Abstract
Sexually reproducing animals require an orchestrated communication between spermatozoa and the egg to generate a new individual. Capacitation, a maturational complex phenomenon that occurs in the female reproductive tract, renders spermatozoa capable of binding and fusing with the oocyte, and it is a requirement for mammalian fertilization. Capacitation encompasses plasma membrane reorganization, ion permeability regulation, cholesterol loss and changes in the phosphorylation state of many proteins. Novel tools to study sperm ion channels, image intracellular ionic changes and proteins with better spatial and temporal resolution, are unraveling how modifications in sperm ion transport and phosphorylation states lead to capacitation. Recent evidence indicates that two parallel pathways regulate phosphorylation events leading to capacitation, one of them requiring activation of protein kinase A and the second one involving inactivation of ser/thr phosphatases. This review examines the involvement of ion transporters and phosphorylation signaling processes needed for spermatozoa to achieve capacitation. Understanding the molecular mechanisms leading to fertilization is central for societies to deal with rising male infertility rates, to develop safe male gamete-based contraceptives and to preserve biodiversity through better assisted fertilization strategies.
Keywords: capacitation, ion channels, lipids, phosphorylation, sperm
Introduction
On September 2010, Dr Robert Edwards was awarded the Nobel Prize for the development of human in vitro fertilization (IVF) therapy. Since the first successful ‘Test-Tube' baby was born in 25 July 1978,1 over four million babies were conceived with this methodology. This outstanding landmark represented by Louise Joy Brown's birth was achieved almost 100 years after the first IVF attempts in mammals. The difficulties to attain mammalian IVF were inherent to a lack of knowledge on how gametes behave in in vitro systems. It was not until the early 1950s that Austin2 and Chang3 independently demonstrated that sperm had to reside in the female reproductive tract for a period of time before becoming able to fertilize an egg. The physiological changes that occur to the sperm in their transit toward the egg were collectively defined as ‘sperm capacitation'. The discovery of capacitation was essential for IVF because this finding made researchers aware that to acquire fertilizing capacity, mammalian sperm need to be in contact with molecules present in the female. Less than 10 years later, this discovery was followed by the first successful mammalian IVF when Chang showed that eggs from a black rabbit fertilized in vitro by capacitated sperm from a black male, and transferred to a white rabbit female, resulted in black offspring.4
The relevance of sperm capacitation for IVF increased the need to further study this process at the molecular level. Inherent to the initial studies on capacitation was the understanding that this process comprises multiple signaling events. For example, as soon as sperm are ejaculated, they become actively motile; however, they are still unable to penetrate the egg layers. In part, this motility activation is regulated by changes in the ionic environment surrounding the sperm. Later on, sperm undergo other modifications in their motility pattern known as hyperactivation. Although the exact role of hyperactivation is not clear yet, this process is believed to be related to the release of sperm from the oviduct reservoirs as well as to help sperm to penetrate the extracellular matrix of the egg.5 Besides changes in the flagellum, capacitation is also related to the ability of sperm to undergo a physiologically induced acrosome reaction. Interestingly, these processes occurring respectively in the tail and the head are temporally coordinated and are activated by similar signaling pathways. Finally, although the acrosome reaction is needed for fertilization and initially this process was considered part of the capacitation process, current views considered that the acrosome reaction is a post-capacitation event.6
In the last years, state-of-the-art techniques are being used to elucidate the molecular basis of sperm capacitation. Among them it is possible to highlight: (i) genetically modified mice models; (ii) global approaches to identify proteins present in different sperm structures; and (iii) whole cell patch clamp techniques to directly measure ion transport in differentiated sperm. Although this review will mention some of the most critical discoveries in the field of capacitation, it will be mostly focused on the analysis of two aspects of capacitation: (i) the regulation of the capacitation-associated changes in ion transport including those that are involved in the modulation of the sperm plasma membrane potential (Em); and (ii) the regulation of the capacitation-associated changes in phosphorylation. For more information, some other excellent reviews on capacitation have been published in recent years.7, 8, 9, 10, 11, 12
Regulation of ion fluxes during sperm capacitation
Cells expend a significant portion of their energetic resources building and maintaining ion concentration gradients across the membrane through the use of pumps and ion transporters. They use these stored resources to decode information from their surroundings and their interior. Ion channels and certain transporters capitalize these resources as their activation can change the electrical potential of the cell and the concentrations of second messengers within a wide time range, depending on the modes of channel regulation. In particular, ion channels can be opened to allow the flow of millions of ions per second across the membrane down their electrochemical gradient by small conformational changes induced by voltage, ligands, phosphorylation changes, membrane pressure and other signaling events.13
As part of their transit through the epididymis, sperm are exposed to conditions very different to the ones that they will encounter in the seminal fluid and in the female tract. In comparison with other body fluids, the epididymis lumen contains lower levels of Na+, higher levels of K+ and lower levels of Cl−. Osmolarity is compensated by organic anions such as carnitin, phosphocoline and glycerylphosphorylcholine.14 Most interestingly, the epididymal fluid is maintained at a low pH by a finely regulated epithelial release of H+ and uptake of HCO3-. This reduced pH is essential to maintain functionally competent sperm.15
After ejaculation, when spermatozoa are transferred to and navigate through the female tract, extracellular ion concentrations and osmolarity change again. In this case, extracellular [K+] ([K+]e) is reduced and [HCO3-]e and [Na+]e are increased in relation to seminal fluid ion concentrations. HCO3- increases from ∼4 mmol l−1 in the epididymis16, 17 to >20 mmol l−1 in the oviduct18 immediately activating mouse sperm flagellar beating. In this new milieu, sperm undergo capacitation, another extra testicular maturational change. As mentioned in the introductory paragraphs, discovery of capacitation made possible mammalian IVF. Initial experiments on this front focused in development of incubation media that support capacitation in vitro. Although each species has specific requirements, there is a consensus that certain components of the capacitation media are universal and close to the composition of the oviductal fluid. Among them, all capacitation media contained close-to-serum levels of Na+, K+, Cl−, Ca2+ and HCO3-.19, 20 In addition, these media contain energy metabolites (such as glucose, pyruvate and lactate) and a protein source that usually is serum albumin. Although the precise role of these molecules in capacitation is still under study, work from several groups is starting to elucidate some of their actions. A schematic representation of the roles and interrelationships between the ion transporters suspected to participate in capacitation is illustrated in Figure 1.
Figure 1.

Ion permeability model. This figure illustrates a schematic representation of the roles and interrelationships between the ion transporters suspected to participate in capacitation. Capacitation and hyperactivation take place during sperm transit through the female tract and have parallel but also intercrossing pathways. Cholesterol removal from the sperm plasma membrane by albumin in the female tract modifies several membrane properties. Albumin can also directly stimulate CatSper channels, increasing [Ca2+]i. The HCO3− increase experienced by sperm upon entering the female reproductive tract elevates intracellular HCO3− possibly through a NBC which would cause a hyperpolarization and stimulation of SACY, producing cAMP. In addition to triggering the phosphorylation cascades (see Figure 2), cAMP may activate the sNHE which together with the proton channel (HV), possibly stimulated by Zn2+ removal in the female reproductive tract,35 would raise pHi and activate CatSper and Slo3 channels. cAMP would also activate CFTR and participate in closing ENaCs which together with K+ channels hyperpolarize sperm. The [Ca2+]i increase may influence glycolysis and the axoneme activity promoting hyperactivated motility. Several Ca2+ mobilizing pumps (PMCA4 and SPCA1) and channels (IP3R, RyR and TRPs) may also participate during Ca2+ signaling. Not all sperm species undergo an Em hyperpolarization associated to capacitation like mouse; the channels and transporters that may contribute to this hyperpolarization are indicated. CFTR, cystic fibrosis transmembrane regulator; Em, membrane potential; IP3R, IP3 receptor; NBC, Na+/HCO3− cotransporter; NKCC, Na+/K+/Cl− cotransporter; PKA, protein kinase A; RyR, ryanodine receptor; SACY, soluble adenylyl cyclase; sNHE, sperm Na+/H+ exchanger; TRP, transient receptor potential.
Intracellular Ca2+ and pH changes
Ca2+ and H+ play a preponderant role during capacitation,20 and both [Ca2+]i and pHi increase during this process.21, 22, 23, 24, 25, 26 Though usually capacitating media contain ∼2 mmol l−1 Ca2+, 100–200 µmol l−1 is enough to capacitate mouse sperm.27, 28 Recent work exploring the [Ca2+]e requirement for the HCO3- stimulation of sperm motility revealed that additionally to its critical role as second messenger, Ca2+ can act as a first messenger. A Ca2+ sensing receptor has been cloned29 and proposed to participate in the HCO3--induced acceleration of mouse sperm flagellar beat frequency.30 However, how the [Ca2+]i increase required for capacitation occurs is not fully answered (Figure 1). Some transporters appear as main candidates responsible for Ca2+ influx: CaV and CatSper channels, Na+/Ca2+ exchangers, Ca2+ ATPases and the novel STIM and ORAI. On the other hand, Ca2+ released from intracellular stores must also be considered. In this regard, the main candidates would be: IP3 receptors, ryanodine receptors, TPC proteins, SERCA and SPM ATPases. We will briefly describe the characteristics and possible relation of CatSpers and CaVs to sperm capacitation.
Ca2+ channels
CatSpers
Bioinformatics and gene-targeting strategies have revealed that mouse spermatozoa possess sperm-specific subunits of a new family of cation channels named CatSper channels (cation channel of spermatozoa). In addition to mammals, these channels are also present in reptiles, tunicates, echinoderms and cnidarians.31, 32 This novel class of Ca2+ channels has been shown to be essential for mammalian hyperactivated sperm motility, sperm detachment from the female reproductive tract33 and egg coat penetration and fertility.32, 34, 35 CatSper mutations in humans have been associated to infertility.36 At present, the family has four members (CatSpers1–4), all most likely found in the principal piece of the flagella, plus two additional auxiliary subunits, CatSperB and G. These later subunits are transmembrane proteins, have large extracellular domains and have unknown functions.34, 37, 38 Interestingly, it has been noted that as in other voltage-dependent ion channels, extracellular ligand binding to auxiliary subunits could modulate CatSper's activity.39
All four CatSper subunits (1–4) are needed for functional expression in mouse sperm, as knockout of any one of them results in absence of the other subunits in mature sperm with the consequent observation of male infertility and the lack of the CatSper associated currents recorded by sealing on cytoplasmic droplet of epididymal sperm.34, 35, 40 CatSper1 null male mice also display defects in cAMP and depolarization-induced Ca2+ entry.34, 41 The pore-forming subunits of CatSper channels are like those of voltage-dependent K+ channels displaying six transmembrane spanning domains with a P-loop between transmembrane domains S5 and S6. It is thought that the channel would be formed by four of these subunits. However, their selectivity filter sequences are closer to those of CaV channels constituted by a single polypeptide with 24 transmembrane domains. CatSper channels are constitutively active, weakly voltage-dependent, Ca2+ selective and strongly potentiated by intracellular alkalinization.34, 42 Unfortunately, it has been impossible to heterologously express CatSper channels; thus their characterization has been restricted to optical, electrophysiological and biochemical experimental strategies in mouse and human sperm.
The high pHi sensitivity of CatSper is likely due to its CatSper1 subunit which contains an intracellular C-terminal domain rich in histidines. Although not yet demonstrated, capacitation-associated pHi changes may activate this channel with the consequent increase in [Ca2+]i. Related to the pHi regulation might be the well-described cyclic nucleotide-induced [Ca2+]i increase,43 as neither cAMP nor cGMP elevate [Ca2+]i in sperm from CatSper null mice. It is worth noting that none of the CatSper subunits have a cyclic nucleotide-binding sequence. Alternatively, cyclic nucleotides may regulate CatSper increasing pHi by activating a sperm specific Na+/H+ exchanger (sNHE) (see below).32, 34 Finally, recent work from the Ren's laboratory suggests that CatSper may contribute to the [Ca2+]i increase that accompanies the acrosome reaction.44 However, though sperm from CatSper null mice lack a zona pellucida (ZP)-induced Ca2+ uptake component, they undergo tyrosine phosphorylation,41 a ZP-induced acrosome reaction and they can fertilize zona-free eggs.34, 35 Interestingly, within the time resolution of Ca2+ measurements in this work, the ZP-induced Ca2+ increase starts in the flagellum where CatSpers are present. How ZP proteins regulate CatSper in the flagellum is not yet known.
