Abstract
For centuries, plants and plant-based products have been used as a valuable and safe natural source of medicines for treating various ailments. The therapeutic potential of most of these plants could be ascribed to their anticancer, antidiabetic, hepatoprotective, cardioprotective, antispasmodic, analgesic and various other pharmacological properties. However, several commonly used plants have been reported to adversely affect male reproductive functions in wildlife and humans. The effects observed with most of the plant and plant-based products have been attributed to the antispermatogenic and/or antisteroidogenic properties of one or more active ingredients. This review discusses the detrimental effects of some of the commonly used plants on various target cells in the testis. A deeper insight into the molecular mechanisms of action of these natural compounds could pave the way for developing therapeutic strategies against their toxicity.
Keywords: male reproduction, natural products, plants, spermatogenesis, steroidogenesis, testis
Introduction
Male reproduction is a multifaceted process that involves the testes, epididymis, accessory sex glands and associated hormones. Testes perform two highly organized and intricate events, called spermatogenesis and steroidogenesis, which are vital for the perpetuation of life. Spermatogenesis, a highly dynamic and synchronized process, takes place within the seminiferous tubules of the testis with the support of somatic Sertoli cells, leading to the formation of mature spermatozoa from undifferentiated stem cells 1. The interstitial compartment, which comprises Leydig cells, are the site of steroidogenesis 2. Subsequent to the process of spermatogenesis, spermatozoa transits from the testis to the ejaculatory ducts, undergoing a sequence of modifications that results in the accomplishment of its ability to move, capacitate and to interact with zona pellucida of the female ovum 3. This complex process is strictly regulated by the hypothalamo–pituitary–testicular axis, which involves the pituitary gonadotropins, luteinizing hormone (LH) and follicle-stimulating hormone (FSH). Apart from LH, FSH and androgens, various growth factors, hormones and estrogens are involved in regulating the testicular functions.
The testis is well equipped with powerful intrinsic defense systems that protect the spermatozoa during its spermatogenic/post-spermatogenic journey and from the injuries caused by other intrinsic or extrinsic factors. Nevertheless, the testis is one of the organs that are very vulnerable to assault, which is reflected by the adverse trend in male reproductive health during the past several years. The male reproductive system is extremely sensitive to various environmental factors such as life style, drugs, radiation, pollution and toxicants, the result of which could be congenital abnormalities in infants and functional alterations in adults 4. Several natural and synthetic products are reported to target the testis at the hormonal level or spermatogenesis or both. In this review, we discuss on some of the commonly used plant products that could hamper the functionality of the testis, thereby leading to infertility.
Plants that impair testicular functions
Plants, since ancient times, have been used globally across varied cultures throughout the known civilizations as a valuable and safe natural source of medicines and as agents of therapeutic, industrial and environmental utilities. From the inception of civilization, humans have relied on plants that could meet their basic necessities such as food, shelter, clothing, fuel and health. Of all the uses ascribed to the plants, their curative abilities played an inevitable part in the lives of primitive societies, as plants comprised their sole source for healing ailments. The sacred knowledge about the healing powers of plants was initially passed down orally through generations, and as civilizations grew written records were prepared for the benefit of the population 5. The Indian and Chinese systems of traditional medicine are well established, with written records dating back to thousand of years 6. A wide majority of herbal plants possess pharmacological principles, which has rendered them useful as curatives for numerous ailments. According to the World Health Organization (WHO) reports, 70%–80% of the world population confide in traditional medicine for primary healthcare 7.
Plants have a long folklore of use in aiding fertility. The Indian sacred text, Rig Veda, describes a 'holy brew' called soma, the intake of which is believed to bestow upon humans infinite powers, including aphrodisiacal qualities 8. The medical historians have recorded plants that could be used as contraceptives, emmenagogues and abortifacients 5. The safety of many of the herbal drugs is only relative, but the population feels more assured because of their long and widespread usage and their familiarity with plants. Plants are known to heal as well as hurt. Several plants are reported to enhance reproductive processes but, on the other hand, to also hinder testicular functions. The effects of plants on testicular functions could be rightly compared with those of a double-edged sword. This review is confined to the toxicological attributes of some of the commonly used plants to the functions of testis.
Plants that affect spermatogenesis
Spermatogenesis is a complex process by which an interdependent population of undifferentiated germ cells undergoes multiplication and maturation to form functional haploid spermatozoa. Spermatogenesis consists of three phases: (a) the spermatogonial phase; (b) the spermatocyte phase; and (c) the spermatid phase. During the spermatogonial phase, the diploid spermatogonium undergoes mitosis to form stem cells and primary spermatocytes. This is followed by the spermatocyte phase, in which the primary spermatocytes undergo two rounds of meiosis to form haploid spermatids. The final phase, also called spermiogenesis, involves the differentiation of immature spermatids into mature spermatozoa. Spermiogenesis comprises polarization of the spermatid, formation of acrosomal cap and flagellum, cytoplasmic remodeling and elongation of the nucleus. Endocrine regulation by testosterone and the architecture of the Sertoli cells and seminiferous tubules also forms an important decisive factor in spermatogenesis 9.
Several plants and plant products are reported to impede various stages of testicular spermatogenesis in many different animal species such as dogs, rats, humans and monkeys 10, 11, 12 (Figure 1). Cannabinoids, one of the oldest narcotic drugs of plant origin, are known to impair human health since olden times 13. Intraperitoneal injection of mice with low doses of Cannabis extracts (40 mg, 60 mg and 80 mg) was reported to induce increased lipid peroxidation in the testis, along with concomitant decrease in the levels of antioxidant enzymes such as superoxide dismutase, catalase and glutathione peroxidase. Damage to the basement membrane, shrinkage of the seminiferous tubules, scanty cytoplasm and shrunken nuclei in the germinal epithelium, and complete arrest of spermatogenesis were also reported. The effects were seen to be reversed on withdrawal of the drug for 45 days, and it was speculated that the endogenous antioxidant system along with the FSH/LH feedback loop was responsible for the observed protective effects on withdrawal 14. A pharmacokinetic study on the plasma, brain and testis of rats was conducted, which measured the concentration of tetrahydrocannabinol in these tissues following exposure to C14Δ-8-labeled tetrahydrocannabinol. Impairment of spermatogenesis before the stage of meiosis and premature apoptosis along with structural and functional anomalies of sperm cells were reported. It was hypothesized that tetrahydrocannabinol, a lipid molecular signal, desynchronizes the membrane signaling and induces a death signal in the lipid bilayer, leading to early apoptosis in sperm cells 15.
Figure 1.
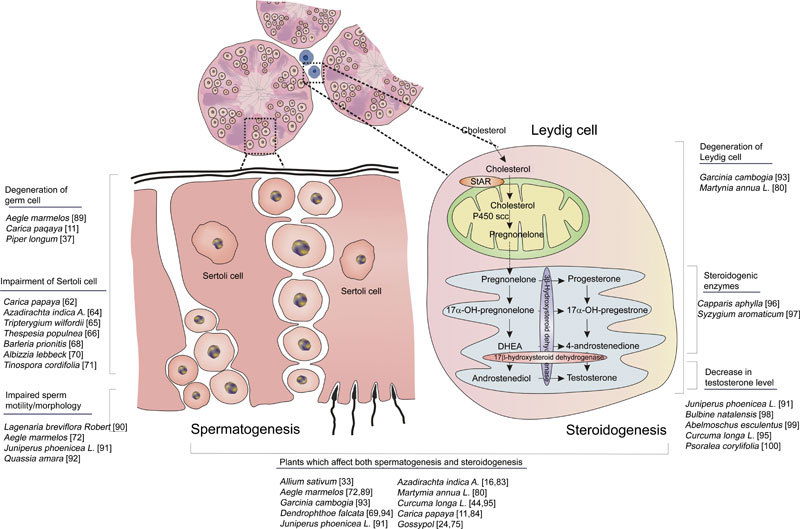
Target sites of plant toxicity in testis.
