Abstract
The clinical features of prostate cancer do not provide an accurate determination of patients undergoing biochemical relapse and are therefore not suitable as indicators of prognosis for recurrence. New molecular markers are needed for proper pre-treatment risk stratification of patients. Our aim was to assess the value of altered expression of syndecan-1 and -2 as a marker for predicting biochemical relapse in patients with clinically localized prostate cancer treated by radical prostatectomy. The expression of syndecan-1 and -2 was examined by immunohistochemical staining in a series of 60 paraffin-embedded tissue samples from patients with localized prostate cancer. Ten specimens from patients with benign prostatic hyperplasia were used as non-malignant controls. Semiquantitative analysis was performed to evaluate the staining patterns. To investigate the prognostic value, Kaplan–Meier survival curves were performed and compared by a log-rank test. In benign samples, syndecan-1 was expressed in basal and secretory epithelial cells with basolateral membrane localisation, whereas syndecan-2 was expressed preferentially in basal cells. In prostate cancer samples, the expression patterns of both syndecans shifted to granular-cytoplasmic localisation. Survival analysis showed a significant difference (P<0.05) between normal and altered expression of syndecan-1 and -2 in free prostate-specific antigen recurrence survival curves. These data suggest that the expression of syndecan-1 and -2 can be used as a prognostic marker for patients with clinically localized prostate cancer, improving the prostate-specific antigen recurrence risk stratification.
Keywords: biochemical recurrence, prostate cancer, syndecans
Introduction
Prostate cancer is the most frequently diagnosed cancer and the second leading cause of cancer mortality in American males.1 Although the majority of these cases are early-stage, localized tumours, patients may have different clinical outcomes after treatment. Several authors have reported that, within 10 years of definitive therapy, up to 50% of patients treated either by radical prostatectomy or radiation therapy will have biochemical failure that is evident upon follow-up.2 According to Pound et al.,3 34% of patients presenting with prostate-specific antigen (PSA) elevation after surgery will develop metastatic disease. At this level of progression, androgen deprivation therapy is indicated due to the androgen sensitivity of prostate cancer cells. Nevertheless, in some patients the disease will become androgen-insensitive, leading to a poor overall survival rate.
Several clinical features have been studied as prognostic parameters for recurrence. However, these factors have not provided an accurate determination of which patients will undergo biochemical relapse. Therefore, new molecular prognostic markers are needed for proper pre-treatment risk stratification.4 Recently, several immunohistochemical markers have been investigated for their prognostic significance for clinically localized disease. Cell adhesion molecules have been suggested as useful markers of recurrence because of their involvement in the transition from a non-invasive phenotype to an invasive one, which is a key step in tumour metastasis in a variety of carcinoma types.5 Among these proteins involved in cell adhesion mechanisms are syndecans, a four-member family of heparan sulphate proteoglycans expressed on adherent and non-adherent cells.6 Syndecans have been evaluated in tumour progression owing to their function in cell proliferation, cell–cell and cell–matrix adhesion, cell motility and invasiveness. These heparan sulphate proteoglycans are expressed in several cell types. Syndecan-1 is expressed mainly in epithelial cells, whereas syndecan-2 has been described in mesenchymal cells.6
Recent evidence indicates that the pattern of syndecan-1 expression is altered in a number of types of carcinomas, and down- or upregulation of syndecan-1 has been associated with progression and a poor prognosis.7 In prostate cancer, the expression of syndecan-1 has been investigated with contradictory results. In a previous study using immunohistochemical analysis, the expression of syndecan-1 was found to be directly associated with a poor prognosis.8 In addition, the overexpression of syndecan-1 was associated with a high Gleason score and a poor clinical outcome.9 Recently, Shariat et al.10 showed that the expression of syndecan-1 was directly correlated with the Gleason score and associated with a high risk of PSA progression after surgery. Nevertheless, the mechanisms involved remain largely unclear, and these differing results need to be addressed.
