Abstract
The androgen receptor (AR) plays a critical role in prostate cancer development and progression. This study aimed to use a computerized docking approach to examine the interactions between the human AR and phyto-oestrogens (genistein, daidzein, and flavone) and xeno-oestrogens (bisphenol A, 4-nonylphenol, dichlorodiphenyl trichloroethane [DDT], diethylstilbestrol [DES]). The predicted three-dimensional structure of AR and androgens was established using X-ray diffraction. The binding of four xeno-oestrogens and three phyto-oestrogens to AR was analysed. The steroids estradiol and dihydrotestosterone (DHT) were used as positive controls and thyroxine as negative control. All the ligands shared the same binding site except for thyroxine. The endogenous hormones DHT and 17β-oestradiol showed the strongest binding with the lowest affinity energy (< −10 kcal mol−1). All three phyto-oestrogens and two xeno-oestrogens (bisphenol A and DES) showed strong binding to AR. The affinities of flavone, genistein, and daidzein were between −8.8 and −8.5 kcal mol−1, while that of bisphenol A was −8.1 kcal mol−1 and DES −8.3 kcal mol−1. Another two xeno-oestrogens, 4-nonylphenol and DDT, although they fit within the binding domain of AR, showed weak affinity (−6.4 and −6.7 kcal mol−1, respectively). The phyto-oestrogens genistein, daidzein and flavone, and the xeno-oestrogens bisphenol A and DES can be regarded as androgenic effectors. The xeno-oestrogens DDT and 4-nonylphenol bind only weakly to AR.
Keywords: androgen receptor, dock, phyto-oestrogens, xeno-oestrogens
Introduction
Since the cloning of its cDNA in 1988, the androgen receptor (AR) has been extensively studied and is considered to play critical roles in prostate cancer development and progression 1, 2, 3. Known as NR3C4 (nuclear receptor subfamily 3, group C, member 4), AR is a xeno-oestrogen nuclear receptor activated by the binding of both of the androgenic hormones, testosterone and dihydrotestosterone (DHT) 4, 5. AR is most closely related to the progesterone receptor, and progestins in higher dosages can block the AR 6, 7. Testosterone and DHT are chemically related sex steroid hormones with a four-ringed carbon backbone. DHT, the metabolic product of testosterone, controls mitotic activity in the prostate by binding to AR and the receptor-ligand complex being translocated to the nucleus of prostate cells to transactivate androgen-responsive genes 8. The proteins translated from these genes drive the cell changes guiding androgen-controlled growth and development 9. Besides androgens, the oestrogens or compounds that mimic oestrogens may also be associated with the tumourigenesis of prostate cancer 10.
Environmental chemicals with oestrogenic activity have an endocrine disruption effect 8. Such substances include natural plant products called phytohormones and synthetic xeno-oestrogens. Xeno-oestrogens, which are now widely dispersed in nature, are encountered in everyday life, including diethylstilbestrol (DES), dichlorodiphenyl trichloroethane (DDT) and other persistent organochlorine pollutants (polychlorinated biphenyls, commonly known as PCBs), as well as the industrial chemicals phthalate and bisphenol A 10. For instance, residues of DDT can enter the human body in vegetables and fruit 11. DES, a growth-promoting hormone for domestic animals, enters the body in meat 12. Other xeno-oestrogens can also be accumulated by exposure to cleaning and polycarbonate plastic products, such as bisphenol A and 4-nonylphenol. Significant levels of BPA have been found in the urine of 93% of the U.S. population in a recent screen by the Centers for Disease Control and Prevention 13, 14. Micro-xeno-oestrogens can harm the reproductive system and promote hormone-related tumourigenesis. Many epidemiological surveys have shown significant induction of testicular cancer and prostate cancer, reduction of sperm quantity, or significant induction of breast cancer and uterus cancer in women. Data from the USA have shown a sudden rise in the incidence of hormone-related cancers from 1973 to 1991: 126% increase in prostate cancer, 41% increase in testis cancer, and 24% increase in breast cancer. Similar trends have been found in investigations in Europe. The incidence of these cancers has doubled every 10 years. These epidemiological data indicate that the increases in certain hormone-related tumours could be ascribed to the prevalence of xeno-oestrogens 15, 16, 17.
As a representative hormone-related tumour, prostate cancer has marked geographic variations between countries. Genetic, epigenetic and environmental factors contribute to the development of the cancer 18. The relationship of xeno-oestrogens with prostate cancer is still obscure and needs further research. Some xeno-oestrogens were found to be associated with prostate cancer, and others were found not to be. For example, conflicting data exist on the effects of bisphenol A with regard to the carcinogenic potential of the prostate gland 19. DDT has shown no positive correlation with prostate cancer 20, 21. Although xeno-oestrogens have similar structures to oestrogens, the reason why different xeno-oestrogens show different associations with prostate cancer is still unclear, and few comparisons among them have been reported.
Phyto-oestrogens, sometimes called 'dietary oestrogens', are a diverse group of naturally occurring non-steroidal plant compounds that, because of their structural similarity with oestradiol (17β-oestradiol), have the ability to cause oestrogenic or/and anti-oestrogenic effects 22. There are three major kinds of phyto-oestrogens: isoflavones, lignans and coumestans, all contained in the plants or the seeds. Phyto-oestrogens have been suggested as cancer preventatives and as treatments for menopausal symptoms and osteoporosis 23, 24. Soybean, a dietary staple in many parts of Asia, is a major source of the isoflavonoids daidzein and genistein 25. Laboratory animal studies and comparisons of Asian and Western human populations suggest that diet plays a large role in these types of health problems, with lower rates of hormone-dependent cancers (breast, prostate) and lower incidences of menopausal symptoms and osteoporosis in Asians than in Westerners 10, 18. A number of epidemiological and experimental studies have found that soybeans, which contain large amounts of isoflavones, including genistein, daidzein, glycitein and equol, have a prophylactic effect on prostate cancer 18, 26. But whether phyto-oestrogens affect androgen-associated diseases or the endocrine system is still controversial 27, 28, 29. Some data suggest that genistein or flavone can block androgen-induced prostate-specific antigen (PSA) induction mediated by AR 30, 31. It is noteworthy that two recent phase II trials showed that isoflavones or soy beverage can decrease PSA levels in prostate cancer patients. It is suggested that AR target genes can be regulated by isoflavones or flavones 32, 33. The binding mechanisms of isoflavones and xeno-oestrogens to AR need to be further investigated.
AutoDock, a widely used molecular docking procedure developed by the Olson Group (Sioux Falls, SD, USA), is an important tool used to reveal the binding of hormones and receptors. AutoDock applies a half-flexible docking method, which permits small molecular conformation changes. Two or more molecules dock using both geometric matching and energy matching. The calculated affiity is based on the AutoDock free energy. This method plays continuously more important roles in many research fields, especially in molecular docking medicine structure analysis 34, 35, 36. To both examine the interactions of human AR with phyto-oestrogens and xeno-oestrogens and evaluate their effectiveness as androgenic effectors, we performed a molecular docking study to investigate the effects of xeno-oestrogen and phyto-oestrogen binding to AR and compared their different affinities for AR.
Materials and methods
Preparation of three-dimensional (3D) structures of the ligands and receptor molecules for docking
All 3D structures of testosterone, 17β-oestradiol, thyroxine, bisphenol A, 4-nonylphenol, DDT, DES, genistein and daidzein and flavone (2-phenyl-1,4-benzopyrone) were obtained from NCBI or the RCSB Protein Data Bank. The 3D spatial structure of the ligand-binding domain (LBD) of AR (676–919 AA) (PDB ID: 2ama) was obtained from RCSB Protein Data Bank.
