Abstract
This study was carried out to determine if exposure to hot environmental temperatures had a direct, detrimental effect on sperm quality. For this the effect of whole-body heat exposure on epididymal spermatozoa of laboratory mice was investigated. C57BL/6 mice (n = 7) were housed in a microclimate chamber at 37°C–38°C for 8 h per day for three consecutive days, while control mice (n = 7) were kept at 23°C–24°C. Cauda epididymal spermatozoa were obtained 16 h after the last heat treatment. The results showed that sperm numbers were similar in the two groups (P = 0.23), but after heat treatment, a significant reduction in the percentage of motile sperm was present (P < 0.0001). Membrane changes of the spermatozoa were investigated by staining with phycoerythrin (PE)-conjugated Annexin V, which detects exteriorization of phosphotidylserine from the inner to the outer leaflet of the sperm plasma membrane, and 7-aminoactinomycin D (7-AAD), which binds to the sperm nucleus when the plasma membrane is damaged. The percentage of spermatozoa showing positive staining with Annexin V–PE or 7-AAD or both, was significantly higher (P < 0.05) in heat-exposed mice compared with controls. These results show that whole-body heat exposure to 37°C–38°C induces membrane changes in the epididymal spermatozoa of mice, which may lead to apoptosis.
Keywords: laboratory mice, sperm membrane damage, whole-body heat
Introduction
In most mammals, testes occur in a scrotum, which has a temperature that is several degrees below that of core body temperature. This lower temperature is brought about by a counter current heat exchange between descending blood in the testicular artery and ascending venous blood in the pampiniform plexus, with the somewhat lower temperature being essential for male germ cell maturation 1. The epididymidis, similar to the testis, is also maintained at a lower temperature than that of core body temperature, and it has been suggested that cooling the epididymidis is the prime mover in the evolution of the scrotum 2. Numerous studies have shown that local heating of the scrotum results in the disruption of spermatogenesis 3, 4. Scrotal heat temperature of 42°C for 30 min also resulted in reduction of epididymal sperm number, viability and motility 5, and an increase in scrotal temperature by 2°C for 16 h a day resulted in fewer embryos in inseminated ewes as early as 4 days of heat treatment 6. The possible effects of whole-body heating on spermatozoa are less clear. There is some suggestive evidence that paternal heat stress of 36°C for 24 h a day may result in abnormalities in early embryo development 7, 8, but these studies did not show any observable changes to the spermatozoa that could have produced such defects.
In ejaculated spermatozoa, studies have shown that temperature changes during freezing and thawing may result in sperm plasma membrane damage 9, 10, whereas heating of spermatozoa to 58°C for 30 min denatures sperm proteins, with an exposure to 100°C resulting in sperm DNA fragmentation 11. Sperm plasmalemma defects, mitochondrial damage and/or DNA fragmentation are all characteristic features of apoptosis 12, 13, but whether whole-body heat exposure produces such adverse effects on the sperm population residing in the epididymis is not known.
The sperm plasmalemma contains phosphatidylserine (PS) and phosphatidylethanolamine in the inner leaflet, whereas sphingomyelin and phosphatidylcholine are present on the outer leaflet 14. Translocation of PS from the inner to the outer leaflet takes place in early apoptosis 15 as well as during sperm capacitation 16 and after incubation with bicarbonate 17. Damaged membranes owing to temperature effects have been shown to bring about impaired sperm motility 18, but whether in vivo heat exposure induces changes in the membrane phospholipid organization that results in subsequent reduced sperm viability appears to be unknown.
The hypothesis that we tested in the current study is that whole-body heating results in changes in membrane phospholipid organization of cauda epididymal spermatozoa. For this study, we exposed mice to a temperature of 37°C–38°C for 8 h per day for 3 consecutive days. This temperature was selected because body temperature of laboratory mice varies between 35°C and 36°C, and to simulate naturally occurring hot environmental conditions, we selected a temperature of 1°C–2°C higher than that of the core body temperature of mice. We chose to expose mice to these high temperatures for 8-h time periods to simulate the approximate period of time the mice would be exposed to high temperature during the day. There are also reports that exposure of mice to 36°C for a 24-h period increases the body temperature by ∼1°C–2°C within 8 h, with an increase in testicular temperature by 3°C 7. Thus in the present study, to ascertain whether short-term increase in body temperature can result in sperm membrane damage, we exposed mice to an elevated temperature of 37°C–38°C for 8 h for 3 consecutive days.
Materials and methods
Animals
For this study, 2- to 3-month-old, C57BL/6 male mice (n = 14) were used. Experimental animals (n = 7) were placed in a microclimate chamber maintained at 37°C–38°C for 8 h per day for 3 consecutive days, while controls (n = 7) were left at 23°C–24°C. Animals had access to food and water at all time periods, and the procedures were approved by the Animal Ethics Committee of the University of Adelaide, Australia (M/39/07).
