Abstract
The measurement or evaluation and clinical significance of human sperm morphology has always been and still is a controversial aspect of the semen analysis for the determination of a male's fertility potential. In this review the background of the development of the evaluation criteria for sperm morphology will be discussed. Aspects of criticism on the strict criteria definition and use of the criteria for sperm morphology evaluation will be discussed as well as possible reasons for the decline in normal sperm morphology values and how we can compromise for this phenomenon resulting in the very low normal reference value as published in the 2010 WHO manual for the Examination and Processing of Human Semen. One of the possible solutions may be to give more attention to a limited number of abnormal sperm morphology categories and the inclusion of sperm morphology patterns. It is concluded in this review that if done correctly and with care and with strict application of existing guidelines as outlined in the 2010 WHO manual, sperm morphology measurement still has a very important role to play in the clinical evaluation of male fertility potential.
Keywords: human sperm morphology, male fertility potential, strict criteria, traditional or liberal criteria, WHO manual for semen analysis
Introduction
The measurement (evaluation) and clinical significance of sperm morphology has always been and still is a controversial subject and there are many reasons for this situation. One of the reasons may be due to the early approach to sperm morphology evaluation as there was no scientific basis on which early human sperm morphology evaluation methodology was based. Furthermore, the evaluation of sperm morphology was and still is regarded as subjective due to the fact that it has to be done by the human eye. Even today most of the modern-day computer-assisted sperm morphology analysis (CAMA) systems still largely depend on human operator skills and are suffering from the same technical problems as manual of sperm morphology evaluation. This is due to preparation, fixation and staining methods of the semen smears, all of which have a severe influence on the sperm morphology evaluation results. As long as (persons working in) laboratories keep looking for shortcuts and the easy way out, especially with staining of the semen smears, and simplifying results to normal or abnormal, sperm morphology will not gain the important recognition as the very sophisticated measurement tool of human fertility potential it should be.
The incorrect assumption that sperm morphology is highly correlated with and depended on sperm concentration and motility also adds to this rejection of sperm morphology as a strong independent semen variable or more commonly called semen parameter. Sperm morphology is a very strong indicator of a person's bodily and thus testicular health, which is strongly reacting to bodily, physiological and environmental stresses, far more than any other organ. Therefore, one cannot expect it to react and be measured as other bodily chemical and blood functions. Unlike abnormal cellular and blood test results indicating a specific illness, abnormal sperm morphology is a reflection of negative stress factors working on the body without affecting the overall health of a specific male.
With the very low percentage of morphologically normal spermatozoa obtained in contemporary laboratory settings, morphology as a tool in the clinical diagnosis of a patient and also as a prognostic and predictive tool for the prediction of male fertility potential with regard to in vivo pregnancies and assisted reproductive technique outcome, sperm morphology evaluation now needs further refinement. Thus, besides a careful or strict approach or application of existing guidelines and adherence to high laboratory standards, the reintroduction of sperm abnormality classes and sperm morphology patterns can be of great clinical significance with regard to treatment, due to, for instance, stress caused by a male urogenital genital tract infection, reflected by a significant increase in the percentage of elongated spermatozoa. Sperm morphology can also help in clinical decision making, for instance, to take couple directly to in vitro fertilisation/intracytoplasmic sperm injection (IVF/ICSI) if genetically caused sperm abnormalities are diagnosed.
In this review these aspects will be discussed with reference to the development of criteria for sperm morphology evaluation, criticism on strict criteria, decreasing normal sperm morphology values, possible reasons for this phenomenon, the question if sperm morphology still has a place to play in today's clinical setting and the refinement of sperm morphology evaluation by the use of sperm morphology patterns.
Development of criteria for morphological normal spermatozoa
Early approach
With the initial evaluation of the role of semen parameters in male fertility and IVF, the main emphasis was on the sperm concentration and to a lesser degree sperm motility while sperm morphology did not receive much attention. This only changed in the first part of the 1900s.
Early contributions to underline the importance of sperm morphology were made in the early 1930s by Cary1 and Moench and Holt,2 by Williams in 19373 and Hammen in 1944,4 all of whom proposed different classification systems and or improved staining methods. Especially Williams made an important contribution to sperm morphology evaluation methodology by pointing out that the evaluation of sperm morphology depended largely upon objective findings, but that comparable results could be obtained by different observers provided that a uniform method of examination was adopted. This would only be possible if spermatozoa were examined minutely and classified into a limited number of groups of no more than six, based on the morphological appearance of the spermatozoa.4 However, it was only with the publications of especially the work from MacLeod in the late 1940s and early 1950s that the role of sperm morphology gained more recognition.5 MacLeod distinguished several classes of abnormal sperm head forms, and those spermatozoa not classified into any of these classes were regarded as normal. Abnormalities of the neck, mid-pieces and tails were not included.
In order to obtain better standardisation, Eliasson in 1971 made an important contribution as he stated that for the complete morphological rating of human spermatozoa, the mid-piece and tail should also be included and that the whole spermatozoon must therefore be taken into consideration with sperm morphology evaluation.6 As far as could be established, Eliasson was the first to put emphasis on the importance of sperm size and measurements in the morphological classification of spermatozoa. Eliasson classified sperm abnormalities in three main groups, viz., those of the sperm head, the mid-piece and the tail. For a spermatozoon to be classified as normal, the whole spermatozoon had to be normal with regard to the head, mid-piece and tail. A normal head had to be a regular oval shape. Borderline forms had to be counted as normal. If in doubt about the actual size of the spermatozoa, it should be measured with the aid of an eyepiece micrometer according to the now well-known measurements with normal head length and width between 3.0–5.0 µm and 2.0–3.0 µm, respectively, length of mid-piece 5.0–7.5 µm and width of mid-piece ∼1 µm and tail length ∼45 µm.