Neem (Azadirachta indica) has long been documented to have antifertility effects 16, 17. The aqueous leaf extract of neem when administered to male mice at a dose of 200 mg kg−1 for 28 days damaged the seminiferous tubules, resulting in the slackening of germinal epithelium, marginal condensation of chromatin in round spermatids, degeneration of germ cells and the derangement of germ cell types from their orderly arrangement in spermatogenesis. The effects were reported to revert back to normalcy after 42 days of withdrawal of the treatment 18. On the contrary, azadiractin, an active ingredient of neem, given at doses of 5 mg, 10 mg and 50 mg per kg body weight (b.w.) did not show any evidence of reproductive toxicity in parent rats or litters (F[1B] and F[2B]) over two generations, implicating the safe use of the compound as a biopesticide 19. Although several studies claim the potential use of neem as a contraceptive, neem oil is reported to induce other toxic effects, such as severe metabolic acidosis 20, 21, encephalopathy 22, ventricular fibrillation 23 and nervous abnormality.
Gossypol, a polyphenolic compound present in the stem, seeds and roots of Gossypium species, is known to exert unique and selective effects upon reproduction in various species such as rats, mice, hamsters, rabbits, monkeys and human beings 24. The contraceptive effect of gossypol was first discovered in China. During the times of drought in China, the cotton cake that was left over after the extraction of the oil from the fiber of the plant was consumed by animals and humans. Shortly after, the contraceptive action of cotton cake was identified and immense quantities of gossypol were extracted from the cotton plant. This discovery led to large-scale testing of gossypol as a male contraceptive in China during the 1970s 25. Gossypol is reported to invoke antifertility effects in rats at 30 mg per kg b.w., whereas a much lesser dose, 0.3 mg per kg b.w., could incite infertility in humans, making the compound very efficient in humans than in rats 12. Later, it was found to reduce blood potassium (hypokalemia) 26, and the sterilization effect was found to be irreversible, although a few studies claim the effect to be reversible. Supplementation with potassium salts has been reported to bring normalcy in the gossypol-treated animals. The WHO investigators claim that gossypol has a slow recovery pattern and irreversible effect, and the safety and efficacy of gossypol as a contraceptive continue to be controversial 27.
Carica papaya is recognized from ancient times for its medicinal properties and the contraceptive characteristics of papaya seed extracts have been reported in the 1970s 28, 29. Degeneration of germ cells and germinal epithelium, reduction in the number of Leydig cells and presence of vacuoles in the seminiferous tubules were observed when crude ripe seeds of papaya were administered orally to male Wistar rats at a dose of 100 mg per kg b.w. 11. The crude chloroform extract of papaya seeds at a dose of 5 mg per animal per day for 40–60 days reduced the fertility potential to 0%, with the suppression of cauda epididymal sperm motility 30. Administration of the chloroform extract of papaya to male rabbits for 150 days caused a decline in sperm concentration with oligospermia on the 75th day and azoospermia after 120 days. Membrane damage in the acrosome, bent mid piece, coiled tail, detached head and arrest of spermatogenesis beyond the level of spermatocytes were also observed 31.
Alcoholic extracts of Momordica charantia have been reported to exhibit antispermatogenic effects in dogs 10. Oral administration of alcohol extracts of the seeds of Momordica charantia to male albino rats at a dose of 25 mg per 100 g b.w. for 35 days caused a decrease in the number of spermatocytes and spermatids, with the effects being more significant when administered through the intraperitoneal route 32. The crude extract of garlic (Allium sativum) when administered to male rats at varying concentrations (5%, 10%, 15% and 30%) for 30 days caused an increase in the percentage of empty seminiferous tubules and brought about a decrease in serum testosterone levels, with the effects being invoked at a dose as low as 10% 33. Furthermore, induction of germ-cell apoptosis through increased expression of caspase inhibitors such as Baculoviral IAP repeat-containing protein 3 (BIRC3) and Baculoviral IAP repeat-containing protein 2 (BIRC2) and activation of caspase-3 were also reported 34. Pepper (Piper longum), a commonly used spice, is reported to induce sterility in laboratory male mice 35. Piperine, an alkaloid extracted from the fruits and roots of black pepper, has been shown to cause damage to the germ cells and seminiferous tubules when administered orally for 30 days 36. Suppression in the levels of antioxidant enzymes, and increase in lipid peroxidation in testis and epididymis along with activation of caspase 3 and Fas apoptotic proteins in testicular germ cells were reported when piperine was administered to male Wistar rats at doses of 10 mg and 100 mg for 30 days 37, 38.
Vincristin and vinblastin, two pharmacologically active compounds isolated from Catharanthus roseus or Vinca rosea, have since long been reported to interfere with reproduction 39, 40. A single injection of 15 μg of vincristine and 40 μg of vinblastin to adult rats was reported to cause degeneration of A4 spermatogonia, 48 h after injection. Changes in the seminiferous tubule and a decline in the percentage of primary spermatocytes, round and elongated spermatids were reported in rats when the extracts of Vinca rosea were administered at various doses 41. Ocimum sanctum or Holy Bail or Tulsi has also been reported to hamper reproduction by targeting spermatogenesis, thereby leading to antifertility 42. Accumulation of sperm in the lumen of seminiferous tubules was observed when rats were administered with ginger (Zingiber officinale) rhizome powder at doses of 50 mg and 100 mg for 20 consecutive days 43. The rhizome extract of Curcuma longa at a dose of 600 mg per kg b.w. for 56 and 84 days caused a reduction in the diameter of seminiferous tubules, loosening of the germinal epithelium, intraperitoneal vacuolation and mixing of spermatids at different stages of spermatogenesis in male Wistar rats, with the effects being reversible following cessation of treatment for 56 days 44.
The extracts from the roots of Tripterygium wilfordii, a Chinese herb that is used in the treatment of various diseases like rheumatoid arthritis, hepatitis, spondylitis and skin disorders, has been shown to exert powerful antifertility effects in rats and human males, with the effects being observed at doses much lower than the ones used to cure rheumatoid arthritis 45. The glycosides of Tripterygium wilfordii at a daily dosage of 10 mg for 7 weeks or 13 weeks and 20 mg for 4 weeks or 10 weeks significantly inhibited spermatogenesis and the turnover of basic nuclear protein synthesis in the late elongated spermatids of rat testis 46. Several other plants are also known to hamper fertility by targeting spermatogenesis at various stages. A list of few recently published articles on plants that impair spermatogenesis is summarized in Table 1.
Table 1. Plants that affect spermatogenesis.
