Abstract
CD133+ prostate cancer stem cells (PCSCs) have recently been identified in human prostate cancer tissues. The present study reports the integrin profile of prostate cancer progenitor cells and the role of α1 and β1 integrins in the homing and differentiation of PCSCs in vitro. PCSCs were isolated from the tissue specimens of patients with prostate cancer and the expression of surface integrins and adhesion patterns were determined. Our analysis of the expression of surface integrins and their adhesion patterns of prostate cancer stem cells derived from prostate cancer tissues revealed that the levels of β1 and α2β1 integrins were significantly higher (P < 0.05) than those of the other integrins. By contrast, peripheral blood-derived CD133+ cells from prostate cancer patients showed a high level of expression (P < 0.01) of α2β1, αvβ3, αvβ5, β1 and α1 integrins and a minimal expression of α4β1 integrins. Moreover, CD133+ cells derived from both prostate cancer tissues and peripheral blood exhibited an increased degree of attachment to extracellular matrix proteins (P < 0.001) and a high expression level of α2β1 integrin. In vitro experiments using blocking antibodies indicated that α1 and β1 integrins have a role in the homing and differentiation of PCSCs. This is the first report to suggest the importance of integrins in mediating the homing and differentiation of PCSCs.
Keywords: androgen receptor, extracellular matrix proteins, focal adhesion kinase, prostate-specific antigen
Introduction
Prostate cancer is a major public health concern in many industrialized countries. It is predominantly a disease of elderly men, with its incidence increasing steeply in the seventh decade of life. Recent studies have indicated that a subpopulation of cells, cancer stem cells (CSCs) 1, is present in prostate cancer. CSCs are capable of self-renewal and are responsible for tumour maintenance and metastasis 2, 3, 4, 5, 6, 7, 8. CSCs can be isolated by observing their ability to efflux Hoechst 33342 dye 9 and are referred to as the 'side population (SP)'. A previous report 10 has indicated that these cells express MDR1. A transmembrane glycoprotein, CD133 (prominin-1), was first recognized in CD34+ progenitor populations from adult blood, bone marrow and foetal liver cells 11, 12, 13, 14. Recently, CD133 has been found to be an important marker for a subpopulation of CSCs in leukaemia, brain tumours, retinoblastomas, renal tumours, pancreatic tumours, colon carcinomas, prostatic carcinomas and hepatocellular carcinomas 15. However, the mechanism underlying the homing and differentiation of CSCs from prostate tumours remains unclear.
The ex vivo expansion of stem cells using extracellular proteins such as fibronectin and laminin enhances the homing and differentiation of these cells and also promotes the up-regulation of β1 integrins 16. Prostate tumour cells have a markedly different surrounding matrix compared with normal prostatic cells and, therefore, changes that occur in the integrin profile may be functionally related to tumour metastasis and growth 14. The altered integrin expression profile and extracellular matrix (ECM) environment that is present in metastatic prostate cancer is likely to affect cell migration. Integrin-activated signalling molecules, such as focal adhesion kinase (FAK), phosphatidylinositol 3-kinase (PI 3-kinase) and members of the extracellular signal-regulated kinase 1 and 2 mitogen-activated protein (ERK1 and 2/MAP) kinase family 15, 17, have been shown to be involved in cell migration. Further study of alterations of the signalling pathways that are controlled by integrins will contribute to a better understanding of the underlying mechanisms that support metastasis establishment 18, 19.
Prostate cancer stem cells (PCSCs) predominantly express CD133, MDR1 and Oct-4 and are found in the prostate cancer tissues and also in the peripheral blood of prostate cancer patients 20, 21. Understanding the homing, adhesion and differentiation characteristics of PCSCs may have significant implications for the development of novel drugs for the treatment of prostate cancer. The full integrin profile of human PCSCs and the ECM proteins that form an adhesion platform for these cells is currently unknown.
Materials and methods
All normal and prostate cancer tissues were obtained from volunteers in Sri Krishna City Hospital, Krishna (District), Andhra Pradesh, India. The study was approved by the Institutional Ethics Committee/Institutional Review Board of Sri Krishna City Hospital.
