Abstract
Globozoospermia is a human infertility syndrome caused by spermatogenesis defects (OMIM 102530). Acrosome plays an important role at the site of sperm-zonapellucida binding during the fertilization process. Thus, malformation of the acrosome is the most prominent feature seen in globozoospermia. Disruption of several mouse genes, including Gopc (Golgi-associated PDZ and coiled-coil motif containing protein), Hrb (HIV-1 Rev binding protein), Csnk2a2 (casein kinase 2, α prime polypeptide) and Pick1 (protein interacting with C kinase 1), results in a phenotype similar to globozoospermia in humans, which suggests their potential role in the disease. However, no mutations with a clear link to globozoospermia have been identified in these genes in humans. In this study, we screened the candidate genes mentioned above in three globozoospermia type I patients and discovered a homozygous missense mutation (G198A) in exon 13 of the PICK1 gene in a Chinese family. The family member affected by this homozygous missense mutation showed a complete lack of acrosome. Using the candidate gene screening strategy, our study is the first to identify an autosomal recessive genetic mutation in PICK1 that was responsible for globozoospermia in humans.
Keywords: acrosome, globozoospermia, PICK1
Introduction
Acrosome is a unique structure of the mature spermatozoon and plays an important role at the site of sperm–zonapellucida binding during the fertilization process. Globozoospermia (also called round-headed spermatozoa) is a rare (incidence < 0.1% among male infertile patients) but severe disorder causing male infertility 1, 2. The most prominent feature of globozoospermia is the malformation of the acrosome, which is totally absent in the most severe cases. Intracytoplasmic sperm injection is a treatment option for these patients, but the fertilization rates are low because of a reduced ability to activate the oocyte 3. In humans, several case reports of globozoospermia have demonstrated that two or more siblings were affected in each family, which suggested a genetic component to this disease 4, 5, 6, 7, 8. In mice, genetic studies have provided direct evidence that disruption of genes, including Gopc (Golgi-associated PDZ and coiled-coil motif containing protein), Hrb (HIV-1 Rev binding protein), Csnk2a2 (casein kinase 2, α prime polypeptide) and Pick1 (protein interacting with C kinase 1), results in a globozoospermia phenotype with decreased fertility. However, there has not been a clear link between homozygous mutations in these genes and globozoospermia in humans 9. One study using a genome-wide scan and sequencing analysis suggested that a homozygous mutation in SPATA16 (spermatogenesis-associated 16) was associated with male infertility in human globozoospermia 4. Here we reported a homozygous missense mutation (G198A) in exon 13 of the PICK1 gene in a Chinese family. The family member affected by this homozygous missense mutation showed a complete lack of acrosome. Therefore, our study is the first to identify a mutation in PICK1 responsible for globozoospermia in humans using the candidate gene screening strategy.
Materials and methods
Clinical information
A total of 100 unrelated, anonymous, fertile men were genotyped by sequencing exon 13 of the PICK1 gene. All subjects were natives of China and had normal somatic karyotypes. No testicular biopsies were performed, and all sperm analyses were performed at least twice in accordance with the recommendations of the World Health Organization (WHO) 10. The subject whose parents were cousins showed typical round-headed spermatozoa (Table 1). The study was approved by the local ethics committee of CITIC-Xiangya Hospital, Changsha, China and informed consent was obtained from all participants.
Table 1. Sperm parameters of the affected individual.
| Sperm characteristics | Parameter | Reference range |
|---|---|---|
| Round-headed spz (%) | 100 | |
| Sperm volume (mL) | 3 | ≥ 2 |
| Number spz × 106 mL−1 | 30 | ≥ 20 |
| Motility (%) | 20 | (A + B) ≥ 50% |
| Grade A spz | 0 | |
| Grade B spz | 16.7 | |
| Grade C spz | 3.3 | |
| Grade D spz | 80 | |
| Acrosome enzyme (μIU per 106 spz) | 25.1 | 48.2–218.7 |
Abbreviation: spz, spermatozoa.
Blood sampling and DNA extraction
Blood samples (5 mL taken into EDTA by venipuncture) were obtained from all subjects. Immediately after collection, whole blood was stored at −80°C until use. Genomic DNA for polymerase chain reaction (PCR) analysis was isolated from thawed whole blood using a phenol/chloroform extraction procedure.