Docking system test
The software AutoDock Vina was used for the docking system test. It was designed and implemented by Dr Oleg Trott in the Molecular Graphics Lab at The Scripps Research Institute (La Jolla, CA, USA) 19. AutoDock tools were used to add polar hydrogen to the receptor. The grid box was set to include the whole receptor region. The receptor output was in PDBQT format, which can be read by using Vina. The ligands were also rewritten into PDBQT format. The AR LBD structure 37 was obtained from RCSB Protein Data Bank, with the ligand extracted by PyMOL software (San Carlos, CA, USA).
To quantify the relative free energy of ligand binding of the different binding patterns, we applied linear-interaction energy (LIE) analysis. LIE quantifies the free energy of a compound in a given binding mode, subtracting electrostatic and van der Waals interaction energies with solvent averaged over the entire simulation from the corresponding energies when bound to the protein: ΔG = α (< Elig-protelec> − < Elig-solvelec>) + β (< Elig-protVdW> − < Elig-solvVdW>) 38, 39, 40, 41, 42.
AutoDock Vina was set with the macromolecule held fixed and the ligands flexible. The region of interest used by AutoDock was initially the whole receptor protein, and then it was defined to include a specific portion of the binding site of the macromolecule, the AR LBD (residues 676–919 aa). A smaller grid, focused on the binding region, was used and the number of simulations was set to 50. Affinity maps for all the atom types present, as well as an electrostatic map, were computed, with a grid spacing of 0.375 Å. In AutoDock Vina, the Broyden–Fletcher–Goldfard–Shanno method was used for load optimization, which used not only the value of the scoring function but also its gradient. Vina avoids imposing artificial restrictions, such as the number of atoms in the input, the number of torsions, the size of the research space, or the exhaustiveness of the search.
The ligands testosterone, 17β-oestradiol, thyroxine, bisphenol A, 4-nonylphenol, DDT, DES, genistein, daidzein and flavone were docked individually into the AR LBD structure. The rotatable bonds remained how they were when the ligand was downloaded. The structural models collected from the lowest-energy docking solution of each cluster of AutoDock were used as input for QXP docking. The algorithm implemented in the QXP program allows for fully flexibility of the inhibitors and simultaneous flexibility of the active-site side chains. The starting structure had previously been optimized by energy minimization. Each docking run included 50 cycles of Monte Carlo perturbation, subsequent fast searching and final energy minimization. For each single-docking QXP simulation the results were evaluated in terms of total estimated binding energy, internal strain energy of the ligand, and van der Waals and electrostatic interaction energies. Lower-affinity energy indicates stronger binding ability.
Results
Reliability analysis of the docking system
The reliability of the AR docking system was tested using the natural androgen DHT as a ligand. The model prediction of the LBD structure of AR binding to DHT in this AutoDock system was compared with that in the X-ray (PDB ID: 2AMA) diffraction system. Figure 1 shows the binding sites of hormones. Lines represent ligands, and ribbons represent the AR LBD. The positional comparison of DHT (shown with a tail) in the LBD of AR predicted by the docking system was highly matched to the one that originated from the X-ray (no tail). A very strong binding with low docking affinity energy (−11.2 kcal mol−1) was also calculated by this method. Figure 1B was derived from Figure 1A, only with the camera zoomed in and AR hidden, demonstrating the accuracy of the 3D structure of AR in this docking system. The reliability of the AR docking system was shown to reliably mimic natural molecular docking and to be suitable for further docking prediction with other ligands.
Figure 1.

Reliability analysis of the docking system. (A): The comparison of position of ligand dihydrotestosterone (DHT) binding to ligand-binding domain of androgen receptor (AR) predicted by this docking system and X-ray (PDBID: 2AMA) diffraction. The lower one is by docking while the upper one is by X-ray diffraction. (B): Derived DHT from Figure 1A only with the camera zoomed in and the AR being hidden. The one with a tail (arrow) is by docking while the the other one is by X-ray diffraction.
Binding of xeno-oestrogens and phyto-oestrogens
A total of 10 ligands were analysed in this study. Two endogenous steroid hormones, DHT and 17β-oestradiol, have a four-ringed carbon backbone, whereas the negative control, non-steroidal thyroxine, is quite different from them (Figure 2A). The xeno-oestrogens bisphenol A, DDT and DES have two additional rings, and 4-nonylphenol is an alkylphenol consisting of a single phenolic ring (Figure 2B). Phyto-oestrogens are a diverse group of naturally occurring non-steroidal plant compounds.
Figure 2.
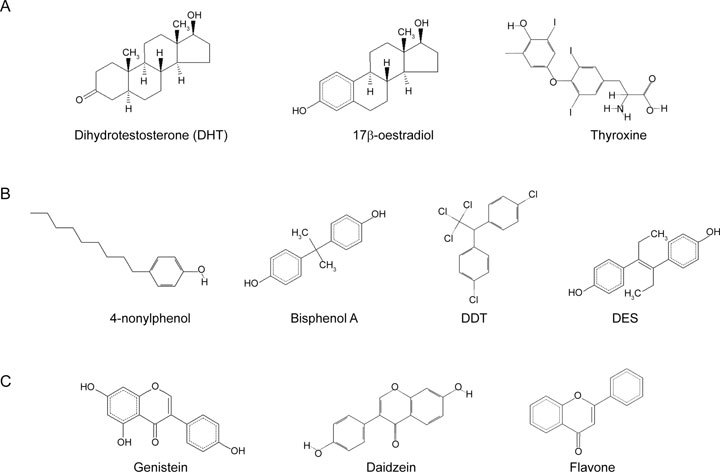
Molecular formulas of ligand compounds. (A): The crystal complexes ligands of two endogenous steroid hormones of dihydrotestosterone (DHT) and 17β-oestradiol, and one endogenous non-steroidal hormone, thyroxin. (B): The crystal complexes ligands of four xenoestrogens of 4-nonylphenol, Bisphenol A, dichlorodiphenyl trichloroethane (DDT) and diethylstilbestrol (DES). (C): The crystal complexes ligands of three phytoestrogen of Genistein, Daidzein and Flavone.
The structures of the isoflavones genistein, daidzein, and flavone showed similar properties: a hydrophobic core and one or two terminal polar groups (Figure 2C), similar to 17β-oestradiol.
All the ligands were docked to the AR LBD separately to calculate their affinities. Thyroxine was set as the negative control, while DHT and 17β-oestradiol as positive control.
AutoDocking of endogenous hormones to AR
The positive-control docking result showed that 17β-oestradiol fit the ligand-binding site of AR, at the same position in AR as its natural ligand, DHT. The negative control, thyroxine, showed a quite different binding position: its docking site was external (Figures 3A and B).
Figure 3.
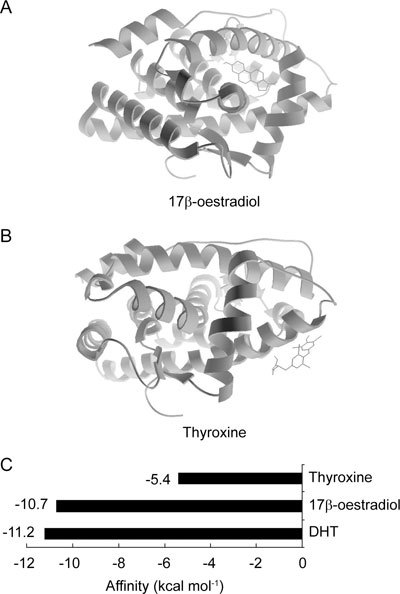
Auto docking results of endogenous hormones binding to androgen receptor. Position of the steroid hormones of 17β-oestradiol (A), and endogenous non-steroid hormone, thyroxin (B) binding to androgen receptor. (C): The affinity energies of endogenous steroid hormones or non-steroid hormone binding to androgen receptor. DHT, dihydrotestosterone.
Comparing the three endogenous ligands, thyroxine was expected to have the weakest binding to AR and the highest affinity energy, which we measured at −5.4 kcal mol−1. Very strong binding to AR with lower affinity energies was expected in the two steroid hormones. We observed affinity energies of −11.2 kcal mol−1 for DHT and −10.7 kcal mol−1 for 17β-oestradiol (Figure 3C).
