Abstract
The small (SK3) and intermediate (IK1) conductance calcium-activated potassium channels could have key roles in the endothelium-dependent hyperpolarization factor pathway, which is believed to contribute to normal penile erection function. We aimed to investigate the expression of SK3 and IK1 in diabetic rodents. The experimental diabetes model was induced in 8-week-old male Sprague–Dawley rats (250–300 g) by a single administration of streptozotocin. Both the diabetes mellitus group (DM group, n = 20) and the control group (NDM group, n = 10) were injected with a low dose of apomorphine to allow for the measurement and comparison of the corresponding penile erections. The mRNA and protein expression levels of SK3 and IK1 were measured by reverse transcription polymerase chain reaction and western blot, respectively. Erectile function was significantly decreased in the DM group compared with control group (P < 0.05). The mRNA and protein expression levels of SK3 and IK1 were reduced in the cavernous tissue of diabetic rats compared with the control group (P < 0.05). Diabetes inhibits mRNA and protein expression of both SK3 and IK1 in the cavernous tissue of diabetic rats. This could play a key role in the development of erectile dysfunction in diabetic rats.
Keywords: diabetes mellitus, endothelium-dependent, hyperpolarization factor, erectile dysfunction, IK1, SK3
Introduction
Erectile dysfunction (ED) is a distressing complication of diabetes. The prevalence of ED among diabetic men varies from 35% to 90% 1, 2, 3. ED is three times more common in diabetic than in non-diabetic men, and may occur 10–15 years earlier in diabetic men. Phosphodiesterase type 5 inhibitors have shown good results in the treatment of ED, but for presently unknown reasons, their efficacy is significantly lowered in diabetic ED 4. Besides the well-established nitric oxide (NO)/cyclic guanosine monophosphate pathway, recent reports have shown the existence of a second, distinct endothelial pathway involving the endothelium-dependent hyperpolarization factor (EDHF). Specifically, this factor has been shown to be involved in the relaxation of the vascular smooth muscle cells in penile arteries 5, 6, 7, 8. Angulo 9 has reported that diabetes can impair endothelium-dependent relaxation of human penile vascular tissues mediated by EDHF, but the causes remain unclear. The calcium-activated potassium channels, particularly the small and intermediate conductance calcium-activated K+-channels (SK3 and IK1), are key players in EDHF-mediated relaxation in small arteries 10. Therefore, we hypothesized that diabetes impairs EDHF-mediated relaxation in penile arteries, and that this impairment may be related to defective expression and/or function of SK3 and IK1 channels. With these premises, we detect the expressions of SK3 and IK1 channels in mRNA and protein levels.
Materials and methods
Establishment and grouping of a diabetes mellitus (DM) rat model
Sprague–Dawley (SD) rats were provided by the Center for Laboratory Animals, Nanjing Medical University (Nanjing, China). The 36 rats were divided into two groups: the control group (NDM group, n = 10) and the experimental group (DM group, n = 26). The diabetes model was induced in male SD rats by a single administration of streptozotocin (STZ) 11, 12. Animals were housed in the laboratory animal unit of Nanjing Medical University, fed with regular chow and given free access to water.
Penile erection experiment
Following Heaton's approach 13, after 8 weeks, rats were set in a transparent observation kit in a tranquil lab for 10 min to allow them to adapt to the new surroundings. Lights were then turned down and each of them was injected with 80 μg kg−1 of apomorphine (APO; Sigma, St. Louis, MO, USA). Close observation to record the status and frequency of penile erection took place after the injection. Each glans engorgement and appearance of the penile shaft indicated one erection. The observation time was 30 min. Erection rate refers to the ratio of the number of rats that presented erections to the total number of rats. Erectile frequency refers to the number of erections in 30 min.
