/0264-410x(95)00128-n. [DOI] [PubMed] [Google Scholar]
- 21.Shimauchi H, Ogawa T, Uchida H, Yoshida J, Ogoh H, Nozaki T, Okada H. Splenic B-cell activation in lipopolysaccharide-non-responsive C3H/HeJ mice by lipopolysaccharide of Porphyromonas gingivalis . Experientia. 1996;52:909. doi: 10.1007/BF01938879. [DOI] [PubMed] [Google Scholar]
- 22.Tanamoto K, Azumi S, Haishima Y, Kumada H, Umemoto T. The lipid A moiety of Porphyromonas gingivalis lipopolysaccharide specifically mediates the activation of C3H/HeJ mice. J. Immunol. 1997;158:4430. [PubMed] [Google Scholar]
- 23.Ogawa T, Uchida H, Amino K. Immunobiological activities of chemically defined lipid A from lipopolysaccharides of Porphyromonas gingivalis . Microbiology. 1994;140:1209. doi: 10.1099/13500872-140-5-1209. [DOI] [PubMed] [Google Scholar]
- 24.Ogawa T. Immunobiological properties of chemically defined lipid A from lipopolysaccharide of Porphyromonas (Bacteroides) gingivalis . Eur. J. Biochem. 1994;219:737. doi: 10.1111/j.1432-1033.1994.tb18552.x. [DOI] [PubMed] [Google Scholar]
- 25.Hirschfeld M, Weis JJ, Toshchakov V, Salkowski CA, Cody MJ, Ward DC, Qureshi N, Michalek SM, Vogel SN. Signaling by Toll-like receptor 2 and 4 agonists results in differential gene expression in murine macrophages. Infect. Immun. 2001;69:1477. doi: 10.1128/IAI.69.3.1477-1482.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Mackler BF, Frostad KB, Robertson PB, Levy BM. Immunoglobulin bearing lymphocytes and plasma cells in human periodontal disease. J. Periodontal Res. 1977;1:37. doi: 10.1111/j.1600-0765.1977.tb00107.x. [DOI] [PubMed] [Google Scholar]
- 27.Okada H, Kida T, Yamagami H. Identification and distribution of immunocompetent cells in inflamed gingiva of human chronic periodontitis. Infect. Immun. 1983;41:365. doi: 10.1128/iai.41.1.365-374.1983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Yamamoto M, Fujihashi K, Hiroi T, McGhee JR, Van Dyke TE, Kiyono H. Molecular and cellular mechanisms for periodontal diseases: role of Th1 and Th2 type cytokines in induction of mucosal inflammation. J. Periodontal Res. 1997;32:115. doi: 10.1111/j.1600-0765.1997.tb01391.x. [DOI] [PubMed] [Google Scholar]
- 29.Cutler CW, Stanford TW, Abraham C, Cederberg RA, Boardman TJ, Ross C. Clinical benefits of oral irrigation for periodontitis are related to reduction of pro-inflammatory cytokine levels and plaque. J. Clin. Periodontol. 2000;27:134. doi: 10.1034/j.1600-051x.2000.027002134.x. [DOI] [PubMed] [Google Scholar]
- 30.Janeway CA., Jr How the immune system works to protect the host from infection: a personal view. Proc. Natl. Acad Sci. USA. 2001;98:7461. doi: 10.1073/pnas.131202998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Medzhitov R, Janeway C., Jr Innate immune induction of the adaptive immune response. Cold Spring Harbor Symp. Quant. Biol. 1999;64:429. doi: 10.1101/sqb.1999.64.429. [DOI] [PubMed] [Google Scholar]
- 32.Medzhitov R, Janeway C., Jr Innate immune recognition: mechanisms and pathways. Immunol. Rev. 2000;173:89. doi: 10.1034/j.1600-065x.2000.917309.x. [DOI] [PubMed] [Google Scholar]
- 33.Aderem A, Ulevitch RJ. Toll-like receptors in the induction of the innate immune response. Nature. 2000;406:782. doi: 10.1038/35021228. [DOI] [PubMed] [Google Scholar]
- 34.Underhill DM, Ozinsky A, Hajjar AM, Stevens A, Wilson CB, Bassetti M, Aderem A. The Toll-like receptor 2 is recruited to macrophage phagosomes and discriminates between pathogens. Nature. 1999;401:811. doi: 10.1038/44605. [DOI] [PubMed] [Google Scholar]