Syndecan-2 represents another candidate prognostic marker because of its relationship with invasiveness, adhesion, cell proliferation and migration.11 Moreover, the overexpression of syndecan-2 in colorectal cancer cell lines triggers the acquisition of a mesenchymal-like phenotype and increases migratory potential.12 However, the expression of syndecan-2 in prostate cancer cells has been poorly studied. Recently, we have found that syndecan-2 is localized to the membrane of epithelial prostate cells, and its expression pattern is altered in association with epithelial–mesenchymal transition markers and Gleason score.13 Other authors have suggested that syndecan-2 expression could serve as an additional prognostic marker to further stratify the risk of disease progression in patients with prostate cancer.14
The aim of this study was to use semiquantitative immunohistochemistry to evaluate the expression of syndecan-1 and -2 in prostate cancer samples from patients with clinically localized disease. We also investigated the relationship between syndecan expression and biochemical recurrence (BCR) to assess the potential role of syndecan expression as a predictor of disease relapse after radical prostatectomy.
Materials and methods
Patient samples
Samples from 60 patients with clinically localized prostate cancer who underwent radical prostatectomy were randomized and included in the study. Paraffin blocks were obtained from the Pathology Service of the Clinical Hospital of the University of Chile. Prostate specimens from 10 patients with benign prostatic hyperplasia (BPH) were used as non-malignant controls. Clinical records were checked for follow-up data. For these patients, the PSA level was measured every 3 months during the first year after surgery, twice a year for the next 3 years and annually thereafter. We considered BCR as two consecutive PSA measurements of greater than 0.4 ng ml−1 at any time during follow-up.
Immunohistochemistry
For immunohistochemical analysis, 5-µm sections of formalin-fixed and paraffin-embedded tissue were placed on slides coated with 3-aminopropyltriethoxysilane (Merk, Hohembrunn, Germany). Areas indicative of carcinoma were identified by a pathologist on corresponding slides stained with haematoxylin and eosin. Slides were deparaffinized in xylene before rehydration in a graded ethanol series. Antigen retrieval was achieved by boiling the slides in EDTA buffer (pH 8.0) in a pressure cooker for 60 min. Endogenous peroxidase activity was blocked by treatment with 3% hydrogen peroxide solution in methanol for 15 min. Non-specific protein binding was inhibited with a blocking solution provided by Zymed Laboratories (San Francisco, CA, USA). Slides were incubated with anti-syndecan-1 (monoclonal, clone 5F7; 1∶100 dilution; Novocastra Co., Newcastle, UK) or anti-syndecan-2 (polyclonal; 1∶1800 dilution; Contreras et al.12) primary antibody for 90 min at 37 °C. Negative immunohistochemical controls were generated by omitting the primary antibody. Tonsil and colon carcinoma samples were used as positive controls for syndecan-1 and -2, respectively. Primary antibody binding was identified by the immunoperoxidase technique using the secondary antibody avidin–biotin complex and a peroxidase substrate kit (Histostain-Plus Bulk Kit; Zymed Laboratories) according to the manufacturer's instructions. Next, the sections were treated with the chromogen 3,3′-diaminobenzidene tetrahydrochloride (Zymed Laboratories) to detect bound antibody complex. Finally, slides were counterstained with Mayer's haematoxylin, dehydrated and covered with cover slips and Entellan mounting medium (Merck, Darmstadt, Germany).
Evaluation of immunostaining
All specimens were analysed independently by two investigators who were unaware of the clinical outcomes of the patients. The evaluation was carried out in three representative zones previously selected by the pathologist, and disagreement during the assessment was overcome by consensus. Immunostaining of cancer cells was compared with that of normal epithelial cells in the same sample as an internal control. A semiquantitative analysis was performed to assess stained cells. To obtain a more accurate evaluation, we considered the localisation, intensity and proportion of positive cells. Localisation patterns were defined as membranous or cytoplasmic according to previous reports.8, 9, 10, 15 Intensity was scored as weak (+/+++), moderate (++/+++) or strong (+++/+++). The proportion of positive cells was evaluated as negative, <40%, 40–80% or >80%. We defined membrane localisation with moderate (++/+++) or strong (+++/+++) intensity and more than 40% of cells staining positively as normal expression. Aberrant expression was defined in cases not meeting one or more of these criteria.
Statistical analysis
The STATA 7.0 software package was used to carry out statistical analyses (Stata Corporation, College Station, TX, USA). Cancer recurrence-free survival analysis was performed using clinical, pathological and immunohistochemistry data through a Kaplan–Meier proportional risk model. Follow-up was calculated as the number of months from treatment until PSA recurrence, which was established as the variable event to construct the curves. The log-rank test was used to evaluate differences in recurrence-free survival probability between the groups. Univariate Cox analysis was used to reveal the ability of the variables to predict survival. In all analyses, P<0.05 was considered statistically significant.