AutoDocking of xeno-oestrogens to AR
Xeno-oestrogens, as endocrine-disrupting chemicals, interfere with endocrine processes by mimicking, blocking, or altering hormones and their signalling systems. Bisphenol A, DDT, 4-nonylphenol and DES all docked to AR. All the four xeno-oestrogens (Figures 4A–D) occupied the same AR binding site as DHT (Figure 1A) and 17β-oestradiol (Figure 3A), suggesting that their structures are similar to those of steroid hormones. However, notable differences were seen in their affinity energies. All four xeno-oestrogens displayed weaker binding to AR than oestradiol but stronger than thyroxine. The affinity energies of bisphenol A and DES were quite different from those of DDT and 4-nonylphenol. As shown in Figure 4E, bisphenol A and DES showed stronger binding to AR with lower energies, −8.1 and −8.3 kcal mol−1, respectively. The stronger binding abilities of these two xeno-oestrogens suggest that they could have deleterious effects on AR function at high enough concentrations. 4-nonylphenol and DDT also fit in the binding domain of AR, but showed weaker binding with higher affinity energies: −6.4 and −6.7 kcal mol−1, respectively. This suggests that they are capable of a limited effect on AR.
Figure 4.
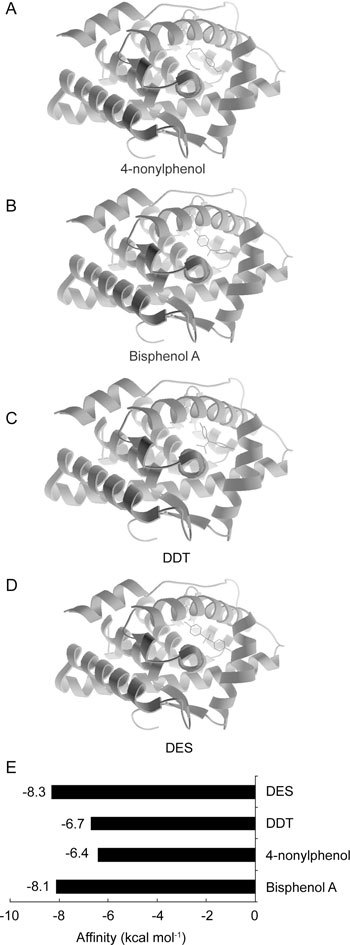
Auto docking results of xeno-oestrogens binding to androgen receptor. Position of 4-nonylphenol (A), Bisphenol A (B), DDT (C) and DES (D) binding to androgen receptor. (E): The affinity energies of xeno-oestrogens binding to androgen receptor. DDT, dichlorodiphenyl trichloroethane; DES, diethylstilbestrol.
AutoDocking of phyto-oestrogens to AR
Phyto-oestrogens, especially the isoflavones, which come from leguminous plants, may substitute for oestrogen and simultaneously prevent the side effects of oestrogen. The isoflavones genistein and daidzein are two phyto-oestrogens found at very high levels in soy formula. Some studies on cancer incidences in different countries suggest that phyto-oestrogens may help protect against certain cancers of the breast, uterus and prostate 18, 30, 31. Isoflavones are the best-known phyto-oestrogens implicated in prostate cancer inhibition.
As shown in Figure 5, all three phyto-oestrogens fit in the middle region of the AR LBD, the same as DHT and 17β-oestradiol. The affinities of the phyto-oestrogens were expected to lie between the affinities of thyroxine and 17β-oestradiol. Two major isoflavones in soybeans, genistein and daidzein, showed affinity energies of −8.5 and −8.7 kcal mol−1, respectively, which were very similar to the affinity energy of flavone of −8.8 kcal mol−1. From this result, we concluded that these three (iso)flavones exhibit similar binding affinities to AR. Considering their sharing of a binding site with oestradiol, their affinities for AR and the quantities potentially consumed in the diet, these phyto-oestrogens could have significant effects on AR and AR-related cancers.
Figure 5.
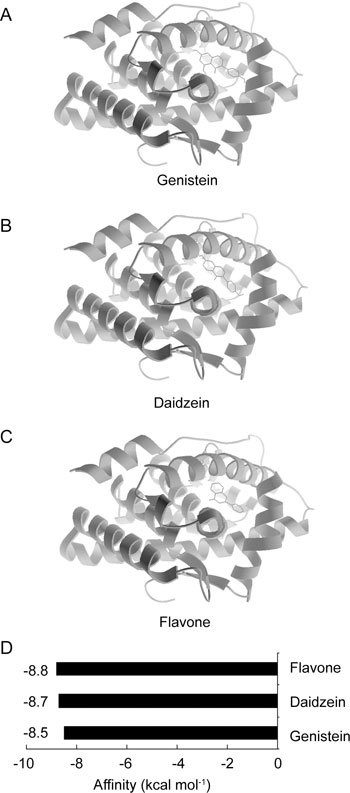
Auto docking results of phytoestrogens binding to androgen receptor. Position of genistein (A), daidzein (B) or flavones (C) binding to androgen receptor. (D): The affinity energies of phyto-oestrogens binding to androgen receptor.
Comparisons of all the ligands binding to AR
The affinities of all the xeno-oestrogens, phyto-oestrogens and related endogenous hormones were summarized in Figure 6. From these data, it can be seen that all the hormones share the same binding site, except for the negative control, thyroxine, which showed the lowest affinity, −5.4 kcal mol−1. As expected, the endogenous hormones DHT and 17β-oestradiol showed the highest binding with the lowest affinity energy, lower than −10 kcal mol−1. All the phyto-oestrogens and two xeno-oestrogens showed strong binding with lower affinity energies: flavone −8.8 kcal mol−1, daidzein −8.7 kcal mol−1, and genistein −8.5 kcal mol−1; bisphenol A −8.1 kcal mol−1; and DES −8.3 kcal mol−1. Interestingly, all these phyto-oestrogens and xeno-oestrogens are reported to be associated with prostate cancer, so we consider them AR-related xeno-oestrogens. Another two xeno-oestrogens, 4-nonylphenol and DDT, while exhibiting the right binding position in AR, showed weak binding, with higher affinity energies of −6.4 and −6.7 kcal mol−1, respectively. This suggests that they have no or very limited effects on AR.
Figure 6.
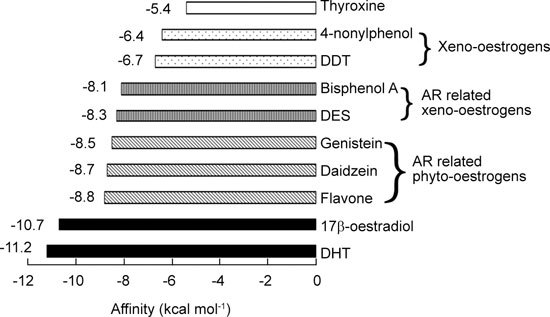
Comparison of the affinity energies of xeno-oestrogens, phytoestrogens and endogenous hormones binding to androgen receptor. AR, androgen receptor; DHT, dihydrotestosterone.
In addition, we summarized some recent data of the effects of xeno-oestrogens and phyto-oestrogens on AR-mediated transcriptional activity and on prostate tumourigenesis to confirm our findings by AutoDock methods. As can be seen from Table 1 presenting part of these data, the xeno-oestrogens DES and BPA and three phyto-oestrogens, genistein, daidzein, flavone implicated as androgenic effectors in our research indeed regulate AR-mediated PSA transcriptional activity. They have been demonstrated previously to either enhance AR-mediated transcriptional activity or inhibit DHT- (or R1881-) induced AR-mediated pPSA activity 30, 31, 32, 43, 44, 45, 46, 47, 48. Moreover, isoflavones or soy beverage has already been shown in phase II trials to decrease PSA levels in prostate cancer patients 32, 33. Although one study found that nonylphenol can inhibit DHT-induced AR-mediated pPSA-luciferase activity, its inhibitory effect was lower than that of BPA 43. No adequate data for DDT and the few effects of nonylphenol are consistent with our observation that 4-nonylphenol and DDT bind only weakly to AR.