| Plant name | Plant part used/extract | Dose and duration | Observed effects | References |
|---|---|---|---|---|
| Allium sativum | Crude extract | 5%, 10%, 15% and 30%; 30 days | Dose-dependent decrease of increase in the percentage empty seminiferous tubules. | 33 |
| Lagenaria breviflora Robert | Whole fruit and ethanol | 1 000, 2 000, 4 000 and 8 000 mg kg−1 day−1; 14 days | Significant decrease in sperm motility and viability. | 90 |
| Azadirachta indica A. | Leaf and powder | 0%, 5%, 10% and 15% of neem leaf meal; 16 weeks | Mild depressive effect on spermatogenesis, sperm quality and seminiferous tubule diameter. | 101 |
| Aegle marmelos | Leaf and ethanol extract | 200 and 300 mg kg day−1; 60 days | Reduces sperm motility, concentration. Morphological changes in the testis. Reduced the surface area of Sertoli and Leydig cells | 72 |
| Garcinia cambogia | Seeds and ethanol extract | 100 and 200 mg kg−1 day−1; 6 days per week for 6 weeks treatment | Distortion in the arrangement of the spermatogenic cells. | 93 |
| Dendrophthoe falcata | Stem and 70% methanol extract | 100 mg kg−1 day−1; 60 days | Inhibition of spermatogenesis by decreasing the weight of testis, epididymis and accessory sex organs. Reduced the Sertoli cell surface area | 69 |
| Juniperus phoenicea L. | Cones and ethanol extract | 400 or 800 mg kg−1 day−1; 21 days | Spermatogenic arrest and decreases sperm motility, count. | 91 |
| Rosmarinus officinalis L. | Leaf and ethanol extract | 250 and 500 mg kg−1 day−1; 63 days | Decreases spermatogenesis by decreasing the primary and secondary spermatocyte in the testis | 102 |
| Quassia amara | Bark and chloroform extract | 12.5%, 25%, 50% and 100%; 15 days | Decreased sperm parameters, epididymal α-galactosidase activity and abnormal sperms. | 92 |
| Martynia annua | Root and ethanol extract | 50, 100 and 200 mg kg−1 day−1 | Spermatogenic arrest by showing degeneration of spermatocytes and a dose-related reduction in sperm parameters. | 80 |
| Mentha arvensis | Leaf and petroleum ether extract | 10 and 20 mg per animal per day; 20, 40 and 60 days | Reduced testis, epididymis weight and spermiogram with normal morphology of sperm. | 103 |
| Hibiscus sabdariffa | Calyx and aqueous extract | 1.15, 2.30 and 4.60 g kg−1 day−1; | Distortion of seminiferous tubules. | 104 |
| Cestrum parqui | Leaf and filtered extract | 40, 62.5, 100, 150 and 250 μg mL−1 | Spermicidal activity at high dose with damage to sperm membrane | 105 |
| Tropaeolum tuberosum | Tubers and aqueous extract | 1 g kg−1 day−1 mL−1; 7, 12, 21 and 42 days | Reduces testicular functions after one spermatogenic cycle by reducing spermatid and sperm number, daily sperm production. | 106 |
| Curcuma longa L. | Rhizome and aqueous extract | 600 mg kg−1 day−1; 56 and 84 days | Suppresses spermatogenesis | 44 |
| Barleria prionitis | Root and methanolic extract | 100 mg kg−1 day−1; 60 days | Spermatogenic cells such as primary spermatocytes, secondary spermatocytes and round spermatocytes were declined | 107 |
Plants that affect sperm motility and morphology
The spermatozoa formed during the process of spermiogenesis are morphologically mature but immotile and gets released into the lumen of the seminerous tubule, which then proceeds into the rete testis via the seminiferous fluid. The peristaltic movements of the adjoining myoid cells of the testis transport the immotile spermatozoa through a series of tubules known as efferent ductules, which connects the retes testis to the head of the epididymis. The passage of the sperm through three segments of the epididymis-caput, corpus and cauda is very essential for the final maturation of the sperm 47. The synthesis and secretion of various proteins by the epididymis, as well as the attainment of various morphological, biochemical and motile properties during the passage through the epididymis are fundamental for the fully fertilizing capabilities of spermatozoa. Reduced sperm number or altered sperm morphology may be indicative of the problems encountered during spermatogenesis or spermiogenesis or the impairment of epididymal environment. Several plant products are reported to alter the morphology of the sperm or to diminish its motility.
Testicular degeneration characterized by reduced number of cells in the epithelium along with reduction in the number of sperm cells was observed when the aqueous extract of Abrus precatorious was administered to male rats at doses of 400 mg, 800 mg and 1 600 mg per kg b.w. for 18 days 48. The alcoholic seed extracts of Abrus precatorious at a dose of 100 mg per kg b.w. for 60 days significantly lowered cauda epididymal sperm motility and brought about a decrease in the levels of succinate dehydrogenase and ATPase in the sperm of male albino rats. Scanning electron microscopic studies on sperm morphology revealed decapitation, acrosomal damage and formation of bulges on the midpiece region of sperms following exposure to Abrus precatorious seed extracts 49. Irreversible impairment of the motility of human spermatozoa at a concentration of 20 mg per mL of the methanol extract of Abrus precatorious seed extracts was reported, which may be due to the decline in cAMP and enhanced generation of reactive oxygen species 50. A significant decrease in the density and motility of the ejaculated spermatozoa were observed in patients receiving the root extracts of Tripterygium wilfordii as treatment for rheumatoid arthritis 45. Increased retained proximal cytoplasmic droplets in the sperm, separation of the heads and tails of the spermatozoa and reduced sperm motility were observed when mature rams were fed with locoweed, Astragalus lentiginosus 51. Incubation of guinea spermatozoa with the crude aqueous extract of Echeveria gibbiflora caused a hypotonic-like effect, which included distention of the plasma membrane over the acrosomal region and formation of a huge head bubble 52. Electron microscopic observation of human spermatozoa revealed the presence of a sticky dense material intercalated along the plasma membrane on exposure to a purified fraction from the crude aqueous extract of Echeveria gibbiflora 53.
Oral administration of ethanolic extracts of neem to adult male mice at 0.5 mg, 1 mg or 2 mg per kg b.w. for 6 weeks interfered with sperm DNA and caused chromosome strand breakage, spindle disturbances and deregulation of genes responsible for sperm morphology. A linear decrease in the percentage of sperm motility was observed with various concentrations (1–50 mg per 1 million sperm) of neem leaf extract, with motility falling to absolute zero within 20 s of exposure to 3 mg dose 54. An in vitro study on hamster sperm showed that piperine interferes with acrosome reaction through the inhibition of calcium influx by stimulation of efflux, thereby impairing fertility 55. In our laboratory, we have demonstrated a reduction in rat sperm motility, viability and count on exposure to piperine at 10 mg and 100 mg per kg b.w. 38. Graded doses of the mormodica seed extract induced abnormalities in the size and shape of rat sperm along with dorsoventral constrictions in the middle region of the sperm head, which was proposed to be due to alterations in cauda epididymal milieu and androgen deficiency 56. An in vitro study on the effects of allitridum, an active principle from garlic, has been reported to inhibit sperm motility and complete immobilization of rat, hamster and human spermatozoa at a dose of 7.5 mg mL−1 of allitridum treatment 57. In vitro studies on the crude aqueous extract of Allium sativum have been reported to reduce sperm viability, membrane disintegration of sperm and irreversible immobilization of ram epididymal and human ejaculated sperm at doses of 0.25 g and 0.5 g per mL, respectively 58. The benzene extract of Ocimum sanctum leaves when administered to male rats at a dose of 250 mg per kg b.w. for 48 days was reported to decrease sperm count, motility and the forward velocity of the sperm. The effects were found to be reversible upon withdrawal of treatment for 2 weeks 59. Although several studies have demonstrated the noxious effects of various plants and/or their products on sperm motility and morphology, the mechanism(s) involved in contributing these effects are poorly understood. Plants may induce deterioration of sperm functions either due to the direct action of the active ingredients of plants on sperm cells and/or by targeting Leydig cells or Sertoli cells and the associated functions. The later part of this review will discuss about the plants that are reported to target Sertoli cell/Leydig cell functions.