Eliasson distinguished six abnormal head classes, viz., too large (length >5.0 µm and width >3.0 µm), too small (length <3.0 µm and width <2.0 µm) and tapering (length >5.0 µm and width <3.0 µm or length <5.0 µm and width <2.0 µm). As long as the shape of the spermatozoa approximates an oval, they had to be counted primarily according to their size as too large, too small and tapering. Then there was a class for amorphous and a class for duplicate heads as well as class for ‘pear-shaped' heads which was to be counted under amorphous or tapering, while borderline forms were to be regarded as normal. The second main class was abnormal mid-pieces when the width of the mid-piece was >2 µm. Cytoplasmic droplets were to be included here when it was larger than half of the sperm head. The third main class was tail defects which included broken or coiled tails but not bended or asymmetrical insertion (into the anterior part of the head). According to Eliasson, the main objective with this classification was (i) to have as few and meaningful alternatives; (ii) to make a standardisation possible; and (iii) to make it easy to instruct other persons. However, there was once again no specific definition to describe a morphological normal spermatozoon and morphological normal spermatozoa were therefore identified indirectly by elimination.
In 1975, Eliasson7 wrote that many different types of spermatozoa had been described in the literature in detail but that there has been an obvious lack of uniformity in the definition of a spermatozoon with a ‘normal' configuration and refers to the work of Freund from 1966.8 The results of a comparative study between 47 laboratories conducted by Freund as based on the then-current head type-classification system for human sperm morphology assessment showed that the method was ‘personality orientated' as well as ‘subjective, qualitative, non-repeatable and difficult to teach to other persons' according to Katz et al..9 Furthermore, Eliasson stated that in order to learn about the relation between ‘morphological patterns' of spermatozoa in the ejaculate and the probability of fertility of the corresponding man one had to rely on probability analyses. This implies strict and reproducible methods for evaluation of sperm morphology and a realistic definition of fertility. Moreover, the criteria for ‘normal configuration' and for specified deviations from this normality must be defined in most precise terms.
In 1981, Eliasson10 expanded on the table with principles for the morphological assessment of human spermatozoa as published in 1971 and 1975.6, 7 Eliasson again stressed that the definitions as provided in the expanded table should not be looked upon as working standards, the relevance of which is in the progress of evaluation, and that the figures in the table did not represent any scientifically proven truth with regard to ‘normal' and ‘abnormal', but that on the other hand, without defined standards which can be used with a certain degree of precision, an accurate evaluation (of sperm morphology) would be impossible.
According to Katz et al.,9 little progress has been made since the publication of these articles in the standardisation for sperm morphology evaluation. According to these authors, the difficulty in classifying human sperm morphology is compounded by the fundamental biological fact that ejaculated spermatozoa do not confound to discrete categories of size and shape. Unlike the haematopoietic cells, for example, spermatozoa appear in an almost infinite variety of forms, and although metric standards have been cited for the dimensions of a ‘normal' human sperm head (by Eliasson6, 7, 10), neither a biological nor clinical basis for these ‘normal values' has been provided. Furthermore, Katz et al.9 stated that no objective morphological criteria for defining normal spermatozoa in human semen at the time of their publication in 1986 have been provided.
Development of the strict criteria definition for morphological normal spermatozoa
In many of the earliest reports on semen analyses that included reports on sperm morphology, it is clear that many of the samples were obtained from the cervical mucus after coitus. This was due to the fact that in many communities masturbation as a means of obtaining semen samples for investigations was frowned upon.11 Based on this technique, Cary1 reported in 1930 that the morphology of spermatozoa bears an definite relation to the success of their ability for migration through the cervical mucus as cells with enlarged and irregular heads are blocked by the selective hazard of the cervical mucus. Cary and Hotchkiss12 stated that sperm with abnormal morphology may process motility when observed in the wet specimen but on postcoital examination these abnormal forms are rarely, if ever found in the upper levels of cervical mucus, and stated that they considered these abnormal forms ineffective for fertilisation. In 1984, Fredricsson and Sennerstam13 reported that spermatozoa found in the cervical mucus are of strong prognostic significance for human fertility. Fredricsson and Björk14 reported that spermatozoa found in the vagina are the same as those in the same semen sample, while spermatozoa in the cervical secretions exhibit significant better morphology than those of the semen sample. Selection in cervical mucus is particular active against spermatozoa with abnormal heads, while abnormalities of the middle pieces and tails as well as tapering forms did not seem to interfere with the ability of spermatozoa to penetrate into cervical mucus. In contrast, Mortimer et al.15 showed that spermatozoa with mid-pieces and tail defects impairing the motility of the spermatozoa were less frequently found in the cervical mucus. Katz et al.16 concluded that spermatozoa migrating through good peri-ovulatory cervical mucus are subjected to a process of selection, but by a mechanism which is not jet fully understood. The result is that spermatozoa found in the mucus at the level of the internal os are usually an apparently homogeneous population, in contrast to the spermatozoa found in the seminal pool.
In an attempt to provide a definition of what could be regarded as a morphological normal spermatozoon based on scientific and biological evidence, Menkveld et al.17 published a description for morphological normal spermatozoa based on spermatozoa obtained from the level of the internal cervix os after penetration through good peri-ovulatory cervical mucus.
The definition for a morphologically normal spermatozoon as proposed by Menkveld et al.17 was as follows: the head requires a smooth oval configuration with a well-defined acrosome comprising about 40–70% of the sperm head. The normal head length should be between 3 and 5 µm and normal width between 2 and 3 µm. The width should be between three-fifths and two-thirds of the head length. So-called borderline normal head forms with no gross abnormalities should be regarded as abnormal. No neck, mid-piece or tail defects may be present. The mid-piece should be axially attached ≤1 µm in width and approximately one and a half time the head length. Cytoplasmic droplets (remnants) which comprise less than half the size of the sperm head can be present. The tail must be uniform, slightly thinner than the mid-piece, uncoiled and 45 µm in length.