Results
Patient characteristics
Clinical and pathological features of the sample donor patients are listed in Table 1. At a median follow-up of 50 months (range: 6–96 months), 30 patients (50%) had undergone BCR. The median interval from surgery to PSA relapse was 36 months (range: 6–60 months).
Table 1. Patient characteristics. Summary of the clinical features of 60 patients with localized prostate carcinoma.
| Patient characteristics | Clinical data |
|---|---|
| Age (years) | |
| Median (range) | 64 (47–73) |
| PSA (ng ml−1) | |
| Median (range) | 8 (2.5–23) |
| ≤4 | 1 (1.66%) |
| 4.1–10 | 39 (65%) |
| 10.1–20 | 17 (28.34%) |
| >20 | 3 (5%) |
| Clinical stage | |
| T1c | 32 (53.34%) |
| T2 | 28 (46.66%) |
| Surgical Gleason score | |
| Median (range) | 6 (5–9) |
| <7 | 31 (51.66%) |
| ≥7 | 29 (48.34%) |
Abbreviation: PSA, prostate-specific antigen.
Expression of syndecans
The immunohistochemical expression patterns of syndecan-1 and -2 are shown in Figures 1 and 2, respectively. There were differences in the expression of both syndecans in prostate cancer tissue. Negative controls without the primary antibody are shown in Figures 1a and 2a. Tonsil and colon carcinoma samples were used as positive controls for syndecan-1 and -2, respectively (Figures 1b and 2b). In BPH tissue samples, syndecan-1 staining was characteristically localized to the membrane of basal cells and the basolateral side of secretory epithelial cells, without expression in the adjacent stroma (Figure 1c). Syndecan-2 was found to be strongly expressed at the plasma membrane of basal cells. Stroma staining was also negative (Figure 2c). In prostate cancer cells, cytoplasmic altered expression of syndecan-1 and -2 was found in 64% and 67% of the samples respectively (Figures 1d and e and 2d and e, and Table 2).
Figure 1.
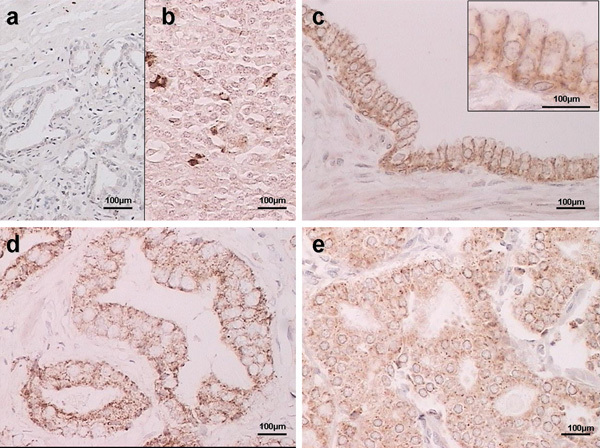
Representative immunostaining results showing syndecan-1 expression in prostate tissue. (a) Negative staining in prostate cancer (no primary antibody); (b) positive control (tonsil); (c) benign prostatic hyperplasia (BPH); (d) membrane staining in prostate cancer; (e) cytoplasmic staining in prostate cancer. Scale bars=100 µm.
Figure 2.
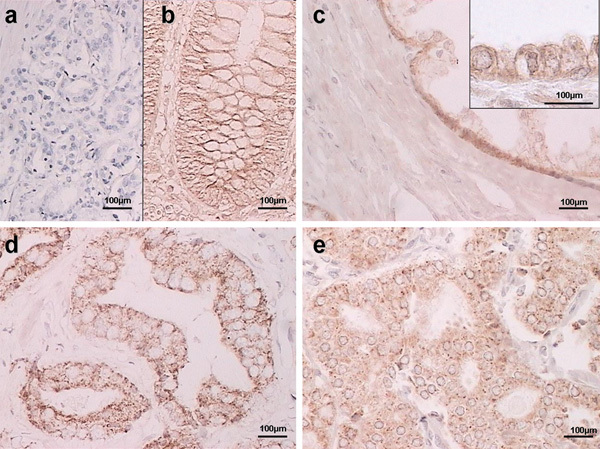
Representative immunostaining results showing syndecan-2 expression in prostate tissue. (a) Negative staining in prostate cancer (no primary antibody); (b) positive control (colon cancer sample); (c) benign prostatic hyperplasia (BPH); (d) membrane staining in prostate cancer; (e) cytoplasmic staining in prostate cancer. Scale bars=100 µm.