Plants that affect Sertoli cells
The somatic Sertoli cells have a very important role in controlling the process of spermatogenesis throughout the adult life. They foster the developing germ cells by regulating the flow of vital nutrients and growth factors through the tight junctions 60. In addition, the rate and quality of spermatogenesis are determined by the number of Sertoli cells present 61. Therefore, any agent that damages the viability and function of Sertoli cells may have profound effects on spermatogenesis. Chloroform extracts of the seeds of Carica papaya when administered to male albino rats and langur monkeys at a dose of 50 mg per kg b.w. for 360 days caused reduction in nuclear and cytoplasmic volume and vacuolization of the Sertoli cells, with the effects being reversible 60–120 days after withdrawal of the treatment 62, 63. Intra-epithelial vacuoles of varying sizes in the cytoplasm of Sertoli cells and disturbances in the co-existence of Sertoli–Sertoli/Sertoli–germ cell were observed when Azadirachta leaf powder was administered to albino rats for 48 days 64. An in vitro study on the effects of mutiglycosides of Tripterygium wilfordii and gossypol acetate at a dose of 3.0 or 30 μg mL−1 on primary cultures of Leydig and Sertoli cells resulted in the complete death of both the cell types within 24 h of exposure. It was concluded that Sertoli cells are more sensitive than Leydig cells to both the compounds 65. Enlargement of the Sertoli cells was observed when 400 mg of the leaf extract of Thespesia populnea was administered to male Swiss mice for 15 days 66. Pure theobromine when administered to male rats at a dose of 500 mg for 7 days inhibited the binding ability of androgen-binding protein and reduced the androgen concentration in seminiferous tubule fluid, signifying Sertoli cells as primary targets for theobromine toxicity 67.
Root extracts of Barleria prionitis (100 mg per kg b.w.), methanolic extracts of Dendrophthoe falcata (100 mg per kg b.w.), methanolic extracts of Albizzia lebbeck bark (100 mg per kg b.w.), methanolic extracts of Tinospora cordifolia (100 mg per kg b.w.) and ethanolic extracts of Aegle marmelos leaves (300 mg per kg b.w.) are reported to reduce the cross-sectional surface area of Sertoli cells when administered orally to male Wistar rats for 60 days 68, 69, 70, 71, 72. There are very few studies that elucidate the molecular mechanism of action by which plants impede the functions of Sertoli cells. Sertoli cells express androgen receptors and require intratesticular testosterone for their normal development and function. It is possible that plants impair Sertoli cell functions by targeting the intratesticular testosterone production by Leydig cells and/or at the level of the hypothalamo–pituitary axis.
Plants that affect Leydig cells and steroidogenesis
Apart from spermatogenesis, the testis performs another important function, the synthesis of androgens that are vital in maintaining spermatogenesis. The hormonal regulation of spermatogenesis is well organized, with a feed-back mechanism involving the hypothalamus, pituitary gland and testis 73. The neurons of the hypothalamus synthesize and secrete gonadotropin-releasing hormone, which induces the production and release of LH and FSH from the pituitary gland. LH causes the synthesis of testosterone in the Leydig cells of the testis, which exerts a negative feedback on hormone release from the hypothalamus and pituitary74. FSH acts on Sertoli cells, resulting in the production of androgen-binding protein, which helps in the passage of testosterone through Sertoli–Sertoli junctional complexes. Any factor that could perturb the LH-stimulated Leydig cell steroidogenesis could have an enormous impact on endocrine regulation of spermatogenesis and could lead to infertility. Numerous plant products are known to target Leydig cells and hinder their functions.
Several studies affirm the undisputable role of gossypol in impairing testicular spermatogeneis 75, 76. Gossypol acetic acid, a polyphenolic compound isolated from the seeds of cotton plant when incubated with isolated rat interstitial cells at a dose of 50 μg mL−1 caused a dramatic decrease in histochemical stain for 3-β-HSD, proving the direct inhibitory effect of the compound 77. Reduction in the levels of testosterone, LH and follicle-stimulating hormone was reported when the crude methanol extract of Quassia amara was administered to male albino rats 78. Administration of the methanol extract of Sarcostemma acidum at a dose of 100 mg to male albino rats for 60 days caused a decrease in the number of mature Leydig cells and an increase in the degeneration of Leydig cell population 79. Ethanolic extracts of the roots of Martynia annua to male rats at doses of 100 and 200 mg per kg b.w. for 60 days caused Leydig cell atrophy and a significant reduction in the serum concentration of LH and testosterone 80. Leydig cell nuclear area and mature Leydig cell numbers were significantly reduced on oral administration of 70% methanolic extract of Tinospora cordifolia stem to male rats at the dose level of 100 mg per rat per day for 60 days 71. Mentha piperita labiatae (20 g L−1) and Mentha spicata labiatae (20 g L−1) herbal teas when fed to Wistar rats increased the FSH and LH levels and decreased total testosterone levels 81. Suppression of the activities of steroidogenic enzymes including the P450 side-chain cleavage enzyme, 3 β-hydroxysteroid dehydrogenase, 17 α-hydroxylase, 20 α-hydroxylase and 17 β-hydroxysteroid dehydrogenase, was observed when primary mouse Leydig cells were incubated with varying concentrations of crude Toona sinensis 82. The leaves of Azadirachta indica when administered orally at a dose of 500 mg per kg b.w. exhibited a regression and decrease in the number of Leydig cells and their nuclear diameter, indicating androgen deficiency 83. Carica papaya seed extracts when administered orally at doses of 50 and 100 mg per kg b.w. for 8 weeks to sexually mature Wistar rats caused pronounced hypertrophy of pituitary gonadotrophs and degeneration of Leydig cells 84. Palmitine hydrochloride isolated from the roots of Berberis chitria at a dose of 30 mg per kg per day when administered orally to dogs for 30 days resulted in 66% and 27% reduction, respectively, in mature and immature Leydig cells 85.
A significant reduction in the levels of serum testosterone and LH was reported when crude extracts of garlic were administered to male rats for 30 days 33. Dose-dependent decrease in the enzyme activity of 3α, 3β, 17β-hydroxysteroid dehydrogenases and degeneration of Leydig cells were reported when Abrus precatorius was administered to male rats 86. Ethanolic extracts of Colebrookea oppositifolia (200 mg) when administered orally for 8–10 weeks was reported to cause a decrease in the nuclear and cytoplasmic surface area of Leydig cells 87. Atrophy of the Leydig cells was observed when the leaf extracts of Azadirachta indica and flower extract of Malvaviscus conzattii were administered to male albino rats 83, 88. Table 2 summarizes a few recent articles on plants that are reported to impair Leydig cell functions. Most of the plants impair steroidogenesis by targeting the enzymes involved in the process at the level of Leydig cells and/or at the level of the hypothalamo–pituitary–gonadal loop. Additional studies are warranted to understand intensely the molecular mechanisms by which plants or their active ingredients hamper steroidogenesis in various species.
Table 2. Plants that affects steroidogenesis.
| Plant name | Plant part used/extract | Dose and duration | Observed effects | References |
|---|---|---|---|---|
| Allium sativum | Crude extract | 5%, 10%, 15% and 30%; 30 days | Reduces testosterone secretion | 33 |
| Aegle marmelos | Leaf and ethanol extract | 200 and 300 mg kg−1 day−1; 60 days | Reduces testosterone levels | 72 |
| Aegle marmelos | Leaf and aqueous extract | 50 mg per 100 g b.w. per day; 28 days | Steroidogenesis was reduced with a reduction in germ cells in testis | 89 |
| Capparis aphylla | Whole plant and ethanol extract | 50, 100 and 200 mg kg−1 day−1; 18 days | Reduces steroidogenic enzymes | 96 |
| Garcinia cambogia | Seeds and ethanol extract | 100 and 200 mg kg−1 day−1; 6 days a week for 6 weeks treatment | Degeneration of the Leydig cells | 93 |
| Dendrophthoe falcate | Stem methanol extract | 50, 100 and 200 mg kg−1 day−1; 60 days | Decreases serum testosterone levels | 94 |
| Juniperus phoenicea L. | Cones and ethanol extract | 400 or 800 mg kg−1 day−1; 21 days | Decreases testosterone levels | 91 |
| Martynia annua | Root and ethanol extract | 50, 100 and 200 mg kg−1 day−1; 60 days | Degeneration of Leydig cells | 80 |
| Syzygium aromaticum L. | Flower buds and hexane | 15, 30 and 60 mg kg−1 day−1; 35 days | Reduction in the steroidogenic enzymes and testosterone levels at higher dose | 97 |
| Bulbine natalensis | Stem and aqueous extract | 25, 50 and 100 mg kg−1 day−1; 7 days | Decreases testosterone and progesterone at high dose | 98 |
| Abelmoschus esculentus | Fruit and methanolic extract | 70 mg kg−1 day−1; 28 days | Decreases serum testosterone levels | 99 |
| Albizia. lebbeck L | Bark and methanolic extract | 100 mg kg−1 day−1; 60 days | Decrease in Leydig cells nuclear area and number of mature Leydig cells Decreases serum testosterone levels | 70 |
| Curcuma longa L. | Crude alcoholic extract | 500 mg kg−1 day−1; 60 days | Decreases serum testosterone levels | 95 |
| Psoralea corylifolia | Crude extract | 10 g kg−1—single dose; 3, 7 days after treatment | Decreases serum testosterone levels | 100 |
| Chromolaena odoratum L. | Leaves and aqueous extract | 250 and 500 mg kg−1 day−1; 14 days | Decreases serum testosterone levels | 108 |
Conclusion
The toxic effects of most of the plants on reproduction were identified while administering them for therapeutic use and/or during contraceptive research. Although a few plants have reached clinical trials, most of them failed the trails due to their toxicity or due to the irreversibility of the effects. Several plants that are reported to have beneficial effects against various ailments were later found to have harmful effects on reproduction. Future research should be directed towards studying the toxic effects of all the commonly used plants. The detailed mechanism of action of natural products in inducing reproductive toxicity should be elucidated.