CaVs
These Ca2+ channels convert Em changes into Ca2+ signals. The subunit of these channels contains the ion permeation pathway which is encoded by a family of at least 10 genes. CaV channels have been cataloged in two major functional classes: high voltage-activated and low voltage-activated. Though transcripts and proteins for both types of CaV have been detected in mammalian sperm,45 electrophysiological evidence has mainly revealed the functional presence of CaV3 channels in mouse and human spermatogenic cells46, 47, 48 and in mouse testicular sperm.49 Weak depolarizations can open CaV3 channels which are encoded by the CaV3 subfamily of genes (CaV3.1 to CaV3.3).49, 50 However, CaV3 channels begin inactivating at relatively hyperpolarized potentials (about −60 mV); at the Em of non-capacitated sperm (about −40 mV) they are inactivated.47, 51, 52 As the pharmacology of the mouse sperm acrosome reaction and at least part of the Ca2+ influx associated to it, are consistent with that of CaV3 channels, it was proposed, though now debated,34 that they participate in this important reaction.53 It turns out that CaV3.1 and CaV3.2 null mice are fertile54, 55 and though CaV3 currents have been recorded in mouse testicular sperm,49 no CaV currents have been detected in epididymal sperm.34 These findings certainly question the role of CaV3 and in general CaV channels in sperm physiology. Resolving this matter requires further studies since [Ca2+]i measurements and their pharmacology are consistent with the presence and participation of certain CaV channels in sperm physiology.56, 57, 58
If CaV channels were involved in the acrosome reaction, sperm Em should hyperpolarize during capacitation to remove their inactivation before they could open and trigger this process.59 Indeed, single-cell Em measurements indicated that the Em of ∼50% of mouse sperm remain close to that of uncapacitated sperm, while the rest hyperpolarize to −80 mV, a potential that can remove inactivation from low voltage-activated Ca2+ channels.51 These experiments suggest that only the hyperpolarized population can undergo the AR when exposed to solubilized ZP (see the section on ‘Em changes').
Intracellular pH regulation
As discussed earlier, mammalian sperm capacitation requires HCO3-. Uptake of this anion contributes to the pHi increase that accompanies this process.21, 22, 60 In addition, HCO3- influx hyperpolarizes mouse sperm depending on the concentration of external Na+, suggesting the involvement of an electrogenic Na+/HCO3- cotransporter during capacitation.60
Regulation of sperm pHi is not only fundamental for capacitation, but also for motility and the acrosome reaction. As mentioned, sperm express a specific member of the mammalian NHE superfamily of Na+/H+ exchangers, sNHE.61, 62, 63 It is localized on the principal piece of the sperm flagellum and predicted to have 14 membrane-spanning helices. sNHE contains a nucleotide-binding domain close to its intracellular C-terminus and four putative transmembrane helices analogous to the voltage sensor of voltage-dependent channels.64 These distinctive characteristics suggest that sNHE may be regulated by cyclic nucleotides and Em,65 in addition to phosphorylation and protein interactions.61 Sperm from sNHE null male mice are immotile and infertile, and can be rescued adding permeable analogs of cAMP or NH4Cl. sNHE and the soluble adenylyl cyclase (SACY) may form a transduction complex to efficiently regulate pHi, HCO3-, cAMP and sperm motility, as the exchanger is required for the full length expression of SACY.63, 65
Recent evidence has revealed that mammalian sperm, particularly those of human, express HV channels in the flagella.35 These channels may participate in the pHi increase necessary for capacitation. Since protein kinase C (PKC) regulates this type of channel in other cell types, an interesting possibility would be that this regulation is conserved in sperm.66 In mouse sperm, it has been shown that HV channels do not play a preponderant role in pHi regulation;35 therefore, it is likely that pHi relies on the regulation of sNHE as suggested by the sterile phenotype of sNHE null mice.63 In this respect, analysis of its sequence predicts that the sNHE is voltage-dependent suggesting that the hyperpolarization that occurs during mouse sperm capacitation could stimulate this exchanger.
Em changes
In sperm, as in most cells, the internal ion concentrations are markedly different from those in the extracellular medium. These differences result from the relative ion permeability of the plasma membrane to each of the ions found in the inner and outer media given by the specific ion channels and transporters present in the cell, to the gradients they establish and the metabolic state of the cell. At rest, the balance of these fluxes, gradients and permeabilities results in an electric potential, known as the resting Em. Before capacitation mouse sperm are relatively depolarized (Em: about from −35 to −45 mV) and become hyperpolarized (about −70 mV) during capacitation.51, 60, 67, 68 This change results from a combination of electrogenic ion permeability alterations that shift the Em towards the K+ equilibrium potential (about −90 mV).60, 69 Several types of K+ channels have been detected in testis, spermatogenic cells and sperm using molecular, electrophysiological and biochemical tools (see the section on ‘K+ channels').67, 70, 71, 72, 73 It has been mentioned that capacitation-associated hyperpolarization may increase pHi-stimulating sNH and consequently CatSper.32 However, a pHi increase shifts the CatSper current–voltage curve toward more negative membrane potential values.42 Considering that CatSper is activated by membrane depolarization, hyperpolarization may have a dual effect on this channel regulated by a subtle balance between these variables, further influenced by cAMP levels. Because capacitation prepares the sperm to undergo the AR, it has been proposed that the capacitation-associated hyperpolarization could also regulate the ability of sperm to generate transient [Ca2+]i elevations during the AR induced by physiological agonists (e.g., ZP).46, 47, 51
In the absence of bovine serum albumin and HCO3-, mouse sperm at pH 7.2 do not hyperpolarize which suggests that cholesterol removal and HCO3- somehow regulate the sperm Em changes that occur during capacitation. As in vitro capacitation also depends on the presence of Na+ and Cl− in the medium,74, 75 other ion channels and transporters are likely to regulate sperm Em and intervene in this maturational process. Furthermore, 50 mmol l−1 external KCl inhibits hyperpolarization and capacitation in mouse sperm.51
Na+ influences the sperm Em
ENaCs
Addition of Na+ to the external media depolarizes mouse sperm more before than after capacitated. This depolarization is very sensitive to amiloride. Indeed, ENaCs, amiloride-sensitive Na+ channels that are expressed in many invertebrate and vertebrate cell types, are present in mouse and human sperm.76 These channels may be regulated by pH, Ca2+, Na+, Cl− and phosphorylation,77 parameters that change during capacitation. The ENaC α- and δ-subunits were detected in the sperm flagellar mid-piece and acrosome region, respectively.76 These channels are Na+-selective, amiloride-sensitive and contribute to the resting Em in cells by displacing it towards the Na+ equilibrium potential.78 Both mouse spermatogenic cells76 and testicular sperm display amiloride-sensitive inward Na+ currents whose characteristics match those of ENaCs. Notably, permeable cAMP analogs which induce in vitro capacitation decrease the sperm depolarization induced by addition of external Na+. These experiments are consistent with the hypothesis that elevation of cAMP levels during sperm capacitation decreases ENaC activity and contribute to the hyperpolarization needed for this process to occur.
K+ channels
As mentioned earlier, mouse sperm hyperpolarize from about −40 to about −70 mV during capacitation.51, 67 The involvement of K+ channels in this change was proposed since it was affected by external K+ and K+-channel blockers.34, 51, 67, 70 Spermatogenic cells and sperm have been reported to possess Ca2+-activated K+ channels,71 voltage-gated K+ channels,72, 73 two-pore domain K+ channels,79 inwardly rectifying K+ (Kir) channels34, 67, 70 and Slo3 K+ channels.49, 80, 81, 82, 83, 84 Below we will discuss the K+ channels that have been proposed to participate in capacitation.
Kirs
Kir channels move K+ easier into the cell than out. The Kir family is constituted by seven subfamilies (Kir1–7). These subfamilies can be further divided into four functional groups: constitutively active classical Kir channels (Kir2), G protein-gated Kir channels (Kir3), ATP-sensitive K+ channels (Kir6) linked to cellular metabolism and K+ transport channels (Kir1, 4, 5 and 7). Among many cell functions, Kir channels are important in maintaining Em and K+ homeostasis, membrane excitability, vascular tone, heart rate and insulin secretion. Intracellular substances such as Mg2+ and polyamines block the pore causing inward rectification. Ions, phospholipids and proteins modulate Kir channel activity. A Kir subunit is basically made up of two transmembrane helices with cytoplasmic NH2 and COOH termini and an extracellular loop which folds back to form the pore-lining ion selectivity filter. Four of these homo- or heteromeric subunits constitute a functional Kir channel.80
Patch-clamp recordings in mouse spermatogenic cells revealed Kir currents sensitive to Ba2+, glucose, tolbutamide and glibenclamide.34, 67, 70 More recently these compounds were shown to block K+ currents in mouse testicular sperm;81 they also inhibited the hyperpolarization associated to mouse sperm capacitation and, at least partially the AR.34, 67, 70 Tolbutamide and glibenclamide are sulfonylureas known to block Kir channels regulated by ATP (KATP channels). KATP channels are formed from Kir6 subunits and ATP-binding cassette sulfonylurea receptor (SUR) proteins.85 Transcripts for the KATP channel subunits SUR1, SUR2, Kir6.1 and Kir6.2 were detected in elongated spermatids and the expressed proteins immunolocalized in mature mouse sperm.70
Kir channels are in general inhibited by low pHi.80 As sperm pHi is relatively acidic before capacitation, it could maintain Em depolarized keeping CaV channels inactivated and unable to cause unregulated Ca2+ entry and the acrosome reaction.22 In spite of the structural similarities between different Kirs, cholesterol affects them differentially. Its depletion increases Kir2 current density, while it inhibits Kir4 channels.86 Sperm capacitation is accompanied by activation of the cAMP/protein kinase A (PKA) signaling pathway, cholesterol depletion and a pHi increase.87 It is therefore feasible that Kir channels such as the KATP channels contribute to the capacitation-associated hyperpolarization in mouse sperm.
Slo3
The Slo gene family of K+ channels are formed by an α subunit with a large cytosolic C-terminal containing potential ligand regulation sites and a β-auxiliary subunit. There are four α subunits, where each type can apparently form a functional tetramer,88 and four different β-subunits that regulate the function and expression of Slo channels.89 Specific cytosolic ions regulate each of the subunits: Ca2+ for Slo1, Na+ for both Slo2.1 (Slick) and Slo2.2 (Slack), and H+ for Slo3.90
The Slo3 subunit, cloned many years ago and expressed only in spermatogenic cells and sperm,80, 82 has only recently been shown to be fundamental for sperm function;49, 83 Slo3 null male mice are infertile.84 Slo3 currents in mouse testicular sperm are similarly activated by intracellular alkalinization, depolarization and cAMP, blocked by 1 mmol l−1 Ba2+ and high [TEA] (60 mmol l−1), and poorly K+ selective (PK+/PNa+: ∼8), as they are when heterologously expressed in oocytes.49 These currents are absent in testicular sperm from Slo3 null mice, confirming their identity.84
As discussed earlier, under capacitating conditions wild-type mouse sperm hyperactivate, their pHi increase and their Em hyperpolarize. In contrast, sperm from Slo3 null mice are unable to swim progressively, to hyperpolarize and to undergo the acrosome reaction. Remarkably, these sperm cannot acrosome react even when exposed to Ca2+ ionophore. These results show that Slo3 channel activation is pivotal for the capacitation-associated processes necessary for fertilization. These considerations also point to this channel as an attractive target for male contraception.84
Recent findings revealed that Slo3 channels heterologously expressed are stimulated by PIP2. This also occurs to the corresponding currents in epididymal mouse sperm. These Slo3 currents are inhibited by EGF acting through the EGF receptor, in a PIP2-dependent manner.91 Further studies are needed regarding the selectivity, voltage dependence and β-subunit regulation of Slo3 channels in mammalian sperm to fully understand their participation in capacitation and fertilization.