Table 2. Changes in the localisation and expression of syndecan-1 and -2 in prostate cancer tissue.
| Localisation | Expression | |
|---|---|---|
| Normal/altered | Weak/strong | |
| Syndecan-1 | 36/64 | 46/54 |
| Syndecan-2 | 33/67 | 21/79 |
Normal localisation: membrane staining. Altered localisation: cytoplasmic staining. Weak expression: 0 or +. Strong expression: ++ or +++. Data are expressed as the percentage of total patients.
Cancer survival rates
Previous studies have reported that the altered expression of syndecan-1 is associated with BCR. Hence, we analysed whether the expression of syndecan-1 and -2 was associated with BCR. As shown in Figure 3, the altered expression of both syndecan-1 (P<0.05) and syndecan-2 (P<0.05) was predictive of BCR. To assess whether syndecan-1 and/or syndecan-2 can be considered independent predictors, a Cox proportional hazards regression model was used with univariate analysis (Table 3). The univariate model indicated that altered syndecan-1 (P=0.045) and syndecan-2 (P=0.044) expression and elevated pre-treatment level of PSA (P=0.019) were predictors for BCR.
Figure 3.
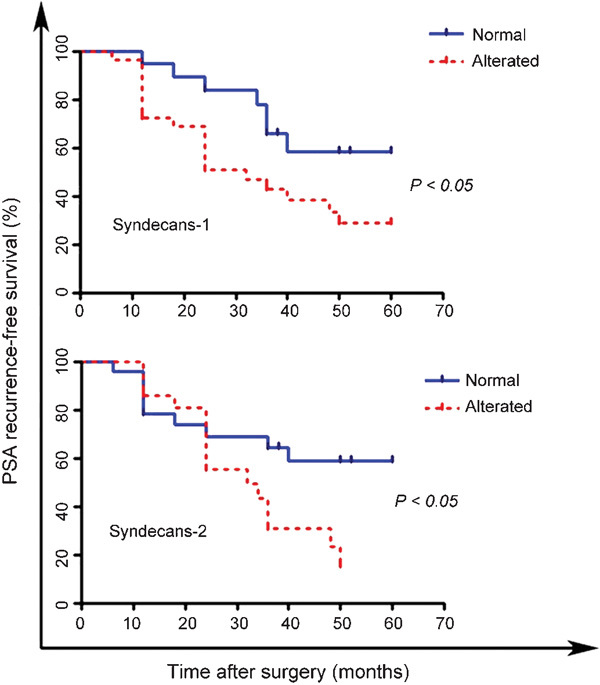
Kaplan–Meier analysis of patients with clinically localized prostate cancer showing normal or altered expression patterns of syndecan-1 and -2 expression. Patients with altered expression of both syndecans had a more rapid rate of PSA recurrence compared with patients with normal syndecan expression at follow-up (P<0.05, log-rank test). PSA, prostate-specific antigen.
Table 3. Univariate analysis. Several variables were analysed with a Cox proportional hazards regression model with univariate analysis. Altered expressions of syndecan-1 and syndecan-2 and elevated pre-treatment level of PSA were predictors of BCR by univariate analysis.
| Variable | Hazard ratio (95% CI) | P value |
|---|---|---|
| Pre-treatment PSA (>20 versus 10–20 versus <10 ng ml−1) | 2.34 (1.15–4.76) | 0.019* |
| Gleason score (≥8 versus 7 versus ≤6) | 1.56 (0.87–2.81) | 0.134 |
| Tumour stage (T1 versus T2) | 2.10 (0.96–4.59) | 0.063 |
| Syndecan-1 expression (altered versus normal) | 2.54 (1.02–6.36) | 0.045* |
| Syndecan-2 expression (altered versus normal) | 2.35 (1.02–5.41) | 0.044* |
Abbreviations: BCR, biochemical recurrence; CI, confidence interval; PSA, prostate-specific antigen.
*Statistically significant.