This definition is essentially the same as that described by Eliasson in 19716 with regard to size and form but provides more descriptive detail with regard to the acrosome, and axially attachment of the neck. However, in an effort to keep the interpretation of normality as simple as possible, the rule that ‘if one is not sure about the classification of the spermatozoon the sperm should be considered as normal' as proposed by Eliasson was changed to the principle that in these cases the spermatozoa should be classified as abnormal.
Thus, although the concept of strong selection for certain types of spermatozoa through the cervical mucus and the importance of these specific type of spermatozoa has been known, it would appear from the available literature that the concept to use the morphological appearance of these spermatozoa to define a morphological normal sperm, based on the functional ability of spermatozoa, has not been used before. The principle has been underlined by the observations by Menkveld et al.18 and Liu and Baker19 that the human zona pellucida selectively binds spermatozoa with normal morphology as defined by strict criteria. This observation has been supported by the findings of Garrett et al.,20 who illustrated that in different semen samples each showing certain separate sperm morphology patterns, like too small, or too large or tapering, only those spermatozoa conforming the nearest to the description of morphologically normal spermatozoa will bind to the zona pellucida.
Criticism on the principles and use of strict criteria
Initially, the traditional or so-called the liberal approach21 was adopted in the 1980 and 1987 WHO manuals,22, 23 but after the publication of strict criteria methodology in 1990,17 the strict criteria principles were accepted in part in the 1992 WHO manual.24 In the 1999 WHO manual,25 strict criteria became the recommended method and were confirmed as the standard method of sperm morphology evaluation in the new 2010 WHO manual.26
However, there has been criticism on the concept and use of strict criteria from time to time, as not being scientific, not being evidence-based and unsuitable for use in the clinical laboratory. Furthermore, it was claimed that strict criteria morphology evaluations are less reproducible and less accurate than the liberal approach.21, 27, 28, 29 The earliest criticism against strict criteria came from Comhaire et al.21 as well as Morgentaler et al..27 Morgentaler et al.27 reported that a comparison of the tradition evaluation method and strict criteria with regard to IVF outcome favoured the traditional method in several areas. In part, low morphology scores with the traditional method were more predictive than strict criteria results. The strongest criticism, however, came from Eliasson as recently as 2003 and 2010.28, 29 Eliasson's principle arguments against the definition and use of strict criteria are inter alia the following:
1. The definition is not logical: according to Eliasson, ‘criterion' basically means ‘standards' and Eliasson argues that standard cannot be more or less strict, but that criteria can be applied in a more or less stricter manner. According to Eliasson, the Tygerberg group wanted to be stricter in its application and therefore placed cells with minor deviations in the “abnormal” group. However, the message that Menkveld et al.17 wanted to bring across was that if one wants to do sperm morphology evaluation on a high standard, standards can be set low (poor standards) or high (excellent), then one must adhere strictly to the guidelines set by the WHO manuals. In order to keep variations in sperm morphology evaluations as small as possible, it was suggested that the WHO recommendation should be changed to ‘borderline normal sperm should be regarded as abnormal—or if not sure regard the sperm cells as abnormal' rather than regard it as normal.
2. Form and function are separate properties: Eliasson argues that it is impossible to claim that ‘nice looking' or ‘ideal' spermatozoa found in cervical mucus are functionally better than those with the same appearance, form and morphometric measures in the seminal plasma. This is difficult to understand. The appearance of spermatozoa after penetration through good peri-ovulatory cervical mucus was only used to provide a definition for a morphological normal spermatozoon. If a normal looking spermatozoon is not able to penetrate through cervical mucus, then the spermatozoon must have a functional disability, as can be concluded from the first part of the section on the development of strict criteria definition for a morphologically normal spermatozoon. According to Eliasson, it is also impossible to accept the frequently used statement that when ‘strict criteria' is not applied one will have two populations: one ‘true' normal and one ‘misjudged' normal. Eliasson argued that when one (a sperm) is judged morphologically normal by any of the systems, it is by definition normal for that system and that in any system morphologically normal spermatozoa can be functionally abnormal. The whole point we have tried to make with our definition and strict criteria methodology was that the traditional definition was wrong. In fact, there was no definition for a normal spermatozoon based on scientific evidence. Due to the fact that spermatozoa were primary first classified according to known abnormalities, and what was left was to be considered as normal, the range for these left over spermatozoa was too wide and therefore included spermatozoa, which according to strict criteria, will be classified as abnormal. A definition by itself can be a wrong definition if not based on scientific facts.
3. Variations in measurement are not a function of borderline forms: by traditional or liberal criteria, borderline forms were rated as normal. By strict criteria, borderline normal forms are regarded as abnormal. The reason for this is to keep variations in assessments within and between observers as small as possible. According to Eliasson, borderline forms have nothing to do with the magnitude of internal variations in the assessment of sperm morphology and with internal quality control in the laboratory for morphology assessment. According to Eliasson, it is a serious misunderstanding to believe that the statement ‘borderline form be regarded as normal', according to the traditional approach, means that one has not to be less consistent (=strict) in the assessment. Our own experience found that the inclusion of borderline forms led to greater differences in results between observers as published previously.30 Kruger et al.31 proposed the inclusion of borderline normal form as an extra parameter in their 1988 article, but this has rarely been adopted in practice.