- 35.Takeuchi O, Hoshino K, Kawai T, Sanjo H, Takada H, Ogawa T, Takeda K, Akira S. Differential roles of TLR2 and TLR4 in recognition of Gramnegative and Gram-positive bacterial cell wall components. Immunity. 1999;11:443. doi: 10.1016/s1074-7613(00)80119-3. [DOI] [PubMed] [Google Scholar]
- 36.Hemmi H, Takeuchi O, Kawai T, Kaisho T, Sato S, Sanjo H, Matsumoto M, Hoshino K, Wagner H, Takeda K, Akira S. A Toll like receptor recognizes bacterial DNA. Nature. 2000;408:659. doi: 10.1038/35047123. [DOI] [PubMed] [Google Scholar]
- 37.Hayashi F, Smith KD, Ozinsky A, Hawn TR, Yi EC, Goodlett DR, Eng JK, Akira S, Underhill DM, Aderem A. The innate immune response to bacterial flagellin is mediated by Toll-like receptor 5. Nature. 2001;410:1099. doi: 10.1038/35074106. [DOI] [PubMed] [Google Scholar]
- 38.Means TK, Lien E, Yoshimura A, Wang S, Golenbock DT, Fenton MJ. The CD14 ligands lipoarabinomannan and lipopolysaccharide differ in their requirement for Toll-like receptors. J. Immunol. 1999;163:6748. [PubMed] [Google Scholar]
- 39.Yoshimura A, Lien E, Ingalls RR, Tuomanen E, Dziarski R, Golenbock D. Cutting edge: recognition of Gram-positive bacterial cell wall components by the innate immune system occurs via Toll-like receptor 2. J. Immunol. 1999;163:1. [PubMed] [Google Scholar]
- 40.Cutler CW, Eke PI, Genco CA, Van Dyke TE, Arnold RR. Hemin-induced modifications of the antigenicity and hemin-binding capacity of Porphyromonas gingivalis lipopolysaccharide. Infect. Immun. 1996;64:2282. doi: 10.1128/iai.64.6.2282-2287.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Westphal O, Jann K. Bacterial lipopolysaccharides: extraction with phenol water and further applications of the procedure. Methods Carbohydr. Chem. 1965;5:83. [Google Scholar]
- 42.Barnden MJ, Allison J, Heath WR, Carbone FR. Defective TCR expression in transgenic mice constructed using cDNA-based α- and β-chain genes under the control of heterologous regulatory elements. Immunol. Cell Biol. 1998;76:34. doi: 10.1046/j.1440-1711.1998.00709.x. [DOI] [PubMed] [Google Scholar]
- 43.Kearney ER, Walunas TL, Karr RW, Morton PA, Loh DY, Bluestone JA, Jenkins MK. Antigen-dependent clonal expansion of a trace population of antigen-specific CD4+ T cells in vivo is dependent on CD28 costimulation and inhibited by CTLA-4. J. Immunol. 1995;155:1032. [PubMed] [Google Scholar]
- 44.Pulendran B, Smith JL, Jenkins M, Schoenborn M, Maraskovsky E, Maliszewski CR. Prevention of peripheral tolerance by a dendritic cell growth factor: flt3 ligand as an adjuvant. J. Exp. Med. 1998;188:2075. doi: 10.1084/jem.188.11.2075. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Hogquist KA, Jameson SC, Heath WR, Howard JL, Bevan MJ, Carbone FR. T cell receptor antagonist peptides induce positive selection. Cell. 1994;76:17. doi: 10.1016/0092-8674(94)90169-4. [DOI] [PubMed] [Google Scholar]
- 46.Martin S, Bevan MJ. Antigen-specific and nonspecific deletion of immature cortical thymocytes caused by antigen injection. Eur. J. Immunol. 1997;27:2726. doi: 10.1002/eji.1830271037. [DOI] [PubMed] [Google Scholar]
- 47.Reis e Sousa C, Germain RN. Analysis of adjuvant function by direct visualization of antigen presentation in vivo: endotoxin promotes accumulation of antigen-bearing dendritic cells in the T cell areas of lymphoid tissue. J. Immunol. 1999;162:6552. [PubMed] [Google Scholar]
- 48.Allison AC. Immunological adjuvants and their modes of action. Arch. Immunol. Ther. Exp. 1997;45:141. [PubMed] [Google Scholar]
- 49.Raychaudhuri S, Rock KL. Fully mobilizing host defense: building better vaccines. Nat. Biotechnol. 1998;16:1025. doi: 10.1038/3469. [DOI] [PubMed] [Google Scholar]