Discussion
As a result of the increasing incidence rates of prostate cancer, management of patients with BCR has become a large and growing challenge in clinical practice.16 Nevertheless, determining the risk of rapid progression to bone metastasis is an issue that remains unsolved. Therefore, the introduction of markers that provide new insights into the understanding of prostate cancer progression is needed to avoid unnecessary treatments, predict disease course and develop more effective therapies.4
In this study, we assessed variation in the expression patterns of two heparan sulphate proteoglycans, syndecan-1 and -2, in prostate tissue. We confirmed that syndecan-1 and, interestingly, syndecan-2, were both expressed in epithelial cells of normal and pathological prostate tissue. Popovic et al.14 have also reported syndecan-2 expression in prostate cancer and prostatic intraepithelial neoplasia samples but not in normal prostate tissue. In our work, we found expression of syndecan-2 at the membranes of basal cells in non-malignant tissue (BPH samples). Differences with the work of Popovic may be related to the control tissue selected (normal versus BPH) and/or the different antibodies used in each study. The staining pattern of syndecan-2 observed in BPH samples shifts to cytoplasmic staining in malignant samples. In addition, the altered expression pattern of syndecan-2 was associated with a high risk of BCR by univariate analysis, suggesting that this syndecan is a predictor of prostate cancer progression following surgery.
In contrast, Shimada et al.17 reported low expression of syndecan-1 in basal cells of normal prostate glands and in cancerous prostate tissue from patients without hormonal treatment. However, patients with androgen deprivation therapy showed increased expression of syndecan-1, suggesting that this syndecan may be associated with androgen sensitivity.17 In the present work, syndecan-1 was found to be expressed in both BPH and prostate cancer samples. In BPH samples, the staining was observed mainly at the membrane, shifting to a cytoplasmic localisation in most of the prostate cancer samples. All prostate cancer samples used in this study were obtained from patients with localized carcinoma and without hormonal treatment. However, the antibody against syndecan-1 used here recognizes the external domain of the protein, staining newly synthesized cytoplasmic protein and membrane protein. There is no information about the epitope recognized by the antibody used in Shimada et al.'s work. Our results are in agreement with other reports that show an inverse correlation between plasma membrane localisation of syndecan-1 and high Gleason score,13, 15, 18 suggesting that this syndecan changes its subcellular localisation rather than its overall protein level during the progression of prostate cancer.
The decrease of syndecan-1 localized to the plasma membrane of cancer cells may be explained by the ability of syndecan-1 to undergo shedding of its ectodomain, a proteolytic cleavage event occurring in vivo as a highly regulated process during development, neoplasia and wound repair.19 Heparanase (HPSE), an extracellular enzyme involved in this shedding, has been shown to be overexpressed in several tumours, increasing the metastatic potential. HPSE regulates both the level and localisation of syndecan-1 within the tumour microenvironment by inducing its shedding from the tumour cell surface and its de novo synthesis. The regulation of syndecan-1 by HPSE has been observed in both human myeloma and breast cancer cell lines.20 In addition, Mahtouk et al.21 have reported, using cultured myeloma cells, that the expression of HPSE correlates with an increase in syndecan-1 gene expression and detection in the soluble fraction. However, knockdown of HPSE using siRNA results in a decrease of syndecan-1 production.22 Because there is evidence that prostate cancer cells overexpress HPSE, it is possible that the production of syndecan-1 is upregulated in these cells, and therefore, the low membrane expression observed may be due to activation of shedding mechanisms. In addition, the increased cytoplasmic localisation of syndecan-1 can be explained by de novo synthesis. This scenario would explain our results obtained using an antibody that binds to the external domain of syndecan-1. Moreover, an elevated level of serum syndecan-1 has been demonstrated to be associated with a poor clinical outcome in cases of lung cancer.23 The evidence presented in this work supports the idea that the syndecan-1 serum level may represent a novel clinical marker for prostate cancer.13, 14, 18
Unexpectedly, we were not able to find a coordinated downregulation of syndecan-1 membrane expression followed by an upregulation of syndecan-2 membrane expression as part of the epithelial–mesenchymal transition. Nonetheless, we observed increased syndecan-2 cytoplasmic expression in prostate cancer cells, which correlates with a high risk of biochemical recurrence. Syndecan-2 may undergo shedding, and the corresponding ectodomain may be acting as an angiogenic factor in gliomas.24 In addition, overexpression of syndecan-2 in colon cancer cells has been associated with downregulation of E-cadherin, a long-recognized feature of the epithelial–mesenchymal transition.12 However, the mechanisms involved in the cytoplasmic overexpression of syndecan-2 and the metastatic process remain unclear.