4. Strict criteria are not compatible with meaningful reference limits: according to Eliasson, it is impossible with strict criteria to establish a lower cutoff limit as would be the case with the tradition or liberal method. It will appear as if this is one of the main reasons for Eliasson's opposition towards strict criteria—results of morphology evaluation according to strict criteria do not follow or allow for statistical rules or patterns. In this regard, Eliasson refers to the paper of Morgentaler et al.27 who published a study where morphology was evaluated according to the traditional or liberal and strict criteria methods by two independent persons. The traditional evaluation method followed the traditional score diagram, while the results obtained with strict criteria did not, and therefore, the traditional method should be regarded as superior in relation to strict criteria methodology. However, Morgentaler et al. themselves stated that morphology assessment is a subjective test, with the absence of uniform standards which render a study as performed by themselves vulnerable to criticism that their morphology assessment may have been performed improperly. Indeed, they state that their results were lower than those reported by Kruger et al. in 198632 and 198831 and by Grow et al.33 and that they (Morgentaler et al.) have been too strict. Another criticism by Eliasson on strict criteria is that with the publication of strict criteria morphology results for the first time in 1986 and 1988,31, 32 the results were not compared to the results of another sperm evaluation methodology. Although not done in the first two clinical papers published on strict criteria,31, 32 Oehninger et al.34 published a paper in which they compared the morphology evaluation results as obtained by strict criteria and the traditional sperm morphology evaluation method, showing irrefutably the advantages of strict criteria morphology evaluation over the traditional method as a diagnostic and prognostic tool with regard to IVF outcome.
5. Strict criteria are useless in the study of testicular health: Eliasson made the statement that one can have good reason to believe that ‘health of the testis' is of importance for fertility and that health cannot be studied by counting the number of ‘ideal cells' but that one should try to understand the message brought to the investigator via the abnormal cells (sperm morphology patterns—own interpretation) to help to classify the underlying illness or pathology and that the ‘exfoliative cytology' of a semen sample is not different from the message or diagnosis made by observations of abnormal cervical or blood cells. In addition, a ‘normal' sperm is not identical to a functionally healthy sperm and the WHO manuals went wrong by suggesting to classify spermatozoa only as normal or abnormal, a system that was not originally described by Menkveld et al.17 for strict criteria. Eliasson stresses once again that the functionality of spermatozoa is of more importance than its morphological appearance, and therefore that abnormal categories should be reintroduced.29 However, we have repeatedly stated in previous publications that one needs morphologically normal spermatozoa for normal sperm functions and, as Eliasson has reported, that morphologically normal spermatozoa by strict criteria do not imply functionally normal spermatozoa. We have also repeatedly stated that, especially in patients with a high percentage of apparently morphologically normal spermatozoa, it is of importance to investigate the functional abilities of these spermatozoa.35
In his article of 2010,29 Eliasson once again argued that no data were presented to demonstrate that strict criteria represented an improvement over the liberal or traditional WHO morphology evaluation approach as published in 1980 and 1987.22, 23 Eliasson stated that among samples evaluated according to the traditional method with normal spermatozoa concentrations and motility, a diagnosis of only abnormal sperm morphology was associated with reduced fertilisation, while with strict criteria this was not. The results suggest that the liberal WHO sperm morphology evaluation method can serve as an independent factor with regard to IVF rate outcome, while the evaluations by strict criteria could not provide this information. However, in the first publications by Kruger et al.31, 32 demonstrating the strong prognostic value of strict criteria, only patients with a sperm concentration of more than 20 million sperm ml−1 and progressive motility of more than 30% were included so as to eliminate the possible influence of sperm concentration and motility on the results.
In the 2010 publication, Eliasson29 repeats his main criticism that strict criteria provides very low normative reference values which are, according to Eliasson, too low and therefore can provide little scope for diagnosis, making it a meaningless end point (see point 4 above). As a remedy, Eliasson suggests that different criteria for categorizing spermatozoa as morphologically abnormal (and borderline forms considered normal), as in earlier editions of the WHO manuals,22, 23 should be re-implemented. This should provide higher percentages of morphologically normal spermatozoa and thus increase the possibility of distinguishing (more clearly) men with lower percentage of such forms. Thus, more workable and informative reference limits of around 40–60% morphologically normal spermatozoa would be provided by using the less restrictive assessments.36
In response to these statements, Handelsman and Cooper36 argued that unless good evidence supports the criteria that determine whichever morphological criteria are chosen as reference thresholds, it seems to be putting the cart before the horse: choosing end points for its ability to distinguish subgroups rather than to separate groups on biological function that comprise authentic clinical fertility rather than laboratory surrogate end points.
In the same journal, Auger37 comments that the innovative nature of the strict criteria definition for describing sperm morphology should be stressed and that before strict criteria was developed, several classification methods were used with vague definitions or no definitions at all, making it difficult to obtain consistent results, from different observers. Such absent or vague definitions often resulted in percentage of morphologically normal spermatozoa being as high as 80% with differences of 30% or more between two observers scoring the same slide. Auger37 also argued that it cannot be denied that with the current very low normal cutoff values with strict criteria, there is little room for any groups with lower percentages of morphologically normal forms to be distinct from fertile groups or men, which naturally reduces the diagnosis value of the low sperm morphology reference limits as published in the 2010 WHO manual26 and that it is thus not a parameter worth examining and that other alternatives should be considered, like total normal sperm concentration.
Decreasing normal sperm morphology values
As mentioned above, there is now a very strong decreasing trend in the reported percentages of morphologically normal spermatozoa. One can argue about the fact if the introduction of strict criteria and especially the guideline that, contrary to those of the traditional approach, ‘borderline normal forms should be taken as abnormal', may be a major contribution factor towards this trend. However, before the introduction of strict criteria, there were already reports that together with the possible decrease in mean sperm concentrations, possible decreases for normal sperm morphology also occurred.