Reverse transcription-polymerase chain reaction (RT-PCR) analysis
Rats were anesthetized with intraperitoneal pentobarbital sodium (70 mg kg−1). This was followed by dissection of the isolated penile tissue, which included the skin, head and corpus spongiosum of the removed penis. The penis was stored in liquid nitrogen. Total RNA was extracted from the cavernous tissue segments using TRIzol reagent (Gibco, Gaithersburg, MD, USA) as described elsewhere, and 4.5 μg of total RNA was reverse-transcribed using first-strand cDNA synthesis kit according to the manufacturer's instructions. Subsequent PCR was performed at an annealing temperature of 58°C in 33 cycles using the following forward and reverse primers (Gapdh, 5′-AAGGTCGGAGTCAACGGATTT-3′, 5′-AGATGATGACCCTTTTGGCTC-3′ SK3, 5′-CACCAGACTCTGCTCCATCA-3′, 5′-GACGAATCGGGTGTTGAAGT-3′ IK1, 5′-CGGCTCTACTATTGGCTGTG-3′, 5′-AGCCTGATTCTTCTGTGGGT C-3′).
Amplification products were separated on 1.5% standard agarose gels with ethidium bromide staining. Densitometry was performed at nonsaturating exposures, and the SK3/Gadph and IK1/Gadph ratios were determined. All results are representative of at least three independent experiments.
Western blot analysis
Penile corpus cavernosum tissues were homogenized with the dounce homogenizer and resuspended in a preparation of modified radio immunoprecipitation buffer (50 mmol L−1 Tris-HCl [pH 7.4], 150 mmol L−1 NaCl, 1 mmol L−1 PMSF, 1 mmol L−1 EDTA , 1% Triton X100, 1% sodium deoxycholate and 0.1% sodium dodecyl sulphate). A bicinchoninic acid protein assay kit (Bio-Rad, Hercules, CA, USA) was used to determine total protein concentration. Samples containing 50 μg of total protein were drawn from each group to be separated by SDS-PAGE. The gel was then left to equilibrate in transfer buffer. Tissues were immersed in twice-distilled water for 10 min and transferred to the transfer buffer for 5 min. The filter and nitrocellulose (NC) membrane (Amersham Biosciences, Uppsala, Sweden) were then processed together. The filter, gel, NC membrane and a second filter were placed on a mat, which was then put into the transfer tank at 100 mA for 3 h with the membrane placed towards the positive pole and the gel towards the negative pole. The transferred NC membrane was then incubated for 1.5 h in 5% degrease milk powder reagent, before it was taken out to be washed with phosphate-buffered saline (PBS) three times, for 5–10 min each time. The NC membrane was then immerged into a plate or a small bag with appropriately diluted SK3 and IK1 rabbit polyclonal anti-rat antibodies (Santa Cruz Biotech, Santa Cruz, CA, USA) at room temperature for 1.5 h. It was then washed with PBS three times, for 10 min each time. Subsequently, the NC membrane was immerged in appropriately diluted peroxidase-conjugated secondary antibodies and goat anti-rabbit IgG (Beijing Zhong Shan-Golden Bridge Biological Technology CO., LTD, Beijing, China) at room temperature for 1 h, and was then washed with PBS four times, each time for 10 min. The membrane was then put into the diaminobenzidine colour development liquid until the effects were satisfactory. Once the colour had been developed, the membrane was washed with twice-distilled water to stop colour development. The dilution of the β-actin (Santa Cruz Biotech) was 1:2 000. The expression of β-actin was used as the internal control for equal loading. The Western blot bands were compared by densitometry using an Eastman Kodak Image Station 440CF (Kodak, New Haven, CT, USA), and the data were analysed using Kodak ID V.3.5.4 (Scientific Imaging System, Rockville, MD, USA). All results were representative of at least three independent experiments.
Statistical analysis
The results were expressed as mean ± SD. The statistical significance of any differences in the measured quantities was determined using Mann–Whitney U test with SPSS 10.0 (SPSS Inc, Chicago, IL, USA). P < 0.05 was considered statistically significant.
Results
Animal model
At the beginning of the study, the two groups of rats presented comparable body weight. Eight weeks later, the control group had gained weight, whereas the STZ-treated rats (DM group) exhibited a significant weight loss (P < 0.05). In addition, the glucose level of the DM group was significantly higher than that of the control group (P < 0.05, Table 1). There were six rat deaths in the DM group and no death in the NDM group.