Table 1. Effects of xeno-oestrogens and phyto-oestrogens on AR-mediated transcriptional activity in published studies.
| Findings | Study type | Reference | |
|---|---|---|---|
| Xeno-oestrogens | |||
| DES | Decline of serum PSA | Clinical | 44, 45 |
| BPA | Inhibition of AR-mediated pPSA-luc induced by | Reporter assay | 43 |
| DHT-elevated PSA of AR-T877A | Xenograft or reporter assay | 46, 55 | |
| DDT | No adequate data | ||
| Nonylphenol | Inhibition of DHT-induced AR-mediated pPSA-luc activity (inhibitor effect lower than BPA) | reporter assay | 43 |
| Phyto-oestrogens | |||
| Genistein | Inhibition of R1881-induced AR-mediated pPSA-luc activity decreased AR binding to ARE | Reporter assay EMSA | 30 |
| Inhibition of R1881-induced AR-mediated pPSA-luc activity enhanced AR-mediated pPSA/ARE/Probasin/MMTV-luc | Reporter assay | 48 | |
| Daidzein | Enhanced AR- (with ARA) mediated MMTV-luc | Reporter assay | 47 |
| Flavone | Inhibition of DHT-induced AR-mediated pPSA-luc activity | Reporter assay | 31 |
| Soy food | Decreased serum PSA | Phase II trial | 32, 33 |
Abbreviations: ARA, androgen receptor- associated protein; ARE: androgen response elements; BPA, bisphenol A; DDT, dichlorodiphenyl trichloroethane; DES, diethylstilbestrol; DHT, dihydrotestosterone; EMSA, electrophoretic mobility shift assay; MMTV, mouse mammary tumor virus.
The epidemiological and animal data on the effects of xeno-oestrogens and phytoestrogens on prostate carcinogenesis confirm our results from another point of view. We summarized part of these data in Table 2. The association between the mortality rate from prostate or testicular cancer and environmental exposure to DDT and para,para'-DDE in the USA during 1971–1994 has been explored by multiple linear regression analysis. That analysis provided no support for the hypothesis of a link between environmental exposure to DDT derivatives and cancer of the male reproductive tract 8, 20, 49. Other data suggest that nonylphenol pretreatment has no effect on prostate carcinogenesis either during the late neonatal period or during the gestation/lactation period in F344 rats 50, 51. By contrast, foetal or developmental exposure to bisphenol A was found to increase susceptibility to prostate carcinogenesis or increase the proliferation of basal cells of the prostate in mice or rats 46, 52, 53, 54, 55, 56. DES is still used as an anti-prostate cancer drug despite its cardiovascular toxicity 57, 58, 59, 60. These data support our findings that bisphenol A and DES but not DDT and 4-nonylphenol are androgenic effectors.
Table 2. Effects of xeno-oestrogens and phyto-oestrogens on prostate carcinogenesis in epidemiological and experimental studies.
| Humans |
Animals |
||||||
|---|---|---|---|---|---|---|---|
| Findings | Study type | OR/RR | Ptrend | Reference | Findings | Reference | |
| Xeno-oestrogens | |||||||
| DES | Therapy | Clinical | 57, 59 | ND | |||
| BPA | ND | Promotion | 52, 53 | ||||
| DDT | No effect | Multiple linear regression analysis | 20, 49 | ND | |||
| Nonylphenol | ND | No effect | 50, 51 | ||||
| Phyto-oestrogens | |||||||
| Genistein | Protection | Cohort | 0.52 (0.30–0.90) | 0.030 | 60 | Protection | 73, 74 |
| Case-control | 0.58 (0.34–0.97) | 0.040 | 66 | ||||
| Case-control | 0.53 (0.29–0.97) | 0.058 | 68 | ||||
| Promoting | Serum case-control | 82 | |||||
| Daidzein | Protection | Cohort | 0.50 (0.28–0.88) | 0.040 | 60 | Protection | 75, 78 |
| Case-control | 0.55 (0.32–0.93) | 0.020 | 66 | ||||
| Case-control | 0.56 (0.31–1.04) | 0.116 | 68 | ||||
| Promotion | Serum case-control | 82 | |||||
| Flavone | ND | Protection | 79, 81 | ||||
| Soy food | Protection | Cohorts and case-controls | 0.3–0.52 | <0.05 | 18 | Protection | 76, 77 |
Abbreviations: BPA, bisphenol A; DES, diethylstilbestrol; DDT, dichlorodiphenyl trichloroethane; ND, no adequate data; OR, odds ratio; RR, relative risk.
Most of the epidemiological studies evaluating the inhibitory effects of soybean isoflavones in prostate cancer are consistent 18. To date, more than five cohorts and eight case-control studies suggest that soy food and soy isoflavones, such as genistein and daidzein, have prophylactic effects on prostate cancer 18, 60, 61, 62, 63, 64, 65, 66, 67, 68, 69, 70, 71, 72. In recent years, various in vitro and in vivo experimental studies have demonstrated that these isoflavones indeed inhibit prostate cancer 73, 74, 75, 76, 77, 78. The natural flavones apigenin and acacetin and the flavone flavopiridol also display a protective effect on prostate cancer 79, 80, 81. However, a case-control study 82 that measured serum levels of isoflavonoids in Japanese men showed that the serum concentrations of genistein and daidzein in both inpatients and outpatients with prostate cancer were higher than in controls. Some other case-control studies showed no association or protective effect for serum isoflavones reviewed by Jian 18. In recent decades, an accumulating body of evidence from laboratory studies have suggested that diets rich in isoflavones are associated with a lower risk of prostate cancer 18. Limited epidemiological studies have also provided promising results that increasing consumption of soy products and isoflavones may result in reduced risk of localized prostate cancer 18. The present study shows that the phyto-oestrogens (genistein, daidzein and flavone) and the xeno-oestrogens (bisphenol A and DES) can bind to AR in an AutoDock model and can be regarded as androgenic effectors, suggesting important roles for them in AR-mediated cancers.
Our results provide a viable explanation of the experimental data associating xeno-oestrogens and phyto-oestrogens with prostate cancer. Some evidences indicate that the risk of prostate cancer is associated with bisphenol A 52, 53, 54, 55, 56, 46 (shown to have an AR affinity energy of −8.1 kcal mol−1), while DES, as an oestrogenic agonist, was an early oestrogen treatment for prostate cancer [57–60] (affinity energy = −8.3 kcal mol−1). 4-nonylphenol and DDT are reported not to be associated with prostate cancer 8, 20, 83, 84 (affinity energies = −6.4 and −6.7 kcal mol−1, respectively). These data suggest that an affinity energy between −8 and −7 kcal mol−1 may be a good cut-off value to predict an association of a ligand with the risk of prostate cancer, whether harmful or preventative. An affinity energy lower than −8 kcal mol−1 indicates a stronger association, while affinity energies higher than −7 kcal mol−1 suggest a weaker association with prostate cancer.
Discussion
Environmental compounds are often presumed to play important roles in modulating prostate cancer growth, but epidemiological and experimental results are controversial, and their exact effects and mechanisms remain largely obscure. DES, an oestrogenic agonist, is a formerly standard drug for prostate cancer. In some cases, DES retains its activity and is still regarded as a reasonable option for castration-resistant prostate cancer because it induces a decline in serum PSA 44, 45, 57. Bisphenol A, also called BPA or 4,4′-(propan-2-ylidene)diphenol, has been used primarily to make plastic products for more than 50 years. According to an experimental study with human prostate tumours implanted into mice, bisphenol A facilitates the bypass of androgen ablation therapy in prostate cancer. Tumour size and PSA levels are significantly greater in exposed animals just 1 month after treatment 46. Data from rats link bisphenol A exposure during critical periods of early development to later prostate cancer 53, 54, 55, 56. However, 4-nonylphenol, a compound of concern as an oestrogenic xenobiotic, was found to lack effects on rat prostate carcinogenesis in male offspring exposed prenatally and neonatally 50, 51. DDT is one of the most well-known synthetic pesticides. Some epidemiological evidence demonstrates that DDT causes cancer of the liver, pancreas and breast but does not cause cancers of the prostate, lung, bladder or stomach 29, 30. These data suggest differences between various xeno-oestrogens in prostate carcinogenesis, but the mechanisms are unclear. Here, by a computer-based AutoDock model, we provide a viable explanation to many experimental findings and identify bisphenol A and DES as AR-related xeno-oestrogens, while 4-nonylphenol and DDT are not.