In 1974, Nelson and Bunge38 speculated that there may be a decreasing trend in human semen parameters possibly due to an adverse environmental factor to which the entire population is exposed to. To investigate this, they studied semen specimens of a large number of men before these men underwent vasectomies as they presumed these men to be representative of a normal population. The average sperm concentration of these men was 48 million ml−1 semen which was significant lower than the 107 million ml−1 semen of 1000 fertile men reported by MacLeod and Gold in 1951.39 The average percentage of abnormal forms of 26% (74% normal) was also significantly (P<0.005) lower compared to the 21% (79% normal) abnormal forms reported in another article by MacLeod and Gold5 in 1952.
Although we could not find a decreasing trend in the mean sperm concentrations of the patients visiting our infertility clinic over a 15-year period of time, from 1968 to 1982, we also observed a significant decreasing trend in the mean percentage of morphologically normal spermatozoa.40 The decreasing trend was stronger in our coloured patient group (r=−0.4156) compared to our white population group (r=−0.3404) with our black population in between (r=−0.3864). We postulated that sperm morphology was a very sensitive semen parameter and that any adverse (e.g. environmental) factor will first be reflected in the sperm morphology as a temporary decrease and after repeated exposure as a permanent decrease in normal sperm morphology. The fact that the decrease was of a greater magnitude in our coloured population was ascribed to the possibility that men from this group were more exposed to adverse environmental conditions. In an additional analysis of our data up to 1995, extending the time period up to 28 years, this trend was continued with regression values (r) of −0.9506, −0.9462 and −0.8505 for our coloured, white and black populations, respectively.
Other more recent reports on decreasing sperm morphology values were published inter alia by Auger et al.41 who found a 0.7% decrease in percentage of morphologically normal spermatozoa per year over a 20-year period, from 1973 to 1992, in the semen samples of health fertile men. Benshushan et al.42 found a decrease of 1.04% per year over a 15-year period, from 1980 to 1995, in a population of healthy students who donated semen for artificial inseminations. A steady decline of 0.3% per year from 1989 to 2000 in percentage of morphologically normal spermatozoa was also found by Chen et al.43 from Boston in the United States over this 11-year period as seen in semen samples from patients attending their clinic. In their study, the decline in the percentage of morphologically normal spermatozoa was mainly due to increased head defects and to a lesser extend to tail defects. Morphology evaluation was done according to strict criteria.
It can be speculated that the reason for the drastic decrease in the normal sperm morphology cutoff values over the years, are mainly threefold, viz., (i) the implementation of strict criteria with the unfortunate response that sperm morphology evaluation became over critical with regard to normality; (ii) the fact that with years more criteria for sperm morphological abnormalities were identified and introduced into the evaluation system; and (iii) a true decline due to negative environmental factors. These points have been discussed in detail in a previous publication by Menkveld.35
Clinical relevance of strict criteria
It may be argued that in itself the very low normal sperm morphology cutoff value of >4% morphological normal spermatozoa as given in the 2010 WHO manual26 and also in the recently published articles by Menkveld et al.44 and Haugen et al.45 may be of limited prognostic value. However, it must be kept in mind that the 2010 WHO manual cutoff value is based on the lower fifth percentile of several combined studies of so-called fertile male populations. This means that in practice most fertile men will have a higher percentage of morphologically normal spermatozoa. This is illustrated in Table 1, providing data of several recent publications where semen parameters of so-called fertile and infertile populations have been compared. Although there is a big overlap in the percentage of morphologically normal spermatozoa, the mean percentage of morphologically normal spermatozoa in the two groups are clearly different from each other.44, 45, 46, 47, 48, 49, 50, 51
Table 1. Recent published data of sperm morphology (% morphological normal) in fertile and subfertile populations.
| Publication | Sperm morphology (% normal) | |||
|---|---|---|---|---|
| Fertile population | Subfertile population | |||
| Mean (s.d.) | Range | Mean (s.d.) | Range | |
| Ombelet et al., 199746 | 12.0 | 1.0–27.0 | 6.6 | 0.0–20.0 |
| Zinaman et al., 200047 | 6.2 (3.7) | 0.2–20.5 | 4.1(3.5)a | 0.0–16.4 |
| Günalp et al., 200148 | 14.9 (5.9) | 2.0–30.0 | 10.1 (8.3) | 0.0–32.0 |
| Guzick et al., 200149 | 14.0 (5.0) | ND | 11.0 (6.0) | ND |
| Menkveld et al., 200144 | 6.5 (3.9) | 1.0–19.0 | 3.0 (2.6)b | 0.0–12.0 |
| Haugen et al.c, 200645 | 13.9 (7.6) | 2.0–34.0 | N/A | N/A |
| Jedrzejczak et al., 200750 | 15.9 (6.5) | 3.0–29.0 | 9.3 (4.9) | 0.0–19.0 |
| Ombelet et al.d, 200951 | 7.4 | 0.0–23.0 | N/A | N/A |
ND = Not done or given.
N/A = Not applicable.
Non-pregnant group.
Selected on an initial count <20 million ml−1.
Fertile population.
Women with anovulation, presumed to represent men from a general population.
This is also illustrated by our own (unpublished) data of three studies which initially started at the IVF Unit of the Department of Obstetrics and Gynecology of the Free University of Amsterdam, and continued at the Department of Obstetrics and Gynecology, Bronovo Hospital, The Hague, The Netherlands. The one group consists of data captured in 2007 from men who recently fathered a child (n=112) and from two groups of men from couples complaining of infertility as captured in 1998 (n=95) and in 2009 (n=100). Summary statistics are presented in Table 2. There was a decreasing trend (P<0.001) in the mean percentages of morphologically normal spermatozoa between the two infertile groups from 1998 and 2009. The mean percentage of morphologically normal spermatozoa for the fertile group was significantly higher compared to the means of the two infertile groups as illustrated in Figure 1. It is therefore clear that notwithstanding the decrease in mean values for morphological normal spermatozoa over years, there is still a clear trend for higher normal values in the fertile population compared to those of the infertile populations.