Isolation of the population of CD133+ cells from prostate tissue biopsy specimens
Normal prostate tissues were collected from multiple organ donors who suffered accidental deaths. Organ donor samples from accidental death victims were removed after obtaining consent from next of kin. In all cases, the normal prostate tissues were free from prostate intraepithelial neoplasia and cancer on histological examination. CD133+ cells were isolated from the prostate tissue of controls (prostate-specific antigen levels < 4 ng mL−1) and cancer patients (prostate-specific antigen levels > 4 ng mL−1) who were 60–70 years of age. Biopsy samples from both control (n = 6) and prostate cancer patients (n = 10) were digested with a trypsin-collagenase mixture (Sigma, St. Louis, MO, USA) to dissociate the cells. The CD133+ cells were selected using magnetic beads and analysed for the presence of CD133 (AC133; Miltenyi Biotech, Bergisch Gladbach, Germany). The purity of CD133+ in the sorted samples was routinely higher than 96%. The CD133+ cells were also used for integrin profile analysis. The immunolabelling of CD133 was performed in prostate cancer tissues using a monoclonal CD133/1 antibody (AC133, Miltenyi Biotech) conjugated to biotin, and avidin-fluorescein isothiocyanate (FITC) was used to detect the biotin-CD133/1 antibody. The expression profile of the androgen receptor in these cells was studied by flow cytometry using a FITC-labelled rabbit anti-human androgen receptor antibody (Sigma), and the expression profiles of CD44 and p53 were characterized using an avidin-labelled rabbit anti-human CD44 antibody (Pharmingen, San Jose, CA, USA) and an avidin-labelled rabbit anti-human p53 antibody (Roche, Mannheim, Germany) using the western blot method.
Isolation of a CD133+ cell population from peripheral blood samples
Mononuclear cells were isolated from the blood samples of control (n = 6) and prostate cancer patients (n = 10) using a Ficoll gradient (Biocoll 1077; Sigma). The cells were selected for CD133 expression on a magnetic column using magnetic beads and were then tested for the presence of CD133 (AC133). The purity of CD133+ cells in the sorted samples was routinely higher than 94%. The CD133+ cells were also used for integrin profile analysis. The expression profile of CD133 was assessed using a monoclonal CD133/1 antibody (AC133) conjugated to biotin, and avidin-FITC was used to detect the biotin-CD133/1 antibody. The expression profile of the androgen receptor was studied by flow cytometry using an FITC-labelled rabbit anti-human androgen receptor antibody (Sigma), and the expression profiles CD44 and p53 were characterized by western blot analysis using an avidin-labelled rabbit anti-human CD44 antibody (Pharmingen) and an avidin-labelled rabbit anti-human p53 antibody (Roche).
Analysis of cell-surface integrins
Fluorescence-activated cell sorter (FACS) analysis was performed to identify fluorescently labelled cell-surface integrins, including FITC-labelled monoclonal anti-human α1, FITC-labelled monoclonal anti-human αvβ3, FITC-labelled monoclonal anti-human αvβ5, FITC-labelled monoclonal anti-human α2β1, phycoerythrin (PE)-labelled monoclonal anti-human β1, PE-labelled monoclonal anti-human β2 and PE-labelled monoclonal anti-human α4β1 (all were procured from Pharmingen) in prostate tissue-derived and peripheral blood-derived CD133+ cells from both control and prostate cancer patients. The same expression profile was analysed in the prostate cancer cell lines PC-3 and DU-145, as well as in freshly isolated peripheral blood-derived mononuclear cells from both control and prostate cancer patients.
Culturing prostate tissue-derived and peripheral blood-derived CD133+ cells
The CD133+ cells that were derived from peripheral blood and from the prostate tissues of prostate cancer patients were cultured on plates coated with fibronectin (50 μg mL−1) and laminin (50 μg mL−1) in Iscove's modified Dulbecco's medium containing bovine serum albumin (2 mg mL−1), bovine transferrin (200 μg mL−1), human insulin (10 μg mL−1), hydrocortisone (2 μmol L−1), hepatocyte growth factor (HGF) (20 ng mL−1), and GM-CSF (granulocyte-monocyte colony-stimulating factor) (20 ng mL−1). Cells were cultured in serum-free basal medium (all from PeproTech Asia, Rehovot, Israel) for 7–10 days, at which point half of the medium was replaced with fresh medium every other day. The cultured cells were trypsinized and analysed for the expression profiles of the androgen receptor, prostate specific antigen, CD57, FAK and α2β1 using an FITC-labelled rabbit anti-human androgen receptor antibody, an FITC-labelled rabbit anti-human prostate-specific antigen antibody, a rabbit anti-human FAK antibody (all procured from Sigma), a rabbit anti-human CD57 clone NK-1 antibody (Pharmingen) and a rabbit anti-human β-actin antibody (Sigma). In some experiments, blocking rabbit anti-human α1 (3 μg mL−1) and β1 antibodies (3 μg mL−1) were used to block α1 and β1 antigens in cell culture.