Table 1. The body weight and fasting blood glucose of all rats.
| Group | n | Weight (g) | Glucose (mmol L−1) | ||
|---|---|---|---|---|---|
| Start | 8 weeks | 72 h | 8 weeks | ||
| DM | 20 | 245.0 ± 18.2 | 216.0 ± 21.5* | 25.1 ± 4.8* | 22.0 ± 3.8* |
| NDM | 10 | 241.0 ± 19.4 | 412.0 ± 26.5 | 5.1 ± 1.1 | 5.9 ± 1.5 |
Data were expressed as mean ± SD.
DM, Diabetes mellitus group; NDM, control group.
P < 0.05, compared with the control group (NDM).
Impact of DM on rats' penile erection
After APO injection, the DM group had less penile erection than the NDM group (P < 0.05; Table 2).
Table 2. Penis erection of rats receiving injections of apomorphine.
| Group | n | Erection frequency | Erection rate (%) |
|---|---|---|---|
| DM | 20 | 0.58 ± 0.56* | 54* |
| NDM | 10 | 1.90 ± 0.70 | 100 |
Data were expressed as mean ± SD.
DM, diabetes mellitus group: NDM, control group.
P < 0.05, compared with the control group (NDM).
Impact of DM on SK3 and IK1 expressions
SK3 and IK1 expression at the mRNA and protein levels was scarce in the cavernous tissue of diabetic rats compared with the control group (P < 0.05; Figures 1, 2, 3).
Figure 1.
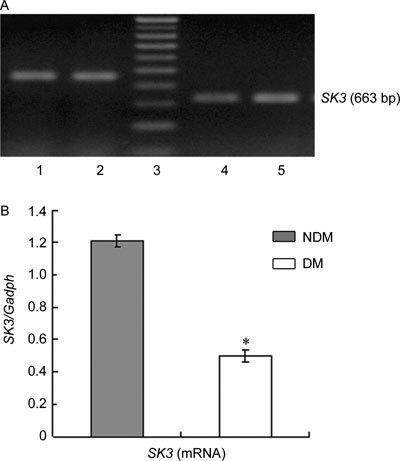
(A): Agarose gel analysis of RT-PCR products encoding SK3 fragments in rats' corpus cavernosum. The Gadph amplification primers were used as controls, 1, 2: Gadph; 3: marker; 4: DM group; 5: NDM group. (B): Densitometry was performed at nonsaturating exposures,and the SK3/Gadph ratios were determined, The data were expressed as mean ± SD (NDM group: n =10, DM group: n = 20). All results are representative of three independent experiments. *P < 0.05; compared with the control group. DM, diabetes mellitus group; NDM, control group.
Figure 2.
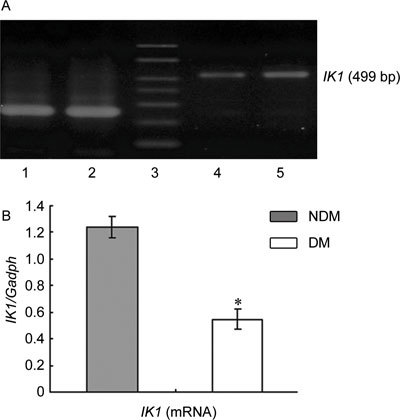
(A): Agarose gel analysis of RT-PCR products encoding IK1 fragments in rats' corpus cavernosum. The Gadph amplification primers were used as controls. Lane 1, 2: Gadph; Lane 3: marker; Lane 4: DM group; Lane 5: NDM group. (B): Densitometry was performed at nonsaturating exposures, and the IK1/Gadph ratios were determined. The data were expressed as mean ± SD (NDM group: n =10, DM group: n = 20). All results are representative of three independent experiments. *P < 0.05; compared with the control group. DM, diabetes mellitus group: NDM, control group.
Figure 3.
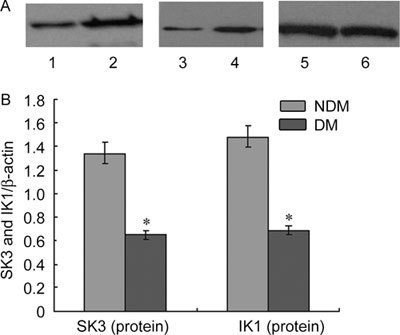
(A): The expression of SK3 and IK1 protein in rats' corpus cavernosum. Proteins were normalised for loading with β-actin, Lane 1: IK1 in DM group; Lane 2: IK1 in NDM group; Lane 3: SK3 in DM group; Lane 4: SK3 in NDM group; Lane 5, 6: β-actin. (B): SK3, IK1 and β-actin bands were subject to densitometry on an Eastman Kodak Co. Image Station 440 CF, and the ratio of SK3 and β-actin, IK1 and β-actin was plotted for quantification of the blots. The data were expressed as mean ± SD (NDM group: n =10, DM group: n = 20). All results are representative of three independent experiments. *P < 0.05; compared with the control group. DM, diabetes mellitus group: NDM, control group.
