Abstract
Sperm maturation in the epididymis may involve differences between mature and immature spermatozoa in their volume regulatory osmolyte response. Spermatozoa obtained from the rat caput and cauda epididymidis were examined for their ability to regulate volume after transfer from in situ epididymal osmolality (measured to be 343 ± 13 and 365 ± 19 mmol kg−1, respectively) to that of the female tract in single- and multiple-step protocols. Cells withstood the single-step treatment better than the multistep protocol. Sperm volume estimates by flow cytometric measurements of forward scatter of cells with intact head membranes was more sensitive than those by assessing cell coiling microscopically. At osmolalites below 210 mmol kg−1 both caput and cauda cells ruptured, limiting the use of flow cytometry. Above this critical value, the use of quinine showed that both caput and cauda cells could regulate volume, but cauda cells were the more effective. Of several organic osmolytes studied, myo-inositol, glutamate and KCl caused only temporary and slight swelling of spermatozoa cells in hypotonic medium. Spermatozoa of both maturities seemed to use potassium as the preferred osmolyte for regulating volume.
Keywords: rat sperm, sperm maturation, volume regulation
Introduction
Sperm maturation within the epididymis of the rat has been shown by the greater ability of spermatozoa collected from the cauda epididymidis, over those from the caput, to fertilize eggs after insemination into the uterus 1, 2. In this species, ejaculation is into the cervix 3 and sperm passage through the uterus is aided by uterine movements 4; hence, the first barrier to active spermatozoa is the uterotubal junction, beyond which caput spermatozoa do not travel 5. After intrauterine insemination, only motile spermatozoa from the cauda are seen to emerge from the junction when viewed from the oviductal side 6. The low fertilizing ability of caput spermatozoa has been, in part, explained by their reduced motility and their propensity to move in circles 5 and consequent inability to move forwards 7, 8. The necessity of motility for spermatozoa to reach the oocytes and penetrate the cumulus oophorus and zona pellucida could also explain why caput spermatozoa are also less effective than cauda spermatozoa at fertilization in vitro 9.
Another hypothesis has recently been presented to explain sperm maturation in terms of the reduced ability of immature spermatozoa to regulate their volume in the female tract 10. The increasing osmolality with which spermatozoa are confronted in the epididymis, if acting to reduce cell volume, should encourage the uptake of secreted permeant epididymal osmolytes during the process of regulatory volume increase 11. These osmolytes would then be released during the process of regulatory volume decrease (RVD), induced after swelling caused when spermatozoa are exposed to the lower osmolality of the female tract fluids. If caput spermatozoa have a lower osmolyte content than that of cauda spermatozoa, they would be less equipped to counter any osmotic challenge imposed during artificial insemination. As a result, the cells would swell and passage through the uterotubal junction would be hampered when inseminated into the uterus. Evidence from several transgenic mouse models indeed indicates that passage through the uterotubal junction depends on straight flagellar morphology and that angulated spermatozoa are unable to negotiate the uterotubal junction 12. Another site of selection of spermatozoa with different volume-regulating abilities is the oviduct, where only cells with superior RVD bind well 13.
Whether immature rat spermatozoa do contain fewer osmolytes than mature, and what these osmolytes are, is not known. There are increases in the carnitine and glutamate content of spermatozoa as they mature in the porcine epididymis 14, 15, 16. The swelling of spermatozoa in hypotonic medium containing many of the organic solutes found at high concentration in epididymal fluid suggests that myo-inositol, L-carnitine, D-glutamate and D-sorbitol are used by spermatozoa obtained from the mouse during RVD 17, and this view is supported by the infertile c-ros knockout mouse model whose spermatozoa contain a less than normal glutamate and myo-inositol content 18, are swollen 19, display flagellar angulation 20 and fail to enter the uterus 21.
In this paper, the rat was used as a model as there are more spermatozoa to be obtained than from the mouse and comparison between caput and cauda spermatozoa can be made in the same animal. However, nothing is known about sperm volume regulation in this species, although the difference between male and female tract fluid osmolalities 22, 23 suggests that volume regulation is important in this species. Transfer of caudal spermatozoa to anisosmolal solutions, as well as after return to isosmolal conditions, is damaging to sperm motility and acrosomal integrity, but head plasma membrane integrity is the less affected 24. Examination of the volume increase in response to the penetrating cryoprotectant glycerol, indicated by flagellar coiling, has shown that rat cauda epididymidal spermatozoa are stiffer than those from the caput 25 and may be less able to bend in hypotonic medium without membrane rupture.
This study was designed to investigate whether there is a difference between caput and cauda spermatozoa in their response to osmotic challenge, and the nature of the osmolytes used in RVD.
Materials and methods
Animals and reagents
A total of 26 rats of the Sprague-Dawley CD strain (Retired Breeders; Harlan-Winkelman, Paderborn, Germany) were asphyxiated with CO2 and killed by cervical dislocation in accordance with the German Federal Law on the Care and Use of Laboratory Animals (license No. 39.32.7.1). The epididymides were cleaned of adipose tissue, rinsed in saline and kept moist in Biggers-Whitten-Whittingham (BWW) medium 26 until use. All chemicals were obtained from Sigma-Aldrich (Taufkirchen, Germany) unless otherwise stated.
Measurement of epididymal fluid osmolality
Undiluted fluid was collected from the caput and cauda epididymidis (regions 2 and 6, respectively 27) by the expressing luminal contents onto the surface of the capsule and withdrawing a known volume directly into the tip of a Gilson, 2–10 μL, positive-displacement pipette. Fluid (2 μL) was transferred onto 3 mm diameter pieces of Wescor filter tissue on the small (2 μL content) chamber of a Wescor Vapro vapour pressure osmometer (model 5520, Kreienbaum Scientific Measuring Systems, Langenfeld, Germany).
Measurements were made after a delay of 15–20 min, to ensure chamber saturation, and consecutive measurements were made every 2 min thereafter, until two estimates agreed to within 3 mmol kg−1. The mean of the last two values was taken as the measured value. Commercially available standard solutions (100, 290 and 1 000 mmol kg−1 from Kreienbaum Scientific Measuring Systems; 400 mmol kg−1 from Serva Electrophoresis GmbH, Heidelberg, Germany; and 550 and 700 mmol kg−1 produced by mixing) were measured under the same time-delay conditions, and the data were used to construct a graph revealing a positive and linear discrepancy between the given and recorded values (mean 115.25 ± SEM; 7.8 mmol kg−1; n = 4), the mean of which was subtracted from all fluid measurements to obtain the reported value.
Media
The BWW medium first mentioned above was modified by addition of NaCl (to 335 or 360 mmol kg−1) to produce isotonic fluids for preincubation of rat caput (BWW335) and cauda (BWW365) spermatozoa, respectively. Medium of 290 mmol kg−1 (BWW290) was used to mimic the osmolality of the female rat oviductal fluid tract (287 mmol kg−1 23). Putative sperm osmolytes were added at concentrations given below, replacing the osmotic equivalent of NaCl to maintain a fixed osmolality of 290 mmol kg−1. These solutions were expected to eliminate the concentration difference of the osmolytes between the sperm cytoplasm and the medium 18.