In vitro adhesion of CD133+ cells to fibronectin and laminin
The adhesion capacities of prostate cancer tissue-derived and peripheral blood-derived CD133+ cells to ECM proteins were analysed on coated 24-well plates in vitro. CD133+ cells were stained with cell tracker green CMFDA (5-chloromethylfluorescein diacetate) (CTG; 1 μL per 4 mL media; Molecular Probes, Eugene, OR, USA) and plated at a density of 2 × 105 cells per well. After 1 h of incubation at 37°C, unattached cells were removed by washing with phosphate buffered saline for 4 min, and the number of attached cells was estimated by measuring the fluorescence of each well by hemocytometer counting 5, 22.
Western blot analysis to determine CD44, p53, CD57, FAK and β-actin expression levels
The isolated cells from control and prostate cancer patients' biopsy specimens were lysed with sodium dodecyl sulphate sample buffer containing 1 mmol L−1 orthovanadate, and the samples were then processed for western blotting 8. Expression of CD44, p53, FAK, CD57 and β-actin was detected in CD133+ cells derived from normal and prostate cancer patients' biopsies. Avidin-labelled rabbit anti-human CD44 (Pharmingen), avidin-labelled rabbit anti-human p53 (Roche), rabbit anti-human FAK (Sigma), rabbit anti-human CD57 clone NK-1 (Pharmingen) and rabbit anti-human β-actin (Sigma) antibodies were used to detect protein expression profiles by Western blot analysis.
Flow cytometry
A total of 5 × 105 cells out of freshly isolated cells from patient biopsies and peripheral blood samples were stained by incubation with 50 μL of diluted primary antibodies at 4°C for 45 min. The cells were washed with 1% phosphate buffered saline with Tween-20 for 4 min and stained with secondary antibody, then washed again for 4 min. They were then fixed with 0.2% paraformaldehyde for 30 min at room temperature. The primary antibodies used in the study were biotin-labelled CD133 (Miltenyi Biotech), FITC-labelled monoclonal anti-human α1, FITC-labelled monoclonal anti-human αvβ3, FITC-labelled monoclonal anti-human αvβ5, FITC-labelled monoclonal anti-human α2β1, PE-labelled monoclonal anti-human β1, PE-labelled monoclonal anti-human β2, PE-labelled monoclonal anti-human α4β1, FITC-labelled rabbit anti-human CD57 clone NK-1, and rabbit-anti human α1 and β1. The secondary antibodies used in this study were anti-biotin-PE and streptavidin-FITC (all procured from Pharmingen), FITC-labelled rabbit anti-human androgen receptor, and FITC-labelled rabbit anti-human prostate-specific antigen (both procured from Sigma). Cells were analysed on a customized BD FACSCalibur™ flow cytometer (BD Biosciences, San Jose, CA, USA) using filters of 530/28 BP for FITC and 575/26 BP for PE and a 488-nm argon laser to excite both PE and FITC.
Statistical analysis
Data were analysed by one-way ANOVA followed by Dunnett's t-test using GraphPad InStat version 3.05 (GraphPad software Inc., San Diego, CA, USA). All P-values < 0.05 were considered to be statistically significant.