Discussion
The endothelium is an important contributor to smooth muscle relaxation of the corpus cavernosum and penile small arteries, and it is thought to have a key role in erectile physiology and ED 14, 15, 16. Under physiological conditions, the endothelium exerts regulatory effects on vasodilatation, as it releases numerous vasoactive substances, such as NO, bradykinin, prostaglandin I2, substance P and so on. EDHF is another vasoactive substance and it has been found in rat, horse and human penile small arteries 5, 6, 7, 8, 17. Moreover, in systemic arteries, several EDHF candidates have been suggested, including potassium ions, products of the cytochrome P450 pathway, C-type natriuretic peptide, hydrogen peroxide and K+ 7, 18, 19. EDHF-mediated relaxation is dependent on the activation of endothelial SK3 and IK1, which can be blocked by the combination of apamin and charybdotoxin. Furthermore, it can cause hyperpolarization of the underlying smooth muscle layer 10.
The small conductance calcium-activated potassium channel family consists of SK1, SK2 and SK3 subtypes. SK1 mRNA has been detected almost exclusively in neuronal tissues, whereas SK2 mRNA has been found in the adrenal gland, prostate, bladder, brain, liver and heart. In addition, SK3 mRNA has been detected in almost every tissue examined, especially in the brain and smooth muscle-rich tissues, including the clitoris and the corpus cavernosum 20. IK1 mRNA has been shown to be present in surface-rich, secretory and inflammatory cell-rich tissues, and to be particularly high in the trachea, prostate, placenta and salivary glands 20. Expression of IK1, SK2 and SK3 mRNA can be detected by RT-PCR. The SK3 protein is abundant in porcine coronary endothelial cells, and immunofluorescent labelling confirms that IK1 and SK3 are expressed at the plasmalemma of porcine coronary endothelial cells 21. In addition, it is well documented that in a number of cell types such as lymphocytes 22 and fibroblasts 23, 24, IK1 expression is highly variable during the different phases of cell proliferation. By contrast, in human colonic and cavernosal vascular endothelium, no IK1 immunoreactivity was detected 20. It is possible that the IK1 expression levels in these cell types vary across species, tissues and physiological status, although this requires further investigation. Overall, these observations demonstrate that SK3 and IK1 have a key role in mediating EDHF's effects and that they are located on the endothelial plasma membrane.
Hilgers and Webb 25 reported that SK3 expression was significantly reduced in mesenteric arteries (MAs) of angiotensin II-induced hypertension rats. Relative mRNA expression levels of IK1 were significantly reduced in the MAs of hypertension rats, whereas protein levels of IK1 were not, but tended to be lower. Zhou et al.26 investigated the role of the endogenous NO synthase inhibitor asymmetric dimethylarginine (ADMA) in the down-regulation of expression of endothelial SK3 in atherosclerotic mice and in cultured human umbilical vein endothelial cells. They found that either lysophosphatidylcholine or ADMA notably decreased the SK3 protein and mRNA expression levels in a concentration-dependent manner. These observations suggest that a reduction in the levels of the SK3 channel may contribute to the defective endothelium-dependent vasodilation.
Diabetes can also impair endothelium-dependent relaxation of human penile vascular tissues mediated by EDHF 9, but the mechanism remains unclear. In our study, we have demonstrated that diabetes can inhibit mRNA and protein expression of SK3 and IK1, which could be related to the observed impairment in the EDHF pathway. In addition, the reduced expression of both molecules in the cavernous tissues might have a key role in the development of ED in diabetic rats. These findings emphasize the importance of the EDHF pathway for normal erectile function. They also provide additional support to the in vitro observation that diabetes can impair EDHF-dependent responses 9. Activating endothelial SK3 and IK1 channels or increasing their expression could constitute a novel therapeutic strategy for the treatment of ED in diabetic men.