Mutation analysis
Primers were designed according to the PICK1 sequence (Table 2) and used in a PCR assay with Advantage 2 DNA polymerase (Clontech, Mountain View, CA, USA). PCR was performed as follows: (1) initial denaturation at 95°C for 1.5 min, (2) 35 cycles of 94°C for 10 s, anneal for 30 s, and 72°C for 1 min, (3) 72°C for 5 min, and (4) hold at 4°C. The PCR fragments were separated by size in a 2% agarose gel in 1 × TAE buffer (40 mmol L−1 Tris, 20 mmol L−1 glacial acetic acid, 1 mmol L−1 EDTA, pH 8.0) and purified using an Agarose Gel DNA Fragment Recovery Kit (Takara, Tokyo, Japan) if necessary. Primers for other genes, including GOPC, HRB and CSNK2A2, were synthesized according to a previously published method 9. Sequencing reactions were carried out using BigDye Terminator v3.1 Cycle Sequencing Kit (Applied Biosystem, Foster City, CA, USA).
Table 2. Summary of PCR primers of Pick1.
| Gene–exon | Annealing T | PCR primers (5′–3′) |
Product sides (bp) | |
|---|---|---|---|---|
| Forward | Reverse | |||
| Pick1–Ex1 | 60 | GGGAAGGGAAGGATTGTCTGC | CAAGTGCCTAAATGCCAACGCC | 395 |
| Pick1–Ex2 | 60 | GAGGGGTGGCGTTGGCATTTAG | AAGGGTCTGTGGGACTGGGAAG | 252 |
| Pick1–Ex3 | 58 | CAGTGGAGCCCCTCAGGATGGTTAG | CAGGTGGTCAGAAAGCCCCTCTG | 341 |
| Pick1–Ex4 | 63 | GAGCAGAGGGTAGAGTGGAAGACAG | ACAAGGAAGGGGGCGGTGAG | 358 |
| Pick1–Ex5 | 62 | AGGAGTCTCAGTCCAGAACAGTCTTG | TTGGTCAGAGGTCAGAGCCCAC | 301 |
| Pick1–Ex6 | 61 | CCACAGACTCACCAGGTCCTTTG | TGGTGACTTCTCAGTTCCACGG | 268 |
| Pick1–Ex7 | 58 | TGTTAGAAGTGTGGGAGCTTTGC | TGCTGGGATTACAGGCGTGAG | 508 |
| Pick1–Ex8 | 61 | TCGGACTGAGCTTTTACCCCTG | CATCGCAAATCCCAGCACCTGG | 373 |
| Pick1–Ex9 | 61 | GCTTCTCCCCAACAAACCCCTG | CTCCAGCATACGACCTTCTCTGC | 295 |
| Pick1–Ex10 | 61 | AGTCCACCAACAAGGGTGACGC | AGCATGGCTGACTGAAGTGGGG | 263 |
| Pick1–Ex11 | 61 | GCCCTGTCATCCCTCAGCAC | GGAACGAGAGTCCAGCCAAGTC | 320 |
| Pick1–Ex12 | 60 | AGGTCTCAGGAATGAAGAACAGCC | TTTCCCACCTCTGAAATGGAGAG | 288 |
| Pick1–Ex13 | 61 | CTCCTGCGTTCCCTGAACTG | CCTCGTGTATCCCTGGACGG | 430 |
Abbreviations: PCR, polymerase chain reaction; T, temperature.
Tracking PICK1 mutation with restriction digest
We prepared Pvu II digests of PCR products of the subjects by individually adding the required components to a clean microtube in the following order: 5.6 μL distilled water, 1 μL Buffer G (10 mmol L−1 Tris-HCl, pH 7.5, 10 mmol L−1 MgCl2, 50 mmol L−1 NaCl and 0.1 mg mL−1 BSA), 3 μL of PCR product and 0.4 μL of Pvu II restriction enzyme. The tube was mixed by flicking and spun for 5 s in the microfuge to bring all the components to the bottom. This digest mixture was then incubated at 37°C for 2 h (in a 37°C water bath). DNA fragments were separated by size through an 8% PAGE gel in 0.5 × TBE buffer (45 mmol L−1 Tris, 45 mmol L−1 boric acid, 1 mmol L−1 EDTA, pH 8.0).