Results
Isolation and characterization of prostate tissue-derived CD133+ cells
CD133+ cells were isolated from prostate tissue samples of six control and ten prostate cancer patients. To characterize isolated CD133+ cells from control and prostate cancer patients, flow cytometry and Western blot analysis were used to detect the expression profile of prostate cancer markers. The immunolabelling of CD133 revealed a difference in the expression level of CD133 between the prostate tissues of control and prostate cancer patients (Figures 1A and B). A high percentage (97%) of CD133+ cells were isolated from the prostate cancer tissues and peripheral blood (PC-CD133+) of prostate cancer patients (Figures 1C and D). The analysis of the expression levels of CD44 and p53 in both prostate cancer tissues and peripheral blood-derived CD133+ cells indicated that CD44 was up-regulated and p53 was down-regulated (Figures 1E and F). In contrast to this, CD133+ cells derived from normal prostate tissues had low levels of CD44 expression and high levels of p53 expression (Figures 1E and F). However, the level of expression of the androgen receptor in both the prostate tissue- and peripheral blood-derived CD133+ cells of prostate cancer patients was low (Figures 1G and H).
Figure 1.
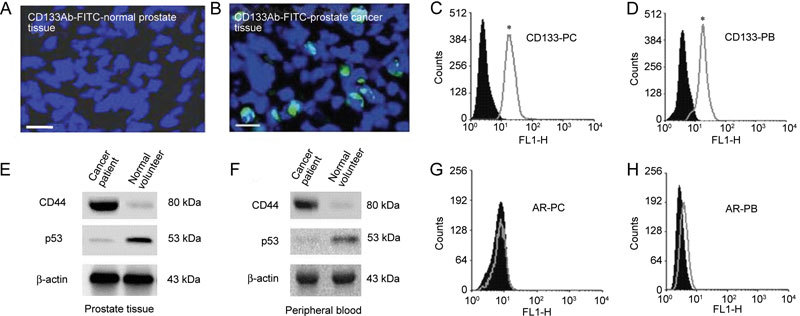
Characterization of prostate cancer stem cells. (A) and (B): The immunolabelling of CD133 in normal and prostate cancer tissues, which was stained with FITC (green). Nuclei were labelled with DAPI (blue). Scale bars = 50 μm. (C) and (D): The purity of CD133+ cells isolated from the prostate cancer tissue (PC) and the peripheral blood (PB). (E) and (F): The expression profiles of CD44, p53 and β-actin in the prostate tissue and peripheral blood of both control and prostate cancer patients. (G) and (H): The expression profile of androgen receptor (AR) in CD133+ cells isolated from prostate cancer tissues and the peripheral blood. In each case, the filled histogram represents an isotype-matched IgG control antibody. Histograms were analyzed with the intensity of the detected signal on the x-axis and the number of counts (cells) on the y-axis. Experiments were performed for each antibody on at least five separate occasions (*P < 0.05, compared with the respective IgG control).
Integrin profile of prostate tissue- and peripheral blood-derived CD133+ cells
Prostate tissue-derived CD133+ cells exhibited a high level of expression of β1 integrin (P < 0.05), a moderate level of expression of α1 integrin, low level of expression of αvβ3 integrin, and no expression of the αvβ5, β2, α2β1 and α4β1 integrins (Table 1). By contrast, peripheral blood derived-CD133+ cells exhibited a high level of expression of α2β1, αvβ3, αvβ5, β1 and α1 integrins (P < 0.01) and minimal expression of α4β1 integrin (Table 1). Cells from the prostate cancer cell lines PC-3 and DU-145 as well as mononuclear cells expressed β2 integrin, which differentiated these cell types from those listed above (Table 1).
Table 1. Integrin profile of prostate tissue and peripheral blood-derived CD133+ cells, prostate cancer cell lines and the mononuclear cells of control and prostate cancer patients (mean ± SEM).