Collection of epididymal spermatozoa
Spermatozoa were removed from the cauda region (region 6 27) by tearing the capsule, exposing a loop of tubule and transferring it to 10 μL of isotonic medium (BWW365). The short segments of cauda tubule were emptied of their contents by squeezing with fine forceps and the empty tubule was removed. The sperm suspension was transferred to a 1.5-mL tube containing a medium of the same osmolality. Caput tubule segments (regions 2–2a 27) were minced by fine forceps in BWW335 and centrifuged at 40 × g for 1 min.
Osmosensitivity of epididymal spermatozoa
As rat sperm cytoplasmic droplets are fragile 11 and the flow cytometer subjects the assessed cells to shear forces, it was necessary to establish the osmotic conditions under which mature and immature epididymal spermatozoa could be subjected without rupture. This was determined first by determining the osmotic sensitivity of maturing spermatozoa to anisosmolal solutions in single-step (abrupt challenge) and multistep (gradual challenge) procedures. Cells were examined for their flagellar form, as well as the flow cytometric dot plot patterns that reflect cell volumes, to establish gating windows containing separate populations of cells of different size.
Spermatozoa from the caput and cauda epididymidis were transferred from preincubation media of 300 mmol kg−1 (caput) and 330 mmol kg−1 (cauda) (osmolalities based on literature values of epididymal fluid osmolality) to 150 mmol kg−1 in sequential decrements of 30 mmol kg−1 ('multistep') or of increasingly larger size ('one step'; 300 to 270; to 240; to 210; to 180; to 150 for caput and an additional step of 330 to 300 for the cauda). A volume of 10 μL of caput epididymidal fluid was taken directly using a Gilson (2–10 μL) positive-displacement pipette into 990 μL of 300 mmol kg−1 preincubation media for caput (330 mmol kg−1 preincubation media for cauda). After an equilibration time of 15 min at 37°C, cells were centrifuged at 300 × g for 5 min and the supernatant was removed. A volume of 1 mL of the medium of next lower osmolality was added and gently mixed without vortexing. After each challenge, the forward scatter (FS) of propidium iodide (PI)-negative cells was measured by flow cytometry and aliquots of the same samples were fixed 1 + 1 with 7% (v/v) glutaraldehyde (Wissenschaftliche Gerätebau GmbH, Zehlendorf, Germany) in isotonic BWW for light microscopic examination of flagellar morphology.
Choice of method for estimating sperm volume
The standard method for measuring cell volume is electronic sizing. For somatic cells, it has the advantage that dead cells are detected simultaneously as their volume is drastically reduced following membrane rupture and cytoplasmic loss. This does not apply to spermatozoa, which have low cytoplasmic volume, and this necessitates an additional method for determination of cell viability 28. The cylindrical, rather than spherical shape, of spermatozoa presents multiple cell populations, especially in species whose spermatozoa have long tails 29. In this work, a flow cytometer was used because simultaneous measurement of cell membrane intactness can be achieved by the inclusion of an impermeant dye. The method has been validated by showing parallel changes in FS and cell volume measurements determined by electronic sizing in the mouse 29 and man 30. This method should be equally applicable to rat spermatozoa, which are even longer than those of the mouse 31.
Flow cytometry
Standards beads (Flow–Check Fluorospheres, Beckman Coulter, Krefeld, Germany) of a nominal diameter of 10 μm were used for daily calibration of the machine. The software only accepted data when the intra-run CV was < 2%, and throughout the experiments (n = 30) the CV of FS was 1.2% and PI-fluorescence (FL 3) was 3.2%. After preincubation in isotonic media for 10 min at 37°C, 2.5 μL of PI (0.5 mg mL−1 in phosphate-buffered saline) to was added to 250 μL samples to stain cells with damaged head membranes. A Cytomics FC 500 MCL flow cytometer (Beckman Coulter) was used to detect FS (voltage 220; gain 5.0), side scatter (SS; voltage 126; gain 2.0, presented on a log scale for clarity) and FL 3 (voltage 442; gain 1.0) of incident laser (power: 20 mW) at 488 nm from 5 000 PI-negative particles per sample. Debris, nonsperm samples and PI-positive cells were gated out by setting FS, SS and FL 3 windows. The mean FS was taken to reflect the volume of these untreated samples 32. Other fluorescent spheres of diameter of precisely 3, 4, 5 and 10 μm were used for calibration of volume and gave mean FS readings of 178, 234, 292.6 and 539.3, respectively. A spline curve best fitted the data and was used to provide values of sperm volume.
Microscopy
An Olympus microscope (BX-40; Olympus, Hamburg, Germany) was used to examine the presence of the various categories of angulated flagella in phase contrast optics at × 40 magnification. The coiled spermatozoa observed included both 'hairpin' angulation (with the flagellum bending acutely at the annulus) and 'pigtail' forms (in which the tip of the tail formed a small sphere). A total of 200 spermatozoa were assessed for the percentage of each of these three forms.
Effects of extracellular osmolytes on rat spermatozoa under hypotonic conditions
Spermatozoa were preincubated in 80 μL isotonic BWW media (BWW335 for caput and BWW365 for cauda) for 15 min at 37°C in 5% (v/v) CO2. A physiological insult was then provided by transferring 80 μL of sperm suspension directly to 920 μL BWW290 containing either an osmolyte (carnitine 50 mmol L−1; glutamate 10 and 50 mmol L−1; sorbitol 50 mmol L−1; taurine 10 mmol L−1; myo-inositol 50 mmol L−1; or KCl 20, 50 and 100 mmol L−1), quinine (0.8 mmol L−1; as positive control) or BWW290 alone (as blank control). At 1, 15, 30 and 45 min after this osmotic challenge, 240 μL aliquots were added to 3 μL PI and read in a flow cytometer until 5 000 nonstained particles were detected.
Statistics
Statistical analysis was made with SigmaStat (v3.5; SysStat Software GmbH, Erkrath, Germany) and significance was accepted when P < 0.05 for the tests stated in the text.
Results
Osmolality of epididymal fluid
The osmolality of epididymal contents declined with time within the chamber. Values fell over 17–19 min to reach consecutive values that agreed to within 3 mmol kg−1. The mean ± SEM osmolality (mmol kg−1) of epididymal contents from eight caput samples was 343.3 ± 13.2 and from eight cauda samples was 365.0 ± 18.7 mmol kg−1. There was no significant difference between these values (paired t-test; P = 0.278).
Osmosensitivity of immature and mature rat spermatozoa determined by microscopy
Reducing the osmolality of fluid in one step led to a significantly greater increase in the percentage of coiled caput than cauda spermatozoa (one-way analysis of variance [ANOVA]; P ≤ 0.001; Figure 1A). Quinine, which should prevent RVD in those cells treated with it, had no significant effect on either caput or cauda cells, although the mean percentage of swollen cells was lower in its presence. A more gentle multistep hypotonic challenge, with intervening centrifugation steps, led to swelling of fewer coiled caput and cauda spermatozoa, but again caput cells responded significantly more than those from the cauda and the effects of quinine were negative, although insignificant (Figure 1B). Although the cells may have experienced slight osmotic changes from the lower-than-epididymal osmolality of the preincubation media before the experimental challenge, these studies suggested that an osmolality of no less that 210 mmol kg−1 was warranted for screening studies of osmolyte influence on cell swelling to limit the occurrence of spermolysis during flow cytometric measurement unrelated to RVD mechanisms.
Figure 1.