Collection of spermatozoa
Mice were killed by an intraperitoneal injection of pentobarbitone, 16 h after the end of the last heat exposure. One cauda epididymidis was removed from each animal from control and heat-treated groups, then blotted on filter paper and placed in equal volume of 500 μL prewarmed modified Biggers, Whitten and Whittingham (BWW) medium 19. Spermatozoa were extruded by retrograde perfusion of BWW into the vas deferens after making a small incision in the cauda epididymidis 20. They were allowed to disperse into the medium in an incubator, maintained at 37°C with 5% CO2 in air, for 10 min.
Assessment of sperm count and motility
Number of sperm extruded from the cauda epididymidis was determined using a Neubauer hemocytometer by the formula:
Sperm number = (dilution factor) × (sperm count in five squares) × 0.05 × 106
All spermatozoa with noticeable tail movements were recorded as motile and the percentage of motile spermatozoa was calculated as
Number of motile spermatozoa in 25 squares × 100/total number of spermatozoa in 25 squares.
Annexin V-phycoerythrin (PE) and 7-aminoactinomycin D (7-AAD) staining
Dual fluorescent staining of spermatozoa with Annexin V–phycoerythrin (PE) and 7-AAD (ApoScreen Annexin V Apoptosis Kit-PE, Beckman Coulter, Fullerton, CA, USA) for determination of changes in cell membrane PS was carried out as follows: spermatozoa were diluted in warm modified BWW medium to a concentration of ∼10 × 106 sperm per mL. A volume of 50 μL of diluted sperm suspension was stained with 10 μL of Annexin V–PE and/or 7-AAD. The samples were incubated in darkness at room temperature (RT) for 15 min and then 500 μL of modified BWW was added before carrying out flow cytometry. Annexin V-positive controls were prepared by treating spermatozoa with dimethyl sulfoxide (Me2SO, 1:1 v/v) for 10 min at RT. The 7-AAD-positive controls were prepared by mixing sperm suspensions with 100% ethanol (1:1 v/v) for 10 min on ice to induce DNA and membrane damage. Both Me2SO- and ethanol-treated sperm were washed twice and resuspended in 50 μL of BWW. Unstained cells were used as negative controls.
Flow cytometry
Dual color flow cytometry was carried out with an EPICS-PROFILE II XL (Coulter, Miami, FL, USA), and data were analyzed using CXP software (version 2.2; Applied Cytometry System, Sheffield, UK). For each sample from control (Figure 1F) and heat treated (Figure 1G) mice, a minimum of 10 000 spermatozoa were assessed. Gates were set for forward and side scatter with unstained sperm, to exclude debris from the analysis. The intensity of staining with Annexin V–PE and 7-AAD was determined for spermatozoa in gate 'A', control (Figure 1A), and heat-treated (Figure 1B) mice. Membrane changes were assessed by staining with Annexin V–PE and 7-AAD. The fluorochromes were excited with an argon ion laser emission at 488 nm and fluorescent detection was carried out with FL2 (576/26 nm) and FL4 (695/40 nm) filters, respectively. Unstained spermatozoa were first run to set negative controls (Figure 1C), following which positive controls, treated with Me2SO and stained with Annexin V–PE, were analyzed to set the gates for Annexin V–PE staining (Figure 1D). To avoid Annexin V–PE spill over into FL4, compensation was set. Subsequently, positive controls treated with 100% ethanol and stained with 7-AAD were analyzed to set gates for 7-AAD staining (Figure 1E). Thus, four different subpopulations of spermatozoa were defined in control (Figure 1F) and heat treated mice (Figure 1G), on the basis of the classification by Pena et al. 21. The D3 quadrant (Annexin V−/7-AAD−) contained live spermatozoa that did not show fluorescence, whereas the D4 quadrant contained spermatozoa stained with Annexin V–PE but not with 7-AAD (Annexin V+/7-AAD−), representing early apoptotic spermatozoa in which the DNA did not stain but exteriorization of the PS on the plasma membrane had taken place. Spermatozoa in the D2 quadrant (Annexin V+/7-AAD+) had both exteriorized PS and DNA staining, which may reflect late stages of apoptosis. Those in D1 quadrant (Annexin V−/7-AAD+) had DNA staining with no detectable PS exteriorization and were assumed to be dead.
Figure 1.