| Integrin name | Prostate-derived CD133+ cells (%) |
Peripheral blood-derived CD133+ cells (%) |
PCa cell lines (%) |
Mononuclear cells (%) |
||||
|---|---|---|---|---|---|---|---|---|
| Controls | PCa patients | Controls | PCa patients | PC-3 | DU-145 | Controls | PCa patients | |
| α1 | 15.0 ± 5.6 | 85.0 ± 3.2 | 18.0 ± 4.6 | 94.0 ± 4.1# | 89.0 ± 3.1 | 90.0 ± 2.3 | 91.0 ± 4.3 | 90.0 ± 2.6 |
| β1 | 27.0 ± 7.4 | 95.0 ± 1.8* | 21.0 ± 5.5 | 97.0 ± 1.5# | 90.0 ± 5.6 | 89.0 ± 6.1 | 93.0 ± 3.2 | 94.0 ± 1.7 |
| αv β3 | 11.0 ± 2.7 | 40.0 ± 8.6 | 10.0 ± 3.7 | 93.0 ± 3.6# | 50.0 ± 4.5 | 49.0 ± 3.6 | 70.0 ± 4.7 | 68.0 ± 5.1 |
| αv β5 | 10.0 ± 3.1 | 10.0 ± 3.2 | 12.0 ± 2.2 | 95.0 ± 4.1# | 52.0 ± 3.8 | 51.0 ± 3.4 | 72.0 ± 5.1 | 71.0 ± 7.6 |
| α2β1 | 8.0 ± 2.6 | 95 .0± 1.3 | 13.0 ± 3.6 | 90.0 ± 1.6# | 58.0 ± 5.6 | 54.0 ± 2.6 | 65.0 ± 6.1 | 60.0 ± 3.2 |
| α4β1 | 10.0 ± 2.3 | 17.0 ± 4.1 | 17.0 ± 4.7 | 30.0 ± 3.2 | 61.0 ± 2.4 | 60.0 ± 6.5 | 79.0 ± 4.1 | 80.0 ± 2.6 |
| β2 | 7.0 ± 4.2 | 20.0 ± 5.6 | 10.0 ± 4.6 | 26.0 ± 3.1 | 41.0 ± 2.9 | 44.0 ± 8.6 | 40.0 ± 5.3 | 23.0 ± 6.7 |
Abbreviation: PCa, Prostate cancer.
P < 0.05,
P < 0.01, compared with the control.
Role of α1 and β1 in the adhesion of CD133+ cells to ECM proteins
As shown in Table 1, α1, β1 and α2β1 integrins are majorly expressed on prostate cancer tissue and peripheral blood derived CD133+ cells. To investigate the role of α1 and β1 integrins in the attachment of CD133+ cells to ECM proteins, we added rabbit anti-human α1 and β1 blocking antibodies to 8–10 days culture of tissue and peripheral blood-derived CD133+ cells (Figure 2A). Collins et al. 19 suggested the role of α2β1 in adhesion of CD133+ prostate cancer stem cells. To support the data, the expression profile of α2β1 prostate cancer tissue-derived and peripheral blood-derived CD133+ cells in culture is shown in Figures 2B and C.
Figure 2.
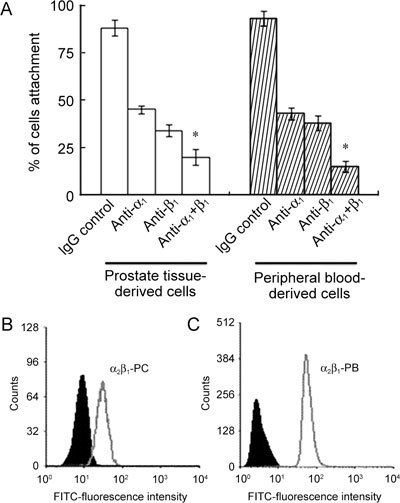
Adhesion properties of CD133+ cells to extracellular matrix proteins. (A): Data regarding the adhesion of prostate cancer tissue- and peripheral blood-derived CD133+ cells to extracellular matrix (ECM) proteins in the presence of various anti-integrin antibodies (α1, β1 and α1 + β1) as compared with an IgG control antibody (3 μg mL−1 of each antibody) (*P < 0.001, compared with the respective IgG control). (B) and (C): The expression profile of α2β1 integrin in the prostate cancer tissue (PC)- and peripheral blood (PB)-derived CD133+ cells with the respective immunoglobulin IgG control antibody. In each case, the filled histogram indicates the isotype-matched IgG control antibody. Histograms were analyzed with the intensity of the detected signal on the x-axis and the number of counts (cells) on the y-axis.
Role of α1 and β1 in differentiation of CD133+ cells
To investigate the role of α1 and β1 integrins in the differentiation of CD133+ cells, we used blocking rabbit antihuman α1 and β1 blocking antibodies in 8–10 days culture of prostate cancer tissue- and peripheral blood derived CD133+ cells and checked for differentiation. The differentiation markers, androgen receptor, prostate-specific antigen, focal adhesion kinase (FAK) and CD57 were down-regulated upon blocking with α1 and β1 antibodies separately and in combination in cultured cells. The results are shown in Figure 3.
Figure 3.