HCO3-, Cl− and cystic fibrosis transmembrane regulator (CFTR)
HCO3- influences mouse sperm Em in addition to its role regulating SACY. Upon addition of HCO3-, the electrogenic influx of this anion causes a temporal hyperpolarization of mouse sperm that is distinct from the slowly developing hyperpolarization associated to capacitation. In these experiments, Na+ replacement from the external media by the non-permeant cation choline+ does not allow the hyperpolarization induced by adding HCO3-, and inhibits the capacitation-associated increase in protein tyrosine phosphorylation and the preparation for the ZP-induced acrosome reaction. Addition of permeable analogs of cAMP to the choline incubation medium restores protein tyrosine phosphorylation. These findings suggest that a Na+/HCO3- cotransporter is present in mouse sperm and is coupled to events regulating capacitation.60
ENaCs and the CFTR have been found to regulate each other and colocalize in several cell types.92 Cystic fibrosis, the most widespread human genetic disease, is caused by mutations in CFTR, a cAMP-modulated, ATP-dependent Cl− channel.93 ENaCs and CFTRs have been detected in the mid-piece of mouse and human sperm flagella.69, 94 Em measurements have indicated that cAMP may close ENaCs by activating CFTR and thus contribute to the capacitation associated hyperpolarization.69
Cl− transport across the plasma membrane is important for the regulation of cell Em, volume, pHi and for HCO3- fluxes. Different Cl− channels, exchangers and cotransporters move this anion.13 Sperm capacitation and fertilization require external Cl−.95, 96 Evidence indicates that [Cl−]i increases during capacitation;69 its influx may be necessary to allow a raise in intracellular HCO3- that would stimulate SACY through Cl−/HCO3- antiporters.69, 96 As sperm Em is close to the Cl− equilibrium potential, opening Cl− channels would not allow accumulation of this anion. These considerations point out that it is likely that other Cl− transport systems are present in sperm. From a battery of Cl− transport antagonists tested in capacitation assays, only general Cl− transport blockers that do not allow identification of specific transporters, such as stilbenes (i.e., DIDS), inhibited tyrosine phosphorylation, hyperactivation and fertilization. These findings are consistent with the presence and importance of Cl− transporters in sperm physiology. Furthermore, as observed in external media without Cl−, bumetanide and furosemide, two Na+/K+/Cl− cotransporter (NKCC) inhibitors, reduced sperm tyrosine phosphorylation and hyperactivation. It is worth noting that higher concentrations were needed for this inhibition than those required to antagonize NKCCs.97 In addition, external K+, an ion needed for NKCC function, was not required for the increase in tyrosine phosphorylation associated to capacitation. This and the high bumetanide concentrations needed to inhibit may indicate that this antagonist could affect other targets in addition to NKCC.96
On the other hand, the ZP-induced acrosome reaction which depends on external Cl−, K+ and Na+, is inhibited at a much lower concentration of bumetanide and furosemide, suggesting that NKCC might participate in the capacitation-dependent preparation of sperm for the acrosome reaction or directly in this reaction. The messenger for this cotransporter and its protein were detected in mouse spermatogenic cells and sperm, respectively.96 Interestingly, other conditions that do not inhibit either hyperactivation or the increase in tyrosine phosphorylation, such as CFTR antagonists DPC and inh-172, can block the ZP-induced acrosome reaction. These results suggest that capacitation requires nonlinear signaling paths. In accordance with this hypothesis, although sperm from SACY null mice fail to hyperactivate and to increase their tyrosine phosphorylation, they are able to undergo the ZP-induced acrosome reaction.98 Noteworthy, the general Cl− channel inhibitor niflumic acid inhibited the mouse acrosome reaction initiated by different stimuli, including ZP and glycine.99 In addition, the presence of glycine receptor/Cl− channel has been shown to be involved in the human zona-induced acrosome reaction.100 The inhibitor strychnine was also able to inhibit the Ca2+ influx associated with addition of recombinant human ZP3,101 further substantiating the key role of Cl− influx in the acrosome reaction.
Is sperm hyperpolarization needed for capacitation and if so why?
Originally it was argued that mouse sperm needed to hyperpolarize during capacitation to remove inactivation from CaV channels so they could open when stimulated by ZP to trigger the acrosome reaction.46, 47, 51 However, the functional involvement of CaV channels in sperm is being questioned. It is worth considering that this hyperpolarization could be required to activate sNHE and increase pHi, which would in turn stimulate Slo3 and CatSper channels,32 and possibly SACY.65 In addition, a hyperpolarization would increase the driving force for Ca2+ uptake through other Ca2+ permeable channels such as transient receptor potentials, some of which are activated by alkalinization.
Phosphorylation events linked to sperm capacitation
cAMP, HCO3- and Ca2+ in SACY regulation
As mentioned, one critical change in the sperm surrounding milieu after ejaculation is the change in HCO3- concentration. In addition to the aforementioned relevance of this anion to regulate pHi and Em, there is a connection between HCO3- and the cAMP pathway.102 This landmark observation indicated that Ca2+ upregulated cAMP levels only when HCO3- was present in the incubation medium. This initial finding was followed by experiments in boar sperm showing that HCO3- in the seminal fluid was needed for motility initiation17 and that this anion was able to increase cAMP levels through direct stimulation of a unique type of adenylyl cyclase present in sperm. More than a decade later, this unique cyclase was cloned from rat testis. Since it is found in a soluble fraction of the testis, it was called SACY.103 Although it is not clear whether SACY has a role in germ cell differentiation, SACY knockout mice are sterile.104 The sterility phenotype is mapped to lack of capacitation; in particular sperm from the SACY null mice are not able to move actively and cannot hyperactivate.105 Unexpectedly, these sperm have no obvious problems with their ability to undergo a ZP-induced acrosome reaction, suggesting that this event can be dissociated from other capacitation-associated processes.104
Role of PKA as a target of cAMP
Over the years the role of cAMP in the regulation of sperm has been supported by biochemical and pharmacological approaches. It is well established that inhibitors of PKA such as H89, rpScAMP and the peptide protein kinase inhibitor106, 107 block sperm motility and other downstream signaling pathways (see below). Although cAMP has multiple targets, these experiments suggested that PKA has a relevant role in capacitation. Consistent with this hypothesis, mammalian sperm contain a specific splicing variant of the PKA catalytic subunit alpha, Cα2.108, 109 When the unique Cα2 exon is eliminated by homologous recombination, mice are sterile without presenting other problems. The sterility is due to defects in sperm capacitation that are phenotypically similar to the ones observed for the SACY knockout.109 More recently, the same group has engineered another mouse model in which exon 5 of the PKA catalytic α-subunit was replaced with a mutant form displaying a point mutation in the ATP-binding pocket.110 Although this mutation is silent and PKA in this mice is able to perform its normal functions, this mutant, named CαM120A, is now sensitive to a bulky inhibitor such as 1NM-PP1, which is inactive against the wild-type PKA. Using sperm of these mice in vitro, the authors clearly showed PKA is required for the activation of flagellar beat and for the flagellar waveform asymmetry associated with hyperactivation.110
Events downstream of PKA activation
Using the mouse as experimental model, it was shown that sperm capacitation is associated with an increase in protein tyrosine phosphorylation.111 This work indicated that HCO3-, Ca2+ and bovine serum albumin (or other cholesterol acceptors) were needed to support capacitation as well as the increase in tyrosine phosphorylation. Following studies demonstrated that glucose,112 Na+ and Cl− were also essential for capacitation and for the increase in phosphorylation76, 96. Interestingly, the lack of protein tyrosine phosphorylation in the absence of each of these capacitation medium elements was reversed by activation of a cAMP pathway.96 Moreover, in complete medium, PKA inhibitors such as H89 or Rp-cAMPS were able to block the increase in tyrosine phosphorylation.107 Altogether, these data indicated that the increase in protein tyrosine phosphorylation was downstream of cAMP synthesis and activation of PKA. The role of cAMP in the increase in tyrosine phosphorylation has been further analyzed using genetically modified mice models.
SACY, Cα2 and AKAP4 are three genes essential for the function of the cAMP/PKA pathway in sperm. Elimination of each of these genes resulted in a male sterile phenotype.113 All these null mice presented defects in the regulation of the protein tyrosine phosphorylation pathway as well as in several other capacitation-related events such as the onset of hyperactivated motility.104, 114, 115 In addition, sperm lacking AKAP4 presented morphological flagellar defects likely due to the role of this protein in tail assembly.115 Consistent with a role of PKA upstream the capacitation-associated increase in tyrosine phosphorylation, the aforementioned sperm from CαM120A-expressing mouse lines did not undergo capacitation-associated changes in tyrosine phosphorylation when the mutated PKA was blocked by the bulky inhibitor 1NM-PP1.110
Despite the fact that many groups have shown similar regulatory pathways in sperm from other species, there is still a limited knowledge on the identity and the role of proteins phosphorylated in tyrosine residues. One of the first proteins to be identified as a substrate for tyrosine phosphorylation was human AKAP4.116 Tyrosine phosphorylation of this protein was also demonstrated in hamster sperm117 and the exact sites of tyrosine phosphorylation were mapped.118 Other proteins known to be tyrosine phosphorylated in mammalian sperm are the valosin-containing protein, AKAP3 and CABYR,118, 119 a sperm-specific aldolase and pyruvate hydrogenase.74, 120 The role of these phosphorylations has not yet been elucidated; however, it is speculated that tyrosine phosphorylation may regulate either the activity or the location of these enzymes.
Regulation of tyrosine phosphorylation: role of Src kinase family and ser/thr phosphatases
Another area of inquiry in the last years has been the identification of the tyrosine kinase(s) responsible for the observed changes in protein tyrosine phosphorylation accompanying the capacitation process. Recently, several groups have demonstrated the presence of cSrc in mouse and human sperm.121, 122, 123, 124 These publications also presented evidence that cSrc inhibitors blocked the capacitation-associated increase in tyrosine phosphorylation. These results were consistent with the hypothesis that cSrc plays a role in the regulation of tyrosine phosphorylation in sperm. Challenging this conclusion, experiments conducted with sperm from cSrc null mice demonstrated that at least in this species, cSrc either is not essential for the increase in tyrosine phosphorylation or it can be replaced by other kinase in the cSrc null sperm.125 Other results in this work indicated that cSrc family kinase inhibitors such as Bosutinib (SKI606) and SU6656 significantly reduced sperm motility parameters, and blocked IVF. These compounds blocked the increase in tyrosine phosphorylation; although this action was expected, these inhibitors also prevented direct phosphorylation by PKA, an event upstream of the increase in tyrosine phosphorylation. This observation was not explained by an unspecific effect of the cSrc inhibitors on PKA activity as shown using an in vitro kinase assay. Altogether, these results suggested the participation of a member of cSrc family kinase in the regulation of capacitation; however, they also indicated that a cSrc family member was upstream or parallel to the PKA activation and not an effector of this signaling pathway. It has been established that cSrc kinases can regulate the activity of ser/thr phosphatases. In particular, the PP2A catalytic subunit exhibits a cSrc consensus phosphorylation site (TPDYFL) in its C-terminal domain that when phosphorylated downregulates its activity. If this pathway was active in sperm, it is predicted that inhibition of cSrc would result in upregulation of PP2A activity with a consequent dephosphorylation of ser/thr-phosphorylated substrates. Indeed, addition of low concentrations of okadaic acid or calyculin, two specific ser/thr phosphatase inhibitors, completely reversed the action of the cSrc inhibitors strongly suggesting that the role of a cSrc family kinase was mediated by a ser/thr phosphatase125 (Figure 2).
Figure 2.