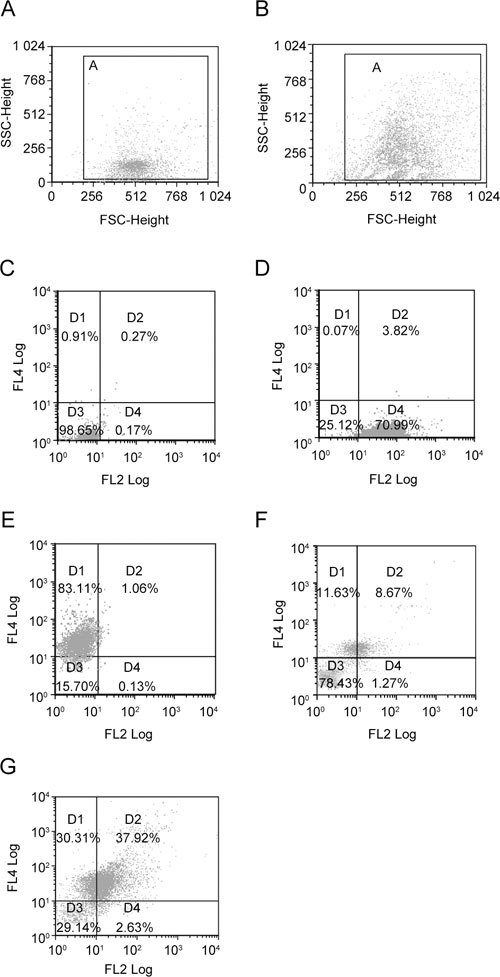
Representative forward (FSC) and side scatter (SSC) dot plots of unstained spermatozoa from control (A) and heat-treated (B) mice. Gate 'A' in dot plots was selected to exclude debris before flow cytometric analysis. In control (A), spermatozoa were mostly concentrated in the lower region of gate 'A', whereas in heat-treated group (B), an upward shift is apparent. Panels C–E show the quadrant limits set with unstained (C), Annexin V–PE-positive (D) and 7-AAD-positive (E) controls. Panels F and G show four different subpopulations of stained spermatozoa in four different quadrants (D1–D4) in control (F) and heat-treated (G) groups. D1, Annexin V−/7-AAD+ (dead spermatozoa); D2, Annexin V+/7-AAD+ (late apoptotic spermatozoa); D3, Annexin V−/7-AAD− (live spermatozoa); and D4, Annexin V+/7-AAD− (early apoptotic spermatozoa). The percentages shown in the quadrants represent values from a single control and heat-treated mouse.
Mean intensity of fluorescence (MIF) of Annexin V–PE and 7-AAD staining
A shift in the MIF of spermatozoa stained with Annexin V–PE and 7-AAD from heat-treated (n = 7) and control (n = 7) groups was also evaluated with the aid of overlay plots in CXP software (version 2.2; Applied Cytometry System). Unstained spermatozoa were analyzed to set peaks for negative controls (black peak, Figure 2), following which the shift in MIF of Annexin V–PE-positive spermatozoa from control (red line, Figure 2A) and heat-treated (blue peak, Figure 2A) groups was evaluated. Similar shifts in MIF of 7-AAD-stained spermatozoa from control (green line, Figure 2B) and heat-treated (red peak, Figure 2B) groups were also analyzed. The values of MIF were obtained from the CXP software.
Figure 2.
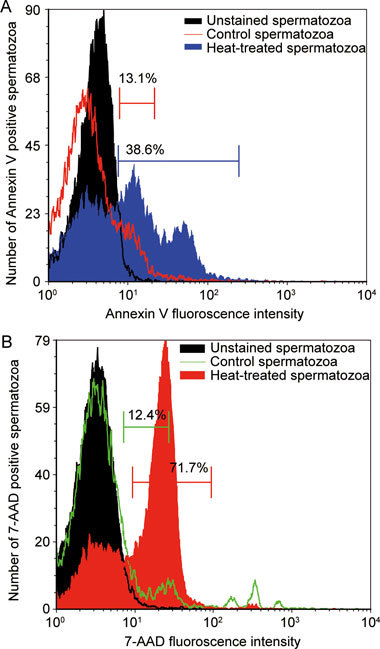
Representative overlay plots showing changes in mean intensity of fluorescence (MIF) in control and heat-treated groups after staining with Annexin V–PE and 7-AAD. In panels A and B, negative controls are shown in black. Panel A shows a positive shift in the MIF of Annexin V–PE staining in heat-treated (blue) compared with control (red line) mice. Similarly, panel B shows a positive shift in the MIF of 7-AAD staining in heat-treated (red) compared with control (green line) mice. The percentages shown in the overlay plots represent values from a single animal from control and heat-treated groups.
Fluorescence microscopy
The sperm suspensions stained with Annexin V–PE and 7-AAD from control and heat-treated groups were used for fluorescence microscopy. For this, sperm were washed twice with phosphate-buffered saline, smeared on slides and examined by fluorescent microscopy with images captured at × 100 magnification using Olympus BX 51 microscope coupled with a CCD camera (Olympus, Tokyo, Japan).