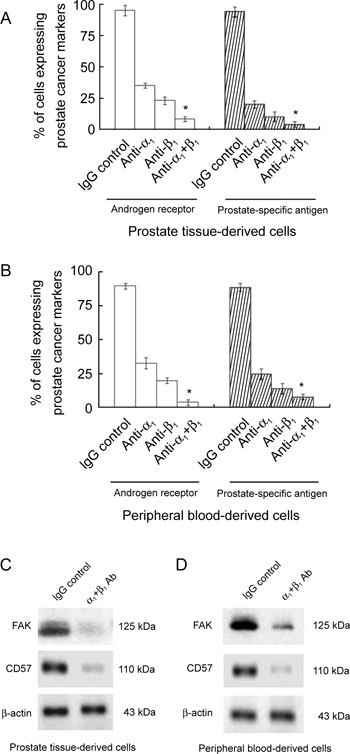
The role of integrins in the differentiation of CD133+ cells. The expression levels of the androgen receptor, and prostate-specific antigen in prostate cancer tissue-derived (A) and peripheral blood-derived (B) CD133+ cells were induced to differentiate with cytokines for 8–10 days in the presence of various anti-integrin antibodies (α1, β1 and α1 + β1). These results were compared with those obtained using an IgG control antibody (3 μg mL−1 of each antibody). Results are expressed as mean ± SEM (*P < 0.01, compared with the respective control). The expression of differentiation markers (CD57 and focal adhesion kinase) and a control (β-actin) indicate that prostate tissue-derived cancer stem cells (C) and peripheral blood-derived prostate cancer stem cells (D) had differentiated into prostatic epithelial cells.
Discussion
A distinct surface integrin expression profile of CD133+ cells derived from prostate cancer tissues and peripheral blood was observed in this study and is shown in Table 1. Our previous studies 20, 21 indicated that 80%–90% of prostate cancer tissue- and peripheral blood-derived CD133+ cells were also positive for MDR1, which is also expressed in the 'side population' of progenitor cells. These CD133+ cells have also been shown to be involved in cell adhesion to specific ECM proteins such as fibronectin and laminin. This may have implications for our understanding of in vivo cancer stem cells and for the development of novel cellular targets for the treatment of adenocarcinoma of the prostate. The components of the prostate tissue that are involved in the homing of cancer cells are currently unknown. Homing may involve the exit of progenitor cells from the circulation 11, 12, 13 followed by their attachment to ECM proteins and incorporation into surrounding tissues. Integrins are known to have a key role in mediating the attachment between prostate-derived progenitor cells and ECM proteins, as well as in potentiating cell differentiation, migration and proliferation. In vitro models suggest that different progenitor cells express several integrins in common that have potentially overlapping matrix-binding characteristics, and indeed, the constituents of the matrix environment are a strong determinant of specific integrin–ECM interactions 15, 17, 20. High levels of expression of α1 and β1 integrins, which are known facilitators of fibronectin binding, were found in prostate cancer tissue- and peripheral blood-derived CD133+ cells in our present study. These integrins are likely to be involved in the interaction between CD133+ cells and fibronectin. Indeed, our CD133+ cells binding data support this concept, as the addition of anti-β1 and α1 integrin-blocking antibodies led to considerable inhibition of the binding of prostate tissue-derived CD133+ cells to fibronectin. The use of blocking anti-human α1 and β1 antibodies in CD133+ cells led to considerable interference (P < 0.001) of the ability of these cells to adhere to ECM proteins, such as fibronectin and laminin. On the contrary, only a 0%–2% interference was observed in the ECM adhesion capabilities of CD133+ cells derived from normal non-cancerous prostate tissues (data not shown) after the addition of these blocking antibodies. Differentiation of CD133+ cells was found to be inhibited in the presence of β1 and α1 integrin blocking antibodies. Flow cytometry and western blot analysis indicated that the expression of the androgen receptor, prostate-specific antigen, CD57 and FAK was inhibited in the presence of blocking antibodies. Prostate tissue-derived CD133+ cells lacked notable expression of αvβ3 and αvβ5 integrins, which have been shown to have a key role in angiogenesis, whereas peripheral blood-derived CD133+ cells strongly expressed these integrins at higher