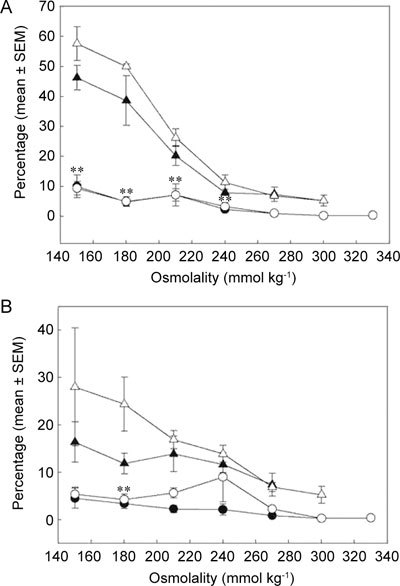
Percentages of coiled rat caput (▵▴) and cauda (○•) sperm tails (ordinate: mean ± SEM) during single-step (A) and multistep (B) immersion into decreasing osmolality (abscissa) with (▴•) or without (▵○) 0.8 mmol L−1 quinine (determined by microscopy; n = 4). **Significant differences between untreated caput and cauda spermatozoa at osmolalities of 240 mmol kg−1 and below (one-way ANOVA, P ≤ 0.001 [A]; P = 0.006 [B]).
Setting the flow cytometer windows
All results generated by the flow cytometer are given as dot plots of values for FS and SS as the ordinate and abscissa, respectively. During preliminary experiments, all cells appearing within the window 'all sperm' were analyzed together, to produce a mean FS value as an indication of cell swelling or shrinkage. However, it was noted that many different treatments gave two major populations of cells within the 'all sperm' window, both of which warranted separate analysis. The analysis protocol was thus changed to provide gatings for each subpopulation and two new windows were generated that enveloped the smaller cells, found in control solutions (the 'not swollen' window), and the larger cells, found in the presence of quinine (the 'swollen' window). Recent reports of which we were unaware at the time 33, 34 indicate that FSC/SSC windows and a single stain alone cannot eliminate all debris and nonsperm particles. The values here may thus be incorrect estimates, but presumably equally so in all treatments.
These consensus windows were used to assess the percentage of cells in each window and their mean FS values. Examples of cauda spermatozoa are shown in Figure 2 and caput spermatozoa in Figure 3. Cauda cells seemed able to regulate volume, as fewer cells were swollen in the absence of quinine (10.5%, Figure 2B), than those from the caput (15.2%, Figure 3B). Nevertheless, quinine nearly doubled the percentage of swollen spermatozoa of both maturities (Figures 2D and 3D).
Figure 2.

Dot plots (forward scatter ordinate, side scatter abscissa) of spermatozoa from the rat cauda epididymidis during flow cytometry (same sample used with both old [A, C] and new [B, D] protocols) when subjected to single-step hypotonicity (290 mmol kg−1) in the absence (A, B) and presence (C, D) of 0.8 mmol L−1 quinine. Percentages of 5 000 propidium iodide (PI)-negative spermatozoa within each window are given.
Figure 3.
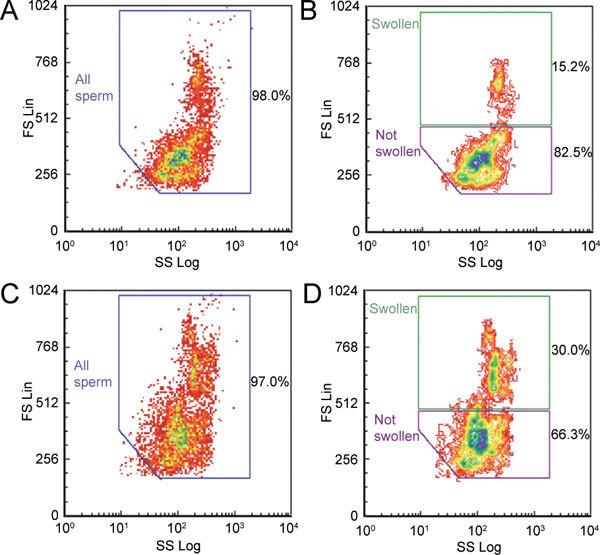
Dot plots (forward scatter ordinate, side scatter abscissa) of spermatozoa from the rat caput epididymidis during flow cytometry (same sample used with both old [A, C] and new [B, D] protocols) when subjected to single-step hypotonicity (290 mmol kg−1) in the absence (A, B) and presence (C, D) of 0.8 mmol L−1 quinine. Percentages of 5 000 PI-negative spermatozoa within each window are given.
Osmosensitivity of immature and mature rat spermatozoa determined by flow cytometry
With the new analytical protocol, the percentages of swollen spermatozoa were calculated on hypotonic challenge. As osmolality was reduced in a single step below the physiological, cauda spermatozoa lost their ability to regulate volume, as shown by the fewer cells appearing in the 'swollen' window with quinine treatment: 2.1-fold at 270 mmol kg−1 (Figures 4A, D), 1.2-fold at 210 mmol kg−1 (Figures 4B, E), but only 0.9-fold at 150 mmol kg−1 (Figures 4C, F). In contrast, caput spermatozoa cells increased in size in response to quinine at all tonicities: 1.8-fold at 270 mmol kg−1 (Figures 5A, D), 1.8-fold at 210 mmol kg−1 (Figures 5B, E) and 1.5-fold at 150 mmol kg−1 (Figures 5C, F).
Figure 4.

Dot plots (forward scatter ordinate, side scatter abscissa) of spermatozoa from the rat cauda epididymidis in the absence (A–C) and presence (D–F) of 0.8 mmol L−1 quinine in solutions of single-step decreasing osmolality: 270 mmol kg−1 (A, D), 210 mmol kg−1 (B, E) and 150 mmol kg−1 (C, F). Percentages of 5 000 PI-negative spermatozoa within each window are given.
Figure 5.

Dot plots (forward scatter ordinate, side scatter abscissa) of spermatozoa from the rat caput epididymidis in the absence (A–C) and presence (D–F) of 0.8 mmol L−1 quinine in solutions of single-step decreasing osmolality: 270 mmol kg−1 (A, D), 210 mmol kg−1 (B, E) and 150 mmol kg−1 (C, F). Percentages of 5 000 PI-negative spermatozoa within each window are given.
With the single-step imposition of osmotic challenge, the percentage of cells in the 'swollen' window increased as osmolality decreased from 270 to 240 mmol kg−1 for caput spermatozoa, but below 270 mmol kg−1, the percentage of such swollen cells decreased (Figure 6A). The percentage of swollen cauda cells was similarly increased when osmolality was reduced, but from 330 to 300 mmol kg−1 and below 300 mmol kg−1, the percentage of swollen cells decreased (Figure 6A). For spermatozoa of both maturities, the presence of quinine significantly promoted swelling (one-way ANOVA, caput (Figure 6A, B), P = 0.01–0.007; cauda (Figure 6A, B), P ≤ 0.001). The basal extent of swelling was higher for caput than that for cauda spermatozoa (one-way repeated measure (RM) ANOVA; P = 0.015 (Figure 6A).
Figure 6.