Acknowledgments
This study was supported by Nanjing Medical University, China (No. 08NJMUZ048).
References
- Malavige LS, Levy JC. Erectile dysfunction in diabetes mellitus. J Sex Med. 2009;6:1232–47. doi: 10.1111/j.1743-6109.2008.01168.x. [DOI] [PubMed] [Google Scholar]
- Malavige LS, Jayaratne SD, Kathriarachchi ST, Sivayogan S, Fernando DJ, et al. Erectile dysfunction among men with diabetes is strongly associated with premature ejaculation and reduced libido. J Sex Med. 2008;5:2125–34. doi: 10.1111/j.1743-6109.2008.00907.x. [DOI] [PubMed] [Google Scholar]
- Cho NH, Ahn CW, Park JY, Ahn TY, Lee HW, et al. Prevalence of erectile dysfunction in Korean men with Type 2 diabetes mellitus. Diabet Med. 2006;23:198–203. doi: 10.1111/j.1464-5491.2005.01789.x. [DOI] [PubMed] [Google Scholar]
- Moore CR, Wang R. Pathophysiology and treatment of diabetic erectile dysfunction. Asian J Androl. 2006;8:675–84. doi: 10.1111/j.1745-7262.2006.00223.x. [DOI] [PubMed] [Google Scholar]
- Prieto D, Simonsen U, Hernandez M, Garcia-Sacristan A. Contribution of K+ channels and ouabain-sensitive mechanisms to the endothelium dependent relaxations of horse penile small arteries. Br J Pharmacol. 1998;123:1609–20. doi: 10.1038/sj.bjp.0701780. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Angulo J, Cuevas P, Fernandez A, Gabancho S, Videla S, et al. Calcium dobesilate potentiates endothelium-derived hyperpolarizing factor-mediated relaxation of human penile resistance arteries. Br J Pharmacol. 2003;139:854–62. doi: 10.1038/sj.bjp.0705293. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kun A, Kiraly I, Pataricza J, Marton Z, Krassoi I, et al. C-Type natriuretic peptide hyperpolarizes and relaxes human penile resistance arteries. J Sex Med. 2008;5:1114–25. doi: 10.1111/j.1743-6109.2008.00775.x. [DOI] [PubMed] [Google Scholar]
- Kun A, Martinez AC, Tankó LB, Pataricza J, Papp JG, et al. Ca2+-activated K+ channels in the endothelial cell layer involved in modulation of neurogenic contractions in rat penile arteries. Eur J Pharmacol. 2003;474:103–15. doi: 10.1016/s0014-2999(03)02004-1. [DOI] [PubMed] [Google Scholar]
- Angulo J, Cuevas P, Fernández A, Gabancho S, Allona A, et al. Diabetes impairs endothelium-dependent relaxation of human penile vascular tissues mediated by NO and EDHF. Biochem Biophys Res Commun. 2003;312:1202–8. doi: 10.1016/j.bbrc.2003.11.034. [DOI] [PubMed] [Google Scholar]
- Félétou M, Vanhoutte PM. EDHF: new therapeutic targets. Pharmacol Res. 2004;49:565–80. doi: 10.1016/j.phrs.2003.10.017. [DOI] [PubMed] [Google Scholar]
- Pu XY, Hu LQ, Wang HP, Luo YX, Wang XH. Improvement in erectile dysfunction after insulin-like growth factor-1 gene therapy in diabetic rats. Asian J Androl. 2007;9:83–91. doi: 10.1111/j.1745-7262.2007.00215.x. [DOI] [PubMed] [Google Scholar]
- Armagan A, Uz E, Yilmaz HR, Soyupek S, Oksay T, et al. Effects of melatonin on lipid peroxidation and antioxidant enzymes in streptozotocin-induced diabetic rat testis. Asian J Androl. 2006;8:595–600. doi: 10.1111/j.1745-7262.2006.00177.x. [DOI] [PubMed] [Google Scholar]
- Heaton JP, Varrin S, Morales A. The characterization of bioassay of erectile function in rat model. J Urol. 1991;145:1099–102. doi: 10.1016/s0022-5347(17)38543-9. [DOI] [PubMed] [Google Scholar]