Results
Identification of homozygous mutation in PICK1 in a patient with globozoospermia
We identified three globozoospermia type I patients in our clinical study. To assess the potential genetic basis for globozoospermia in humans, we performed PCR and DNA sequencing analysis on four candidate genes (GOPC, HRB, CSNK2A2 and PICK1) in these patients. We chose these four genes because disruption of these genes results in a globozoospermia phenotype in mice 9. The subjects were screened for these genes, and we found a homozygous missense mutation in the PICK1 gene in one of these patients. The affected individual showed only round-headed spermatozoa (Figure 1) with decreased motility and acrosomal enzyme activity (Table 1). We sequenced exons 1–13 of PICK1 and the flanking sequences (primers shown in Table 2) in the affected individual and found a homozygous G→A transition at nucleotide 1567 according to the Genebank entry NM_012407 (Figure 2A). The transition generated a missense substitution (G393R) located in the C-terminal acidic domain. Thus, we have identified a homozygous mutation in the PICK1 gene that is associated with globozoospermia in humans.
Figure 1.
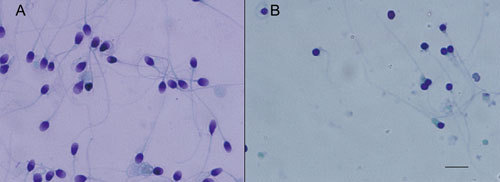
Morphology of sperm by Papanicolaou staining. (A) Normal human sperm and (B) globozoospermia. Globozoospermia results in the loss of the typical acrosome of normal sperm; instead, they have round ball-like heads. Bar = 10 μm.
Figure 2.
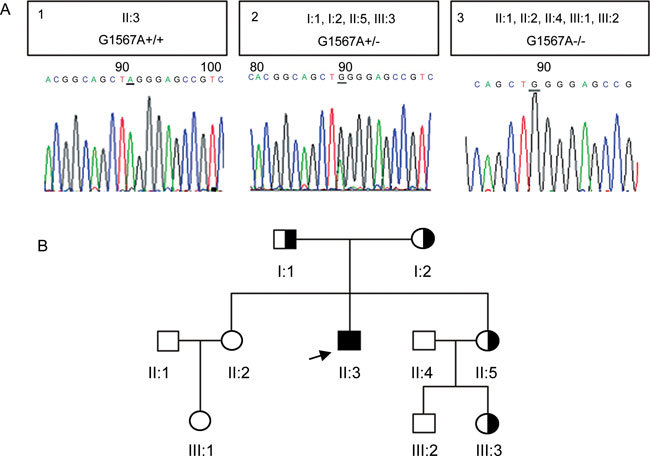
Sequence analysis and pedigree. (A1)–(A3) PICK1 sequence analysis in the proband and his family. The mutation position of the sequence is underlined. The transition generates a missense substitution (G393R) located in the C-terminal acidic domain of the PICK1 protein. (A1): In the proband (II:3), automated sequencing revealed a homozygous G→A transition at the base pair 1 567 of the PICK1 gene. (A2): The heterozygous G→A transition at the same position of the PICK1 gene was observed in the subject's parents (I:1 and I:2), a sister (II:5) and a niece (III:3). (A3): Brothers in law (II:1 and II:4), sister (II:2), niece (III:1) and nephew (III:2) were homozygous for the wild-type allele. (B): The pedigree of the family.
Tracking PICK1 mutations across generations of the patient's family members
To determine the mode of inheritance of this mutated gene, we tracked PICK1 mutations across multiple generations of the patient's family members. As shown in Figure 2, we also found a heterozygous mutation at the same location in his parents, a sister and a niece. These findings demonstrated that the mutation was transmitted in an autosomal recessive mode.
Finally, this mutation created G198A in exon 13 and disrupted a Pvu II site, which was subsequently used to confirm and identify the mutation in the pedigree of this patient and other individuals. Figure 3 shows that the homozygous mutation was not present in 100 control chromosomes.
Figure 3.