These results suggest that syndecan-1 and -2 are involved in the biology of prostate cancer, probably by promoting an adequate environment for the metastatic process. This regulation is correlated with the biochemical progression of the disease in clinically localized prostate cancer.
Author contributions
RL and FC contributed to biopsies selection and immunohistochemistry, IG contributed to histopathological analysis of biopsies, JF contributed to quantitative and statistical analysis of data, EO contributed to classification and analysis of clinical data of patients, and EAC and HRC contributed to experimental design, data analysis and manuscript writing.
Acknowledgments
This work was supported by Fondo Nacional de Ciencia y Tecnología (FONDECYT) project 11060500 (to HRC) and by PG/043/2006 from the University of Chile (to RL).
The authors declare no competing financial interest.
References
- Jemal A, Center MM, DeSantis C, Ward EM. Global patterns of cancer incidence and mortality rates and trends. Cancer Epidemiol Biomarkers Prev. 2010;19:1893–907. doi: 10.1158/1055-9965.EPI-10-0437. [DOI] [PubMed] [Google Scholar]
- Han M, Partin A, Pound C, Epstein J, Walsh PC. Long-term biochemical disease-free and cancer-specific survival following anatomic radical retropubic prostatectomy. The 15-years Johns Hopkins experience. Urol Clin North Am. 2001;28:555–65. doi: 10.1016/s0094-0143(05)70163-4. [DOI] [PubMed] [Google Scholar]
- Pound CR, Partin AW, Eisenberger MA, Chan DW, Pearson JD, et al. Natural history of progression after PSA elevation following radical prostatectomy. JAMA. 1999;281:1591–7. doi: 10.1001/jama.281.17.1591. [DOI] [PubMed] [Google Scholar]
- Tricoli JV, Schoenfeldt M, Conley B. Detection of prostate cancer and prediction progression: current and future diagnostic markers. Clin Cancer Res. 2004;10:3943–53. doi: 10.1158/1078-0432.CCR-03-0200. [DOI] [PubMed] [Google Scholar]
- Stewart D, Cooper C, Sikes R. Changes in extracellular matrix (EMC) and EMC-associated proteins in the metastatic progression of prostate cancer. Reprod Biol Endocrinol. 2004;2:1–13. doi: 10.1186/1477-7827-2-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu W, Litwack ED, Stanley MJ, Langford JK, Lander AD, et al. Heparan sulfate proteoglycans as adhesive and anti-invasive molecules. Syndecans and glypican have distinct functions. J Biol Chem. 1998;273:22825–32. doi: 10.1074/jbc.273.35.22825. [DOI] [PubMed] [Google Scholar]
- Mennerich D, Vogel A, Klaman I, Dahl E, Lichtner RB, et al. Shift of syndecan-1 expression from epithelial to stromal cells during progression of solid tumours. Eur J Cancer. 2004;40:1373–82. doi: 10.1016/j.ejca.2004.01.038. [DOI] [PubMed] [Google Scholar]
- Chen D, Adenekan B, Chen L, Vaughan ED, Gerald W, et al. Syndecan-1 expression in locally invasive and metastatic prostate cancer. Urology. 2004;63:402–7. doi: 10.1016/j.urology.2003.08.036. [DOI] [PubMed] [Google Scholar]
- Zellweger T, Ninck C, Mirlacher M, Annefeld M, Glass AG, et al. Tissue microarray analysis reveals prognostic significance of syndecan-1 expression in prostate cancer. Prostate. 2003;55:20–9. doi: 10.1002/pros.10209. [DOI] [PubMed] [Google Scholar]
- Shariat SF, Svatek RS, Kabbani W, Walz J, Lotan Y, et al. Prognostic value of syndecan-1 expression in patients treated with radical prostatectomy. BJU Int. 2008;101:232–7. doi: 10.1111/j.1464-410X.2007.07181.x. [DOI] [PubMed] [Google Scholar]