The epidemiological studies on the correlations between serum phyto-oestrogens and prostate cancer are controversial. One study suggests a causal relationship between isoflavones and prostate cancer 8. Recently a European prospective investigation of plasma phyto-oestrogens and prostate cancer was performed, finding that higher plasma concentrations of genistein were associated with a lower risk of prostate cancer 85. Most experimental and epidemiological studies suggest that genistein and daidzein are promising agents for cancer chemoprevention and/or treatment 18. Eight case-control studies 65, 66, 67, 68, 69, 70, 71, 72 and five cohort studies 60, 61, 62, 63, 64 have reported the protective effect of soy food, with odds ratios or relative risks ranging from 0.3 to 0.69, including in China and Japan, where people consume more soybean food, tofu, soymilk and natto. In vivo and in vitro experimental data have also shown the protective effect of the isoflavones genistein and daidzein and the natural flavones apigenin and acacetin against prostate cancer 73, 74, 75, 76, 77, 78, 79, 80, 81. Here, we demonstrated the AR-binding mechanism of three abundant phyto-oestrogens (genistein, daidzein and flavone).
AR plays a critical role in prostate cancer development and progression 2, 3. One approach to understanding the role of environmental compounds in prostate cancer is docking them to AR to evaluate their effects. The chemical structure-based ligand and receptor binding is the most important primary step in generating downstream signal transduction. AutoDock, based on a complex 'lock-and-key model', is an excellent method to reveal ligand–receptor binding. AutoDock applies a half-flexible docking method. The ligand and the receptor are flexible, and their conformation can be changed in the program. This requested docking operation adapts mutually to achieve optimum matching. Furthermore, the docking results are appraised based on the affinity energy. The molecular docking needs to satisfy not only the spatial shape match but also the energy match. The binding intensities of ligand and receptor depend on affinity and position in the process of complex formation.
Here, using the AutoDock method, the interactions between human AR and phyto-oestrogens and xeno-oestrogens were studied. The visualization of the intermolecular function was advantageous in understanding their mechanisms. Of all the ligands, the endogenous oestradiol showed the strongest binding to AR with an affinity energy of −10.7 kcal mol−1, which is very close to the −11.2 kcal mol−1 of DHT, with the same binding position. Three phyto-oestrogens (flavone, genistein and daidzein), and two xeno-oestrogens (bisphenol A and DES) also exhibited strong binding affinities to AR, much stronger than the negative control, thyroxine. Their binding position was the same one used by oestradiol and DHT. Therefore, they may exert significant effects on AR, which leads us to refer to them as AR-related xeno-oestrogens or AR-related phyto-oestrogens. Three phyto-oestrogens even showed stronger binding than DES, which was formerly widely used as an oestrogenic agonist in the clinic. Another xeno-oestrogen, bisphenol A, is similar in structure to DES, so all five of these oestrogenic compounds are extremely likely to influence the organism at sufficient concentrations. Although another two xeno-oestrogens, DDT and 4-nonylphenol, used the same binding site as DHT and oestradiol, their lower binding energies approached that of thyroxine. Therefore, we concluded that they had no or limited effects on AR function.
The binding position and affinity energy are two key aspects of evaluating ligand–receptor binding. The negative-control, non-steroid hormone thyroxine was deficient in both binding position and energy. DDT and 4-nonylphenol displayed the right position but not the energy, while bisphenol A, DES, and the phyto-oestrogens flavone, genistein, and daidzein were strong in both, so they are regarded as AR-related factors.
Asian populations generally eat large quantities of soy products compared with Western populations. One study found that Asian populations have lower rates of hormone-dependent cancers (breast, prostate) and lower incidences of menopausal symptoms and osteoporosis than Westerners 85. The geometric mean levels of plasma total isoflavonoids were 7–10 times higher in Japanese men than in Finnish men. Asian immigrants living in Western nations also have increased risk of these maladies as they 'Westernize' their diets to include more protein and fat and reduce their fibre and soy intake 86. Interestingly, we observed that phyto-oestrogens had greater binding abilities than the xeno-oestrogens. Considering the differences in the consumption methods and quantities between phyto-oestrogens and xeno-oestrogens, much higher concentrations of (iso)flavones can be consumed from the diet. These competitive ingredients could possibly reduce the prostate's exposure to endogenous DHT or extraneous xeno-oestrogens, thus reducing prostate cancer risk. This provides a good explanation for much of the epidemiological data, indicating the significant protective effect of (iso)flavones on prostate cancer. These high phyto-oestrogen levels could hypothetically inhibit the growth of prostate cancer in Chinese and Japanese men, and they might explain the low incidence and mortality from prostate cancer in Japan 87, 88, 89. One possibility may be that phyto-oestrogens can lower lifetime exposure to natural oestrogens or xeno-oestrogens by binding to AR. Our report indicates that each xeno-oestrogen and phyto-oestrogen examined can bind to the LBD of AR. The mechanisms by which the ligands trigger AR-dependent signalling in different cell lines need further research.
Our study should be valuable for understanding some of the contradictory experimental data on the different effects of xeno-oestrogens on prostate cancer. It also emphasizes the importance of phyto-oestrogens in prostate cancer prevention. AutoDock technology can substantially narrow the focus of research involving ligand-receptor interactions. As a parallel supplement to experimental results, AutoDock data can play a critical role in understanding prostate cancer development and progression.
Acknowledgments
This study was supported by Ministry of Science and Technology (No. 2010DFA31430), the National Natural Science Foundation of China (No. 30871301, 30700827), Ministry of Education of China (No. 108047), Jilin Provincial Science & Technology Department (No. 20070719, 20080731, 200905116). We thank Mr Michael Hoyt, who critically read and revised our manuscript.
References
- Chang C, Kokontis J, Liao ST. Molecular cloning of human and rat complementary DNA encoding androgen receptors. Science. 1988;240:324–6. doi: 10.1126/science.3353726. [DOI] [PubMed] [Google Scholar]
- Heinlein CA, Chang C. Androgen receptor in prostate cancer. Endocr Rev. 2004;5:276–308. doi: 10.1210/er.2002-0032. [DOI] [PubMed] [Google Scholar]
- Rahman M, Miyamoto H, Chang C. Androgen receptor coregulators in prostate cancer: mechanisms and clinical implications. Clin Cancer Res. 2004;10:2208–19. doi: 10.1158/1078-0432.ccr-0746-3. [DOI] [PubMed] [Google Scholar]
- Lu NZ, Wardell SE, Burnstein KL, Defranco D, Fuller PJ, et al. International Union of Pharmacology. LXV. The pharmacology and classification of the nuclear receptor superfamily: glucocorticoid, mineralocorticoid, progesterone, and androgen receptors. Pharmacol Rev. 2006;58:782–97. doi: 10.1124/pr.58.4.9. [DOI] [PubMed] [Google Scholar]
- Roy AK, Lavrovsky Y, Song CS, Chen S, Jung MH, et al. Regulation of androgen action. Vitam Horm. 1999;55:309–52. doi: 10.1016/s0083-6729(08)60938-3. [DOI] [PubMed] [Google Scholar]
- Bardin CW, Brown T, Isomaa VV, Jänne OA. Progestins can mimic, inhibit and potentiate the actions of androgens. Pharmacol Ther. 1983;23:443–59. doi: 10.1016/0163-7258(83)90023-2. [DOI] [PubMed] [Google Scholar]
- Raudrant D, Rabe T. Progestogens with antiandrogenic properties. Drugs. 2003;63:463–92. doi: 10.2165/00003495-200363050-00003. [DOI] [PubMed] [Google Scholar]
- Bogert CJ, Strauss MA, Harbison RD.Reproductive toxicology and occupational exposureIn: Zenz C, Dickerson OB, Horvath EP, editors. Occupational Medicine3rd edn. St Louis: Mosby-Year Book, Inc.; 1994p836–69.