levels. Together, these data suggest a difference in the underlying mechanism of the integrin–matrix binding interaction of prostate cancer tissue-derived CD133+ cells compared with peripheral blood-derived CD133+ cells. Figures 2B and C shows that expression of α2β1 was up-regulated in cultured peripheral blood- and prostate tissue-derived CD133+ cells. Moreover, the α2β1 integrin, which is a significant mediator of collagen binding, was expressed at high levels in both peripheral blood- and prostate tissue-derived CD133+ cells. Collins et al. 19, 23 suggested that α2β1hi progenitor cells from prostate tissues later became differentiated into androgen receptor-positive prostate cancer cells. Further studies are required to elucidate the role that α2β1 integrin in prostate cancer tissue-derived and peripheral blood-derived CD133+ cells have in cell signalling pathways. It has been previously shown that the tyrosine phosphorylation and activation of pp125FAK, in association with β1 and α1 integrins, are both increased when integrins bind to ECM proteins. This binding triggers cytokine induction followed by the differentiation of CD133+ cells into prostatic epithelial cells that express CD57, androgen receptor and prostate-specific antigen. We believe that the interaction between integrin molecules and ECM proteins is responsible for the increased attachment and differentiation capacities of prostate cancer tissue- and peripheral blood-derived CD133+ cells. However, in vivo experiments are required to determine whether or not the phenotype of in vivo tumour-initiating cells is comparable to the in vitro phenotype.
Conclusion
In summary, we were able to identify and isolate a population of cells from prostate tissues that is highly enriched for a phenotype that is consistent with that of a CSC, which appears to have attachment and differentiation capabilities that allow these cells to recapitulate the original tumour phenotype. The tumour heterogeneity and attachment of β1 and α1 integrins to ECM proteins that occur in human prostate cancer appear to be a consequence of the differentiation of tumour cells from androgen receptor-negative stem cells to androgen receptor-positive prostate cancer epithelial cells. This study provides evidence that prostate cancer can originate from CD133-enriched cells that express α1 and β1 integrins, which are responsible for the regulation of cellular attachment to ECM and cellular differentiation.
Acknowledgments
This work was supported in part by grants from the Institute of Science and Technology of Jawaharlal Nehru Technological University, Hyderabad, India. We thank Dr A. Mukhopadhyay for his assistance in writing and editing this paper.
References
- Jordan CT, Guzman ML, Noble M. Cancer stem cells. N Engl J Med. 2006;355:1253–61. doi: 10.1056/NEJMra061808. [DOI] [PubMed] [Google Scholar]
- Okita K, Ichisaka T, Yamanaka S. Generation of germline-competent induced pluripotent stem cells. Nature. 2007;448:313–7. doi: 10.1038/nature05934. [DOI] [PubMed] [Google Scholar]
- Park IH, Zhao R, West JA, Yabuuchi A, Huo H, et al. Reprogramming of human somatic cells to pluripotency with defined factors. Nature. 2008;451:141–6. doi: 10.1038/nature06534. [DOI] [PubMed] [Google Scholar]
- Yu J, Vodyanik MA, Smuga-Otto K, Antosiewicz-Bourget J, Frane JL, et al. Induced pluripotent stem cell lines derived from human somatic cells. Science. 2007;318:1917–20. doi: 10.1126/science.1151526. [DOI] [PubMed] [Google Scholar]
- Jin T, Branch DR, Zhang X, Qi S, Youngson B, et al. Examination of POU homeobox gene expression in human breast cancer cells. Int J Cancer. 1999;81:104–12. doi: 10.1002/(sici)1097-0215(19990331)81:1<104::aid-ijc18>3.0.co;2-q. [DOI] [PubMed] [Google Scholar]
- Monk M, Holding C. Human embryonic genes re-expressed in cancer cells. Oncogene. 2001;20:8085–91. doi: 10.1038/sj.onc.1205088. [DOI] [PubMed] [Google Scholar]