Ultrastructure of Annexin V–PE and 7-AAD-stained spermatozoa
Spermatozoa from control and heat-treated groups were separated by a cell sorter (BDFACS Aria, San Jose, CA, USA) into two subpopulations, Annexin V–PE+/7-AAD+ and Annexin V–PE−/7-AAD−. Sperm pellets (∼4 000 spermatozoa) from each subpopulation from heat-treated and control animals were fixed in 4% paraformaldehyde and 3% glutaraldehyde, made up in 0.1 mol L-1 phosphate buffer (pH 7.4) and processed for transmission electron microscopy (TEM) for morphological studies.
Statistical analysis
Data from control and heat-treated groups were compared by unpaired t-test using Graph Pad Prism software (version 5.01; Aberystwyth, Wales, UK) and presented as mean ± SEM, with level of statistical significance set at P < 0.05.
Results
Sperm numbers and motility
There were no significant differences in numbers of caudal epididymal sperm between control and experimental animals (mean ± SEM, 29.0 × 106 ± 4.3 × 106 vs. 24.8 × 106 ± 3.1 × 106; P = 0.23). The percentage of motile spermatozoa, however, was significantly reduced in heat-treated compared with the control group (mean ± SEM, 85.0% ± 2.6% vs. 42.5% ± 1.5%; P < 0.0001).
Subpopulations of spermatozoa
Flow cytometry showed that exposure to heat significantly increased the percentage of spermatozoa stained with either Annexin V–PE (P = 0.015) or 7-AAD alone (P < 0.001) or both Annexin V–PE and 7-AAD (P < 0.001) (D4, D1 and D2, respectively, Figure 3).
Figure 3.
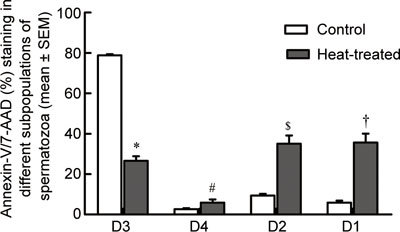
Histograms showing percentage of Annexin V–PE and 7-AAD-stained cells in different subpopulations of spermatozoa in control (n = 7) and heat-treated (n = 7) mice. There were significantly reduced percentages of live spermatozoa (D3) in heat-treated mice compared with controls (mean ± SEM, 26.6% ± 2.2% vs. 78.8% ± 0.6%; *P < 0.0001) and a significant increase in percentage of Annexin V–PE-positive (5.8% ± 1.6% vs. 2.6% ± 0.4%; #P = 0.015; D4), Annexin V–PE and 7-AAD-positive (35.0% ± 4.1% vs. 9.4% ± 0.8%; $P < 0.001; D2) and Annexin V–PE negative and 7-AAD-positive spermatozoa (35.7% ± 4.4% vs. 5.9% ± 0.9%; †P < 0.001; D1).
A noticeable positive shift in the MIF of spermatozoa stained with Annexin V–PE and 7-AAD from heat-treated compared with control groups was estimated with the overlay plots. In Annexin V–PE-stained spermatozoa from heat-treated groups, the MIF was significantly higher compared with control mice (mean ± SEM, 1.50% ± 0.15% vs. 0.57% ± 0.07%; P = 0.0001). Similarly, in 7-AAD-stained spermatozoa from heat-treated group the MIF was significantly higher in heat-treated compared with control groups (mean ± SEM, 2.12% ± 0.11% vs. 0.86% ± 0.20%; P = 0.0002).
The percentage of spermatozoa showing staining with Annexin V–PE was significantly higher in heat-exposed mice compared with controls (mean ± SEM, 37.7% ± 4.3% vs. 15.4% ± 1.2%; P = 0.002). In addition, the percentage of 7-AAD-positive spermatozoa was significantly higher in heat-exposed mice compared with controls (mean ± SEM, 70.5% ± 2.4% vs. 14.8% ± 1.5%; P = 0.0001).
Structure of the Annexin V–PE and 7-AAD-stained spermatozoa
Spermatozoa stained with Annexin V–PE and 7-AAD did not appear to differ in their ultrastructural morphology, as observed by TEM, compared with the unstained controls. The sperm nuclei invariably had condensed chromatin; they also had an intact acrosome, subacrosomal space, plasma membrane and sperm tail. Flourescence microscopy of stained sperm from control and heat-treated groups showed speckled staining of PS by Annexin V–PE on the plasma membrane of head and tail regions, whereas 7-AAD stained the sperm nuclei (Figure 4A) indicating a dead population of spermatozoa. Speckled staining of PS on the plasma membrane of sperm head and tail suggests early apoptosis (Figures 4B and C), whereas sperm stained with both Annexin V–PE and 7-AAD were probably in late apoptosis (Figure 4D). In the figure, Annexin V–PE staining is only evident on the sperm tail. Staining of PS on the head region is not clear because of similar color emissions from Annexin V–PE and 7-AAD.