- Cato AC, Nestl A, Mink S. Rapid actions of steroid receptors in cellular signaling pathways. Science's STKE. 2002;138:re9. doi: 10.1126/stke.2002.138.re9. [DOI] [PubMed] [Google Scholar]
- Prins GS. Endocrine disruptors and prostate cancer risk. Endocr Relat Cancer. 2008;15:649–56. doi: 10.1677/ERC-08-0043. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Clapp RW, Jacobs MM, Loechler EL. Environmental and occupational causes of cancer: new evidence 2005–2007. Rev Environ Health. 2008;23:1–37. doi: 10.1515/reveh.2008.23.1.1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rumsey TS, Tyrrell HF, Dinius DA, Moe PW, Cross HR. Effect of diethylstilbestrol on tissue gain and carcass merit of feedlot beef steers. J Anim Sci. 1981;53:589–600. doi: 10.2527/jas1981.533589x. [DOI] [PubMed] [Google Scholar]
- Calafat AM, Ye X, Wong LY, Reidy JA, Needham LL. Exposure of the U.S. population to bisphenol A and 4-tertiary-octylphenol: 2003–2004. Environ Health Perspect. 2008;116:39–44. doi: 10.1289/ehp.10753. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Klotz DM, Beckman BS, Hill SM, Mclachlan JA, Walters MR, et al. Identification of environmental chemicals with estrogenic activity using a combination of in vitro assays. Environ Health Perspect. 1996;104:1084–9. doi: 10.1289/ehp.961041084. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Merrill RM, Brawley OW. Prostate cancer incidence and mortality rates among white and black men. Epidemiology. 1997;8:126–31. doi: 10.1097/00001648-199703000-00001. [DOI] [PubMed] [Google Scholar]
- Ries LAG, Miller BA, Hankey BF, Kosary CL, Harras A, et al. National Cancer Institute's Surveillance, Epidemiology, and End Results (SEER) Cancer Statistics Review1973–1991, NIH Publication No. 94–2789. Bethesda: National Cancer Institute; 1994p1
- Henley DV, Korach KS. Endocrine-disrupting chemicals use distinct mechanisms of action to modulate endocrine system function. Endocrinology. 2006;147:S25–32. doi: 10.1210/en.2005-1117. [DOI] [PubMed] [Google Scholar]
- Jian L. Soy, isoflavones, and prostate cancer. Mol Nutr Food Res. 2009;53:217–26. doi: 10.1002/mnfr.200800167. [DOI] [PubMed] [Google Scholar]
- Keri R, Ho SM, Hunt PA, Knudsen KE, Soto AM, et al. An evaluation of evidence for the carcinogenic activity of bisphenol A. Reprod Toxicol. 2007;24:240–52. doi: 10.1016/j.reprotox.2007.06.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cocco P, Benichou J. Mortality from cancer of the male reproductive tract and environmental exposure to the anti-androgen p,p'-dichlorodiphenyldichloroethylene in the United States. Oncology. 1998;55:334–9. doi: 10.1159/000011872. [DOI] [PubMed] [Google Scholar]
- Takayama S, Sieber SM, Dalgard DW, Thorgeirsson UP, Adamson RH. Effects of long-term oral administration of DDT on nonhuman primates. J Cancer Res Clin Oncol. 1999;125:219–25. doi: 10.1007/s004320050266. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yildiz F.Phytoestrogens in Functional FoodsBoca Raton: CRC Press Taylor & Francis Ltd; 2005p3
- Adlercreutz H, Mazur W. Phyto-oestrogens and Western diseases. Ann Med. 1997;29:95–120. doi: 10.3109/07853899709113696. [DOI] [PubMed] [Google Scholar]
- Messina M, Gardner C, Barnes S. Gaining insight into the health effects of soy but a long way still to go: Commentary on the fourth International Symposium on the Role of Soy in Preventing and Treating Chronic Disease. J Nutr. 2002;132:547S–51S. doi: 10.1093/jn/132.3.547S. [DOI] [PubMed] [Google Scholar]
- Denis L, Morton MS, Griffiths K. Diet and its preventive role in prostatic disease. Eur Urol. 1999;35:377–87. doi: 10.1159/000019912. [DOI] [PubMed] [Google Scholar]
- Hwang YW, Kim SY, Jee SH, Kim YN, Nam CM. Soy food consumption and risk of prostate cancer: a meta-analysis of observational studies. Nutr Cancer. 2009;61:598–606. doi: 10.1080/01635580902825639. [DOI] [PubMed] [Google Scholar]
- Sharma P, Wisniewski A, Braga-Basaria M, Xu X, Yep M, et al. Lack of an effect of high dose isoflavones in men with prostate cancer undergoing androgen deprivation therapy. J Urol. 2009;182:2265–72. doi: 10.1016/j.juro.2009.07.030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eustache F, Mondon F, Canivenc-Lavier MC, Lesaffre C, Fulla Y, et al. Chronic dietary exposure to a low-dose mixture of genistein and vinclozolin modifies the reproductive axis, testis transcriptome, and fertility. Environ Health Perspect. 2009;117:1272–9. doi: 10.1289/ehp.0800158. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tanaka M, Fujimoto K, Chihara Y, Torimoto K, Yoneda T, et al. Isoflavone supplements stimulated the production of serum equol and decreased the serum dihydrotestosterone levels in healthy male volunteers. Prostate Cancer Prostatic Dis. 2009;12:247–52. doi: 10.1038/pcan.2009.10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Davis JN, Kucuk O, Sarkar FH. Expression of prostate-specific antigen is transcriptionally regulated by genistein in prostate cancer cells. Mol Carcinog. 2002;34:91–101. doi: 10.1002/mc.10053. [DOI] [PubMed] [Google Scholar]
- Rosenberg Zand RS, Jenkins DJ, Brown TJ, Diamandis EP. Flavonoids can block PSA production by breast and prostate cancer cell lines. Clin Chim Acta. 2002;317:17–26. doi: 10.1016/s0009-8981(01)00698-2. [DOI] [PubMed] [Google Scholar]
- Pendleton JM, Tan WW, Anai S, Chang M, Hou W, et al. Phase II trial of isoflavone in prostate-specific antigen recurrent prostate cancer after previous local therapy. BMC Cancer. 2008;8:132. doi: 10.1186/1471-2407-8-132. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kwan W, Duncan G, Van Patten C, Liu M, Lim J. A phase II trial of a soy beverage for subjects without clinical disease with rising prostate-specific antigen after radical radiation for prostate cancer. Nutr Cancer. 2010;62:198–207. doi: 10.1080/01635580903305318. [DOI] [PubMed] [Google Scholar]
- He YP, Hu HR, Xu LS, Meng G, Fan KN, et al. Structure-activity relationship studies on 6-naphthylmethyl substituted HEPT derivatives as non-nucleoside reverse transcriptase inhibitors based on molecular docking. Chem J Chin Univ. 2005;26:254–8. [Google Scholar]
- Chen HF, Gao K, Fan BT, Yuan SG, Jia ZJ, et al. Virtual screening and rational design of phenylpropanoid glycosides analogues based on molecular docking. Acta Chim Sin. 2002;60:1860–6. [Google Scholar]
- Zhao LF, Ding XQ, Ding JJ, Chen JS. Homology modeling and docking studies of the α2A-adrenergic receptor. Acta Phys Chim Sin. 2005;21:151–5. [Google Scholar]