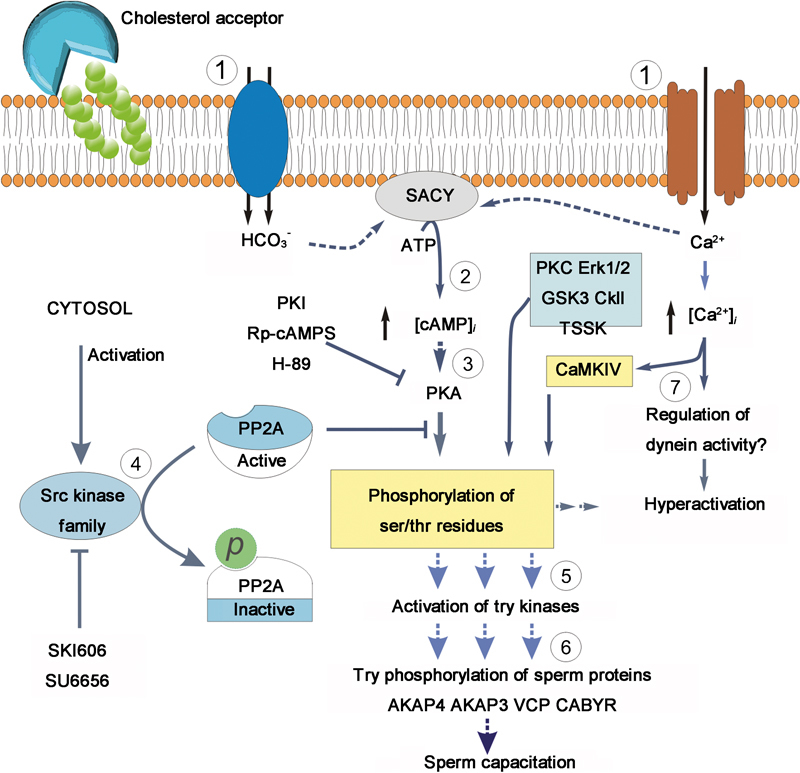
Model of the regulation of phosphorylation pathways during mammalian sperm capacitation. The overall pathway is modulated by cholesterol removal of sperm plasma membrane.  Influx of HCO3− and Ca2+ stimulates an SACY.
Influx of HCO3− and Ca2+ stimulates an SACY.  Increasing intracellular cAMP concentrations.
Increasing intracellular cAMP concentrations.  High cAMP levels activate PKA. This activation can be blocked in vitro by addition of the inhibitors H-89, Rp-cAMPs and the peptide PKI.
High cAMP levels activate PKA. This activation can be blocked in vitro by addition of the inhibitors H-89, Rp-cAMPs and the peptide PKI.  In vivo, phosphorylation of PKA substrates is subjected to regulation by a ser/thr phosphatase, likely PP2A. This phosphatase is in turn downregulated by a member of the Src kinase family by still unknown mechanisms. Activation of Src kinase family members can be blocked by in vitro addition of the inhibitors SU6656 or SKI-606 (Bosutinib). The onset of PKA substrates phosphorylation is followed by activation of unidentified tyrosine kinases
In vivo, phosphorylation of PKA substrates is subjected to regulation by a ser/thr phosphatase, likely PP2A. This phosphatase is in turn downregulated by a member of the Src kinase family by still unknown mechanisms. Activation of Src kinase family members can be blocked by in vitro addition of the inhibitors SU6656 or SKI-606 (Bosutinib). The onset of PKA substrates phosphorylation is followed by activation of unidentified tyrosine kinases  and the promotion of tyrosine phosphorylation of sperm proteins
and the promotion of tyrosine phosphorylation of sperm proteins  (e.g., AKAP4, AKAP3, VCP and CABYR).
(e.g., AKAP4, AKAP3, VCP and CABYR).  Intracellular Ca2+ increase might affect the axoneme directly, through selective regulation of dyneins. In addition, Ca2+ upregulates CaMKIV, leading to hyperactivation. PKA, protein kinase A; PKC, protein kinase C; PKI, protein kinase inhibitor; SACY, soluble adenylyl cyclase; VCP, valosin-containing protein.
Intracellular Ca2+ increase might affect the axoneme directly, through selective regulation of dyneins. In addition, Ca2+ upregulates CaMKIV, leading to hyperactivation. PKA, protein kinase A; PKC, protein kinase C; PKI, protein kinase inhibitor; SACY, soluble adenylyl cyclase; VCP, valosin-containing protein.
Other phosphorylation pathways observed during sperm capacitation
Although most is known about the regulation of the cAMP/PKA pathway and the downstream increase in tyrosine phosphorylation, several other protein kinases are present in sperm and are likely to play a role in the regulation of sperm capacitation. Different PKC isoforms have been described in mammalian sperm.126, 127 For example, phorbol esters stimulate these kinases and change sperm motility,128 the acrosome reaction129 and cAMP levels.130 Even though these phorbol ester effects are likely to be mediated by PKC, the possibility that other phorbol ester receptors such as chimaerins are present in sperm cannot be discarded.131 Other ser/thr protein kinases reported in sperm are: Erk1/2;132, 133 GSK3;134 CaMKIV;135 and casein kinase II,136 members of the testis-specific serine kinase family.137 Interestingly, proline-directed phosphorylation appears to be regulated by cholesterol efflux.138 Altogether, this evidence suggests that in addition to PKA, other kinases are involved in sperm capacitation; however, more research is needed to explain how they interact with other capacitation-associated pathways.
Model of the regulation of phosphorylation during capacitation
During the last years, the textbook model of sperm capacitation has been linear. In this model, Ca2+ and HCO3- were involved in the activation of a cAMP/PKA pathway through the regulation of SACY. The finding that cSrc inhibitors obliterated phosphorylation of PKA substrates, without inhibiting PKA activation, has the implication that capacitation is also dependent on the activation of a second pathway. As part of this pathway, it is predicted that capacitation will also require the activation of a cSrc family kinase and that this activation will result in phosphorylation and downregulation of a ser/thr phosphatase. In parallel, activation of SACY with the consequent increase in cAMP synthesis will activate PKA. Both processes are needed to induce the increase in phosphorylation observed during sperm capacitation. How different components of the capacitation media interact to activate these pathways warrant future investigation (Figure 2).
Lipids in sperm capacitation
Phospholipid transferases
It is now well established that phospholipids in the bilayer structure of cell membranes are distributed asymmetrically.139 Under normal conditions, lipid distribution among the inner and the outer leaflet of the plasma membrane is maintained by the combined activity of several phospholipid transferases: an aminophospholipid transferase (‘flippase') that moves phosphatidylserine and phosphatidylethanolamine from the outer to the inner leaflet;140 a nonspecific phospholipid transferase (‘floppase') that transfers phospholipids from the inner to the outer leaflet;141 and a bidirectional carrier (‘scramblase') that moves all phospholipids in both directions across the membrane bilayer.142 Under certain conditions, the normal phospholipid asymmetry maintained throughout cell life collapses, with the result that phosphatidylserine and phosphatidylethanolamine appear in the outer leaflet. Among other situations, such collapse (also known as ‘scrambling') is seen during lymphocyte activation, red blood cell ageing and apoptosis.143, 144, 145
In sperm, scrambling is observed as an early capacitation event.146, 147 Analysis of phospholipid movements strongly suggests that the collapse of phospholipid asymmetry in these cells is due to an increase in the activity of a sperm scramblase.146 Moreover, the change in phospholipid distribution appears to be stimulated by HCO3-, mediated by a cAMP/PKA pathway and enhanced by protein phosphatase inhibitors.146, 147 Importantly, while scrambling is a well-known characteristic of apoptosis (programmed cell death), there is evidence suggesting that the HCO3--induced scrambling in sperm is not related to this process.148 Although the role of phospholipid scrambling in sperm capacitation is not well understood, it is likely to be connected with the need for serum albumin in this process. Phospholipid scrambling may facilitate albumin-mediated cholesterol extraction.146, 149 Though serum albumin may have other roles during capacitation such as promoting Ca2+ influx,150, 151 its ability to facilitate cholesterol efflux is required for capacitation. Capacitation is blocked by addition of cholesterol and/or cholesterol analogs to the capacitation medium.152 Furthermore, serum albumin can be substituted by cholesterol-binding compounds such as high-density lipoproteins152, 153 and β-cyclodextrins154, 155, 156, 157 to induce capacitation in vitro.
Cholesterol removal and lipid rafts
In spite of the fact that cholesterol efflux is involved in sperm capacitation, how this efflux modulates sperm physiology is not well understood. Possibly cholesterol concentrates in specialized sperm plasma membrane microdomains, also known as rafts.154, 155, 156, 157, 158, 159 Despite controversies regarding the methods used to obtain these rafts,160 alternative approaches have emerged to identify proteins in these domains.161 Several protein markers for these structures have been found in the sperm such as caveolin1 and cavelin2, as well as flotilin 1 and 2.161, 162, 163, 164 Most interestingly, sphingolipids such as GM1 and GM3 are also present in these cells. In the case of GM1, a capacitation-associated movement of this sphingolipid has been observed.165, 166 Using fluorescent cholera toxin-B subunit to track GM1 in mouse sperm may allow following capacitation in the female tract.166 In addition, it has recently been proposed that GM1 binds decapacitation factors that are released as part of the capacitation process.167
In other cell types, lipid rafts bring protein assemblies together; it is predicted that changes in the cholesterol/phospholipid ratio within these structures can modulate their size and behavior.168, 169 In somatic cells, cholesterol-binding reagents such as beta-cyclodextrins, are capable of activating signaling pathways, for example, those involving tyrosine kinases, G proteins and/or other molecules.170, 171, 172 As mentioned, the release of cholesterol accompanies sperm capacitation and correlates with an increase in protein phosphorylation. A working hypothesis consistent with this observation is that activation of raft complexes by cholesterol release and the regulation of phosphorylation pathways are coordinated and form part of the same signaling pathway. Alternatively or complementary to this hypothesis, changes in lateral mobility of membrane proteins as a consequence of cholesterol loss might be responsible for capacitation.173
Final remarks
In view of the notion that the maturational process undergone by mammalian sperm in the female reproductive tract known as capacitation is essential for fertilization, there is a need to unravel the molecular processes involved in this complex and fundamental process. The discovery of sperm capacitation over 50 years ago opened new possibilities and a whole new era of reproductive medicine. However, while key aspects of capacitation including ionic transport, PKA stimulation and tyrosine phosphorylation of sperm proteins have significantly advanced, their functional interrelation is still not clearly understood. An initial fast bicarbonate-induced PKA activation appears to trigger capacitation both in the female tract and in in vitro systems. However, further work is needed to identify the ionic fluxes involved, the spatial PKA substrate arrangements and their signaling roles, as well as the sperm cAMP-dependent processes that are PKA-independent. Exciting new imaging, proteomic and electrophysiological tools are allowing researchers to tackle these and other open questions in the field: What are the identities of the sperm proteins involved in capacitation that change their phosphorylation state? Which kinase(s) and phosphatases are responsible for modulating their function and interaction, and how are they regulated? Which are the identities of the transporters responsible for the key ion fluxes necessary for capacitation and how are they intertwined with the modulation of second messengers and phosphorylation changes? Are hyperactivation and capacitation parallel or independent and what is the role of the sperm-specific ionic channels CatSper and Slo3 in both processes? Is the concept of capacitation extensive to other species? Addressing such unsolved questions will bring fundamental progress into this fascinating field helping to set a better ground base for developing new infertility treatments, diagnostics and contraceptive techniques.
Acknowledgments
This work was supported by Dirección General de Asuntos del Personal Académico (DGAPA): IN211809 (to AD), CONACyT: 49113 (to AD), NIH: R01 HD44044 (to PEV) and HD038082 (to PEV and AD).
The authors declare no competing financial interest.