Figure 4.
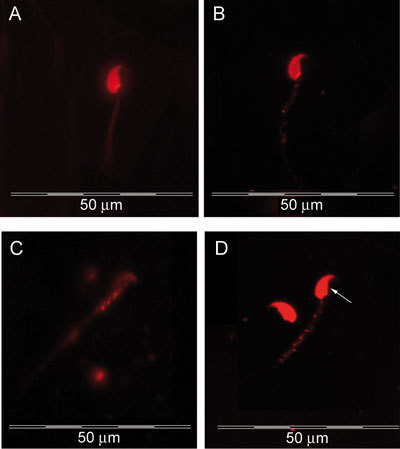
Fluorescent images of laboratory mouse spermatozoa stained with Annexin V–PE and 7-AAD. (A): Nucleus stained with 7-AAD represents a dead spermatozoon; (B): speckled staining of phosphatidylserine on the plasma membrane of sperm head and tail (C) in an early apoptotic spermatozoon; (D): staining with both Annexin V–PE (speckled staining in tail region) and 7-AAD (→ stained sperm nucleus) represents a late apoptotic spermatozoon.
Discussion
The results of this study indicate that, when male mice are exposed to 37°C–38°C for 8 h per day for 3 consecutive days and examined 16 h after the last heat exposure, there is impaired sperm motility and an increased number of spermatozoa with plasma membrane changes within the cauda epididymidis.
As it takes, in laboratory mice, about 7 days for the sperm to migrate from caput to cauda epididymidis 22, the population of spermatozoa affected by heat exposure would have been already present within either the corpus or cauda epididymidis at the time of heat exposure, as the first exposure to heat took place only 3 days before the retrieval of sperm from the cauda.
In an earlier study, 3 days after exposure to 36°C for two 12-h periods, no changes in sperm motility was found to occur, whereas changes were observed 14 days after heat exposure 23. This suggested that testicular germ cells are likely to have been the source of deleterious effects of heat on sperm. In contrast, in the present study, sperm with impaired motility and apoptotic-like changes were found 96 h after the start and 16 h after end of the heat treatment, suggesting direct affects of heating on the extratesticular sperm population. This decrease in motility could have been due to either the high temperature having a direct effect on the spermatozoa and/or an adverse effect of altered epididymal environment in which the sperm reside. This could have come about by either an influx of free radicals producing cellular damage 24 and/or the sperm mitochondria producing reactive oxygen species (ROS), with an increase in ROS reducing the percentage of motile sperm 25. It is also possible that deep body temperature could have altered the ionic and protein composition of caudal epididymal fluid as a result of changes in the secretory and absorptive behavior of the surrounding epithelium 26, 27. Future studies are required to tease out these various options.
Membrane changes, as evident from PS translocation from inner to outer leaflet of plasma membrane, DNA fragmentation 28, defects in mitochondria 29 and activation of caspases 30, are all characteristic features of apoptosis. In the present study, in the sperm of heat-treated mice there was an increase in Annexin V–PE-positive spermatozoa (Annexin V+/7-AAD−), suggestive of a temperature-dependent exteriorization of the PS. These findings are similar to those of several other studies in which temperature effects on PS translocation in the sperm plasma membrane have been found to occur 31, 32, but the previous studies were mostly related to sperm plasma membrane damage caused during freezing and thawing.
The population of spermatozoa stained with Annexin V+/7-AAD− may either be in early apoptosis 15, 33 or have undergone premature capacitation and/or the acrosome reaction 16. Heat-induced premature capacitation has been found to occur in cryptepididymal spermatozoa 34, but, in the current study, the possibility of capacitation and/or acrosomal reaction bringing about these changes is unlikely, as fluorescence microscopy showed the presence of PS exteriorization over both the sperm head and tail plasma membrane and not just over the apical head plasma membrane as occurs during capacitation 17, 35. The exteriorization of PS in this sperm population is thus highly suggestive of early apoptotic membrane damage due to heating.
A previous study has shown DNA fragmentation in apoptotic mouse spermatozoa after local heat treatment to the scrotum 5. In addition, apoptotic nonviable human sperm subpopulations have been shown to have DNA fragmentation by TUNEL assay 36. However, positivity with TUNEL does not differentiate apoptosis from other forms of cellular damage such as necrosis and autolytic cell death 37. It has previously been suggested that ultrastructural studies need to be performed for documentation of apoptosis 38, although TEM studies have shown that TUNEL-positive spermatozoa may not have DNA fragmentation 39. Similarly, in the current study, we found that the mouse spermatozoa stained positively with Annexin V–PE and 7-AAD had normal nuclear morphology under TEM. This supports the view that sperm DNA fragmentation, determined by apoptotic markers, may not be reflected in chromatin ultrastructure. Clearly, further study needs to be performed to conclusively show that the results are indicative of apoptosis.