- Villena J, Berndt C, Granes F, Reina M, Vilaro S. Syndecan-2 expression enhances adhesion and proliferation of stably transfected Swiss 3T3 cells. Cell Biol Int. 2003;27:1005–10. doi: 10.1016/j.cellbi.2003.09.004. [DOI] [PubMed] [Google Scholar]
- Contreras HR, Fabre M, Granes F, Casaroli-Marano R, Rocamora N, et al. Syndecan-2 expression in colorectal cancer-derived HT-29 M6 epithelial cells induces a migratory phenotype. Biochem Biophys Res Commun. 2001;286:742–51. doi: 10.1006/bbrc.2001.5459. [DOI] [PubMed] [Google Scholar]
- Contreras HR, Ledezma RA, Vergara J, Cifuentes F, Barra C, et al. The expression of syndecan-1 and -2 is associated with Gleason score and epithelial–mesenchymal transition markers, E-cadherin and beta-catenin, in prostate cancer. Urol Oncol. 2009;28:534–40. doi: 10.1016/j.urolonc.2009.03.018. [DOI] [PubMed] [Google Scholar]
- Popovic A, Demirovic A, Spajic B, Stimac G, Kruslin B, et al. Expression and prognostic role of syndecan-2 in prostate cancer. Prostate Cancer Prostatic Dis. 2010;13:78–82. doi: 10.1038/pcan.2009.43. [DOI] [PubMed] [Google Scholar]
- Kiviniemi J, Kallajoki M, Kujala I, Matikainen MT, Alanen K, et al. Altered expression of syndecan-1 in prostate cancer. APMIS. 2004;112:89–97. doi: 10.1111/j.1600-0463.2004.apm1120202.x. [DOI] [PubMed] [Google Scholar]
- Ward JF, Moul JW. Rising prostate-specific antigen after primary prostate cancer therapy. Nat Clin Pract Urol. 2005;2:174–82. doi: 10.1038/ncpuro0145. [DOI] [PubMed] [Google Scholar]
- Shimada K, Nakamura M, de Velasco MA, Tanaka M, Ouji Y, et al. Syndecan-1, a new target molecule involved in progression of androgen-independent prostate cancer. Cancer Sci. 2009;100:1248–54. doi: 10.1111/j.1349-7006.2009.01174.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brimo F, Vollmer R, Friszt M, Corcos J, Bismar T. Syndecan-1 expression in prostate cancer and its value as biomarker for disease progression. BJU Int. 2010;106:418–23. doi: 10.1111/j.1464-410X.2009.09099.x. [DOI] [PubMed] [Google Scholar]
- Sanderson RD, Yang Y, Kelly T, MacLeod V, Dai Y, et al. Enzymatic remodeling of heparan sulfate proteoglycans within the tumor microenvironment: growth regulation and the prospect of new cancer therapies. J Cell Biochem. 2005;96:897–905. doi: 10.1002/jcb.20602. [DOI] [PubMed] [Google Scholar]
- Yang Y, Macleod V, Miao HQ, Theus A, Zhan F, et al. Heparanase enhances syndecan-1 shedding: a novel mechanism for stimulation of tumor growth and metastasis. J Biol Chem. 2007;282:13326–33. doi: 10.1074/jbc.M611259200. [DOI] [PubMed] [Google Scholar]
- Mahtouk K, Hose D, Raynaud P, Hundemer M, Jourdan M, et al. Heparanase influences expression and shedding of syndecan-1, and its expression by the bone marrow environment is a bad prognostic factor in multiple myeloma. Blood. 2007;109:4914–23. doi: 10.1182/blood-2006-08-043232. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lerner I, Baraz L, Pikarsky E, Meirovitz A, Edovitsky E, et al. Function of heparanase in prostate tumorigenesis: potential for therapy. Clin Cancer Res. 2008;14:668–76. doi: 10.1158/1078-0432.CCR-07-1866. [DOI] [PubMed] [Google Scholar]
- Joensuu H, Anttonen A, Eriksson M, Makitaro I, Alfthan H, et al. Soluble syndecan-1 and serum basic fibroblast growth factor are new prognostic factors in lung cancer. Cancer Res. 2002;62:5210–17. [PubMed] [Google Scholar]
- Fears CY, Gladson CL, Woods A. Syndecan-2 is expressed in the microvasculature of gliomas and regulates angiogenic processes in microvascular endothelial cells. J Biol Chem. 2006;281:14533–6. doi: 10.1074/jbc.C600075200. [DOI] [PubMed] [Google Scholar]