References
- Steptoe PC, Edwards RG. Birth after the reimplantation of a human embryo. Lancet. 1978;2:366. doi: 10.1016/s0140-6736(78)92957-4. [DOI] [PubMed] [Google Scholar]
- Austin CR. The capacitation of the mammalian sperm. Nature. 1952;170:326. doi: 10.1038/170326a0. [DOI] [PubMed] [Google Scholar]
- Chang MC. Fertilizing capacity of spermatozoa deposited into the fallopian tubes. Nature. 1951;168:697–8. doi: 10.1038/168697b0. [DOI] [PubMed] [Google Scholar]
- Chang MC. Fertilization of rabbit ova in vitro. Nature. 1959;184 Suppl 7:466–7. doi: 10.1038/184466a0. [DOI] [PubMed] [Google Scholar]
- Suarez SS. Regulation of sperm storage and movement in the mammalian oviduct. Int J Dev Biol. 2008;52:455–62. doi: 10.1387/ijdb.072527ss. [DOI] [PubMed] [Google Scholar]
- Florman HM, Jungnickel MK, Sutton KA. Regulating the acrosome reaction. Int J Dev Biol. 2008;52:503–10. doi: 10.1387/ijdb.082696hf. [DOI] [PubMed] [Google Scholar]
- Bailey JL. Factors regulating sperm capacitation. Syst Biol Reprod Med. 2010;56:334–48. doi: 10.3109/19396368.2010.512377. [DOI] [PubMed] [Google Scholar]
- Aitken RJ, Baker MA. The role of proteomics in understanding sperm cell biology. Int J Androl. 2008;31:295–302. doi: 10.1111/j.1365-2605.2007.00851.x. [DOI] [PubMed] [Google Scholar]
- Brewis IA, Gadella BM. Sperm surface proteomics: from protein lists to biological function. Mol Hum Reprod. 2010;16:68–79. doi: 10.1093/molehr/gap077. [DOI] [PubMed] [Google Scholar]
- Darszon A, Labarca P, Nishigaki T, Espinosa F. Ion channels in sperm physiology. Physiol Rev. 1999;79:481–510. doi: 10.1152/physrev.1999.79.2.481. [DOI] [PubMed] [Google Scholar]
- Fraser LR. The ‘switching on' of mammalian spermatozoa: molecular events involved in promotion and regulation of capacitation. Mol Reprod Dev. 2010;77:197–208. doi: 10.1002/mrd.21124. [DOI] [PubMed] [Google Scholar]
- Abou-Haila A, Tulsiani DR. Signal transduction pathways that regulate sperm capacitation and the acrosome reaction. Arch Biochem Biophys. 2009;485:72–81. doi: 10.1016/j.abb.2009.02.003. [DOI] [PubMed] [Google Scholar]
- Hille B. Sunderland, MA; Sinauer Associates Inc.; 2001. Ion Channels of Excitable Membranes. [Google Scholar]
- Turner TT.Necessity's potion: inorganic ions and small organic molecules in the epididymal lumenIn: Robaire B, Hinton B, editors. The Epididymis: From Molecules to Clinical Practice. A Comprehensive Survey of the Efferent Ducts, the Epididymis, and the Vas Deferens New York; Kluwer Academic/Plenum; 2002p131 [Google Scholar]
- Shum WW, da Silva N, Brown D, Breton S. Regulation of luminal acidification in the male reproductive tract via cell–cell crosstalk. J Exp Biol. 2009;212:1753–61. doi: 10.1242/jeb.027284. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Asari M, Sasaki K, Miura K, Ichihara N, Nishita T. Immunohistolocalization of the carbonic anhydrase isoenzymes (CA-I, CA-II, and CA-III) in the reproductive tract of male horses. Am J Vet Res. 1996;57:439–43. [PubMed] [Google Scholar]
- Okamura N, Tajima Y, Soejima A, Masuda H, Sugita Y. Sodium bicarbonate in seminal plasma stimulates the motility of mammalian spermatozoa through direct activation of adenylate cyclase. J Biol Chem. 1985;260:9699–705. [PubMed] [Google Scholar]
- Maas DH, Storey BT, Mastroianni L., Jr Hydrogen ion and carbon dioxide content of the oviductal fluid of the rhesus monkey (Macaca mulatta) Fertil Steril. 1977;28:981–5. [PubMed] [Google Scholar]
- Visconti PE, Westbrook VA, Chertihin O, Demarco I, Sleight S, et al. Novel signaling pathways involved in sperm acquisition of fertilizing capacity. J Reprod Immunol. 2002;53:133–50. doi: 10.1016/s0165-0378(01)00103-6. [DOI] [PubMed] [Google Scholar]
- Yanagimachi R.Physiology of reproductionIn: Knobil E, Neill J, editors. Mammalian Fertilization New York; Raven Press; 1994p189 [Google Scholar]
- Parrish JJ, Susko-Parrish JL, First NL. Capacitation of bovine sperm by heparin: inhibitory effect of glucose and role of intracellular pH. Biol Reprod. 1989;41:683–99. doi: 10.1095/biolreprod41.4.683. [DOI] [PubMed] [Google Scholar]
- Zeng Y, Oberdorf JA, Florman HM. pH regulation in mouse sperm: identification of Na+-, Cl−-, and HCO3−-dependent and arylaminobenzoate-dependent regulatory mechanisms and characterization of their roles in sperm capacitation. Dev Biol. 1996;173:510–20. doi: 10.1006/dbio.1996.0044. [DOI] [PubMed] [Google Scholar]
- Baldi E, Casano R, Falsetti C, Krausz C, Maggi M, et al. Intracellular calcium accumulation and responsiveness to progesterone in capacitating human spermatozoa. J Androl. 1991;12:323–30. [PubMed] [Google Scholar]
- Dasgupta S, Mills CL, Fraser LR. Ca2+-related changes in the capacitation state of human spermatozoa assessed by a chlortetracycline fluorescence assay. J Reprod Fertil. 1993;99:135–43. doi: 10.1530/jrf.0.0990135. [DOI] [PubMed] [Google Scholar]
- Galantino-Homer HL, Florman HM, Storey BT, Dobrinski I, Kopf GS. Bovine sperm capacitation: assessment of phosphodiesterase activity and intracellular alkalinization on capacitation-associated protein tyrosine phosphorylation. Mol Reprod Dev. 2004;67:487–500. doi: 10.1002/mrd.20034. [DOI] [PubMed] [Google Scholar]
- Lishko PV, Botchkina IL, Fedorenko A, Kirichok Y. Acid extrusion from human spermatozoa is mediated by flagellar voltage-gated proton channel. Cell. 2010;140:327–37. doi: 10.1016/j.cell.2009.12.053. [DOI] [PubMed] [Google Scholar]
- Marin-Briggiler CI, Gonzalez-Echeverria F, Buffone M, Calamera JC, Tezon JG, et al. Calcium requirements for human sperm function in vitro. . Fertil Steril. 2003;79:1396–403. doi: 10.1016/s0015-0282(03)00267-x. [DOI] [PubMed] [Google Scholar]
- Fraser LR. Minimum and maximum extracellular Ca2+ requirements during mouse sperm capacitation and fertilization in vitro. . J Reprod Fertil. 1987;81:77–89. doi: 10.1530/jrf.0.0810077. [DOI] [PubMed] [Google Scholar]
- Bouschet T, Henley JM. Calcium as an extracellular signalling molecule: perspectives on the calcium sensing receptor in the brain. C R Biol. 2005;328:691–700. doi: 10.1016/j.crvi.2004.10.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carlson AE, Hille B, Babcock DF. External Ca2+ acts upstream of adenylyl cyclase SACY in the bicarbonate signaled activation of sperm motility. Dev Biol. 2007;312:183–92. doi: 10.1016/j.ydbio.2007.09.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cai X, Clapham DE. Evolutionary genomics reveals lineage-specific gene loss and rapid evolution of a sperm-specific ion channel complex: CatSpers and CatSperbeta. PLoS One. 2008;3:e3569. doi: 10.1371/journal.pone.0003569. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Navarro B, Kirichok Y, Chung JJ, Clapham DE. Ion channels that control fertility in mammalian spermatozoa. Int J Dev Biol. 2008;52:607–13. doi: 10.1387/ijdb.072554bn. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ho K, Wolff CA, Suarez SS. CatSper-null mutant spermatozoa are unable to ascend beyond the oviductal reservoir. Reprod Fertil Dev. 2009;21:345–50. doi: 10.1071/rd08183. [DOI] [PubMed] [Google Scholar]
- Ren D, Xia J. Calcium signaling through CatSper channels in mammalian fertilization. Physiology (Bethesda) 2010;25:165–75. doi: 10.1152/physiol.00049.2009. [DOI] [PubMed] [Google Scholar]
- Lishko PV, Kirichok Y. The role of Hv1 and CatSper channels in sperm activation. J Physiol. 2010;588:4667–72. doi: 10.1113/jphysiol.2010.194142. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hildebrand MS, Avenarius MR, Fellous M, Zhang Y, Meyer NC, et al. Genetic male infertility and mutation of CATSPER ion channels. Eur J Hum Genet. 2010;18:1178–84. doi: 10.1038/ejhg.2010.108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu J, Xia J, Cho KH, Clapham DE, Ren D. CatSperbeta, a novel transmembrane protein in the CatSper channel complex. J Biol Chem. 2007;282:18945–52. doi: 10.1074/jbc.M701083200. [DOI] [PubMed] [Google Scholar]
- Wang H, Liu J, Cho KH, Ren D. A novel, single, transmembrane protein CATSPERG is associated with CATSPER1 channel protein. Biol Reprod. 2009;81:539–44. doi: 10.1095/biolreprod.109.077107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang H, Liu J, Cho KH, Ren D. A novel, single, transmembrane protein CATSPERG is associated with CATSPER1 channel protein. Biol Reprod. 2009;81:539–44. doi: 10.1095/biolreprod.109.077107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Qi H, Moran MM, Navarro B, Chong JA, Krapivinsky G, et al. All four CatSper ion channel proteins are required for male fertility and sperm cell hyperactivated motility. Proc Natl Acad Sci USA. 2007;104:1219–23. doi: 10.1073/pnas.0610286104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carlson AE, Westenbroek RE, Quill T, Ren D, Clapham DE, et al. CatSper1 required for evoked Ca2+ entry and control of flagellar function in sperm. Proc Natl Acad Sci USA. 2003;100:14864–8. doi: 10.1073/pnas.2536658100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kirichok Y, Navarro B, Clapham DE. Whole-cell patch-clamp measurements of spermatozoa reveal an alkaline-activated Ca2+ channel. Nature. 2006;439:737–40. doi: 10.1038/nature04417. [DOI] [PubMed] [Google Scholar]
- Wiesner B, Weiner J, Middendorff R, Hagen V, Kaupp UB, et al. Cyclic nucleotide-gated channels on the flagellum control Ca2+ entry into sperm. J Cell Biol. 1998;142:473–84. doi: 10.1083/jcb.142.2.473. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xia J, Ren D. Egg coat proteins activate calcium entry into mouse sperm via CATSPER channels. Biol Reprod. 2009;80:1092–8. doi: 10.1095/biolreprod.108.074039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Darszon A, Acevedo JJ, Galindo BE, Hernández-González EO, Nishigaki T, et al. Sperm channel diversity and functional multiplicity. Reproduction. 2006;131:977–88. doi: 10.1530/rep.1.00612. [DOI] [PubMed] [Google Scholar]
- Arnoult C, Cardullo RA, Lemos JR, Florman HM. Activation of mouse sperm T-type Ca2+ channels by adhesion to the egg zona pellucida. Proc Natl Acad Sci USA. 1996;93:13004–9. doi: 10.1073/pnas.93.23.13004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liévano A, Santi CM, Serrano CJ, Treviño CL, Bellvé AR, et al. T-type Ca2+ channels and alpha1E expression in spermatogenic cells, and their possible relevance to the sperm acrosome reaction. FEBS Lett. 1996;388:150–4. doi: 10.1016/0014-5793(96)00515-7. [DOI] [PubMed] [Google Scholar]