The absence of any significant difference in the sperm counts between heat-treated and control mice differs from our earlier study on the effects of heating on spermatozoa 23. This is likely to be because of the time difference in obtaining the sperm samples for counting after heating. Previous counts were performed as late as 3–14 days after the last heat treatment, whereas in the present study, sperm counts were estimated 16 h after the last heat treatment. Therefore, absence of any change in the sperm numbers, in spite of increased numbers of heat-affected spermatozoa, could be explained by the length of time it takes for these spermatozoa to be removed from the cauda epididymidis. It has been estimated that it takes days to weeks to remove nonviable spermatozoa from the cauda epididymidis because of their resistance to degradation 40. In contrast, heat stress has been shown to reduce the length and diameter of the epididymis, thereby decreasing its storage capacity and consequently the sperm number stored within it 26. Future studies on epididymal morphology, may throw some light on sperm count defects after whole-body heat treatment.
In conclusion, the present investigation has shown significant increases in PS exteriorization and membrane permeability of mouse epididymal spermatozoa after whole-body heat exposure. Our observations indicate that, after whole-body heat exposure, damage occurs to at least some of the epididymal spermatozoa population. These findings support the suggestion that, in humans, there could be adverse effects of exposure to high body temperature on male fertility.
Acknowledgments
We are grateful to Dr Devendra Hiwase and Dr Smita Hiwase, Division of Hematology, Royal Adelaide Hospital, Adelaide, South Australia for their help in standardizing the flow cytometry techniques and for assistance in the analysis of the data and Mr Alan Bishop for technical assistance in flow cytometry. We also thank Dr Ravinder Anand Ivell for assistance with media preparation and Mr Tavik Morgenstern of the School of Medical Sciences, University of Adelaide in the production of images. We also would like to express our extreme gratitude to the reviewers for a number of very constructive and useful comments, the inclusion of which has markedly improved the quality of this paper. Finally, we thank the University of Adelaide for financial assistance.
References
- Setchell BP, Breed WG.Anatomy, vasculature and innervation of the male reproductive tractIn: Neill JD, editor. Physiology of Reproduction. Amsterdam: Elsevier; 2006p771–825.
- Bedford JM. Anatomical evidence for the epididymis as the prime mover in the evolution of the scrotum. Am J Anat. 1978;152:483–507. doi: 10.1002/aja.1001520404. [DOI] [PubMed] [Google Scholar]
- Setchell BP. The parkes lecture. Heat and the testis. J Reprod Fertil. 1998;114:179–94. doi: 10.1530/jrf.0.1140179. [DOI] [PubMed] [Google Scholar]
- Setchell BP. The effects of heat on the testes of mammals. Anim Reprod. 2006;3:81–91. [Google Scholar]
- Perez-Crespo M, Pintado B, Gutierrez-Adan A. Scrotal heat stress effects on sperm viability, sperm DNA integrity, and the offspring sex ratio in mice. Mol Reprod Dev. 2008;75:40–7. doi: 10.1002/mrd.20759. [DOI] [PubMed] [Google Scholar]
- Mieusset R, Quintana Casares P, Sanchez Partida LG, Sowerbutts SF, Zupp JL, et al. Effects of heating the testes and epididymides of rams by scrotal insulation on fertility and embryonic mortality in ewes inseminated with frozen semen. J Reprod Fertil. 1992;94:337–43. doi: 10.1530/jrf.0.0940337. [DOI] [PubMed] [Google Scholar]
- Zhu B, Walker SK, Oakey H, Setchell BP, Maddocks S. Effect of paternal heat stress on the development in vitro of preimplantation embryos in the mouse. Andrologia. 2004;36:384–94. doi: 10.1111/j.1439-0272.2004.00635.x. [DOI] [PubMed] [Google Scholar]
- Zhu BK, Setchell BP. Effects of paternal heat stress on the in vivo development of preimplantation embryos in the mouse. Reprod Nutr Dev. 2004;44:617–29. doi: 10.1051/rnd:2004064. [DOI] [PubMed] [Google Scholar]