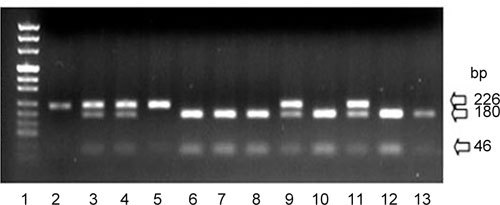
The agarose gel of the product from a Pvu II enzyme cut (Lane 1: Puc Mix Marker 8, Lane 2: PCR product without an enzyme cut, Lane 3: I:1, Lane 4: I:2, Lane 5: II:3, Lane 6: II:1, Lane 7: II:2, Lane 8: III:1, Lane 9: II:5, Lane 10: II:4, Lane 11: III:3, Lane 12: III:2 and Lane 13: normal man). The G198A mutation in exon 13 disrupted a Pvu II site. Thus, the homozygous individual (Lane 5) and the PCR product without an enzyme cut (Lane 2) showed one band (226 bp); heterozygous individuals (Lanes 3, 4, 9 and 11) showed three bands (226, 180 and 46 bp); and individuals homozygous for the wild-type allele (Lanes 6, 7, 8, 10, 12 and 13) showed two bands (180 and 46 bp). The positions of the molecular mass markers and enzyme cut product are indicated (bp).
Discussion
Screens for mutations of candidate genes in the pedigree of patients and other individuals are a common method to investigate the function of a gene 11, 12. In this study, we screened four candidate genes in three globozoospermia type I patients. We did not observe any mutations in GOPC, HRB or CSNK2A2, which may be due to limited samples or the controversial mechanism of CSNK2A213. Fortunately, we discovered a homozygous missense mutation (G198A) in exon 13 of the PICK1 gene, which resulted in the complete lack of acrosome in one patient. Therefore, we have provided evidence for the first time that a PICK1 mutation transmitted by an autosomal recessive mode was responsible for globozoospermia in humans.
The human PICK1 gene contains 13 exons encoding a 415 amino-acid protein. The cytosolic protein interacts with many membrane proteins via its postsynaptic density-95/Discs large/zona occludens-1 (PDZ) domain. Its interactions with membrane proteins regulate their subcellular targeting and surface expression. PICK1 has been repeatedly reported to be involved in the neuronal system 14. In neurons, synaptic targeting and surface expression of AMPA (α-amino-3-hydroxy-5-methyl-isoxazole-4-propionic acid) glutamate receptors were found to be critical for synaptic plasticity. PICK1 is a cytosolic protein that interacts with many membrane proteins, including AMPA receptors, via its PDZ domain. Lipid binding of the PICK1 BAR (Bin/amphiphysin/Rvs) domain was positively regulated by its PDZ domain and negatively regulated by its C-terminal acidic domain. Recently, Xiao et al. 15 reported that male mice deficient in PICK1 were infertile and had a phenotype resembling the human disease globozoospermia. The primary defect in the testes of Pick1-knockout mice was fragmentation of acrosomes in the early stages of spermiogenesis. This fragmentation was followed by defects in nuclear elongation and mitochondrial sheath formation, which led to round-headed sperm, reduced sperm count and severely impaired sperm motility. However, the genetic mutations in Pick1 genes were not identified, and the correlation with globozoospermia remains unknown.
Here, we found a homozygous mutation in the C-terminal domain of PICK1 in a globozoospermia patient, and observed heterozygous mutations in family members. This is the first reported mutation in the PICK1 gene that has resulted in globozoospermia in humans.
In the present study, the G198A homozygous patient did not show any obvious physiological or anatomical defects other than the infertility, which suggested that the altered amino acid G393R in the C-terminal domain did not result in alterations of somatic functions. The heterozygous parents, who were cousins, did not have reduced fecundity. In addition, his heterozygous sister bore a heterozygous son. Similar to humans, homozygous mutant mice had no apparent somatic defects, and both male and female heterozygous mice showed normal fertility. Only homozygous males failed to produce pups. The PICK1 mutant phenotype in the patient in this study was consistent with the phenotype observed in Pick1-deficient mice. In our opinion, the homozygosity for an autosomal gene defect underlies this phenotype. The altered amino acid G393R in the C-terminal domain may influence the negative regulation with the BAR domain and affect the formation of the acrosome because of the perturbation of lipid-BAR domain binding. We conclude that the homozygous mutation of PICK1 results in globozoospermia.
Specifications
All DNA and amino acid numeration in this manuscript referred to PICK1 isoform 1, which is the longest isoform (references NM_012407 and NP_036539.1, respectively). All three variants encoded the same protein. Thus, the described mutation (G198A) affects all three isoforms in the same manner.