- Publicover SJ, Barratt CL. Voltage-operated Ca2+ channels and the acrosome reaction: which channels are present and what do they do. Hum Reprod. 1999;14:873–9. doi: 10.1093/humrep/14.4.873. [DOI] [PubMed] [Google Scholar]
- Martínez-López P, Santi CM, Treviño CL, Ocampo-Gutiérrez AY, Acevedo JJ, et al. Mouse sperm K+ currents stimulated by pH and cAMP possibly coded by Slo3 channels. Biochem Biophys Res Commun. 2009;381:204–9. doi: 10.1016/j.bbrc.2009.02.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Perez-Reyes E. Molecular physiology of low-voltage-activated t-type calcium channels. Physiol Rev. 2003;83:117–61. doi: 10.1152/physrev.00018.2002. [DOI] [PubMed] [Google Scholar]
- Arnoult C, Kazam IG, Visconti PE, Kopf GS, Villaz M, et al. Control of the low voltage-activated calcium channel of mouse sperm by egg ZP3 and by membrane hyperpolarization during capacitation. Proc Natl Acad Sci USA. 1999;96:6757–62. doi: 10.1073/pnas.96.12.6757. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Santi CM, Darszon A, Hernandez-Cruz A. A dihydropyridine-sensitive T-type Ca2+ current is the main Ca2+ current carrier in mouse primary spermatocytes. Am J Physiol. 1996;271:C1583–93. doi: 10.1152/ajpcell.1996.271.5.C1583. [DOI] [PubMed] [Google Scholar]
- José O, Hernández-Hernández O, Chirinos M, González-González ME, Larrea F, et al. Recombinant human ZP3-induced sperm acrosome reaction: evidence for the involvement of T- and L-type voltage-gated calcium channels. Biochem Biophys Res Commun. 2010;395:530–4. doi: 10.1016/j.bbrc.2010.04.059. [DOI] [PubMed] [Google Scholar]
- Choi S, Na HS, Kim J, Lee J, Lee S, et al. Attenuated pain responses in mice lacking CaV3.2 T-type channels. Genes Brain Behav. 2007;6:425–31. doi: 10.1111/j.1601-183X.2006.00268.x. [DOI] [PubMed] [Google Scholar]
- Stamboulian S, Kim D, Shin HS, Ronjat M, de Waard M, et al. Biophysical and pharmacological characterization of spermatogenic T-type calcium current in mice lacking the CaV3.1 (alpha1G) calcium channel: CaV3.2 (alpha1H) is the main functional calcium channel in wild-type spermatogenic cells. J Cell Physiol. 2004;200:116–24. doi: 10.1002/jcp.10480. [DOI] [PubMed] [Google Scholar]
- Escoffier J, Boisseau S, Serres C, Chen CC, Kim D, et al. Expression, localization and functions in acrosome reaction and sperm motility of CaV3.1 and CaV3.2 channels in sperm cells: an evaluation from CaV3.1 and CaV3.2 deficient mice. J Cell Physiol. 2007;212:753–63. doi: 10.1002/jcp.21075. [DOI] [PubMed] [Google Scholar]
- Wennemuth G, Westenbroek RE, Xu T, Hille B, Babcock DF. CaV2.2 and CaV2.3 (N- and R-type) Ca2+ channels in depolarization-evoked entry of Ca2+ into mouse sperm. J Biol Chem. 2000;275:21210–7. doi: 10.1074/jbc.M002068200. [DOI] [PubMed] [Google Scholar]
- Darszon A, Nishigaki T, Beltran C, Trevino CL. Calcium channels in the development, maturation and function of spermatozoa. Physiol Rev. 2011. [DOI] [PubMed]
- Florman HM, Arnoult C, Kazam IG, Li C, O'Toole CM. A perspective on the control of mammalian fertilization by egg-activated ion channels in sperm: a tale of two channels. Biol Reprod. 1998;59:12–6. doi: 10.1095/biolreprod59.1.12. [DOI] [PubMed] [Google Scholar]
- Demarco IA, Espinosa F, Edwards J, Sosnik J, de la Vega-Beltran JL, et al. Involvement of a Na+/HCO3− cotransporter in mouse sperm capacitation. J Biol Chem. 2003;278:7001–9. doi: 10.1074/jbc.M206284200. [DOI] [PubMed] [Google Scholar]
- Casey JR, Grinstein S, Orlowski J. Sensors and regulators of intracellular pH. Nat Rev Mol Cell Biol. 2010;11:50–61. doi: 10.1038/nrm2820. [DOI] [PubMed] [Google Scholar]
- Quill TA, Wang D, Garbers DL. Insights into sperm cell motility signaling through sNHE and the CatSpers. Mol Cell Endocrinol. 2006;250:84–92. doi: 10.1016/j.mce.2005.12.031. [DOI] [PubMed] [Google Scholar]
- Wang D, King SM, Quill TA, Doolittle LK, Garbers DL. A new sperm-specific Na+/H+ exchanger required for sperm motility and fertility. Nat Cell Biol. 2003;5:1117–22. doi: 10.1038/ncb1072. [DOI] [PubMed] [Google Scholar]
- Catterall WA. Structure and regulation of voltage-gated Ca2+ channels. Annu Rev Cell Dev Biol. 2000;16:521–55. doi: 10.1146/annurev.cellbio.16.1.521. [DOI] [PubMed] [Google Scholar]
- Wang D, Hu J, Bobulescu IA, Quill TA, McLeroy P, et al. A sperm-specific Na+/H+ exchanger (sNHE) is critical for expression and in vivo bicarbonate regulation of the soluble adenylyl cyclase (sAC) Proc Natl Acad Sci USA. 2007;104:9325–30. doi: 10.1073/pnas.0611296104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Musset B, Capasso M, Cherny VV, Morgan D, Bhamrah M, et al. Identification of Thr29 as a critical phosphorylation site that activates the human proton channel Hvcn1 in leukocytes. J Biol Chem. 2010;285:5117–21. doi: 10.1074/jbc.C109.082727. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Munoz-Garay C, de la Vega-Beltran JL, Delgado R, Labarca P, Felix R, et al. Inwardly rectifying K+ channels in spermatogenic cells: functional expression and implication in sperm capacitation. Dev Biol. 2001;234:261–74. doi: 10.1006/dbio.2001.0196. [DOI] [PubMed] [Google Scholar]
- Zeng Y, Clark EN, Florman HM. Sperm membrane potential: hyperpolarization during capacitation regulates zona pellucida-dependent acrosomal secretion. Dev Biol. 1995;171:554–63. doi: 10.1006/dbio.1995.1304. [DOI] [PubMed] [Google Scholar]
- Hernández-González EO, Treviño CL, Castellano LE, de la Vega-Beltrán JL, Ocampo AY, et al. Involvement of cystic fibrosis transmembrane conductance regulator in mouse sperm capacitation. J Biol Chem. 2007;282:24397–406. doi: 10.1074/jbc.M701603200. [DOI] [PubMed] [Google Scholar]
- Acevedo JJ, Mendoza-Lujambio I, de la Vega-Beltran JL, Trevino CL, Felix R, et al. KATP channels in mouse spermatogenic cells and sperm, and their role in capacitation. Dev Biol. 2006;289:395–405. doi: 10.1016/j.ydbio.2005.11.002. [DOI] [PubMed] [Google Scholar]
- Chan HC, Wu WL, Sun YP, Leung PS, Wong TP, et al. Expression of sperm Ca2+-activated K+ channels in Xenopus oocytes and their modulation by extracellular ATP. FEBS Lett. 1998;438:177–82. doi: 10.1016/s0014-5793(98)01298-8. [DOI] [PubMed] [Google Scholar]
- Felix R, Serrano CJ, Treviño CL, Muñoz-Garay C, Bravo A, et al. Identification of distinct K+ channels in mouse spermatogenic cells and sperm. Zygote. 2002;10:183–8. doi: 10.1017/s0967199402002241. [DOI] [PubMed] [Google Scholar]
- Salvatore L, D'Adamo MC, Polishchuk R, Salmona M, Pessia M. Localization and age-dependent expression of the inward rectifier K+ channel subunit Kir 5.1 in a mammalian reproductive system. FEBS Lett. 1999;449:146–52. doi: 10.1016/s0014-5793(99)00420-2. [DOI] [PubMed] [Google Scholar]
- Arcelay E, Salicioni AM, Wertheimer E, Visconti PE. Identification of proteins undergoing tyrosine phosphorylation during mouse sperm capacitation. Int J Dev Biol. 2008;52:463–72. doi: 10.1387/ijdb.072555ea. [DOI] [PubMed] [Google Scholar]
- Darszon A, Treviño CL, Wood C, Galindo B, Rodríguez-Miranda E, et al. Ion channels in sperm motility and capacitation. Soc Reprod Fertil Suppl. 2007;65:229–44. [PubMed] [Google Scholar]
- Hernández-González EO, Sosnik J, Edwards J, Acevedo JJ, Mendoza-Lujambio I, et al. Sodium and epithelial sodium channels participate in the regulation of the capacitation-associated hyperpolarization in mouse sperm. J Biol Chem. 2006;281:5623–33. doi: 10.1074/jbc.M508172200. [DOI] [PubMed] [Google Scholar]
- Kellenberger S, Schild L. Epithelial sodium channel/degenerin family of ion channels: a variety of functions for a shared structure. Physiol Rev. 2002;82:735–67. doi: 10.1152/physrev.00007.2002. [DOI] [PubMed] [Google Scholar]
- Awayda MS. Regulation of the epithelial Na+ channel by intracellular Na+. . Am J Physiol. 1999;277:C216–24. doi: 10.1152/ajpcell.1999.277.2.C216. [DOI] [PubMed] [Google Scholar]
- Hur CG, Choe C, Kim GT, Cho SK, Park JY, et al. Expression and localization of two-pore domain K+ channels in bovine germ cells. Reproduction. 2009;137:237–44. doi: 10.1530/REP-08-0035. [DOI] [PubMed] [Google Scholar]
- Hibino H, Inanobe A, Furutani K, Murakami S, Findlay I, et al. Inwardly rectifying potassium channels: their structure, function, and physiological roles. Physiol Rev. 2010;90:291–366. doi: 10.1152/physrev.00021.2009. [DOI] [PubMed] [Google Scholar]
- Martínez-López P, Treviño CL, de la Vega-Beltrán JL, Blas GD, Monroy E, et al. TRPM8 in mouse sperm detects temperature changes and may influence the acrosome reaction J Cell Physiole-pub ahead of print 10 November 2010; doi: 10.1002/jcp.22493. [DOI] [PubMed]
- Schreiber M, Wei A, Yuan A, Gaut J, Saito M, et al. Slo3, a novel pH-sensitive K+ channel from mammalian spermatocytes. J Biol Chem. 1998;273:3509–16. doi: 10.1074/jbc.273.6.3509. [DOI] [PubMed] [Google Scholar]
- Navarro B, Kirichok Y, Clapham DE. KSper, a pH-sensitive K+ current that controls sperm membrane potential. Proc Natl Acad Sci USA. 2007;104:7688–92. doi: 10.1073/pnas.0702018104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Santi CM, Martínez-López P, de la Vega-Beltrán JL, Butler A, Alisio A, et al. The SLO3 sperm-specific potassium channel plays a vital role in male fertility. FEBS Lett. 2010;584:1041–6. doi: 10.1016/j.febslet.2010.02.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zingman LV, Alekseev AE, Hodgson-Zingman DM, Terzic A. ATP-sensitive potassium channels: metabolic sensing and cardioprotection. J Appl Physiol. 2007;103:1888–93. doi: 10.1152/japplphysiol.00747.2007. [DOI] [PubMed] [Google Scholar]
- Levitan I. Cholesterol and Kir channels. IUBMB Life. 2009;61:781–90. doi: 10.1002/iub.192. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Salicioni AM, Platt MD, Wertheimer EV, Arcelay E, Allaire A, et al. Signalling pathways involved in sperm capacitation. Soc Reprod Fertil Suppl. 2007;65:245–59. [PubMed] [Google Scholar]
- Salkoff L, Butler A, Ferreira G, Santi C, Wei A. High-conductance potassium channels of the SLO family. Nat Rev Neurosci. 2006;7:921–31. doi: 10.1038/nrn1992. [DOI] [PubMed] [Google Scholar]
- Yang CT, Zeng XH, Xia XM, Lingle CJ. Interactions between beta subunits of the KCNMB family and Slo3: beta4 selectively modulates Slo3 expression and function. PLoS One. 2009;4:e6135. doi: 10.1371/journal.pone.0006135. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Salkoff L, Butler A, Ferreira G, Santi C, Wei A. High-conductance potassium channels of the SLO family. Nat Rev Neurosci. 2006;7:921–31. doi: 10.1038/nrn1992. [DOI] [PubMed] [Google Scholar]