Acknowledgments
Dr PP Mathur acknowledges the receipt of financial support from the Department of Science and Technology, Govt. of India under the projects (1) SP/SO/B-65/99, (2) DST-FIST and (3) the Indian Council of Medical Research. Shereen Cynthia D'Cruz acknowledges the Indian Council of Medical Research, New Delhi, India for the Senior Research Fellowship. The authors also thank the staff of Bioinformatics Centre, Pondicherry University, Pondicherry, India for providing various facilities.
References
- Hess RA, Renato de Franca L. Spermatogenesis and cycle of the seminiferous epithelium. Adv Exp Med Biol. 2008;636:1–15. doi: 10.1007/978-0-387-09597-4_1. [DOI] [PubMed] [Google Scholar]
- Ge R, Chen G, Hardy MP. The role of the Leydig cell in spermatogenic function. Adv Exp Med Biol. 2008;636:255–69. doi: 10.1007/978-0-387-09597-4_14. [DOI] [PubMed] [Google Scholar]
- Litscher ES, Williams Z, Wassarman PM. Zona pellucida glycoprotein ZP3 and fertilization in mammals. Mol Reprod Dev. 2009;76:933–41. doi: 10.1002/mrd.21046. [DOI] [PubMed] [Google Scholar]
- Saradha B, Mathur PP. Effect of environmental contaminants on male reproduction. Environ Toxicol Pharmacol. 2006;21:34–41. doi: 10.1016/j.etap.2005.06.004. [DOI] [PubMed] [Google Scholar]
- Kritiker KR, Basu BD.Indian Medicinal PlantsNew Canaught Place, Dehradun, India: Bishen Singh, Mahendra Pal Singh; 1975p785–8.
- Patwardhan BWD, Pushpangadan P, Bhatt N. Ayurveda and Traditional Chinese Medicine: A comparative overview. Evid Based Compl Alt. 2005;2 doi: 10.1093/ecam/neh140. [DOI] [PMC free article] [PubMed] [Google Scholar]
- WHO. Traditional Medicine Strategy 2002–2005Geneva: WHO; 2002
- McDonald A.A Botanical Perspective on the Identity of Soma (Nelumbo nucifera Gaertn.) Based on Scriptural and Iconographic RecordsNew York: Botanical Garden Press; Supplement (Winter, 2004). pp. S147—50+S51+S52–73.
- Saez JM, Avallet O, Lejeune H, Chatelain PG. Cell-cell communication in the testis. Horm Res. 1991;36:104–15. doi: 10.1159/000182142. [DOI] [PubMed] [Google Scholar]
- Dixit VP, Khanna P, Bhargava SK. Effects of Momordica charantia L. fruit extract on the testicular function of dog. Planta Med. 1978;34:280–6. doi: 10.1055/s-0028-1097451. [DOI] [PubMed] [Google Scholar]
- Udoh P, Kehinde A. Studies on antifertility effect of pawpaw seeds (Carica papaya) on the gonads of male albino rats. Phytother Res. 1999;13:226–8. doi: 10.1002/(SICI)1099-1573(199905)13:3<226::AID-PTR396>3.0.CO;2-E. [DOI] [PubMed] [Google Scholar]
- Liu GZ, Lyle KC, Cao J. Clinical trial of gossypol as a male contraceptive drug. Part I. Efficacy study. Fertil Steril. 1987;48:459–61. doi: 10.1016/s0015-0282(16)59418-7. [DOI] [PubMed] [Google Scholar]
- Dixit VP, Gupta CL, Agrawal M. Testicular degeneration and necrosis induced by chronic administration of Cannabis extract in dogs. Endokrinologie. 1977;69:299–305. [PubMed] [Google Scholar]
- Mandal TK, Das NS. Testicular toxicity in Cannabis extract treated mice: association with oxidative stress and role of antioxidant enzyme systems. Toxicol Ind Health. 2010;26:11–23. doi: 10.1177/0748233709354553. [DOI] [PubMed] [Google Scholar]
- Nahas GG, Frick HC, Lattimer JK, Latour C, Harvey D. Pharmacokinetics of THC in brain and testis, male game-totoxicity and premature apoptosis of spermatozoa. Hum Psychopharmacol. 2002;17:103–13. doi: 10.1002/hup.369. [DOI] [PubMed] [Google Scholar]
- Joshi AR, Ahamed RN, Pathan KM, Manivannan B. Effect of Azadirachta indica leaves on testis and its recovery in albino rats. Indian J Exp Biol. 1996;34:1091–4. [PubMed] [Google Scholar]
- Choudhary DN, Singh JN, Verma SK, Singh BP. Antifertility effects of leaf extracts of some plants in male rats. Indian J Exp Biol. 1990;28:714–6. [PubMed] [Google Scholar]
- Mishra RK, Singh SK. Effect of aqueous leaf extract of Azadirachta indica on the reproductive organs in male mice. Indian J Exp Biol. 2005;43:1093–103. [PubMed] [Google Scholar]
- Srivastava MK, Raizada RB. Lack of toxic effect of technical azadirachtin during postnatal development of rats. Food Chem Toxicol. 2007;45:465–71. doi: 10.1016/j.fct.2006.09.010. [DOI] [PubMed] [Google Scholar]
- Dhongade RK, Kavade SG, Damle RS. Neem oil poisoning. Indian Pediatr. 2008;45:56–7. [PubMed] [Google Scholar]
- Sutton NM, Bates N, Campbell A. Apparent adverse reactions to neem (margosa) oil in cats. Vet Rec. 2009;164:592–3. doi: 10.1136/vr.164.19.592. [DOI] [PubMed] [Google Scholar]
- Lai SM, Lim KW, Cheng HK. Margosa oil poisoning as a cause of toxic encephalopathy. Singapore Med J. 1990;31:463–5. [PubMed] [Google Scholar]
- Balakrishnan V, Pillai NR, Santhakumari G. Ventricular fibrillation and cardiac arrest due to neem leaf poisoning. J Assoc Physicians India. 1986;34:536. [PubMed] [Google Scholar]