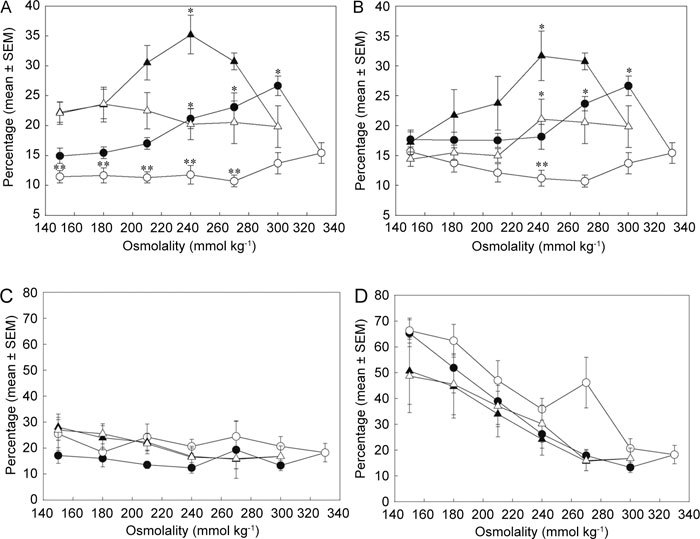
Percentages (ordinate) of swollen (A, B) and PI-positive (C, D) caput (▵▴) and cauda (○•) rat spermatozoa during single-step (A, C) and multistep (B, D) transfer to decreasing osmolality (abscissa) with (▴•) or without (▵○) 0.8 mmol L−1 quinine (from flow cytometric observations; n = 4). *Significant differences between quinine-treated and control groups with osmolality for spermatozoa from each epididymal region (one-way ANOVA, caput [A] P = 0.01; caput [B] P = 0.007; cauda [A] P ≤ 0.001; cauda [B] P ≤ 0.001). **Significant differences between control values of caput (▵) and cauda (○) spermatozoa with osmolality (one-way RM ANOVA, P = 0.015 [A]; P ≤ 0.001 [B]).
A similar biphasic pattern of quinine- and hypo-osmolality-induced cell swelling was observed when the multistep procedure was used for cells of both maturation states; again caput cells were more responsive at lower osmolality than cauda cells in the absence of quinine (Figure 6B). The swelling of cauda spermatozoa shown by flow cytometry here contrasts with the lack of coiling observed microscopically under similar conditions (Figure 1).
The percentage of caput epididymidal cells stained by PI did not change much as osmolality was decreased; percentages rose from 20% to 30% whether or not quinine was present and whether the single- or multistep protocol was used (Figure 6C). In contrast, cauda epididymidal spermatozoa were more susceptible to osmolality-induced head membrane leakage. Whether quinine was present or not, and whether the single- or multistep protocol was used, the percentage of PI-permeant cells increased in a linear manner when the osmolality decreased below 260 mmol kg−1 from about 20% to 70% (Figure 6D).
Initial volumes of rat caput and cauda epididymidal spermatozoa
The mean ± SD volumes of caput and cauda epididymidal spermatozoa placed in solutions of osmolality (335 mmol kg−1 caput and 365 mmol kg−1 cauda) similar to those in the epididymal canal, were 142 ± 36 and 83 ± 14 fL, respectively (n = 14) (P < 0.001; paired t-test).
Effects of dithiothreitol (DTT) on swelling of rat spermatozoa
The permeant reducing agent DTT was initially added to the incubations of rat spermatozoa to break disulfide bonds within sperm structures and soften the especially stiff flagellum of rat cauda spermatozoa 35. The percentage of cells swollen in single step (290 mmol kg−1) was increased by the use of DTT by 2.2-fold for cauda spermatozoa (Figures 7A, D), more than that for caput cells (1.3-fold; Figures 7D, B), in the absence of quinine.
Figure 7.
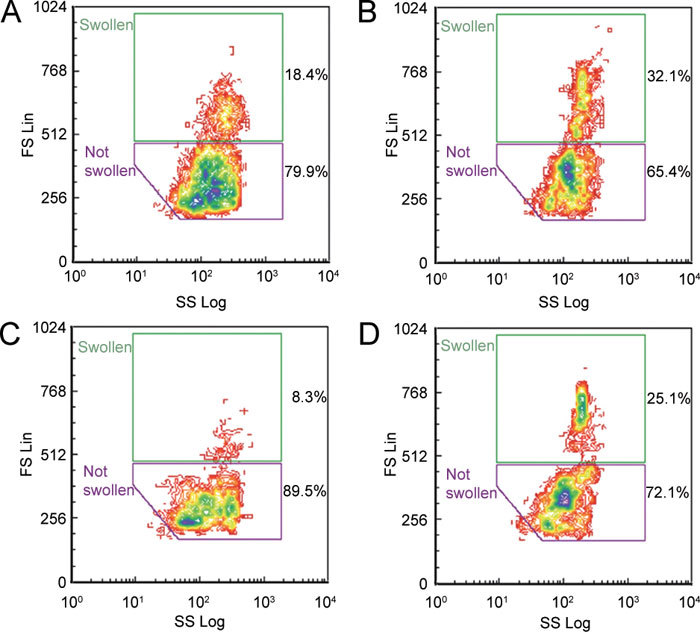
Dot plots (forward scatter ordinate, side scatter abscissa) of spermatozoa from the rat cauda (A, C) and caput (B, D) epididymidis subjected to single-step hypotonicity (290 mmol kg−1) in the presence (A, B) and absence (C, D) of 2 mmol L−1 dithiothreitol (DTT). Percentages of 5 000 PI-negative spermatozoa within each window are given.
Effect of extracellular osmolytes on spermatozoa under hypotonic conditions
Hypotonic conditions were applied to spermatozoa in the presence of high concentrations of putative osmolytes involved in RVD to see whether this process could be blocked by eliminating the concentration difference of osmolytes in the cell and extracellular fluid. Figure 8B shows that myo-inositol increased the size of caput spermatozoa by 2.0-fold over the controls, compared with a 3.2-fold increase with quinine.
Figure 8.
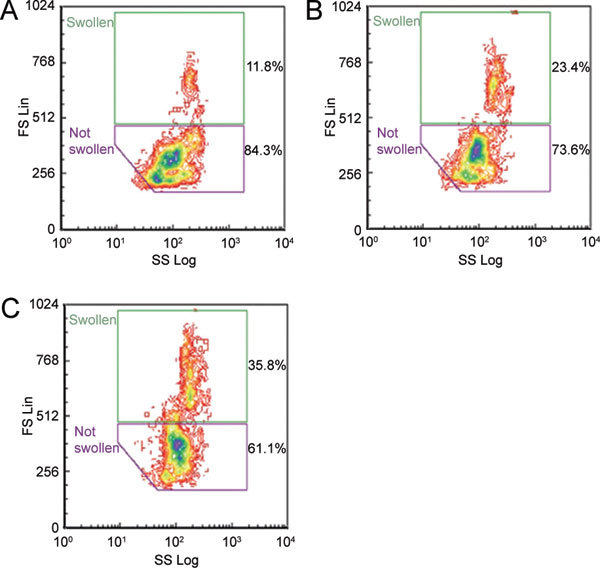
Dot plots (forward scatter ordinate, side scatter abscissa) of untreated control (A), 50 mmol L−1myo-inositol-treated (B) and 0.8 mmol L−1 quinine-treated positive control (C) caput epididymidal spermatozoa 15 min after being subjected to single-step hypotonicity (290 mmol kg−1). Percentages of 5 000 PI-negative spermatozoa within each window are given.