- Tang QY, Zhang Z, Xia J, Ren D, Logothetis DE. Phosphatidylinositol 4,5-bisphosphate activates Slo3 currents and its hydrolysis underlies the epidermal growth factor-induced current inhibition. J Biol Chem. 2010;285:19259–66. doi: 10.1074/jbc.M109.100156. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Berdiev BK, Qadri YJ, Benos DJ. Assessment of the CFTR and ENaC association. Mol Biosyst. 2009;5:123–7. doi: 10.1039/b810471a. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rogers CS, Abraham WM, Brogden KA, Engelhardt JF, Fisher JT, et al. The porcine lung as a potential model for cystic fibrosis. Am J Physiol Lung Cell Mol Physiol. 2008;295:L240–63. doi: 10.1152/ajplung.90203.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xu WM, Shi QX, Chen WY, Zhou CX, Ni Y, et al. Cystic fibrosis transmembrane conductance regulator is vital to sperm fertilizing capacity and male fertility. Proc Natl Acad Sci USA. 2007;104:9816–21. doi: 10.1073/pnas.0609253104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen WY, Xu WM, Chen ZH, Ni Y, Yuan YY, et al. Cl− is required for HCO3− entry necessary for sperm capacitation in guinea pig: involvement of a Cl−/HCO3− exchanger (SLC26A3) and CFTR. Biol Reprod. 2009;80:115–23. doi: 10.1095/biolreprod.108.068528. [DOI] [PubMed] [Google Scholar]
- Wertheimer EV, Salicioni AM, Liu W, Trevino CL, Chavez J, et al. Chloride Is essential for capacitation and for the capacitation-associated increase in tyrosine phosphorylation. J Biol Chem. 2008;283:35539–50. doi: 10.1074/jbc.M804586200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Russell JM. Sodium–potassium–chloride cotransport. Physiol Rev. 2000;80:211–76. doi: 10.1152/physrev.2000.80.1.211. [DOI] [PubMed] [Google Scholar]
- Hess KC, Jones BH, Marquez B, Chen Y, Ord TS, et al. The ‘soluble' adenylyl cyclase in sperm mediates multiple signaling events required for fertilization. Dev Cell. 2005;9:249–59. doi: 10.1016/j.devcel.2005.06.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Espinosa F, de la Vega-Beltran JL, Lopez-Gonzalez I, Delgado R, Labarca P, et al. Mouse sperm patch-clamp recordings reveal single Cl− channels sensitive to niflumic acid, a blocker of the sperm acrosome reaction. FEBS Lett. 1998;426:47–51. doi: 10.1016/s0014-5793(98)00305-6. [DOI] [PubMed] [Google Scholar]
- Sato Y, Son JH, Meizel S. The mouse sperm glycine receptor/chloride channel: cellular localization and involvement in the acrosome reaction initiated by glycine. J Androl. 2000;21:99–106. [PubMed] [Google Scholar]
- Bray C, Son JH, Kumar P, Harris JD, Meizel S. A role for the human sperm glycine receptor/Cl− channel in the acrosome reaction initiated by recombinant ZP3. Biol Reprod. 2002;66:91–7. doi: 10.1095/biolreprod66.1.91. [DOI] [PubMed] [Google Scholar]
- Garbers DL, Tubb DJ, Hyne RV. A requirement of bicarbonate for Ca2+-induced elevations of cyclic AMP in guinea pig spermatozoa. J Biol Chem. 1982;257:8980–4. [PubMed] [Google Scholar]
- Braun T, Dods RF. Development of a Mn2+-sensitive, ‘soluble' adenylate cyclase in rat testis. Proc Natl Acad Sci USA. 1975;72:1097–101. doi: 10.1073/pnas.72.3.1097. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xie F, Garcia MA, Carlson AE, Schuh SM, Babcock DF, et al. Soluble adenylyl cyclase (sAC) is indispensable for sperm function and fertilization. Dev Biol. 2006;296:353–62. doi: 10.1016/j.ydbio.2006.05.038. [DOI] [PubMed] [Google Scholar]
- Esposito G, Jaiswal BS, Xie F, Krajnc-Franken MA, Robben TJ, et al. Mice deficient for soluble adenylyl cyclase are infertile because of a severe sperm-motility defect. Proc Natl Acad Sci USA. 2004;101:2993–8. doi: 10.1073/pnas.0400050101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vijayaraghavan S, Goueli SA, Davey MP, Carr DW. Protein kinase A-anchoring inhibitor peptides arrest mammalian sperm motility. J Biol Chem. 1997;272:4747–52. doi: 10.1074/jbc.272.8.4747. [DOI] [PubMed] [Google Scholar]
- Visconti PE, Moore GD, Bailey JL, Leclerc P, Connors SA, et al. Capacitation of mouse spermatozoa. II. Protein tyrosine phosphorylation and capacitation are regulated by a cAMP-dependent pathway. Development. 1995;121:1139–50. doi: 10.1242/dev.121.4.1139. [DOI] [PubMed] [Google Scholar]
- Desseyn JL, Burton KA, McKnight GS. Expression of a nonmyristylated variant of the catalytic subunit of protein kinase A during male germ-cell development. Proc Natl Acad Sci USA. 2000;97:6433–8. doi: 10.1073/pnas.97.12.6433. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nolan MA, Babcock DF, Wennemuth G, Brown W, Burton KA, et al. Sperm-specific protein kinase A catalytic subunit Calpha2 orchestrates cAMP signaling for male fertility. Proc Natl Acad Sci USA. 2004;101:13483–8. doi: 10.1073/pnas.0405580101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morgan DJ, Weisenhaus M, Shum S, Su T, Zheng R, et al. Tissue-specific PKA inhibition using a chemical genetic approach and its application to studies on sperm capacitation. Proc Natl Acad Sci USA. 2008;105:20740–5. doi: 10.1073/pnas.0810971105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Visconti PE, Bailey JL, Moore GD, Pan D, Olds-Clarke P, et al. Capacitation of mouse spermatozoa. I. Correlation between the capacitation state and protein tyrosine phosphorylation. Development. 1995;121:1129–37. doi: 10.1242/dev.121.4.1129. [DOI] [PubMed] [Google Scholar]
- Urner F, Leppens-Luisier G, Sakkas D. Protein tyrosine phosphorylation in sperm during gamete interaction in the mouse: the influence of glucose. Biol Reprod. 2001;64:1350–7. doi: 10.1095/biolreprod64.5.1350. [DOI] [PubMed] [Google Scholar]
- Burton KA, McKnight GS. PKA, germ cells, and fertility. Physiology (Bethesda) 2007;22:40–6. doi: 10.1152/physiol.00034.2006. [DOI] [PubMed] [Google Scholar]
- Eddy EM. The scaffold role of the fibrous sheath. Soc Reprod Fertil Suppl. 2007;65:45–62. [PubMed] [Google Scholar]
- Miki K, Willis WD, Brown PR, Goulding EH, Fulcher KD, et al. Targeted disruption of the Akap4 gene causes defects in sperm flagellum and motility. Dev Biol. 2002;248:331–42. doi: 10.1006/dbio.2002.0728. [DOI] [PubMed] [Google Scholar]
- Carrera A, Moos J, Ning XP, Gerton GL, Tesarik J, et al. Regulation of protein tyrosine phosphorylation in human sperm by a calcium/calmodulin-dependent mechanism: identification of A kinase anchor proteins as major substrates for tyrosine phosphorylation. Dev Biol. 1996;180:284–96. doi: 10.1006/dbio.1996.0301. [DOI] [PubMed] [Google Scholar]
- Jha KN, Shivaji S. Identification of the major tyrosine phosphorylated protein of capacitated hamster spermatozoa as a homologue of mammalian sperm a kinase anchoring protein. Mol Reprod Dev. 2002;61:258–70. doi: 10.1002/mrd.1155. [DOI] [PubMed] [Google Scholar]
- Ficarro S, Chertihin O, Westbrook VA, White F, Jayes F, et al. Phosphoproteome analysis of capacitated human sperm. Evidence of tyrosine phosphorylation of a kinase-anchoring protein 3 and valosin-containing protein/p97 during capacitation. J Biol Chem. 2003;278:11579–89. doi: 10.1074/jbc.M202325200. [DOI] [PubMed] [Google Scholar]
- Li YF, He W, Kim YH, Mandal A, Digilio L, et al. CABYR isoforms expressed in late steps of spermiogenesis bind with AKAPs and ropporin in mouse sperm fibrous sheath. Reprod Biol Endocrinol. 2010;8:101. doi: 10.1186/1477-7827-8-101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mitra K, Rangaraj N, Shivaji S. Novelty of the pyruvate metabolic enzyme dihydrolipoamide dehydrogenase in spermatozoa: correlation of its localization, tyrosine phosphorylation, and activity during sperm capacitation. J Biol Chem. 2005;280:25743–53. doi: 10.1074/jbc.M500310200. [DOI] [PubMed] [Google Scholar]
- Baker MA, Hetherington L, Aitken RJ. Identification of SRC as a key PKA-stimulated tyrosine kinase involved in the capacitation-associated hyperactivation of murine spermatozoa. J Cell Sci. 2006;119:3182–92. doi: 10.1242/jcs.03055. [DOI] [PubMed] [Google Scholar]
- Lawson C, Goupil S, Leclerc P. Increased activity of the human sperm tyrosine kinase SRC by the cAMP-dependent pathway in the presence of calcium. Biol Reprod. 2008;79:657–66. doi: 10.1095/biolreprod.108.070367. [DOI] [PubMed] [Google Scholar]
- Mitchell LA, Nixon B, Baker MA, Aitken RJ. Investigation of the role of SRC in capacitation-associated tyrosine phosphorylation of human spermatozoa. Mol Hum Reprod. 2008;14:235–43. doi: 10.1093/molehr/gan007. [DOI] [PubMed] [Google Scholar]
- Varano G, Lombardi A, Cantini G, Forti G, Baldi E, et al. Src activation triggers capacitation and acrosome reaction but not motility in human spermatozoa. Hum Reprod. 2008;23:2652–62. doi: 10.1093/humrep/den314. [DOI] [PubMed] [Google Scholar]
- Krapf D, Arcelay E, Wertheimer EV, Sanjay A, Pilder SH, et al. Inhibition of Ser/Thr phosphatases induces capacitation-associated signaling in the presence of Src kinase inhibitors. J Biol Chem. 2010;285:7977–85. doi: 10.1074/jbc.M109.085845. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lax Y, Rubinstein S, Breitbart H. Subcellular distribution of protein kinase C alpha and betaI in bovine spermatozoa, and their regulation by calcium and phorbol esters. Biol Reprod. 1997;56:454–9. doi: 10.1095/biolreprod56.2.454. [DOI] [PubMed] [Google Scholar]
- Rotman T, Etkovitz N, Spiegel A, Rubinstein S, Breitbart H. Protein kinase A and protein kinase Cα/PPP1CC2 play opposing roles in the regulation of phosphatidylinositol 3-kinase activation in bovine sperm. Reproduction. 2010;140:43–56. doi: 10.1530/REP-09-0314. [DOI] [PubMed] [Google Scholar]
- Rotem R, Paz GF, Homonnai ZT, Kalina M, Naor Z. Protein kinase C is present in human sperm: possible role in flagellar motility. Proc Natl Acad Sci USA. 1990;87:7305–8. doi: 10.1073/pnas.87.18.7305. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Naor Z, Breitbart H. Protein kinase C and mammalian spermatozoa acrosome reaction. Trends Endocrinol Metab. 1997;8:337–42. doi: 10.1016/s1043-2760(97)00134-3. [DOI] [PubMed] [Google Scholar]
- Visconti PE, Tezon JG. Phorbol esters stimulate cyclic adenosine 3', 5'-monophosphate accumulation in hamster spermatozoa during in vitro capacitation. Biol Reprod. 1989;40:223–31. doi: 10.1095/biolreprod40.2.223. [DOI] [PubMed] [Google Scholar]