- Coutinho EM. Gossypol: a contraceptive for men. Contraception. 2002;65:259–63. doi: 10.1016/s0010-7824(02)00294-9. [DOI] [PubMed] [Google Scholar]
- Wang YE, Luo YD, Tang XC. Studies on the anti-fertility actions of cotton seed meal and gossypol (author's transl) Yao Xue Xue Bao. 1979;14:663–9. [PubMed] [Google Scholar]
- Reidenberg MM, Gu ZP, Lorenzo B, Coutinho E, Athayde C, et al. Differences in serum potassium concentrations in normal men in different geographic locations. Clin Chem. 1993;39:72–5. [PubMed] [Google Scholar]
- Waites GM, Wang C, Griffin PD. Gossypol: reasons for its failure to be accepted as a safe, reversible male antifertility drug. Int J Androl. 1998;21:8–12. doi: 10.1046/j.1365-2605.1998.00092.x. [DOI] [PubMed] [Google Scholar]
- Bodhankar SL, Garg SK, Mathur VS. Antifertility screening of plants. Part IX. Effect of five indigenous plants on early pregnancy in female albino rats. Indian J Med Res. 1974;62:831–7. [PubMed] [Google Scholar]
- Chinoy NJ, Ranga Geetha M. Effects of Carica papaya seed extracts on the physiology of the vas deferens of albino rats. Acta Eur Fertil. 1984;15:59–65. [PubMed] [Google Scholar]
- Lohiya NK, Goyal RB. Antifertility investigations on the crude chloroform extract of Carica papaya Linn. seeds in male albino rats. Indian J Exp Biol. 1992;30:1051–5. [PubMed] [Google Scholar]
- Lohiya NK, Mishra PK, Pathak N, Manivannan B, Jain SC. Reversible azoospermia by oral administration of the benzene chromatographic fraction of the chloroform extract of the seeds of Carica papaya in rabbits. Adv Contracept. 1999;15:141–61. doi: 10.1023/a:1006701826831. [DOI] [PubMed] [Google Scholar]
- Naseem MZ, Patil SR, Ravindra, Patil RS. Antispermatogenic and androgenic activities of Momordica charantia (Karela) in albino rats. J Ethnopharmacol. 1998;61:9–16. doi: 10.1016/s0378-8741(98)00006-3. [DOI] [PubMed] [Google Scholar]
- Hammami I, Nahdi A, Mauduit C, Benahmed M, Amri M, et al. The inhibitory effects on adult male reproductive functions of crude garlic (Allium sativum) feeding. Asian J Androl. 2008;10:593–601. doi: 10.1111/j.1745-7262.2008.00358.x. [DOI] [PubMed] [Google Scholar]
- Hammami I, Amara S, Benahmed M, El May MV, Mauduit C. Chronic crude garlic-feeding modified adult male rat testicular markers: mechanisms of action. Reprod Biol Endocrinol. 2009;7:65. doi: 10.1186/1477-7827-7-65. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Munshi SR, Rao SS. Antifertility activity of an indigenous plant preparation (ROC-101) I. Effect on reproduction. Indian J Med Res. 1972;60:1054–60. [PubMed] [Google Scholar]
- Malini T, Manimaran RR, Arunakaran J, Aruldhas MM, Govindarajulu P. Effects of piperine on testis of albino rats. J Ethnopharmacol. 1999;64:219–25. doi: 10.1016/s0378-8741(98)00128-7. [DOI] [PubMed] [Google Scholar]
- D'Cruz SC, Vaithinathan S, Saradha B, Mathur PP. Piperine activates testicular apoptosis in adult rats. J Biochem Mol Toxicol. 2008;22:382–8. doi: 10.1002/jbt.20251. [DOI] [PubMed] [Google Scholar]
- D'Cruz SC, Mathur PP. Effect of piperine on the epididymis of adult male rats. Asian J Androl. 2005;7:363–8. doi: 10.1111/j.1745-7262.2005.00059.x. [DOI] [PubMed] [Google Scholar]
- Joshi MS, Ambaye RY. Effect of alkaloids from Vinca rosea L. on spermatogenesis in male rats. Indian J Exp Biol. 1968;6:256–7. [PubMed] [Google Scholar]
- Murugavel T, Akbarsha MA. Anti-spermatogenic effect of Vinca rosea Linn. Indian J Exp Biol. 1991;29:810–2. [PubMed] [Google Scholar]
- Bustos-Obregon E, Lopez ML. Selective effect of Vinca rosea L. alkaloids on type A4 rat spermatogonium. Andrologie. 1973;5:245–7. doi: 10.1111/j.1439-0272.1973.tb00918.x. [DOI] [PubMed] [Google Scholar]
- Kasinathan S, Ramakrishnan S, Basu SL. Antifertility effect of Ocimum sanctum L. Indian J Exp Biol. 1972;10:23–5. [PubMed] [Google Scholar]
- Khaki A, Fathiazad F, Nouri M, Khaki AA, Ozanci CC, et al. The effects of ginger on spermatogenesis and sperm parameters of rat. Iranian J Reprod Med. 2009;7:7–12. [Google Scholar]
- Mishra RK, Singh SK. Reversible antifertility effect of aqueous rhizome extract of Curcuma longa L. in male laboratory mice. Contraception. 2009;79:479–87. doi: 10.1016/j.contraception.2009.01.001. [DOI] [PubMed] [Google Scholar]
- Zhen QS, Ye X, Wei ZJ. Recent progress in research on Tripterygium: a male antifertility plant. Contraception. 1995;51:121–9. doi: 10.1016/0010-7824(94)00018-r. [DOI] [PubMed] [Google Scholar]
- Lu QX. Effect of glycosides of Tripterygium wilfordii Hook on the reproductive system and major organs of male rats. Zhongguo Yi Xue Ke Xue Yuan Xue Bao. 1990;12:203–7. [PubMed] [Google Scholar]
- Bedford JM.Evolution of the sperm maturation and sperm storage functions of the epididymisBaltimore: Urban and Schwarzenberg; 19797–21.