Calculation of the percentage of swollen cells in the consensus windows indicated that quinine significantly promoted swelling of spermatozoa from 1 to 45 min of incubation (mean values over this period for caput: control [Co] 13.4 ± 0.8 [mean ± SEM] vs. quinine [Qu] 33.0 ± 1.0, n = 68; cauda cells: Co 9.4 ± 0.3 vs. Qu 18.8 ± 0.4, n = 68; one-way ANOVA, P ≤ 0.001). A volume of 50 mmol L−1 of myo-inositol significantly promoted swelling of caput cells only after 15 min incubation (Co 14.5 ± 1.0 vs. myo-inositol 23.3 ± 2.8, n = 9; one-way ANOVA, P ≤ 0.001), and both 50 mmol L−1 glutamate (11.1 ± 1.6, n = 8) and 50 mmol L−1 KCl (9.8 ± 0.5, n = 8) significantly (one-way ANOVA, P ≤ 0.001) stimulated swelling of cauda cells (Co 6.44 ± 0.42), but only after 1 min.
Discussion
The present study examined maturation of rat spermatozoa from the viewpoint of their ability to regulate volume and lose osmolytes. From the assumption that intraluminal osmolality reflects that of the cells in that region, that is the bathing fluid is isotonic 36, suitable hypo-osmotic challenges should initiate volume regulation. The measurements of fluid osmolality recorded here for the rat cauda (365 mmol kg−1) are higher than those reported by Jones (37; 317 mmol kg−1, converted from ΔTf) and Levine and Marsh (22; 329 mmol kg−1), who both used freezing point depression on centrifuged fluid, but lower than that from Ekksitikul and Chulavatnatol (38; 505 mmol kg−1), who used the vapour pressure method used here, although without giving saturation time periods.
The advantage of vapour pressure measurements of osmolality is that samples can be monitored soon after collection, without centrifugation 39, and thus before any evaporation or hydrolysis that can occur during the prolonged (∼45 min) high-speed centrifugation needed to separate spermatozoa from the fluid 40, which may alter osmolality artificially. The demonstration of chamber saturation here validates the present results and shows rat cauda epididymidal fluid osmolality to be lower than that of the mouse 41, but similar to that of other species 11.
That there was no significant difference between the values from the caput and cauda fluid was unexpected, and may mean that osmotically active solutes are secreted more proximally in this species than in others, so that caput spermatozoa have already begun their maturation process and are able to regulate volume to some extent. The difference in osmolality between caput and cauda fluids was not great, but both osmolalities were higher than that of rete testis fluid, which varies around 332 42, 328 43 and 307 mmol kg−1 44. This suggests that regulatory volume increase is necessary for rat epididymal spermatozoa to maintain their volume at female tract fluid osmolality (287 mmol kg−1 23), as it is for spermatozoa from other species 17.
When transferred from the epididymis to fluids with osmolality close to those measured for epididymal fluid, there was a difference in the size of caput and cauda epididymidal spermatozoa. Caput spermatozoa were significantly larger than those from the cauda. As no major osmotic insult should have been experienced by the cells, these volumes should be isotonic cell volumes; the large caput size may reflect the retention of droplets that were lost by the cauda cells. Rat epididymal sperm volume has been measured by electronic sizing after transfer to a solution isotonic with blood (but clearly hypotonic to epididymal fluid) and it varies considerably. Brotherton 45 measured cauda sperm volume to be between 68 and 78 fL and stated 46 that cauda cells were 10% smaller than caput spermatozoa (although data in the table reported the opposite caput 78 fL < cauda 86 fL). Si et al.24 reported volumes between 36 and 37 fL. The differences reflect the difficulty in measuring volumes of nonspherical cells with small cytoplasmic reserves that are susceptible to physical stress.
The gradual application of permeant cryoprotectants at isotonicity is suggested to be less damaging to human and bovine spermatozoa than a single-step addition, by limiting the extent of cell membrane excursion, and thus cell damage, at each step 47, 48, 49. However, gradual exposure to hypotonic media was found to be more damaging to rat spermatozoa than that caused during the single step and caused fewer caput cells to swell, although this could be an effect of the repeated centrifugation. Unlike the general similarity seen in the response of mature and immature spermatozoa to decreasing osmolality on volume regulation, there was a marked difference in sensitivity in head membrane permeability, with cauda head membranes staining with PI to a far greater extent than those of caput cells at osmolalities below ∼240 mmol kg−1. These data parallel the rapid decline in viability of cauda cells below 260 mmol kg−1, shown by Si et al.24 for Fisher and Sprague-Dawley rats, after return to 290 mmol kg−1 after hypotonic challenge. Mature rat spermatozoa are also more susceptible than caput cells to damage by acylcarnitines when swollen in glycerol 35.
The determination of cell swelling by flow cytometry was far more sensitive than that of microscopic observation of flagellar coiling, because subpopulations of swollen caput and cauda spermatozoa were apparent when they were subjected to a physiological insult (at 290 mmol kg−1) in the absence (caput) and presence (cauda) of quinine, when no microscopic coiling was evident. The difference between the results from the microscope and flow cytometer reflects the fact that they measure different aspects of the same phenomenon (morphology and light scattering). Measurement of both of these have drawbacks: flagellar coiling will depend on the stiffness of the flagellum and the flow cytometer subjects the cells (especially to swollen cells) to additional shear stresses as they pass single-file through the detector.
The observations that DTT, a permeant sulfhydryl-reducing agent, increased the size of cauda cells in hypotonic medium in the absence of quinine confirms that the stiffness of cauda spermatozoa resisted volume change; caput spermatozoa stiffen with time in vitro25 and they also respond by swelling in the presence of DTT. Si et al. 24 also showed a reduction in motility and head membrane intactness when rat spermatozoa (from SD and Fisher strains) were subjected in a single step to large hypotonic stresses. Thus, the difference in the ability of cauda and caput spermatozoa to coil may not indicate a difference in their ability to regulate volume, but rather reflect an inability to swell, which in turn reflects the stiffness of the flagellum 25. This most likely is responsible for the loss of droplets when epididymal spermatozoa are released abruptly into hypotonic saline 11, as cell swelling and membrane rupture would be a consequence of the flagellum's failure to bend and to accommodate the increased cytoplasmic volume.
The osmosensitivity of spermatozoa from various species (mice 50, dogs 51, cats 52, horses 53, pigs 54, 55, bulls 56, men 47, eagles 57 and kangaroos 58) is usually determined by examining sperm function (motility or membrane intactness) after a single-step challenge to a hyper- or hypotonic medium that does not contain reducing agents. These studies have shown that mature (cauda) spermatozoa are damaged by hypotonic challenges. Comparison between mature and immature spermatozoa in the kangaroo showed that caput cells were more tolerant of hypotonic conditions than those of the cauda 58, as found here for rat spermatozoa. This could reflect similar in vitro effects on flagellar stiffness in that species.