- Colon-Gonzalez F, Kazanietz MG. C1 domains exposed: from diacylglycerol binding to protein–protein interactions. Biochim Biophys Acta. 2006;1761:827–37. doi: 10.1016/j.bbalip.2006.05.001. [DOI] [PubMed] [Google Scholar]
- Luconi M, Barni T, Vannelli GB, Krausz C, Marra F, et al. Extracellular signal-regulated kinases modulate capacitation of human spermatozoa. Biol Reprod. 1998;58:1476–89. doi: 10.1095/biolreprod58.6.1476. [DOI] [PubMed] [Google Scholar]
- de Lamirande E, Gagnon C. The extracellular signal-regulated kinase (ERK) pathway is involved in human sperm function and modulated by the superoxide anion. Mol Hum Reprod. 2002;8:124–35. doi: 10.1093/molehr/8.2.124. [DOI] [PubMed] [Google Scholar]
- Aquila S, Gentile M, Middea E, Catalano S, Morelli C, et al. Leptin secretion by human ejaculated spermatozoa. J Clin Endocrinol Metab. 2005;90:4753–61. doi: 10.1210/jc.2004-2233. [DOI] [PubMed] [Google Scholar]
- Marín-Briggiler CI, Jha KN, Chertihin O, Buffone MG, Herr JC, et al. Evidence of the presence of calcium/calmodulin-dependent protein kinase IV in human sperm and its involvement in motility regulation. J Cell Sci. 2005;118:2013–22. doi: 10.1242/jcs.02326. [DOI] [PubMed] [Google Scholar]
- Mudgal P, Anand SR. Casein kinase II activity of buffalo sperm chromatin. Mol Reprod Dev. 1998;50:178–84. doi: 10.1002/(SICI)1098-2795(199806)50:2<178::AID-MRD8>3.0.CO;2-H. [DOI] [PubMed] [Google Scholar]
- Li Y, Sosnik J, Brassard L, Reese M, Spiridonov NA, et al. Expression and localization of five members of the testis-specific serine kinase (Tssk) family in mouse and human sperm and testis Mol Hum Reprode-pub ahead of print 20 August 2010; doi: 10.1093/molehr/gaq071. [DOI] [PMC free article] [PubMed]
- Jha KN, Salicioni AM, Arcelay E, Chertihin O, Kumari S, et al. Evidence for the involvement of proline-directed serine/threonine phosphorylation in sperm capacitation. Mol Hum Reprod. 2006;12:781–9. doi: 10.1093/molehr/gal085. [DOI] [PubMed] [Google Scholar]
- Daleke DL. Regulation of transbilayer plasma membrane phospholipid asymmetry. J Lipid Res. 2003;44:233–42. doi: 10.1194/jlr.R200019-JLR200. [DOI] [PubMed] [Google Scholar]
- Tanaka K, Fujimura-Kamada K, Yamamoto T. Functions of phospholipid flippases. J Biochem. 2011;149:131–43. doi: 10.1093/jb/mvq140. [DOI] [PubMed] [Google Scholar]
- Wang L, Beserra C, Garbers DL. A novel aminophospholipid transporter exclusively expressed in spermatozoa is required for membrane lipid asymmetry and normal fertilization. Dev Biol. 2004;267:203–15. doi: 10.1016/j.ydbio.2003.11.004. [DOI] [PubMed] [Google Scholar]
- Bevers EM, Williamson PL. Phospholipid scramblase: an update. FEBS Lett. 2010;584:2724–30. doi: 10.1016/j.febslet.2010.03.020. [DOI] [PubMed] [Google Scholar]
- Balasubramanian K, Schroit AJ. Aminophospholipid asymmetry: a matter of life and death. Annu Rev Physiol. 2003;65:701–34. doi: 10.1146/annurev.physiol.65.092101.142459. [DOI] [PubMed] [Google Scholar]
- Bevers EM, Comfurius P, Dekkers DW, Harmsma M, Zwaal RF. Transmembrane phospholipid distribution in blood cells: control mechanisms and pathophysiological significance. Biol Chem. 1998;379:973–86. [PubMed] [Google Scholar]
- Zachowski A. Phospholipids in animal eukaryotic membranes: transverse asymmetry and movement. Biochem J. 1993;294 Pt 1:1–14. doi: 10.1042/bj2940001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gadella BM, Harrison RA. The capacitating agent bicarbonate induces protein kinase A-dependent changes in phospholipid transbilayer behavior in the sperm plasma membrane. Development. 2000;127:2407–20. doi: 10.1242/dev.127.11.2407. [DOI] [PubMed] [Google Scholar]
- Harrison RA, Miller NG. cAMP-dependent protein kinase control of plasma membrane lipid architecture in boar sperm. Mol Reprod Dev. 2000;55:220–8. doi: 10.1002/(SICI)1098-2795(200002)55:2<220::AID-MRD12>3.0.CO;2-I. [DOI] [PubMed] [Google Scholar]
- de Vries KJ, Wiedmer T, Sims PJ, Gadella BM. Caspase-independent exposure of aminophospholipids and tyrosine phosphorylation in bicarbonate responsive human sperm cells. Biol Reprod. 2003;68:2122–34. doi: 10.1095/biolreprod.102.012500. [DOI] [PubMed] [Google Scholar]
- Flesch FM, Brouwers JF, Nievelstein PF, Verkleij AJ, van Golde LM, et al. Bicarbonate stimulated phospholipid scrambling induces cholesterol redistribution and enables cholesterol depletion in the sperm plasma membrane. J Cell Sci. 2001;114:3543–55. doi: 10.1242/jcs.114.19.3543. [DOI] [PubMed] [Google Scholar]
- Espinosa F, López-González I, Muñoz-Garay C, Felix R, de la Vega-Beltrán JL, et al. Dual regulation of the T-type Ca2+ current by serum albumin and beta-estradiol in mammalian spermatogenic cells. FEBS Lett. 2000;475:251–6. doi: 10.1016/s0014-5793(00)01688-4. [DOI] [PubMed] [Google Scholar]
- Xia J, Ren D. The BSA-induced Ca2+ influx during sperm capacitation is CATSPER channel-dependent. Reprod Biol Endocrinol. 2009;7:119. doi: 10.1186/1477-7827-7-119. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Visconti PE, Ning X, Fornés MW, Alvarez JG, Stein P, et al. Cholesterol efflux-mediated signal transduction in mammalian sperm: cholesterol release signals an increase in protein tyrosine phosphorylation during mouse sperm capacitation. Dev Biol. 1999;214:429–43. doi: 10.1006/dbio.1999.9428. [DOI] [PubMed] [Google Scholar]
- Therien I, Soubeyrand S, Manjunath P. Major proteins of bovine seminal plasma modulate sperm capacitation by high-density lipoprotein. Biol Reprod. 1997;57:1080–8. doi: 10.1095/biolreprod57.5.1080. [DOI] [PubMed] [Google Scholar]
- Choi YH, Toyoda Y. Cyclodextrin removes cholesterol from mouse sperm and induces capacitation in a protein-free medium. Biol Reprod. 1998;59:1328–33. doi: 10.1095/biolreprod59.6.1328. [DOI] [PubMed] [Google Scholar]
- Osheroff JE, Visconti PE, Valenzuela JP, Travis AJ, Alvarez J, et al. Regulation of human sperm capacitation by a cholesterol efflux-stimulated signal transduction pathway leading to protein kinase A-mediated up-regulation of protein tyrosine phosphorylation. Mol Hum Reprod. 1999;5:1017–26. doi: 10.1093/molehr/5.11.1017. [DOI] [PubMed] [Google Scholar]
- Visconti PE, Galantino-Homer H, Ning X, Moore GD, Valenzuela JP, et al. Cholesterol efflux-mediated signal transduction in mammalian sperm. β-cyclodextrins initiate transmembrane signaling leading to an increase in protein tyrosine phosphorylation and capacitation. J Biol Chem. 1999;274:3235–42. doi: 10.1074/jbc.274.5.3235. [DOI] [PubMed] [Google Scholar]
- Cross NL. Effect of methyl-beta-cyclodextrin on the acrosomal responsiveness of human sperm. Mol Reprod Dev. 1999;53:92–8. doi: 10.1002/(SICI)1098-2795(199905)53:1<92::AID-MRD11>3.0.CO;2-Q. [DOI] [PubMed] [Google Scholar]
- Simons K, Ikonen E. How cells handle cholesterol. Science. 2000;290:1721–6. doi: 10.1126/science.290.5497.1721. [DOI] [PubMed] [Google Scholar]
- Simons K, Vaz WL. Model systems, lipid rafts, and cell membranes. Annu Rev Biophys Biomol Struct. 2004;33:269–95. doi: 10.1146/annurev.biophys.32.110601.141803. [DOI] [PubMed] [Google Scholar]
- Shogomori H, Brown DA. Use of detergents to study membrane rafts: the good, the bad, and the ugly. Biol Chem. 2003;384:1259–63. doi: 10.1515/BC.2003.139. [DOI] [PubMed] [Google Scholar]
- Asano A, Selvaraj V, Buttke DE, Nelson JL, Green KM, et al. Biochemical characterization of membrane fractions in murine sperm: identification of three distinct sub-types of membrane rafts. J Cell Physiol. 2009;218:537–48. doi: 10.1002/jcp.21623. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miranda PV, Allaire A, Sosnik J, Visconti PE. Localization of low-density detergent-resistant membrane proteins in intact and acrosome-reacted mouse sperm. Biol Reprod. 2009;80:897–904. doi: 10.1095/biolreprod.108.075242. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sleight SB, Miranda PV, Plaskett NW, Maier B, Lysiak J, et al. Isolation and proteomic analysis of mouse sperm detergent-resistant membrane fractions: evidence for dissociation of lipid rafts during capacitation. Biol Reprod. 2005;73:721–9. doi: 10.1095/biolreprod.105.041533. [DOI] [PubMed] [Google Scholar]
- Trevino CL, Serrano CJ, Beltran C, Felix R, Darszon A. Identification of mouse trp homologs and lipid rafts from spermatogenic cells and sperm. FEBS Lett. 2001;509:119–25. doi: 10.1016/s0014-5793(01)03134-9. [DOI] [PubMed] [Google Scholar]
- Selvaraj V, Buttke DE, Asano A, McElwee JL, Wolff CA, et al. GM1 dynamics as a marker for membrane changes associated with the process of capacitation in murine and bovine spermatozoa. J Androl. 2007;28:588–99. doi: 10.2164/jandrol.106.002279. [DOI] [PubMed] [Google Scholar]
- Bruckbauer A, Dunne PD, James P, Howes E, Zhou D, et al. Selective diffusion barriers separate membrane compartments. Biophys J. 2010;99:L1–3. doi: 10.1016/j.bpj.2010.03.067. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kawano N, Yoshida K, Iwamoto T, Yoshida M. Ganglioside GM1 mediates decapacitation effects of SVS2 on murine spermatozoa. Biol Reprod. 2008;79:1153–9. doi: 10.1095/biolreprod.108.069054. [DOI] [PubMed] [Google Scholar]
- Edidin M. Membrane cholesterol, protein phosphorylation, and lipid rafts. Sci STKE. 2001;2001:pe1. doi: 10.1126/stke.2001.67.pe1. [DOI] [PubMed] [Google Scholar]
- Zajchowski LD, Robbins SM. Lipid rafts and little caves. Compartmentalized signalling in membrane microdomains. Eur J Biochem. 2002;269:737–52. doi: 10.1046/j.0014-2956.2001.02715.x. [DOI] [PubMed] [Google Scholar]
- Brown DA, London E. Functions of lipid rafts in biological membranes. Annu Rev Cell Dev Biol. 1998;14:111–36. doi: 10.1146/annurev.cellbio.14.1.111. [DOI] [PubMed] [Google Scholar]
- Kabouridis PS, Janzen J, Magee AL, Ley SC. Cholesterol depletion disrupts lipid rafts and modulates the activity of multiple signaling pathways in T lymphocytes. Eur J Immunol. 2000;30:954–63. doi: 10.1002/1521-4141(200003)30:3<954::AID-IMMU954>3.0.CO;2-Y. [DOI] [PubMed] [Google Scholar]
- Roy S, Luetterforst R, Harding A, Apolloni A, Etheridge M, et al. Dominant-negative caveolin inhibits H-Ras function by disrupting cholesterol-rich plasma membrane domains. Nat Cell Biol. 1999;1:98–105. doi: 10.1038/10067. [DOI] [PubMed] [Google Scholar]
- Jones R, Howes E, Dunne PD, James P, Bruckbauer A, et al. Tracking diffusion of GM1 gangliosides and zona pellucida binding molecules in sperm plasma membranes following cholesterol efflux. Dev Biol. 2010;339:398–406. doi: 10.1016/j.ydbio.2009.12.044. [DOI] [PubMed] [Google Scholar]