- Simonsen U, Garcia-Sacristan A, Prieto D. Penile arteries and erection. J Vasc Res. 2002;39:283–303. doi: 10.1159/000065541. [DOI] [PubMed] [Google Scholar]
- Sáenz de Tejada I, Angulo J, Cellek S, González-Cadavid N, et al. Physiology of erectile function. J Sex Med. 2004;1:254–65. doi: 10.1111/j.1743-6109.04038.x. [DOI] [PubMed] [Google Scholar]
- Sáenz de Tejada I, Angulo J, Cellek S, González-Cadavid N, Heaton J, et al. Pathophysiology of erectile dysfunction. J Sex Med. 2005;2:26–39. doi: 10.1111/j.1743-6109.2005.20103.x. [DOI] [PubMed] [Google Scholar]
- Prieto D. Physiological regulation of penile arteries and veins. Int J Impot Res. 2008;20:17–29. doi: 10.1038/sj.ijir.3901581. [DOI] [PubMed] [Google Scholar]
- Edwards G, Dora KA, Gardener MJ, Garland CJ, Weston AH. K+ is an endothelium derived hyperpolarizing factor in rat arteries. Nature. 1998;396:269–72. doi: 10.1038/24388. [DOI] [PubMed] [Google Scholar]
- Ellis A, Triggle CR. Endothelium-derived reactive oxygen species: their relationship to endothelium dependent hyperpolarisation and vascular tone. Can J Physiol Pharmacol. 2003;81:1013–28. doi: 10.1139/y03-106. [DOI] [PubMed] [Google Scholar]
- Chen MX, Gorman SA, Benson B, Singh K, Hieble JP, et al. Small and intermediate conductance Ca(2+)-activated K+ channels confer distinctive patterns of distribution in human tissues and differential cellular localisation in the colon and corpus cavernosum. Naunyn Schmiedeberg's Arch Pharmacol. 2004;369:602–15. doi: 10.1007/s00210-004-0934-5. [DOI] [PubMed] [Google Scholar]
- Burnham MP, Bychkov R, Félétou M, Richards GR, Vanhoutte PM, et al. Characterization of an apamin-sensitive small-conductance Ca(2+)-activated K(+) channel in porcine coronary artery endothelium: relevance to EDHF. Br J Pharmacol. 2002;135:1133–43. doi: 10.1038/sj.bjp.0704551. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jensen BS, Hertz M, Christophersen P, Madsen LS. The Ca2+-activated K+ channel of intermediate conductance: a possible target for immune suppression. Expert Opin Ther Targets. 2002;6:623–36. doi: 10.1517/14728222.6.6.623. [DOI] [PubMed] [Google Scholar]
- Ghanshani S, Wulff H, Miller MJ, Rohm H, Neben A, et al. Up-regulation of the IKCa1 potassium channel during T-cell activation. Molecular mechanism and functional consequences. J Biol Chem. 2000;275:37137–49. doi: 10.1074/jbc.M003941200. [DOI] [PubMed] [Google Scholar]
- Pena TL, Chen SH, Konieczny SF, Rane SG. Ras/MEK/ERK up-regulation of the fibroblast KCa channel FIK is a common mechanism for basic fibroblast growth factor and transforming growth factor-beta suppression of myogenesis. J Biol Chem. 2000;275:13677–82. doi: 10.1074/jbc.275.18.13677. [DOI] [PubMed] [Google Scholar]
- Hilgers RH, Webb RC. Reduced expression of SKCa and IKCa channel proteins in rat small mesenteric arteries during angiotensin II-induced hypertension. Am J Physiol Heart Circ Physiol. 2007;292:H2275–84. doi: 10.1152/ajpheart.00949.2006. [DOI] [PubMed] [Google Scholar]
- Zhou Z, Jiang DJ, Jia SJ, Xiao HB, Xiao B, et al. Down-regulation of endogenous nitric oxide synthase inhibitors on endothelial SK3 expression. Vasc Pharmacol. 2007;47:265–71. doi: 10.1016/j.vph.2007.08.003. [DOI] [PubMed] [Google Scholar]