- Trott O, Olson AJ. AutoDock Vina: improving the speed and accuracy of docking with a new scoring function, efficient optimization and multithreading. J Comput Chem. 2010;31:455–61. doi: 10.1002/jcc.21334. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pereira de Jésus-Tran K, Côté PL, Cantin L, Blanchet J, Labrie F, et al. Comparison of crystal structures of human androgen receptor ligand-binding domain complexed with various agonists reveals molecular determinants responsible for binding affinity. Protein Sci. 2006;15:987–99. doi: 10.1110/ps.051905906. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lill MA, Winiger F, Vedani A, Ernst B. Impact of induced fit on ligand binding to the androgen receptor: a multidimensional QSAR study to predict endocrine-disrupting effects of environmental chemicals. J Med Chem. 2005;48:5666–74. doi: 10.1021/jm050403f. [DOI] [PubMed] [Google Scholar]
- D'Ursi P, Salvi E, Fossa P, Milanesi L, Rovida E. Modelling the interaction of steroid receptors with endocrine disrupting chemicals. BMC Bioinformatics. 2005;6 Suppl 4:S10. doi: 10.1186/1471-2105-6-S4-S10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brzozowski AM, Pike AC, Dauter Z, Hubbard RE, Bonn T, et al. Molecular basis of agonism and antagonism in the oestrogen receptor. Nature. 1997;389:753–8. doi: 10.1038/39645. [DOI] [PubMed] [Google Scholar]
- Morris GM, Goodsell DS, Halliday RS, Huey R, Hart WE, et al. Automated docking using a Lamarckian genetic algorithm and empirical binding free energy function. J Comp Chem. 1998;19:1639–62. [Google Scholar]
- Lee HJ, Chattopadhyay S, Gong EY, Ahn RS, Lee K. Antiandrogenic effects of bisphenol A and nonylphenol on the function of androgen receptor. Toxicol Sci. 2003;75:40–6. doi: 10.1093/toxsci/kfg150. [DOI] [PubMed] [Google Scholar]
- Montgomery RB, Nelson PS, Lin D, Ryan CW, Garzotto M, et al. Diethylstilbestrol and docetaxel: a Phase II study of tubulin active agents in patients with metastatic, androgen-independent prostate cancer. Cancer. 2007;110:996–1002. doi: 10.1002/cncr.22917. [DOI] [PubMed] [Google Scholar]
- Takezawa Y, Nakata S, Kobayashi M, Kosaku N, Fukabori Y, et al. Moderate dose diethylstilbestrol diphosphate therapy in hormone refractory prostate cancer. Scand J Urol Nephrol. 2001;35:283–7. doi: 10.1080/003655901750425855. [DOI] [PubMed] [Google Scholar]
- Wetherill YB, Hess-Wilson JK, Comstock CE, Shah SA, Buncher CR, et al. Bisphenol A facilitates bypass of androgen ablation therapy in prostate cancer. Mol Cancer Ther. 2006;5:3181–90. doi: 10.1158/1535-7163.MCT-06-0272. [DOI] [PubMed] [Google Scholar]
- Chen JJ, Chang HC. By modulating androgen receptor coactivators, daidzein may act as a phytoandrogen. Prostate. 2007;67:457–62. doi: 10.1002/pros.20470. [DOI] [PubMed] [Google Scholar]
- Gao S, Liu GZ, Wang Z. Modulation of androgen receptor-dependent transcription by resveratrol and genistein in prostate cancer cells. Prostate. 2004;59:214–25. doi: 10.1002/pros.10375. [DOI] [PubMed] [Google Scholar]
- Cocco P. On the rumors about the silent spring. Review of the scientific evidence linking occupational and environmental pesticide exposure to endocrine disruption health effects. Cad Saude Publica. 2002;18:379–402. doi: 10.1590/s0102-311x2002000200003. [DOI] [PubMed] [Google Scholar]
- Inaguma S, Takahashi S, Imaida K, Suzuki S, Shirai T. p-Nonylphenol pretreatment during the late neonatal period has no effect on 3,2′-dimethyl-4-aminobiphenyl-induced prostate carcinogenesis in male F344 rats. Cancer Lett. 2004;212:159–66. doi: 10.1016/j.canlet.2004.03.028. [DOI] [PubMed] [Google Scholar]
- Yoshino H, Nabae K, Doi Y, Ichihara T, Tamano S. Lack of effects of gestational and lactational exposure to 4-nonylphenol on PhIP-induced rat prostate carcinogenesis. Toxicol Pathol. 2005;18:183–8. [Google Scholar]
- Timms BG, Howdeshell KL, Barton L, Bradley S, Richter CA, et al. Estrogenic chemicals in plastic and oral contraceptives disrupt development of the fetal mouse prostate and urethra. Proc Natl Acad Sci USA. 2005;102:7014–9. doi: 10.1073/pnas.0502544102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ho SM, Tang WY, Belmonte de Frausto J, Prins GS. Developmental exposure to estradiol and bisphenol A increases susceptibility to prostate carcinogenesis and epigenetically regulates phosphodiesterase type 4 variant 4. Cancer Res. 2006;66:5624–32. doi: 10.1158/0008-5472.CAN-06-0516. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Murray TJ, Maffini MV, Ucci AA, Sonnenschein C, Soto AM. Induction of mammary gland ductal hyperplasias and carcinoma in situ following fetal bisphenol A exposure. Reprod Toxicol. 2007;23:383–90. doi: 10.1016/j.reprotox.2006.10.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wetherill YB, Petre CE, Monk KR, Puga A, Knudsen KE. The xenoestrogen bisphenol A induces inappropriate androgen receptor activation and mitogenesis in prostatic adenocarcinoma cells. Mol Cancer Ther. 2002;1:515–24. [PubMed] [Google Scholar]
- Wetherill YB, Fisher NL, Staubach A, Danielsen M, de Vere White RW, et al. Xenoestrogen action in prostate cancer: pleiotropic effects dependent on androgen receptor status. Cancer Res. 2005;65:54–65. [PubMed] [Google Scholar]
- Serrate C, Loriot Y, De La Motte Rouge T, Gross-Goupil M, Massard C, et al. Diethylstilbestrol (DES) retains activity and is a reasonable option in patients previously treated with docetaxel for castration-resistant prostate cancer. Ann Oncol. 2009;20:965. doi: 10.1093/annonc/mdp112. [DOI] [PubMed] [Google Scholar]
- Scherr D, Pitts WR, Jr, Vaughn ED., Jr Diethylstilbesterol revisited: androgen deprivation, osteoporosis and prostate cancer. J Urol. 2002;167:535–8. doi: 10.1016/S0022-5347(01)69080-3. [DOI] [PubMed] [Google Scholar]
- Malkowicz SB. The role of diethylstilbestrol in the treatment of prostate cancer. Urology. 2001;58:108–13. doi: 10.1016/s0090-4295(01)01252-3. [DOI] [PubMed] [Google Scholar]
- Kurahashi N, Iwasaki M, Sasazuki S, Otani T, Inoue M, et al. Soy product and isoflavone consumption in relation to prostate cancer in Japanese men. Cancer Epidemiol Biomarkers Prev. 2007;16:538–45. doi: 10.1158/1055-9965.EPI-06-0517. [DOI] [PubMed] [Google Scholar]
- Allen NE, Sauvaget C, Roddam AW, Appleby P, Nagano J, et al. A prospective study of diet and prostate cancer in Japanese men. Cancer Causes Control. 2004;15:911–20. doi: 10.1007/s10552-004-1683-y. [DOI] [PubMed] [Google Scholar]
- Nomura AM, Hankin JH, Lee J, Stemmermann GN. Cohort study of tofu intake and prostate cancer: no apparent association. Cancer Epidemiol Biomarkers Prev. 2004;13:2277–9. [PubMed] [Google Scholar]