Table 2. Summary statistics for the sperm morphology (% normal) for the three groups investigated as evaluated at Tygerberg Hospital.
| Group | Sperm morphology (% normal) | |||||
|---|---|---|---|---|---|---|
| n | Median | Meana | s.d. | Minimum | Maximum | |
| Fertile (2007) | 112 | 7.0 | 7.2 | 3.9 | 1 | 21 |
| Infertile (1998) | 95 | 4.0 | 6.4 | 5.0 | 1 | 19 |
| Infertile (2009) | 100 | 2.0 | 2.4 | 1.9 | 1 | 9 |
Mann–Whitney test for independent values: Fertile (2007) versus Infertile (1998), P<0.0164; Fertile (2007) versus Infertile (2009), P<0.001; Infertile (1998) versus Infertile (2009), P<0.001.
Figure 1.
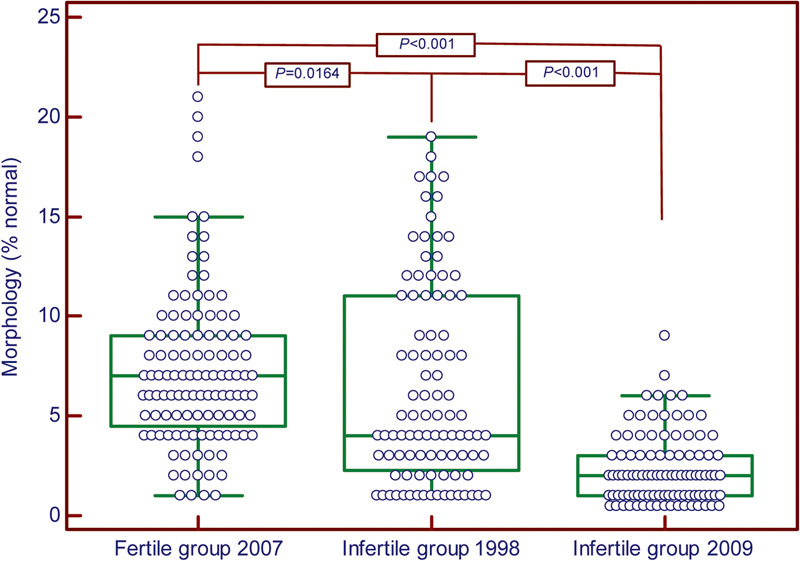
Box and whisker plots for sperm morphology from the three groups investigated. Upper and lower lines from boxes represent the 25 and 75 percentiles and middle lines represent the medians. The statistical significant values (Mann–Whitney test for independent values) are shown on top of boxes.
However, as already stated before, with these very low cutoff values of only 4% morphologically normal spermatozoa, it is becoming of the utmost importance to search for additional tools to identify those men who may have impaired fertility potential due to abnormal sperm morphology. One of these tools which can be employed may be sperm morphology patterns, including sperm size measurements as too small or too big.
Sperm patterns and sperm sizes as additional tools for sperm morphology evaluation
Even in the very early years, several articles have been published stating that sperm morphology patterns are very male-specific. Moreover, under normal circumstances, i.e., stress and illness-free conditions, these specific patterns have even been said to be as unique as the man's fingerprints.52, 53, 54
Certain sperm morphology anomalities are known to be genetically determined like globozoospermia, the short tail syndrome as well as the small headed spermatozoa with small acrosomes. These patterns cannot be altered by environmental influences. Like microcephaly,55, 56 coiled tails around the sperm head also seems to be a genetically determined abnormality as this has also been reported to be consisted in certain males.57 These types of sperm abnormalities are normally easy to recognize. On the other hand, to classify spermatozoa as too large, too small or tapering, one need to make use of sperm measurements to ensure that these spermatozoa are classified in the correct category.
Sperm measurements
From some of the previous sections, it should be clear that sperm size and therefore sperm measurements is a very important aspect of the sperm morphology evaluation process. According to Hammen,4 biometrical examination of spermatozoa, i.e., sperm measurements or morphometrics, as a supplement examination of sperm morphology, was introduced as early as 1915, and thereafter has been advocated by Williams and Salvage in 192558 and by Moench and Holt in 1931.59
An important contribution towards the use of sperm measurements as a routine parameter in the sperm morphology evaluation process was made by Eliasson in 1971.6 However, the measurements as proposed by Eliasson6 and adopted in the first four WHO manuals22, 23, 24, 25 and other publications17 are in need of re-evaluation as the range allowed for, especially the normal head length of 3.0–5.0 µm, is too wide. Our own experience indicated that the head length for normal spermatozoa may vary between 4.0 and 4.5 µm with a mean (s.d.) of 4.07 (0.19) µm and a mean (s.d.) width of 2.98 (0.14) µm as measured with a build-in microscope eyepiece micrometer.35, 60 We have shown in several publications that males presenting with large headed spermatozoa of >5.0 µm in length, and with proportional increase in width, and/or large acrosomes, as seen in Papanicolaou-stained smears, can be associated with poor IVF results32 and decreased sperm functional abilities.61
Alternative measurements have previously been proposed by several authors like Katz et al. in 19869 and Garrett and Baker in 199520 reporting length and width measurements of 4.37×2.83 µm and 4.35×2.89 µm for Papanicolaou- and Shorr-stained smears of donors, respectively. However, these reports received little attention. The new WHO (2010) manual26 does not refer to the ‘old' sperm measurements anymore, but makes the comment that head dimensions of 77 Papanicolaou-stained semen smears, measured with a computerized system, to have a median length of 4.1 µm (95% confidence interval (CI): 3.7–4.7 µm), median width of 2.8 µm (95% CI: 2.5–3.2 µm) and a length-to-width ratio of 1.5 (95% CI: 1.3–1.8). Unfortunately, a comment is made that ‘once a normally shaped spermatozoa is identified, an eye-piece micrometer may be useful for distinguishing between the normal and abnormal (size—own word) spermatozoa, but with this technique the form of the sperm head is much more important than its dimension unless grossly abnormal'. However, it is for the normal oval form that sperm measurements are of the utmost importance. Sperm measurements are an area deserving much more attention in today's sperm morphology assessment methodology and more research is needed to establish more accurate sperm measurements.