- 50.Pulendran B, Lingappa J, Kennedy MK, Smith J, Teepe M, Rudensky A, Maliszewski CR, Maraskovsky E. Developmental pathways of dendritic cells in vivo: distinct function, phenotype, and localization of dendritic cell subsets in FLT3 ligand-treated mice. J. Immunol. 1997;159:2222. [PubMed] [Google Scholar]
- 51.Reis e Sousa C, Hieny S, Scharton-Kersten T, Jankovic D, Charest RN, Germain H, Sher A. In vivo microbial stimulation induces rapid CD40 ligand-independent production of interleukin 12 by dendritic cells and their redistribution to T cell areas. J. Exp. Med. 1997;186:1819. doi: 10.1084/jem.186.11.1819. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Ohteki T, Fukao T, Suzue K, Maki C, Ito M, Nakamura M, Koyasu S. Interleukin 12-dependent interferon γ production by CD8α + lymphoid dendritic cells. J. Exp. Med. 1999;189:1981. doi: 10.1084/jem.189.12.1981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.De Smedt T, Pajak B, Muraille E, Lespagnard L, Heinen E, De Baetselier P, Urbain J, Leo O, Moser M. Regulation of dendritic cell numbers and maturation by lipopolysaccharide in vivo. J. Exp. Med. 1996;184:1413. doi: 10.1084/jem.184.4.1413. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Wysocka M, Kubin M, Vieira LQ, Ozmen L, Garotta G, Scott P, Trinchieri G. Interleukin-12 is required for interferon-γ production and lethality in lipopolysaccharide-induced shock in mice. Eur. J. Immunol. 1995;25:672. doi: 10.1002/eji.1830250307. [DOI] [PubMed] [Google Scholar]
- 55.Magram J, Connaughton SE, Warrier RR, Carvajal DM, Wu CY, Ferrante J, Stewart C, Sarmiento U, Faherty DA, Gately MK. IL-12-deficient mice are defective in IFNγ production and type 1 cytokine responses. Immunity. 1996;4:471. doi: 10.1016/s1074-7613(00)80413-6. [DOI] [PubMed] [Google Scholar]
- 56.Kalinski P, Hilkens CM, Wierenga EA, Kapsenberg ML. T-cell priming by type-1 and type-2 polarized dendritic cells: the concept of a third signal. Immunol. Today. 1999;20:561. doi: 10.1016/s0167-5699(99)01547-9. [DOI] [PubMed] [Google Scholar]
- 57.Lanzavecchia A, Sallusto F. Dynamics of T lymphocyte responses: intermediates, effectors, and memory cells. Science. 2000;290:92. doi: 10.1126/science.290.5489.92. [DOI] [PubMed] [Google Scholar]
- 58.Pulendran B, Palucka K, Banchereau J. Sensing pathogens and tuning immune responses. Science. 2001;293:253. doi: 10.1126/science.1062060. [DOI] [PubMed] [Google Scholar]
- 59.D’Ostiani CF, Del Sero G, Bacci A, Montagnoli C, Spreca A, Mencacci A, Ricciardi-Castagnoli P, Romani L. Dendritic cells discriminate between yeasts and hyphae of the fungus Candida albicans: implications for initiation of T helper cell immunity in vitro and in vivo. J. Exp. Med. 2000;191:1661. doi: 10.1084/jem.191.10.1661. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Kadowaki N, Antonenko S, Yiu-Nam Lau J, Liu YJ. Natural interferon-α/β-producing cells link innate and adaptive immunity. J. Exp. Med. 2000;192:219. doi: 10.1084/jem.192.2.219. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Vieira PL, de Jong EC, Wierenga EA, Kapsenberg ML, Kalinski P. Development of Th1-inducing capacity in myeloid dendritic cells requires environmental instruction. J. Immunol. 2000;164:4507. doi: 10.4049/jimmunol.164.9.4507. [DOI] [PubMed] [Google Scholar]
- 62.Cella M, Salio M, Sakakibara Y, Langen H, Julkunen I, Lanzavecchia A. Maturation, activation, and protection of dendritic cells induced by doublestranded RNA. J. Exp. Med. 1999;189:821. doi: 10.1084/jem.189.5.821. [DOI] [PMC free article] [PubMed] [Google Scholar]