- Guthrie HD, Welch GR. Impact of storage prior to cryopreservation on plasma membrane function and fertility of boar sperm. Theriogenology. 2005;63:396–410. doi: 10.1016/j.theriogenology.2004.09.020. [DOI] [PubMed] [Google Scholar]
- Holt WV, North RD. Effects of temperature and restoration of osmotic equilibrium during thawing on the induction of plasma membrane damage in cryopreserved ram spermatozoa. Biol Reprod. 1994;51:414–24. doi: 10.1095/biolreprod51.3.414. [DOI] [PubMed] [Google Scholar]
- Jiang MX, Zhu Y, Zhu ZY, Sun QY, Chen DY. Effects of cooling, cryopreservation and heating on sperm proteins, nuclear DNA, and fertilization capability in mouse. Mol Reprod Dev. 2005;72:129–34. doi: 10.1002/mrd.20328. [DOI] [PubMed] [Google Scholar]
- Gorczyca W, Traganos F, Jesionowska H, Darzynkiewicz Z. Presence of DNA strand breaks and increased sensitivity of DNA in situ to denaturation in abnormal human sperm cells: analogy to apoptosis of somatic cells. Exp Cell Res. 1993;207:202–5. doi: 10.1006/excr.1993.1182. [DOI] [PubMed] [Google Scholar]
- Barroso G, Taylor S, Morshedi M, Manzur F, Gavino F, et al. Mitochondrial membrane potential integrity and plasma membrane translocation of phosphatidylserine as early apoptotic markers: a comparison of two different sperm subpopulations. Fertil Steril. 2006;85:149–54. doi: 10.1016/j.fertnstert.2005.06.046. [DOI] [PubMed] [Google Scholar]
- Vermes I, Haanen C, Steffens-Nakken H, Reutelingsperger C. A novel assay for apoptosis. Flow cytometric detection of phosphatidylserine expression on early apoptotic cells using fluorescein labelled annexin v. J Immunol Methods. 1995;184:39–51. doi: 10.1016/0022-1759(95)00072-i. [DOI] [PubMed] [Google Scholar]
- Martin SJ, Reutelingsperger CP, McGahon AJ, Rader JA, van Schie RC, et al. Early redistribution of plasma membrane phosphatidylserine is a general feature of apoptosis regardless of the initiating stimulus: inhibition by overexpression of bcl-2 and abl. J Exp Med. 1995;182:1545–56. doi: 10.1084/jem.182.5.1545. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kurz A, Viertel D, Herrmann A, Muller K. Localization of phosphatidylserine in boar sperm cell membranes during capacitation and acrosome reaction. Reproduction. 2005;130:615–26. doi: 10.1530/rep.1.00561. [DOI] [PubMed] [Google Scholar]
- Gadella BM, Harrison RA. Capacitation induces cyclic adenosine 3',5'-monophosphate-dependent, but apoptosis-unrelated, exposure of aminophospholipids at the apical head plasma membrane of boar sperm cells. Biol Reprod. 2002;67:340–50. doi: 10.1095/biolreprod67.1.340. [DOI] [PubMed] [Google Scholar]
- Khan DR, Ahmad N, Anzar M, Channa AA. Apoptosis in fresh and cryopreserved buffalo sperm. Theriogenology. 2009;71:872–6. doi: 10.1016/j.theriogenology.2008.09.056. [DOI] [PubMed] [Google Scholar]
- Biggers J, Whitten W, Whittingham D.The culture of mouse embryos in vitroIn: Daniel JJ, editor. Methods in Mammalian Embryology. San Francisco: Freeman Press; 1971p86–116.
- Walsh A, Whelan D, Bielanowicz A, Skinner B, Aitken RJ, et al. Identification of the molecular chaperone, heat shock protein 1 (chaperonin 10), in the reproductive tract and in capacitating spermatozoa in the male mouse. Biol Reprod. 2008;78:983–93. doi: 10.1095/biolreprod.107.066860. [DOI] [PubMed] [Google Scholar]
- Pena FJ, Johannisson A, Wallgren M, Rodriguez-Martinez H. Assessment of fresh and frozen-thawed boar semen using an annexin-v assay: a new method of evaluating sperm membrane integrity. Theriogenology. 2003;60:677–89. doi: 10.1016/s0093-691x(03)00081-5. [DOI] [PubMed] [Google Scholar]
- Cornwall GA, Vreeburg JT, Holland MK, Orgebin-Crist MC. Interactions of labeled epididymal secretory proteins with spermatozoa after injection of 35s-methionine in the mouse. Biol Reprod. 1990;43:121–9. doi: 10.1095/biolreprod43.1.121. [DOI] [PubMed] [Google Scholar]
- Yaeram J, Setchell BP, Maddocks S. Effect of heat stress on the fertility of male mice in vivo and in vitro. Reprod Fertil Dev. 2006;18:647–53. doi: 10.1071/rd05022. [DOI] [PubMed] [Google Scholar]