- Jacobsen BK, Knutsen SF, Fraser GE. Does high soy milk intake reduce prostate cancer incidence? The Adventist Health Study (United States) Cancer Causes Control. 1998;9:553–7. doi: 10.1023/a:1008819500080. [DOI] [PubMed] [Google Scholar]
- Severson RK, Nomura AM, Grove JS, Stemmermann GN. A prospective study of demographics, diet, and prostate cancer among men of Japanese ancestry in Hawaii. Cancer Res. 1989;49:1857–60. [PubMed] [Google Scholar]
- Li X, Li J, Tsuji I, Nakaya N, Nishino Y, et al. Mass screening-based case-control study of diet and prostate cancer in Changchun, China. Asian J Androl. 2008;10:551–60. doi: 10.1111/j.1745-7262.2008.00384.x. [DOI] [PubMed] [Google Scholar]
- Nagata Y, Sonoda T, Mori M, Miyanaga N, Okumura K, et al. Dietary isoflavones may protect against prostate cancer in Japanese men. J Nutr. 2007;137:1974–9. doi: 10.1093/jn/137.8.1974. [DOI] [PubMed] [Google Scholar]
- Sonoda T, Nagata Y, Mori M, Miyanaga N, Takashima N, et al. A case-control study of diet and prostate cancer in Japan: possible protective effect of traditional Japanese diet. Cancer Sci. 2004;95:238–42. doi: 10.1111/j.1349-7006.2004.tb02209.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee MM, Gomez SL, Chang JS, Wey M, Wang RT, et al. Soy and isoflavone consumption in relation to prostate cancer risk in China. Cancer Epidemiol Biomarkers Prev. 2003;12:665–8. [PubMed] [Google Scholar]
- Kolonel LN, Hankin JH, Whittemore AS, Wu AH, Gallagher RP, et al. Vegetables, fruits, legumes and prostate cancer: a multiethnic case-control study. Cancer Epidemiol Biomarkers Prev. 2000;9:795–804. [PubMed] [Google Scholar]
- Jain MG, Hislop GT, Howe GR, Ghadirian P. Plant foods, antioxidants, and prostate cancer risk: findings from case-control studies in Canada. Nutr Cancer. 1999;34:173–84. doi: 10.1207/S15327914NC3402_8. [DOI] [PubMed] [Google Scholar]
- Strom SS, Yamamura Y, Duphorne CM, Spitz MR, Babaian RJ, et al. Phytoestrogen intake and prostate cancer: a case-control study using a new database. Nutr Cancer. 1999;33:20–5. doi: 10.1080/01635589909514743. [DOI] [PubMed] [Google Scholar]
- Villeneuve PJ, Johnson KC, Kreiger N, Mao Y. Risk factors for prostate cancer: results from the Canadian National Enhanced Cancer Surveillance System. The Canadian Cancer Registries Epidemiology Research Group. Cancer Causes Control. 1999;10:355–67. doi: 10.1023/a:1008958103865. [DOI] [PubMed] [Google Scholar]
- El Touny LH, Banerjee PP. Identification of a biphasic role for genistein in the regulation of prostate cancer growth and metastasis. Cancer Res. 2009;69:3695–703. doi: 10.1158/0008-5472.CAN-08-2958. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang J, Eltoum IE, Lamartiniere CA. Genistein chemopre-vention of prostate cancer in TRAMP mice. J Carcinog. 2007;6:3. doi: 10.1186/1477-3163-6-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhou JR, Gugger ET, Tanaka T, Guo Y, Blackburn GL, et al. Soybean phytochemicals inhibit the growth of transplantable human prostate carcinoma and tumor angiogenesis in mice. J Nutr. 1999;129:1628–35. doi: 10.1093/jn/129.9.1628. [DOI] [PubMed] [Google Scholar]
- Bemis DL, Capodice JL, Desai M, Buttyan R, Katz AE. A concentrated aglycone isoflavone preparation (GCP) that demonstrates potent anti-prostate cancer activity in vitro and in vivo. Clin Cancer Res. 2004;10:5282–92. doi: 10.1158/1078-0432.CCR-03-0828. [DOI] [PubMed] [Google Scholar]
- Zhou JR, Yu L, Zhong Y, Blackburn GL. Soy phytochem-icals and tea bioactive components synergistically inhibit androgen-sensitive human prostate tumors in mice. J Nutr. 2003;133:516–21. doi: 10.1093/jn/133.2.516. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kohen F, Gayer B, Kulik T, Frydman V, Nevo N, et al. Synthesis and evaluation of the antiproliferative activities of derivatives of carboxyalkyl isoflavones linked to N-t-Boc-hexylenediamine. J Med Chem. 2007;50:6405–10. doi: 10.1021/jm070727z. [DOI] [PubMed] [Google Scholar]
- Shukla S, MacLennan GT, Flask CA, Fu P, Mishra A, et al. Blockade of beta-catenin signaling by plant flavonoid apigenin suppresses prostate carcinogenesis in TRAMP mice. Cancer Res. 2007;67:6925–35. doi: 10.1158/0008-5472.CAN-07-0717. [DOI] [PubMed] [Google Scholar]
- Singh RP, Agrawal P, Yim D, Agarwal C, Agarwal R. Acacetin inhibits cell growth and cell cycle progression, and induces apoptosis in human prostate cancer cells: structure-activity relationship with linarin and linarin acetate. Carcinogenesis. 2005;26:845–54. doi: 10.1093/carcin/bgi014. [DOI] [PubMed] [Google Scholar]
- Drees M, Dengler WA, Roth T, Labonte H, Mayo J, et al. Flavopiridol (L86-8275): selective antitumor activity in vitro and activity in vivo for prostate carcinoma cells. Clin Cancer Res. 1997;3:273–9. [PubMed] [Google Scholar]
- Akaza H, Miyanaga N, Takashima N, Naito S, Hirao Y, et al. Is daidzein non-metabolizer a high risk for prostate cancer? A case-controlled study of serum soybean isoflavone concentration. Jpn J Clin Oncol. 2002;32:296–300. doi: 10.1093/jjco/hyf064. [DOI] [PubMed] [Google Scholar]
- Rogan WJ, Chen A. Health risks and benefits of bis(4-chlorophenyl)-1,1,1-trichloroethane (DDT) Lancet. 2005;366:763–73. doi: 10.1016/S0140-6736(05)67182-6. [DOI] [PubMed] [Google Scholar]
- Eskenazi B, Chevrier J, Rosas LG, Anderson HA, Bornman MS, et al. The Pine River Statement: human health consequences of DDT use. Environ Health Perspect. 2009;117:1359–67. doi: 10.1289/ehp.11748. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kao PC, P'Eng FK. How to reduce the risk factors of osteoporosis in Asia. Zhonghua Yi Xue Za Zhi (Taipei) 1995;55:209–13. [PubMed] [Google Scholar]
- Travis RC, Spencer EA, Allen NE, Appleby PN, Roddam AW, et al. Plasma phyto-oestrogens and prostate cancer in the European Prospective Investigation into Cancer and Nutrition. Br J Cancer. 2009;100:1817–23. doi: 10.1038/sj.bjc.6605073. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Adlercreutz H, Markkanen H, Watanabe S. Plasma concentrations of phyto-oestrogens in Japanese men. Lancet. 1993;342:1209–10. doi: 10.1016/0140-6736(93)92188-y. [DOI] [PubMed] [Google Scholar]
- Li X, Tsuji I, Kuwahara M, Zhang H, Wang H, et al. Mass screening of prostate cancer in Changchun City of China. Int Urol Nephrol. 2004;36:541–8. doi: 10.1007/s11255-004-0842-0. [DOI] [PubMed] [Google Scholar]
- Kuwahara M, Tochigi T, Kawamura S, Ogata Y, Xu N, et al. Mass screening for prostate cancer: a comparative study in Natori, Japan and Changchun,China. Urology. 2003;61:137–41. doi: 10.1016/s0090-4295(02)02093-9. [DOI] [PubMed] [Google Scholar]