References
- Lalonde L, Langlais J, Antaki P, Chapdelaine A, Roberts KD, et al. Male infertility associated with round-headed acrosomeless spermatozoa. Fertil Steril. 1988;49:316–21. [PubMed] [Google Scholar]
- Singh G. Ultrastructural features of round-headed human spermatozoa. Int J Fertil. 1992;37:99–102. [PubMed] [Google Scholar]
- Battaglia DE, Koehler JK, Klein NA, Tucker MJ. Failure of oocyte activation after intracytoplasmic sperm injection using round-headed sperm. Ferti Steril. 1997;68:118–22. doi: 10.1016/s0015-0282(97)81486-0. [DOI] [PubMed] [Google Scholar]
- Dam AH, Koscinski I, Kremer JA, Moutou C, Jaeger AS, et al. Homozygous mutation in SPATA16 is associated with male infertility in human globozoospermia. Am J Hum Genet. 2007;81:813–20. doi: 10.1086/521314. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Florke-Gerloff S, Topfer-Petersen E, Muller-Esterl W, Mansouri A, Schatz R, et al. Biochemical and genetic investigation of round-headed spermatozoa in infertile men including two brothers and their father. Andrologia. 1984;16:187–202. doi: 10.1111/j.1439-0272.1984.tb00262.x. [DOI] [PubMed] [Google Scholar]
- Dale B, Iaccarino M, Fortunato A, Gragnaniello G, Kyozuka K, et al. A morphological and functional study of fusibility in round-headed spermatozoa in the human. Fertil Steril. 1994;61:336–40. doi: 10.1016/s0015-0282(16)56528-5. [DOI] [PubMed] [Google Scholar]
- Carrell DT, Emery BR, Liu L. Characterization of aneuploidy rates, protamine levels, ultrastructure, and functional ability of round-headed sperm from two siblings and implications for intracytoplasmic sperm injection. Fertil Steril. 1999;71:511–6. doi: 10.1016/s0015-0282(98)00498-1. [DOI] [PubMed] [Google Scholar]
- Kalahanis J, Rousso D, Kourtis A, Mavromatidis G, Makedos G, et al. Round-headed spermatozoa in semen specimens from fertile and subfertile men. J Reprod Med. 2002;47:489–93. [PubMed] [Google Scholar]
- Christensen GL, Ivanov IP, Atkins JF, Campbell B, Carrell DT. Identification of polymorphisms in the Hrb, GOPC, and Csnk2a2 genes in two men with globozoospermia. J Androl. 2006;27:11–5. doi: 10.2164/jandrol.05087. [DOI] [PubMed] [Google Scholar]
- World Health Organization. WHO Laboratory Manual for the Examination of Human Semen and Sperm–Cervical Mucus Interaction, 4th ed. Cambridge: Cambridge University Press; 1999.
- Garshasbi M, Hadavi V, Habibi H, Kahrizi K, Kariminejad R, et al. A defect in the TUSC3 gene is associated with autosomal recessive mental retardation. Am J Hum Genet. 2008;82:1158–64. doi: 10.1016/j.ajhg.2008.03.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cornier AS, Staehling-Hampton K, Delventhal KM, Saga Y, Caubet JF, et al. Mutations in the MESP2 gene cause spondylothoracic dysostosis/Jarcho-Levin syndrome. Am J Hum Genet. 2008;82:1334–41. doi: 10.1016/j.ajhg.2008.04.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Escalier D, Silvius D, Xu X. Spermatogenesis of mice lacking CK2alpha': failure of germ cell survival and characteristic modifications of the spermatid nucleus. Mol Reprod Dev. 2003;66:190–201. doi: 10.1002/mrd.10346. [DOI] [PubMed] [Google Scholar]
- Jin W, Ge WP, Xu J, Cao M, Peng L, et al. Lipid binding regulates synaptic targeting of PICK1, AMPA receptor trafficking and synaptic plasticity. J Neurosci. 2006;26:2380–90. doi: 10.1523/JNEUROSCI.3503-05.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xiao N, Kam C, Shen C, Jin W, Wang J, et al. PICK1 deficiency causes male infertility in mice by disrupting acrosome formation. J Clin Invest. 2009;119:802–12. doi: 10.1172/JCI36230. [DOI] [PMC free article] [PubMed] [Google Scholar]