Recently a paper by Maree et al.62 clearly demonstrated the effect of different fixation and staining methods on sperm measurements, as has previously been reported by Katz et al.9 Maree et al.62 performed measurements on an unselected population of males and analysed spermatozoa in wet preparations and three different staining methods, viz., Papanicolaou, Rapidiff® and a new stain called SpermBlue®. Results clearly indicated that Papanicolaou staining caused shrinkage of the sperm heads compared to the wet-preparation configurations, confirming the observations of Katz et al..9 The new SpermBlue® stain provided measurements more or less equal to the wet preparation measurements, while the Rapidiff® staining resulted in clearly larger head measurements as has previously been reported for rapid blood stains. The actual measurements are provided in Table 3 together with other reported sperm measurements. According to Maree et al.,62 the observed difference with the different staining techniques are mainly caused by the differences in osmolality of the fixation mediums for each staining technique. It is therefore important that the fixation and staining method should be taken into consideration when performing sperm morphology measurements and staining method indicated on report form.
Table 3. Means (s.d.) of sperm measurements obtained with manuals or CAMA measurements of stained and unstained smears.
| Authors | Methods | Stain | Length (µm) | Width (µm) |
|---|---|---|---|---|
| Menkveld60 | Manual | Papanicolaou | 4.07 (0.19) | 2.98 (0.14) |
| Katz et al.9 | CAMAa,b | Papanicolaou | 4.37 (4.26–4.49) | 2.83 (2.77–2.89) |
| Wet prep | 5.26 (5.18–5.35) | 3.37 (3.26–3.48) | ||
| WHO26 | CAMAa | Papanicolaou | 4.1 (3.7–4.7) | 2.8 (2.5–3.2) |
| Maree et al.62 | CAMA | Wet prep | 4.79 (0.26) | 2.82 (0.23) |
| Papanicolaou | 4.28 (0.27) | 2.65 (0.19) | ||
| SpermBlue® | 4.73 (0.27) | 2.75 (0.24) | ||
| Rapidiff® | 5.17 (0.27) | 3.12 (0.21) |
Abbreviation: CAMA, computer-assisted sperm morphology analysis.
Papanicolaou stain and fertile population.
Median (95% CI).
The importance of sperm morphology measurements, as part of the routine sperm morphology assessment, especially for less experienced observers, is illustrated by a recent (unpublished) study performed by us where it was observed that sperm size measurements could have been one of the reasons for discrepancies in the outcome of sperm morphology evaluation results between personnel from the Bronovo and Tygerberg Hospital laboratories. Semen smears from 100 men attending the IVF clinic at the Bronovo Hospital were compared. The mean (s.d.) percentage of morphologically normal spermatozoa as counted at Bronovo and Tygerberg Hospitals were 2.4% (2.3% range: 1–15%) and 2.4% (1.9% range: 1–9%), respectively. These results did not differ statistically from each other (P=0.3141). As can be observed form the difference in the ranges, there was a tendency by the observers at Bronovo Hospital for slightly higher percentages of morphologically normal spermatozoa compared to the results of the Tygerberg Hospital observer (RM). A careful re-evaluation of the five cases where the largest differences were observed indicated that these differences were mainly due to the fact that spermatozoa that were regarded as too large were counted/classified as normal by the Bronovo Hospital observers due to the fact that these spermatozoa have mostly a very nice looking oval appearance. This observation underlines the importance of the use of a build-in micrometer when performing sperm morphology evaluations, especially when training new personnel.
Clinical significance of sperm size
Small-headed spermatozoa
Small-headed spermatozoa have constantly been observed in the same patients and resulted in poor IVF and ICSI results.55 Poland et al.56 also reported that besides the percentage of morphologically normal spermatozoa, microcephaly was the most stable abnormality when present in the group of patients investigated. Thus, small-headed spermatozoa are a more generally occurring sperm abnormality pattern which is unfortunately not commonly recognized and reported as a severe sperm abnormality. When one is conscious about the occurrence of this abnormality, it is easy to observe. Small spermatozoa are, according to the classification provided by Eliasson,6 <3.5 µm in length and <2.5 µm in width. The same measurements (<3.5 µm by <2.5 µm) have been used by Kihaile et al.55 to classify Diff–Quik-stained spermatozoa as too small. Too small spermatozoa may also present with very small abnormally formed acrosomes. If not aware of this condition, poor results will be obtained with IVF and even ICSI, but with selection of the more normal appearing spermatozoa, ICSI results can be improved.55 Even when larger spermatozoa are present but with small acrosomes, <30% of normal sperm heads, the prognosis for the patient with IVF is poor as these spermatozoa have a low vitality and are not able to undergo the acrosome reaction.63 Gandini et al.64 found a positive correlation between TUNEL-positive spermatozoa and abnormal sperm morphology, with especially small-headed spermatozoa showing a very high degree of DNA fragmentation.