This demonstrated inability of mature rat spermatozoa to alter shape and resist osmotically-induced swelling was confirmed by the observations in hypotonic medium containing quinine, which was anticipated to cause further swelling (as RVD is prevented), but which in fact reduced caput sperm swelling and had no effect on the cauda cells. Quinine would create extra tension on the cell membrane of hypotonically treated cells, promoting rupture of membrane and precluding their recognition as swollen cells. Such swelling would exacerbate the vulnerability of spermatozoa to shear forces experienced in the flow cytometer. An alternative explanation is that premature activation of RVD channels occurs in immature sperm cells, so that RVD is impaired and channel inhibitors reduce cell size 28. This has been shown for porcine ejaculated spermatozoa (already subjected to hypotonic accessory gland fluids before experimentation) that are undergoing necrotic volume increase 59. It is possible that immature spermatozoa exhibit an 'immature RVD' that resembles the 'impaired RVD' of necrotic cells, but any accelerated hypotonic regulation should have been observed as more efficient RVD by the immature cells, and this was not observed.
The results show that if a flow cytometer is to be used to monitor the effects of osmolytes on rat sperm cell volume, then the osmotic challenge should not be below 210 mmol kg−1 to exclude spurious readings associated with ruptured cells. Under these conditions, and by using the FS-determined windows to estimate the percentages of swollen cells, it was clear that both cauda and caput spermatozoa could regulate volume under hypotonic conditions, but that cauda spermatozoa were the more effective, in that fewer cells were swollen in the absence of quinine. The improvement is an evidence for the development of the ability to regulate volume on maturation in the epididymis and thus supports the hypothesis that infertility of immature spermatozoa could reflect failed volume regulation in the female tract 10, 19, 60, 61. The fact that quinine had the ability to block volume regulation by both mature and immature spermatozoa suggests that maturation occurs more proximally in the rat epididymis than that in the mouse. In the mouse, the distinction between caput and cauda cells is more marked, as hypotonically swollen caput spermatozoa fail to swell more in the presence of quinine 19. These results from both species suggest that RVD-related osmolytes are using potassium channels.
Despite the fact that during epididymal transit, rat spermatozoa sequentially encounter high (millimolar) concentrations of GPC 62, carnitine (rising in the distal caput 27) and myo-inositol (rising in the proximal cauda 63), the demonstration that neither caput nor cauda spermatozoa responded markedly or consistently by swelling to the organic osmolytes added to hypotonic medium argues that these compounds are not involved in the process of volume regulation. Rat caput spermatozoa have already been exposed in rete testis fluid to high concentrations of K+, but not such high concentrations of carnitine or myo-inositol (see Cooper and Yeung 11 and Turner 64), so that RVD employing K+ may be sufficient for them to maintain volume.
Differences between caput and cauda spermatozoa in the extent of osmolyte handling was evident here, and recently bovine caput epididymidal spermatozoa were shown to regulate their isotonic volume in a K+-dependent way 60, but less effectively in that of cauda spermatozoa 61. The attractive concept that organic osmolytes produced by the epididymis are involved in sperm volume regulation was not supported by the indirect studies presented here. Direct measurement of sperm osmolytes before and after osmotic challenge may indicate their utilization in this process. Nevertheless, impaired volume regulation, whether or not of epididymal origin, is one cause of male infertility 65, 66 that could be inhibited for post-testicular contraception.
Conflict of interest
The authors declare that there is no conflict of interest that could be perceived as prejudicing the impartiality of the research reported.
Acknowledgments
This work was supported by the Deutsche Forschungsgemeinschaft DFG Grant Co 24812/1.
References
- Dyson AL, Orgebin-Crist MC. Effect of hypophysectomy, castration and androgen replacement upon the fertilizing ability of rat epididymal spermatozoa. Endocrinology. 1973;93:391–402. doi: 10.1210/endo-93-2-391. [DOI] [PubMed] [Google Scholar]
- Paz (Frenkel) G, Kaplan R, Yedwab G, Homonnai ZT, Kraicer PF. The effect of caffeine on rat epididymal spermatozoa: motility, metabolism and fertilizing capacity. Int J Androl. 1978. pp. 145–52.
- Blandau RJ. On the factors involved in sperm transport through the cervix uteri of the albino rat. Am J Anat. 1945;77:253–72. [Google Scholar]
- Rossman I. Uterine contractions and the transport of sperm of the rat. Anat Rec. 1937;69:133–49. [Google Scholar]
- Blandau RJ, Rumery RE. The relationship of swimming movements of epididymal spermatozoa to their fertilizing capacity. Fertil Steril. 1964;15:571–9. doi: 10.1016/s0015-0282(16)35401-2. [DOI] [PubMed] [Google Scholar]
- Gaddum-Rosse P. Some observations on sperm transport through the uterotubal junction of the rat. Am J Anat. 1981;160:333–41. doi: 10.1002/aja.1001600309. [DOI] [PubMed] [Google Scholar]
- Fray CS, Hoffer AP, Fawcett DW. A reexamination of motility patterns of rat epididymal spermatozoa. Anat Rec. 1972;173:301–7. doi: 10.1002/ar.1091730305. [DOI] [PubMed] [Google Scholar]
- Yeung CH, Oberlander G, Cooper TG. Characterization of the motility of maturing rat spermatozoa by computer-aided objective measurement. J Reprod Fertil. 1992;96:427–41. doi: 10.1530/jrf.0.0960427. [DOI] [PubMed] [Google Scholar]
- Moore HD, Akhondi MA. Fertilizing capacity of rat spermatozoa is correlated with decline in straight-line velocity measured by continuous computer-aided sperm analysis: epididymal rat spermatozoa from the proximal cauda have a greater fertilizing capacity in vitro than those from the distal cauda or vas deferens. J Androl. 1996;17:50–60. [PubMed] [Google Scholar]
- Cooper TG. Sperm maturation in the epididymis: a new look at an old problem. Asian J Androl. 2007;9:533–9. doi: 10.1111/j.1745-7262.2007.00285.x. [DOI] [PubMed] [Google Scholar]
- Cooper TG, Yeung CH. Acquisition of volume regulatory response of sperm upon maturation in the epididymis and the role of the cytoplasmic droplet. Microsc Res Tech. 2003;61:28–38. doi: 10.1002/jemt.10314. [DOI] [PubMed] [Google Scholar]