- Adedapo AA, Omoloye OA, Ohore OG. Studies on the toxicity of an aqueous extract of the leaves of Abrus precatorius in rats. Onderstepoort J Vet Res. 2007;74:31–6. doi: 10.4102/ojvr.v74i1.137. [DOI] [PubMed] [Google Scholar]
- Rao MV. Antifertility effects of alcoholic seed extract of Abrus precatorius Linn. in male albino rats. Acta Eur Fertil. 1987;18:217–20. [PubMed] [Google Scholar]
- Ratnasooriya WD, Amarasekera AS, Perera NS, Premakumara GA. Sperm antimotility properties of a seed extract of Abrus precatorius. J Ethnopharmacol. 1991;33:85–90. doi: 10.1016/0378-8741(91)90166-b. [DOI] [PubMed] [Google Scholar]
- Panter KE, James LF, Hartley WJ. Transient testicular degeneration in rams fed locoweed (Astragalus lentiginosus) Vet Hum Toxicol. 1989;31:42–6. [PubMed] [Google Scholar]
- Delgado NM, Taboada Ramirez J, Ortega Hernandez A, Merchant-Larios H, Sanchez-Vazquez ML, et al. Effects of a purified fraction from Echeveria gibbiflora aqueous crude extract on guinea-pig spermatozoa. Phytother Res. 1999;13:46–9. doi: 10.1002/(SICI)1099-1573(199902)13:1<46::AID-PTR373>3.0.CO;2-S. [DOI] [PubMed] [Google Scholar]
- Reyes R, Merchant-Larios H, Ortega-Hernandez A, Delgado NM. Male contraception, IV: hypotonic-like effect from Echeveria gibbiflora on human sperm. Arch Androl. 2002;48:443–9. doi: 10.1080/01485010290099327. [DOI] [PubMed] [Google Scholar]
- Awasthy KS. Genotoxicity of a crude leaf extract of neem in male germ cells of mice. Cytobios. 2001;106 (Suppl 2):151–64. [PubMed] [Google Scholar]
- Piyachaturawat P, Sriwattana W, Damrongphol P, Pholpramool C. Effects of piperine on hamster sperm capacitation and fertilization in vitro. Int J Androl. 1991;14:283–90. doi: 10.1111/j.1365-2605.1991.tb01094.x. [DOI] [PubMed] [Google Scholar]
- Girini MM, Ahamed RN, Aladakatti RH. Effect of graded doses of Momordica charantia seed extract on rat sperm: scanning electron microscope study. J Basic Clin Physiol Pharmacol. 2005;16:53–66. doi: 10.1515/jbcpp.2005.16.1.53. [DOI] [PubMed] [Google Scholar]
- Qian YX, Shen PJ, Xu RY, Liu GM, Yang HQ, et al. Spermicidal effect in vitro by the active principle of garlic. Contraception. 1986;34:295–302. doi: 10.1016/0010-7824(86)90010-7. [DOI] [PubMed] [Google Scholar]
- Chakrabarti K, Pal S, Bhattacharyya AK. Sperm immobilization activity of Allium sativum L. and other plant extracts. Asian J Androl. 2003;5:131–5. [PubMed] [Google Scholar]
- Ahmed M, Ahamed RN, Aladakatti RH, Ghosesawar MG. Reversible anti-fertility effect of benzene extract of Ocimum sanctum leaves on sperm parameters and fructose content in rats. J Basic Clin Physiol Pharmacol. 2002;13:51–9. doi: 10.1515/jbcpp.2002.13.1.51. [DOI] [PubMed] [Google Scholar]
- Griswold MD. The central role of Sertoli cells in spermatogenesis. Semin Cell Dev Biol. 1998;9:411–6. doi: 10.1006/scdb.1998.0203. [DOI] [PubMed] [Google Scholar]
- Petersen C, Soder O. The sertoli cell–a hormonal target and 'super' nurse for germ cells that determines testicular size. Horm Res. 2006;66:153–61. doi: 10.1159/000094142. [DOI] [PubMed] [Google Scholar]
- Manivannan B, Mittal R, Goyal S, Ansari AS, Lohiya NK. Sperm characteristics and ultrastructure of testes of rats after long-term treatment with the methanol subfraction of Carica papaya seeds. Asian J Androl. 2009;11:583–99. doi: 10.1038/aja.2009.25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lohiya NK, Manivannan B, Goyal S, Ansari AS. Sperm motility inhibitory effect of the benzene chromatographic fraction of the chloroform extract of the seeds of Carica papaya in langur monkey, Presbytis entellus entellus. Asian J Androl. 2008;10:298–306. doi: 10.1111/j.1745-7262.2008.00331.x. [DOI] [PubMed] [Google Scholar]
- Aladakatti RH, Ahamed RN. Changes in Sertoli cells of albino rats induced by Azadirachta indica A. Juss leaves. J Basic Clin Physiol Pharmacol. 2005;16:67–80. doi: 10.1515/jbcpp.2005.16.1.67. [DOI] [PubMed] [Google Scholar]
- Lan ZJ, Gu ZP, Lu RF, Zhuang LZ. Effects of multiglycosides of Tripterygium wilfordii (GTW) on rat fertility and Leydig and Sertoli cells. Contraception. 1992;45:249–61. doi: 10.1016/0010-7824(92)90069-6. [DOI] [PubMed] [Google Scholar]
- Krishnamoorthy P, Vaithinathan S. Effect of the extract of Thespesia populnea leaves on mice testis. J Environ Biol. 2003;24:327–30. [PubMed] [Google Scholar]
- Wang Y, Waller DP. Theobromine toxicity on Sertoli cells and comparison with cocoa extract in male rats. Toxicol Lett. 1994;70:155–64. doi: 10.1016/0378-4274(94)90159-7. [DOI] [PubMed] [Google Scholar]
- Gupta RS, Kumar P, Dixit VP, Dobhal MP. Antifertility studies of the root extract of the Barleria prionitis Linn in male albino rats with special reference to testicular cell population dynamics. J Ethnopharmacol. 2000;70:111–7. doi: 10.1016/s0378-8741(99)00150-6. [DOI] [PubMed] [Google Scholar]
- Gupta RS, Kachhawa JB, Sharma A. Effect of methanolic extract of Dendrophthoe falcata stem on reproductive function of male albino rats. J Herbal Pharmacother. 2007;7:1–13. doi: 10.1300/j157v07n02_01. [DOI] [PubMed] [Google Scholar]
- Gupta RS, Kachhawa JB, Chaudhary R. Antispermatogenic, antiandrogenic activities of Albizia lebbeck (L.) Benth bark extract in male albino rats. Phytomedicine. 2006;13:277–83. doi: 10.1016/j.phymed.2004.11.008. [DOI] [PubMed] [Google Scholar]
- Gupta RS, Sharma A. Antifertility effect of Tinospora cordifolia (Willd.) stem extract in male rats. Indian J Exp Biol. 2003;41:885–9. [PubMed] [Google Scholar]
- Chauhan A, Agarwal M. Reversible changes in the antifertility induced by Aegle marmelos in male albino rats. Syst Biol Reprod Med. 2008;54:240–6. doi: 10.1080/19396360802516856. [DOI] [PubMed] [Google Scholar]
- Sofikitis N, Giotitsas N, Tsounapi P, Baltogiannis D, Giannakis D, Pardalidis N. Hormonal regulation of spermatogenesis and spermiogenesis. J Steroid Biochem Mol Biol. 2008;109:323–30. doi: 10.1016/j.jsbmb.2008.03.004. [DOI] [PubMed] [Google Scholar]
- Dufau ML, Winters CA, Hattori M, Aquilano D, Baranao JL, et al. Hormonal regulation of androgen production by the Leydig cell. J Steroid Biochem. 1984;20:161–73. doi: 10.1016/0022-4731(84)90203-6. [DOI] [PubMed] [Google Scholar]
- Udoh P, Patil DR, Deshpande MK. Histopathological and biochemical effects of gossypol acetate on pituitary-gonadal axis of male albino rats. Contraception. 1992;45:493–509. doi: 10.1016/0010-7824(92)90162-m. [DOI] [PubMed] [Google Scholar]
- Zhuang LZ, Gu ZP, Chang CC. Comparison of sensitivities of rat spermatozoa, Sertoli and Leydig cells to gossypol acetic acid in vitro by the LD50. Zhongguo Yao Li Xue Bao. 1986;7:563–7. [PubMed] [Google Scholar]
- Paz GF, Homonnai ZT. Effect of the antifertility agent, gossypol acetic acid, on the metabolism and testosterone secretion of isolated rat interstitial cells in vitro. Contraception. 1984;29:543–52. doi: 10.1016/s0010-7824(84)80016-5. [DOI] [PubMed] [Google Scholar]
- Raji Y, Bolarinwa AF. Antifertility activity of Quassia amara in male rats – in vivo study. Life Sci. 1997;61:1067–74. doi: 10.1016/s0024-3205(97)00615-2. [DOI] [PubMed] [Google Scholar]