- Flanagan SW, Moseley PL, Buettner GR. Increased flux of free radicals in cells subjected to hyperthermia: detection by electron paramagnetic resonance spin trapping. FEBS Lett. 1998;431:285–6. doi: 10.1016/s0014-5793(98)00779-0. [DOI] [PubMed] [Google Scholar]
- Koppers AJ, De Iuliis GN, Finnie JM, McLaughlin EA, Aitken RJ. Significance of mitochondrial reactive oxygen species in the generation of oxidative stress in spermatozoa. J Clin Endocrinol Metab. 2008;93:3199–207. doi: 10.1210/jc.2007-2616. [DOI] [PubMed] [Google Scholar]
- Bedford JM. Effects of elevated temperature on the epididymis and testis: experimental studies. Adv Exp Med Biol. 1991;286:19–32. doi: 10.1007/978-1-4684-5913-5_3. [DOI] [PubMed] [Google Scholar]
- Esponda P, Bedford JM. The influence of body temperature and castration on the protein composition of fluid in the rat cauda epididymidis. J Reprod Fertil. 1986;78:505–14. doi: 10.1530/jrf.0.0780505. [DOI] [PubMed] [Google Scholar]
- Anzar M, He L, Buhr MM, Kroetsch TG, Pauls KP. Sperm apoptosis in fresh and cryopreserved bull semen detected by flow cytometry and its relationship with fertility. Biol Reprod. 2002;66:354–60. doi: 10.1095/biolreprod66.2.354. [DOI] [PubMed] [Google Scholar]
- Marchetti C, Obert G, Deffosez A, Formstecher P, Marchetti P. Study of mitochondrial membrane potential, reactive oxygen species, DNA fragmentation and cell viability by flow cytometry in human sperm. Hum Reprod. 2002;17:1257–65. doi: 10.1093/humrep/17.5.1257. [DOI] [PubMed] [Google Scholar]
- Paasch U, Grunewald S, Fitzl G, Glander HJ. Deterioration of plasma membrane is associated with activated caspases in human spermatozoa. J Androl. 2003;24:246–52. doi: 10.1002/j.1939-4640.2003.tb02669.x. [DOI] [PubMed] [Google Scholar]
- Woelders H. Fundamentals and recent development in cryopreservation of bull and boar semen. Vet Q. 1997;19:135–8. doi: 10.1080/01652176.1997.9694758. [DOI] [PubMed] [Google Scholar]
- Sion B, Janny L, Boucher D, Grizard G. Annexin v binding to plasma membrane predicts the quality of human cryopreserved spermatozoa. Int J Androl. 2004;27:108–14. doi: 10.1046/j.1365-2605.2003.00457.x. [DOI] [PubMed] [Google Scholar]
- Muratori M, Porazzi I, Luconi M, Marchiani S, Forti G, et al. Annexinv binding and merocyanine staining fail to detect human sperm capacitation. J Androl. 2004;25:797–810. doi: 10.1002/j.1939-4640.2004.tb02858.x. [DOI] [PubMed] [Google Scholar]
- Bedford JM, Yanagimachi R. Epididymal storage at abdominal temperature reduces the time required for capacitation of hamster spermatozoa. J Reprod Fertil. 1991;91:403–10. doi: 10.1530/jrf.0.0910403. [DOI] [PubMed] [Google Scholar]
- de Vries KJ, Wiedmer T, Sims PJ, Gadella BM. Caspase-independent exposure of aminophospholipids and tyrosine phosphorylation in bicarbonate responsive human sperm cells. Biol Reprod. 2003;68:2122–34. doi: 10.1095/biolreprod.102.012500. [DOI] [PubMed] [Google Scholar]
- Zhang HB, Lu SM, Ma CY, Wang L, Li X, et al. Early apoptotic changes in human spermatozoa and their relationships with conventional semen parameters and sperm DNA fragmentation. Asian J Androl. 2008;10:227–35. doi: 10.1111/j.1745-7262.2008.00295.x. [DOI] [PubMed] [Google Scholar]
- Grasl-Kraupp B, Ruttkay-Nedecky B, Koudelka H, Bukowska K, Bursch W, et al. In situ detection of fragmented DNA (tunel assay) fails to discriminate among apoptosis, necrosis, and autolytic cell death: a cautionary note. Hepatology. 1995;21:1465–8. doi: 10.1002/hep.1840210534. [DOI] [PubMed] [Google Scholar]
- Baccetti B, Collodel G, Piomboni P. Apoptosis in human ejaculated sperm cells (notulae seminologicae 9) J Submicrosc Cytol Pathol. 1996;28:587–96. [PubMed] [Google Scholar]
- Muratori M, Piomboni P, Baldi E, Filimberti E, Pecchioli P, et al. Functional and ultrastructural features of DNA-fragmented human sperm. J Androl. 2000;21:903–12. [PubMed] [Google Scholar]
- Jones R. Sperm survival versus degradation in the mammalian epididymis: a hypothesis. Biol Reprod. 2004;71:1405–11. doi: 10.1095/biolreprod.104.031252. [DOI] [PubMed] [Google Scholar]