- Cooper TG, Yeung CH, Wagenfeld A, Nieschlag E, Poutanen M, et al. Mouse models of infertility due to swollen spermatozoa. Mol Cell Endocrinol. 2004;216:55–63. doi: 10.1016/j.mce.2003.10.076. [DOI] [PubMed] [Google Scholar]
- Petrunkina AM, Gehlhaar R, Drommer W, Waberski D, Töpfer-Petersen E. Selective sperm binding to pig oviductal epithelium in vitro. Reproduction. 2001;121:889–96. [PubMed] [Google Scholar]
- Jeulin C, Soufir JC, Marson J, Paquignon M, Dacheux JL. Acetylcarnitine and spermatozoa: relationship with epididymal maturation and motility in the boar and man. Reprod Nutr Dev. 1988;28:1317–27. [PubMed] [Google Scholar]
- Pruneda A, Pinart E, Bonet S, Yeung CH, Cooper TG. Study of the polyol pathway in the porcine epididymis. Mol Reprod Dev. 2006;73:859–65. doi: 10.1002/mrd.20481. [DOI] [PubMed] [Google Scholar]
- Pruneda A, Yeung CH, Bonet S, Pinart E, Cooper TG. Concentrations of carnitine, glutamate and myo-inositol in epididymal fluid and spermatozoa from boars. Anim Reprod Sci. 2007;97:344–55. doi: 10.1016/j.anireprosci.2006.01.013. [DOI] [PubMed] [Google Scholar]
- Yeung CH, Barfield JP, Cooper TG. Physiological volume regulation by spermatozoa. Mol Cell Endocrinol. 2006;250:98–105. doi: 10.1016/j.mce.2005.12.030. [DOI] [PubMed] [Google Scholar]
- Yeung CH, Anapolski M, Setiawan I, Lang F, Cooper TG. Effects of putative epididymal osmolytes on sperm volume regulation of fertile and infertile c-ros transgenic mice. J Androl. 2004;25:216–23. doi: 10.1002/j.1939-4640.2004.tb02781.x. [DOI] [PubMed] [Google Scholar]
- Yeung CH, Anapolski M, Sipilä P, Wagenfeld A, Poutanen M, et al. Sperm volume regulation: maturational changes in fertile and infertile transgenic mice and association with kinematics and tail angulation. Biol Reprod. 2002;67:269–75. doi: 10.1095/biolreprod67.1.269. [DOI] [PubMed] [Google Scholar]
- Yeung CH, Sonnenberg-Riethmacher E, Cooper TG. Infertile spermatozoa of c-ros tyrosine kinase receptor knockout mice show flagellar angulation and maturational defects in cell volume regulatory mechanisms. Biol Reprod. 1999;61:1062–9. doi: 10.1095/biolreprod61.4.1062. [DOI] [PubMed] [Google Scholar]
- Yeung CH, Wagenfeld A, Nieschlag E, Cooper TG. The cause of infertility of c-ros tyrosine kinase knockout male mice. Biol Reprod. 2000;63:612–8. doi: 10.1095/biolreprod63.2.612. [DOI] [PubMed] [Google Scholar]
- Levine N, Marsh DJ. Micropuncture studies of the electrochemical aspects of fluid and electrolyte transport in individual seminiferous tubules, the epididymis and the vas deferens in rats. J Physiol. 1971;213:557–70. doi: 10.1113/jphysiol.1971.sp009400. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Waring DW. Rate of formation and osmolality of oviductal fluid in the cycling rat. Biol Reprod. 1976;15:297–302. doi: 10.1095/biolreprod15.3.297. [DOI] [PubMed] [Google Scholar]
- Si W, Benson JD, Men H, Critser JK. Osmotic tolerance limits and effects of cryoprotectants on the motility, plasma membrane integrity and acrosomal integrity of rat sperm. Cryobiology. 2006;53:336–48. doi: 10.1016/j.cryobiol.2006.09.001. [DOI] [PubMed] [Google Scholar]
- Cooper TG. Prevention of hypo-osmotic swelling by detergents provides clues to the membrane structure of rat sperm. Int J Androl. 1985;8:159–67. doi: 10.1111/j.1365-2605.1985.tb00829.x. [DOI] [PubMed] [Google Scholar]
- Biggers JD, Whittem WK, Whittingham DG.The culture of mouse embryos in vitro Daniel JC Jr, editor. Methods in Mammalian EmbryologySan Francisco: Freeman; 197186–116.
- Hinton BT, Snoswell AM, Setchell BP. The concentration of carnitine in the luminal fluid of the testis and epididymis of the rat and some other mammals. J Reprod Fertil. 1979;56:105–11. doi: 10.1530/jrf.0.0560105. [DOI] [PubMed] [Google Scholar]
- Petrunkina AM, Harrison RA, Tsolova M, Jebe E, Töpfer-Petersen E. Signalling pathways involved in the control of sperm cell volume. Reproduction. 2007;133:61–73. doi: 10.1530/rep.1.01137. [DOI] [PubMed] [Google Scholar]
- Yeung CH, Anapolski M, Cooper TG. Measurement of volume changes in mouse spermatozoa using an electronic sizing analyzer and a flow cytometer: validation and application to an infertile mouse model. J Androl. 2002;23:522–8. [PubMed] [Google Scholar]
- Yeung CH, Anapolski M, Depenbusch M, Zitzmann M, Cooper TG. Human sperm volume regulation. Response to physiological changes in osmolality, channel blockers and potential sperm osmolytes. Hum Reprod. 2003;18:1029–36. doi: 10.1093/humrep/deg204. [DOI] [PubMed] [Google Scholar]
- Cummins JM, Woodall PF. On mammalian sperm dimensions. J Reprod Fertil. 1985;75:153–75. doi: 10.1530/jrf.0.0750153. [DOI] [PubMed] [Google Scholar]
- Yeung CH, Anapolski M, Cooper TG. Measurement of volume changes in mouse spermatozoa using an electronic sizing analyzer and a flow cytometer: validation and application to an infertile mouse model. J Androl. 2002;23:522–8. [PubMed] [Google Scholar]
- Petrunkina AM, Harrison RA. Systematic misestimation of cell subpopulations by flow cytometry: a mathematical analysis. Theriogenology. 2010;73:839–47. doi: 10.1016/j.theriogenology.2009.09.007. [DOI] [PubMed] [Google Scholar]
- Petrunkina AM, Waberski D, Bollwein H, Sieme H. Identifying non-sperm particles during flow cytometric physiological assessment: a simple approach. Theriogenology. 2010;73:995–1000. doi: 10.1016/j.theriogenology.2009.12.006. [DOI] [PubMed] [Google Scholar]
- Cooper TG. Osmotic swelling of maturing rat spermatozoa and lysis of caput spermatozoa by acylcarnitine and acylcholines. Gamete Res. 1986;14:47–56. [Google Scholar]
- Cooper TG, Barfield JP, Yeung CH. The tonicity of murine epididymal spermatozoa and their permeability towards common cryoprotectants and epididymal osmolytes. Reproduction. 2008;135:625–33. doi: 10.1530/REP-07-0573. [DOI] [PubMed] [Google Scholar]
- Jones R. Comparative biochemistry of mammalian epididymal plasma. Comp Biochem Physiol. 1978;61:365–70. doi: 10.1016/0305-0491(78)90138-4. [DOI] [PubMed] [Google Scholar]
- Eksittikul T, Chulavatnatol M. Increase in fluid viscosity during epididymal transit and the immobilization of rat epididymal spermatozoa. Int J Androl. 1986;9:229–40. doi: 10.1111/j.1365-2605.1986.tb00886.x. [DOI] [PubMed] [Google Scholar]
- Sweeney TE, Beuchat CA. Limitations of methods of osmometry: measuring the osmolality of biological fluids. Am J Physiol. 1993;264:R469–80. doi: 10.1152/ajpregu.1993.264.3.R469. [DOI] [PubMed] [Google Scholar]