- Venma PK, Sharma A, Mathur A, Sharma P, Gupta RS, et al. Effect of Sarcostemma acidum stem extract on spermatogenesis in male albino rats. Asian J Androl. 2002;4:43–7. [PubMed] [Google Scholar]
- Mali PC, Ansari AS, Chaturvedi M. Antifertility effect of chronically administered Martynia annua root extract on male rats. J Ethnopharmacol. 2002;82:61–7. doi: 10.1016/s0378-8741(02)00084-3. [DOI] [PubMed] [Google Scholar]
- Akdogan M, Ozguner M, Kocak A, Oncu M, Cicek E. Effects of peppermint teas on plasma testosterone, follicle-stimulating hormone, and luteinizing hormone levels and testicular tissue in rats. Urology. 2004;64:394–8. doi: 10.1016/j.urology.2004.03.046. [DOI] [PubMed] [Google Scholar]
- Poon SL, Leu SF, Hsu HK, Liu MY, Huang BM. Regulatory mechanism of Toona sinensis on mouse Leydig cell steroidogenesis. Life Sci. 2005;76:1473–87. doi: 10.1016/j.lfs.2004.08.026. [DOI] [PubMed] [Google Scholar]
- Aladakatti RH, Ahamed RN. Ultrastructural changes in Leydig cells and cauda epididymal spermatozoa induced by Azadirachta indica leaves in albino rats. Phytother Res. 2005;19:756–66. doi: 10.1002/ptr.1710. [DOI] [PubMed] [Google Scholar]
- Udoh P, Essien I, Udoh F. Effects of Carica papaya (paw paw) seeds extract on the morphology of pituitary-gonadal axis of male Wistar rats. Phytother Res. 2005;19:1065–8. doi: 10.1002/ptr.1388. [DOI] [PubMed] [Google Scholar]
- Gupta RS, Dixit VP. Testicular cell population dynamics following palmitine hydroxide treatment in male dogs. J Ethnopharmacol. 1989;25:151–7. doi: 10.1016/0378-8741(89)90016-0. [DOI] [PubMed] [Google Scholar]
- Sinha S, Mathur RS. Effect of steroidal fraction of seeds of Abrus precatorius Linn. on rat testis. Indian J Exp Biol. 1990;28:752–6. [PubMed] [Google Scholar]
- Gupta RS, Yadav RK, Dixit VP, Dobhal MP. Antifertility studies of Colebrookia oppositifolia leaf extract in male rats with special reference to testicular cell population dynamics. Fitoterapia. 2001;72:236–45. doi: 10.1016/s0367-326x(00)00311-7. [DOI] [PubMed] [Google Scholar]
- Chakraborty S, Pakrashi A. Antifertility effect of chronically administered Malviscus conzattii flower extract on fertility of male rats. Contraception. 1991;43:273–85. doi: 10.1016/0010-7824(91)90146-7. [DOI] [PubMed] [Google Scholar]
- Das UK, Maiti R, Jana D, Ghosh D. Effect of aqueous extract of leaf of Aegle marmelos on testicular activities in rats. Iran J Pharmacol Ther. 2006;5:21–5. [Google Scholar]
- Saba AB, Oridupa OA, Oyeyemi MO, Osanyigbe OD. Spermatozoa morphology and characteristics of male wistar rats administered with ethanolic extract of Lagenaria breviflora roberts. Afr J Biotechnol. 2009;8:1170–5. [Google Scholar]
- Shkukani H, Salhab A, Disi A, Shomaf M, Al Quadan F. Antifertility effect of ethanolic extract of Juniperus phoenica (L.) in male albino rats. J Herbal Pharmacother. 2007;7:179–89. doi: 10.1080/15228940802152463. [DOI] [PubMed] [Google Scholar]
- Parveen S, Das S, Kundra CP, Pereira BM. A comprehensive evaluation of the reproductive toxicity of Quassia amara in male rats. Reprod Toxicol. 2003;17:45–50. doi: 10.1016/s0890-6238(02)00080-1. [DOI] [PubMed] [Google Scholar]
- Oluyemi KA, Jimoh OR, Adesanya OA, Omotuyi IO, Josiah SJ, et al. Effects of crude ethanolic extract of Garcinia cambogia on the reproductive system of male wistar rats (Rattus novergicus) Afr J Biotechnol. 2007;6:1236–8. [Google Scholar]
- Gupta RS, Kachhawa JB. Evaluation of contraceptive activity of methanol extract of Dendrophthoe falcata stem in male albino rats. J Ethnopharmacol. 2007;112:215–8. doi: 10.1016/j.jep.2007.02.022. [DOI] [PubMed] [Google Scholar]
- Ashok P, Meenakshi B. Contraceptive effect of Curcuma longa (L.) in male albino rat. Asian J Androl. 2004;6:71–4. [PubMed] [Google Scholar]
- Sarathchandran I, Manavalan R, Akbarsha MA, Kadalmani B, Karar PK. Effect of ethanolic extract of Capparis aphylla (Roth) on testicular steroidogenesis in rat. J Biol Sci. 2007;7:582–4. [Google Scholar]
- Mishra RK, Singh SK. Safety assessment of Syzygium aromaticum flower bud (clove) extract with respect to testicular function in mice. Food Chem Toxicol. 2008;46:3333–8. doi: 10.1016/j.fct.2008.08.006. [DOI] [PubMed] [Google Scholar]
- Yakubu MT, Afolayan AJ. Reproductive toxicologic evaluations of Bulbine natalensis Baker stem extract in albino rats. Theriogenology. 2009;72:322–32. doi: 10.1016/j.theriogenology.2009.01.026. [DOI] [PubMed] [Google Scholar]
- Olatunji-Bello II, Ijiwole2 T, Awobajo FO. Evaluation of the deleterious effects of aqueous fruit extract of Abelmoschus esculentus (Okro fruit) ond some male reproductive parameters in sprague dawley rats. J Phytol. 2009;1:461–8. [Google Scholar]
- Takizawa T, Mitsumori K, Takagi H, Nasu M, Yasuhara K, et al. Sequential analysis of testicular lesions and serum hormone levels in rats treated with a Psoralea corylifolia extract. Food Chem Toxicol. 2004;42:1–7. doi: 10.1016/s0278-6915(03)00220-5. [DOI] [PubMed] [Google Scholar]
- Ogbuewu IP, Okoli IC, Iloeje MU. Semen quality characteristics, reaction time, testis weight and seminiferous tubule diameter of buck rabbits fed neem (Azadirachta indica A. Juss) leaf meal based diets. Iran J Reprod Med. 2009;7:23–8. [Google Scholar]
- Nusier MK, Bataineh HN, Daradkah HM. Adverse effects of rosemary (Rosmarinus officinalis L.) on reproductive function in adult male rats. Exp Biol Med (Maywood) 2007;232:809–13. [PubMed] [Google Scholar]
- Sharma N, Jocob D. Antifertility investigation and toxicological screening of the petroleum ether extract of the leaves of Mentha arvensis L. in male albino mice. J Ethnopharmacol. 2001;75:5–12. doi: 10.1016/s0378-8741(00)00362-7. [DOI] [PubMed] [Google Scholar]
- Orisakwe OE, Husaini DC, Afonne OJ. Testicular effects of sub-chronic administration of Hibiscus sabdariffa calyx aqueous extract in rats. Reprod Toxicol. 2004;18:295–8. doi: 10.1016/j.reprotox.2003.11.001. [DOI] [PubMed] [Google Scholar]
- Souad K, Ali S, Mounir A, Mounir TM. Spermicidal activity of extract from Cestrum parqui. Contraception. 2007;75:152–6. doi: 10.1016/j.contraception.2006.10.006. [DOI] [PubMed] [Google Scholar]
- Cardenas-Valencia I, Nieto J, Gasco M, Gonzales C, Rubio J, et al. Tropaeolum tuberosum (Mashua) reduces testicular function: effect of different treatment times. Andrologia. 2008;40:352–7. doi: 10.1111/j.1439-0272.2008.00868.x. [DOI] [PubMed] [Google Scholar]
- Verma PK, Sharma A, Joshi SC, Gupta RS, Dixit VP. Effect of isolated fractions of Barleria prionitis root methanolic extract on reproductive function of male rats: preliminary study. Fitoterapia. 2005;76:428–32. doi: 10.1016/j.fitote.2005.03.007. [DOI] [PubMed] [Google Scholar]
- Yakubu MT, Akanji MA, Oladiji AT. Evaluation of antiandrogenic potentials of aqueous extract of Chromolaena odoratum (L.) K. R. leaves in male rats. Andrologia. 2007;39:235–43. doi: 10.1111/j.1439-0272.2007.00792.x. [DOI] [PubMed] [Google Scholar]