- Gidekel S, Pizov G, Bergman Y, Pikarsky E. Oct-3/4 is a dose-dependent oncogenic fate determinant. Cancer Cell. 2003;4:361–70. doi: 10.1016/s1535-6108(03)00270-8. [DOI] [PubMed] [Google Scholar]
- Ponti D, Costa A, Zaffaroni N, Pratesi G, Petrangolini G, et al. Isolation and in vitro propagation of tumorigenic breast cancer cells with stem/progenitor cell properties. Cancer Res. 2005;65:5506–11. doi: 10.1158/0008-5472.CAN-05-0626. [DOI] [PubMed] [Google Scholar]
- Goodell MA, Brose K, Paradis G, Conner AS, Mulligan RC. Isolation and functional properties of murine hematopoietic stem cells that are replicating in vivo. J Exp Med. 1996;183:1797–806. doi: 10.1084/jem.183.4.1797. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rentala S, Sagar MMB, Khurana S, Mukhopadhyay A. MDR1 gene expression enhances long-term engraftibility of cultured bone marrow cells. Biochem Biophys Res Commun. 2005;335:957–64. doi: 10.1016/j.bbrc.2005.07.167. [DOI] [PubMed] [Google Scholar]
- Sata M, Saiura A, Kunisato A, Tojo A, Okada S, et al. Hematopoietic stem cells differentiate into vascular cells that participate in the pathogenesis of atherosclerosis. Nat Med. 2002;8:403–9. doi: 10.1038/nm0402-403. [DOI] [PubMed] [Google Scholar]
- Levesque JP, Simmons PJ. Cytoskeleton and integrin-mediated adhesion signaling in human CD34− hemopoietic progenitor cells. Exp Hematol. 1999;27:579–86. doi: 10.1016/s0301-472x(98)00069-1. [DOI] [PubMed] [Google Scholar]
- Hirschi K, Goodell M. Common origins of blood and blood vessels in adults. Differentiation. 2001;68:186–92. doi: 10.1046/j.1432-0436.2001.680406.x. [DOI] [PubMed] [Google Scholar]
- Lawson DA, Witte ON. Stem cells in prostate cancer initiation, and progression. J Clin Invest. 2007;117:2044–50. doi: 10.1172/JCI32810. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lang SH, Frame FM, Collins AT. Prostate cancer stem cells. J Pathol. 2009;217:299–306. doi: 10.1002/path.2478. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sagar BM, Rentala S, Gopal PN, Sharma S, Mukhopadhyay A. Fibronectin and laminin enhance engraftibility of cultured hematopoietic stem cells. Biochem Biophys Res Commun. 2006;350:1000–5. doi: 10.1016/j.bbrc.2006.09.140. [DOI] [PubMed] [Google Scholar]
- Tanentzapf G, Devenport D, Godt D, Brown NH. Integrin dependent anchoring of a stem-cell niche. Nat Cell Biol. 2007;9:1413–8. doi: 10.1038/ncb1660. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Robinson EJ, Neal DE, Collins AT. Basal cells are progenitors of luminal cells in primary cultures of differentiating human prostatic epithelium. Prostate. 1998;37:149–60. doi: 10.1002/(sici)1097-0045(19981101)37:3<149::aid-pros4>3.0.co;2-e. [DOI] [PubMed] [Google Scholar]
- Collins AT, Habib FK, Maitland NJ, Neal DE. Identification and isolation of human prostate epithelial stem cells based on alpha(2)beta(1)-integrin expression. J Cell Sci. 2001;114:3865–72. doi: 10.1242/jcs.114.21.3865. [DOI] [PubMed] [Google Scholar]
- Rentala S, Mangamoori LN. Oct-4 expression maintained stem cell properties in prostate cancer derived CD133+MDR1+ cells. Trop J Pharmaceut Res. 2009;8:3–9. [Google Scholar]
- Rentala S, Yalavarthy PD, Mangamoori LN. Stem cell properties of prostate tissue-derived CD133+MDR1+ cells and Oct-4 expression. Asian J Androl 3APFA Proc. 2009;11:56. [Google Scholar]
- Simper D, Stalboerger PG, Panetta CJ, Wang S, Caplice NM. Smooth muscle progenitor cells in human blood. Circulation. 2002;106:1199–204. doi: 10.1161/01.cir.0000031525.61826.a8. [DOI] [PubMed] [Google Scholar]
- Collins AT, Berry PA, Hyde C, Stower MJ, Maitland NJ. Prospective identification of tumorigenic prostate cancer stem cells. Cancer Res. 2005;65:10946–51. doi: 10.1158/0008-5472.CAN-05-2018. [DOI] [PubMed] [Google Scholar]