- Back DJ, Shenton JC, Glover TD. The composition of epididymal plasma from the cauda epididymidis of the rat. J Reprod Fertil. 1974;40:211–4. doi: 10.1530/jrf.0.0400211. [DOI] [PubMed] [Google Scholar]
- Cooper TG, Barfield JP. Utility of infertile male models for contraception and conservation. Mol Cell Endrocrinol. 2006;250:206–11. doi: 10.1016/j.mce.2005.12.047. [DOI] [PubMed] [Google Scholar]
- Wu YW, Yuan D. A modified method for collection of rete testis fluid from the rat. Int J Androl. 1984;7:362–8. doi: 10.1111/j.1365-2605.1984.tb00793.x. [DOI] [PubMed] [Google Scholar]
- Tuck RR, Setchell BP, Waites GM, Young JA. The composition of fluid collected by micropuncture and catheterization from the seminiferous tubules and rete testis of rats. Pflugers Arch. 1970;318:225–43. doi: 10.1007/BF00593663. [DOI] [PubMed] [Google Scholar]
- Clulow J, Jones RC, Hansen LA. Micropuncture and cannulation studies of fluid composition and transport in the ductuli efferentes testis of the rat: comparisons with the homologous metanephric proximal tubule. Exp Physiol. 1994;79:915–28. doi: 10.1113/expphysiol.1994.sp003817. [DOI] [PubMed] [Google Scholar]
- Brotherton J. The counting and sizing of spermatozoa from ten animal species using a coulter counter. Andrologia. 1975;7:169–85. doi: 10.1111/j.1439-0272.1975.tb00923.x. [DOI] [PubMed] [Google Scholar]
- Brotherton J. Difference in size between spermatozoa from the cauda epididymidis and the caput epididymidis of the rat. J Reprod Fertil. 1975;48:365–6. doi: 10.1530/jrf.0.0480365. [DOI] [PubMed] [Google Scholar]
- Gao DY, Ashworth E, Watson PF, Kleinhans FW, Mazur P, et al. Hyperosmotic tolerance of human spermatozoa: separate effects of glycerol, sodium chloride, and sucrose on spermolysis. Biol Reprod. 1993;49:112–23. doi: 10.1095/biolreprod49.1.112. [DOI] [PubMed] [Google Scholar]
- Setyawan EE, Cooper TG, Widiasih DA, Junaidi A, Yeung CH. Effects of cryoprotectant treatments on bovine sperm function and osmolyte content. Asian J Androl. 2009;11:571–81. doi: 10.1038/aja.2009.44. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Widiasih D, Yeung CH, Junaidi A, Cooper TG. Multistep and single-step treatment of human spermatozoa with cryoprotectants. Fertil Steril. 2009;92:382–9. doi: 10.1016/j.fertnstert.2008.05.046. [DOI] [PubMed] [Google Scholar]
- Du J, Tao J, Kleinhans FW, Mazur P, Critser JK. Water volume and osmotic behaviour of mouse spermatozoa determined by electron paramagnetic resonance. J Reprod Fertil. 1994;101:37–42. doi: 10.1530/jrf.0.1010037. [DOI] [PubMed] [Google Scholar]
- Songsasen N, Yu I, Murton S, Paccamonti DL, Eilts BE, et al. Osmotic sensitivity of canine spermatozoa. Cryobiology. 2002;44:79–90. doi: 10.1016/S0011-2240(02)00009-3. [DOI] [PubMed] [Google Scholar]
- Pukazhenthi B, Noiles E, Pelican K, Donoghue A, Wildt D, et al. Osmotic effects on feline spermatozoa from normospermic versus teratospermic donors. Cryobiology. 2000;40:139–50. doi: 10.1006/cryo.2000.2233. [DOI] [PubMed] [Google Scholar]
- Pommer AC, Rutllant J, Meyers SA. The role of osmotic resistance on equine spermatozoal function. Theriogenology. 2002;58:1373–84. doi: 10.1016/s0093-691x(02)01039-7. [DOI] [PubMed] [Google Scholar]
- Petrunkina AM, Topfer-Petersen E. Heterogeneous osmotic behaviour in boar sperm populations and its relevance for detection of changes in plasma membrane. Reprod Fertil Dev. 2000;12:297–305. doi: 10.1071/rd00087. [DOI] [PubMed] [Google Scholar]
- Caiza De La Cueva FI, Rigau T, Pujol R, Piedrafita J, Rodriguez-Gil JE. Resistance to hyperosmotic stress in boar spermatozoa: the role of the ionic pumps and the relationship with cryosurvival. Anim Reprod Sci. 1997;48:301–15. doi: 10.1016/s0378-4320(97)00061-4. [DOI] [PubMed] [Google Scholar]
- Guthrie HD, Liu J, Critser JK. Osmotic tolerance limits and effects of cryoprotectants on motility of bovine spermatozoa. Biol Reprod. 2002;67:1811–6. doi: 10.1095/biolreprod67.6.1811. [DOI] [PubMed] [Google Scholar]
- Blanco JM, Gee G, Wildt DE, Donoghue AM. Species variation in osmotic, cryoprotectant, and cooling rate tolerance in poultry, eagle, and peregrine falcon spermatozoa. Biol Reprod. 2000;63:1164–71. doi: 10.1095/biolreprod63.4.1164. [DOI] [PubMed] [Google Scholar]
- McClean R, MacCallum C, Blyde D, Holt W, Johnston S. Actin localisation and the effect of cytochalasin D on the osmotic tolerance of cauda epididymidal kangaroo spermatozoa. Cryo Letters. 2006;27:253–60. [PubMed] [Google Scholar]
- Petrunkina AM, Jebe E, Töpfer-Petersen E. Regulatory and necrotic volume increase in boar spermatozoa. J Cell Physiol. 2005;204:508–21. doi: 10.1002/jcp.20317. [DOI] [PubMed] [Google Scholar]
- Sahin E, Petrunkina AM, Waberski D, Harrison RA, Topfer-Petersen E. Control of bull sperm cell volume during epididymal maturation. Reprod Fertil Dev. 2009;21:469–78. doi: 10.1071/rd08162. [DOI] [PubMed] [Google Scholar]
- Sahin E, Petrunkina AM, Ekhlasi-Hundrieser M, Hettel C, Waberski D, et al. Fibronectin type II-module proteins in the bovine genital tract and their putative role in cell volume control during sperm maturation. Reprod Fertil Dev. 2009;21:479–88. doi: 10.1071/rd08209. [DOI] [PubMed] [Google Scholar]
- Hinton BT, Setchell BP. Concentrations of glycerophosphocholine, phosphocholine and free inorganic phosphate in the luminal fluid of the rat testis and epididymis. J Reprod Fertil. 1980;58:401–6. doi: 10.1530/jrf.0.0580401. [DOI] [PubMed] [Google Scholar]
- Hinton BT, White RW, Setchell BP. Concentrations of myo-inositol in the luminal fluid of the mammalian testis and epididymis. J Reprod Fertil. 1980;58:395–9. doi: 10.1530/jrf.0.0580395. [DOI] [PubMed] [Google Scholar]
- Turner TT.Necessity's potion: inorganic ions and small organic molecules in the epididymal lumenIn: Robaire B, Hinton BT, editors. The Epididymis. From Molecules to Clinical Practice. A Comprehensive Survey of the Efferent Ducts, the Epididymis and the Vas Deferens. New York: Kluwer Academic/Plenum publishers, Springer-Verlag; 2002. 131–50.
- Petrunkina AM, Petzoldt R, Stahlberg S, Pfeilsticker J, Beyerbach M, et al. Sperm-cell volumetric measurements as parameters in bull semen function evaluation: correlation with nonreturn rate. Andrologia. 2001;33:360–7. doi: 10.1046/j.1439-0272.2001.00457.x. [DOI] [PubMed] [Google Scholar]
- Petrunkina AM, Harrison RA, Ekhlasi-Hundrieser M, Töpfer-Petersen E. Role of volume-stimulated osmolyte and anion channels in volume regulation by mammalian sperm. Mol Hum Reprod. 2004;10:815–23. doi: 10.1093/molehr/gah106. [DOI] [PubMed] [Google Scholar]