Large- or megalo-headed spermatozoa
Large- or megalo-headed sperm can be due to two reasons. The first may be the results of genetic, chromosomal or DNA aberrations. Spermatozoa with disomy can present with severe abnormal megalo heads and multiple tails. These spermatozoa are observed in the ejaculate and testicular biopsies65 of the same patients and low fertilisation rates, of 43.2% compared to 60.2%, were achieved when using the megalo-headed spermatozoa in ICSI compared to a control group, which had zero morphological normal forms as evaluated by strict criteria. Pregnancy rates of ICSI using megalo-headed spermatozoa were 9.1% compared to 40% of the control group. Low fertilisation and pregnancy rates are achieved with ICSI and this may be due to the high incidence of chromosomal aberrations in the ejaculated megalo-headed spermatozoa66 or due to poor DNA integrity or packaging. Bianchi et al.67 reported that >75% of megalo heads showed poor DNA packaging with chromomycin A3 staining resulting in lower fertilisation rates with subzonal sperm insemination.
Megalo heads can also be caused by the use of medicine such as sulphasalazine for the treatment of ulcerative colitis68 and Crohn's disease.69 When the sulphasalazine treatment is stopped, the semen parameters can return to normal values and the megalo heads can disappear. It is not clear if this will result in fertilisation and viable pregnancies. However, substitution of sulphasalazine with mesalazine has resulted in increased semen parameters and reduction of the megalo heads with subsequent viable pregnancies.70
Elongated spermatozoa
Elongation is generally recognized as a stress-induced sperm morphology aberration and is prevalent especially in males with urogenital gland infections and the presence of a varicocele.71 The classic tapering or narrow forms has been described in detail by Eliasson6 as being a sperm head longer than 5 µm and the width <3 µm or a length of <5 µm and a width of <2 µm. Pyriform heads are also included under elongation of spermatozoa. Sperm elongation is accomplished by severe structural damage as well as severe DNA damage. The increased sperm head length results from an abnormally elongated nucleus that also presents particular membranous layers between the outer and inner leaves of the nuclear envelope. The sperm nuclear anomalies are also associated with anomalies of the neck region, persistence of cytoplasmic residual material and increased frequency of chromosomal aneuploidy rates, together with impaired chromatin compaction, due to possible mechanisms such as meiotic non-disjunction during spermatogenesis.72 Low ICSI fertilisation rates have also been found in men with severely elongated spermatozoa compared to other sperm morphology abnormalities.73 However, when males with urogenital gland infections are treated with long-term antibiotics or a varicocele is corrected by a varicocelectomy, semen parameters including sperm morphology can improve and result in pregnancies.74 In the case of varicocelectomies where no substantial improvement in semen parameters is observed, pregnancies do still occur due to the improvement of sperm DNA quality caused by the reduction of reactive oxygen species after the varicocelectomy.75
Another illustration of the reversibility of elongated forms was presented by Toth76 who published a case study of a male presenting with a prolactin-secreting pituitary adenoma. Semen analyses showed a very low percentage (10%) of morphological normal spermatozoa due to a rather particular abnormality. The sperm cells had an overall elongated head with a ballooned acrosomal segment. A horizontal line was very often apparent around the equatorial segment, and a typical elongation of the post-equatorial segment was observed. These cells were previously interpreted as tapering forms but presented more like an extreme form of pyriform. Almost all of the extreme pyriforms had large amounts of cytoplasmic residual material over the mid-piece section, and several tail segments were observed to be abnormally formed. The patient underwent a trans-sphenoidal exploration of the pituitary fossa and the removal of a well-demarcated chromophobe adenoma. A semen analysis 2 months after the operation showed a marked improvement in normal morphology, followed by a pregnancy of his wife and total disappearance of the elongated typed spermatozoa about 1 year after the operation.
Eliasson's tables6, 7, 10 with the principles for morphological assessment (classification) of human spermatozoa describe two classes of elongated spermatozoa, the classical tapering form and the pear-shaped form. In the table from his 1981 publication, the pear-shaped form is described as ‘between oval and tapering, regular shape'.10 Eliasson mentions that the ‘pear-shaped head' is regarded as a major sperm defect related to subfertility in the bull by Blom77 and that it may be possible that a high percentage of ‘pear-shaped heads' may be related to human male subfertility and this served as a motivation to included this special subgroup in the assessment of human sperm morphology, but unfortunately no relationship between human infertility and this abnormality could at that stage has been presented. A relationship between ‘pear-shaped' sperm heads and male infertility has been published in later years by several other authors.76, 78
Rousso et al.78 stated that pyriform spermatozoa are a frequent but little studied sperm abnormality since described by Blom77 and Eliasson.6, 7, 10 Like Eliasson, Rousso et al.78 did not give a detailed description of the appearance of a pyriform spermatozoon but provided an illustration by means of a photograph. From this it is clear that pyriform spermatozoa consist of a normally shaped acrosomal structure which can be normal in size or enlarged, while the post-acrosomal region shows severe elongation and an acute narrowing to the posterior end, ending in about the same thickness as the connecting mid-piece. They found that the greater the number of spermatozoa with pyriform heads, the higher the percentage of other morphologically abnormal spermatozoa, especially spermatozoa with broken necks and cytoplasmic residues as well as coiled tails. There was also a significant positive correlation with acephalous sperms and acrosome-less sperm. Rousso et al. speculated that pyriform spermatozoa and the other above mentioned abnormal forms may all originate from the same abnormal morphogenetic process. These authors found a mean (s.d.) of 13% (7.8; range: 0–70%) and 22% (14.9% range: 1–31%) of pyriform spermatozoa (P<0.001) in fertile and subfertile populations, respectively. Unfortunately, the occurrence of this abnormality was not correlated with any fertilisation or pregnancy outcome studies.78 Fenster et al.79 reported that the psychological stress of a recent death of a close family member caused significant reductions in sperm movement characteristics and a marginal increase of spermatozoa with larger and more tapered nuclei. Therefore, it is clear that any type of stress can cause temporary changes of sperm morphology.
Conclusion
There is a concern about the fecundity of today's population. As a measure of fecundity, time to pregnancy and daily sperm production has been suggested as measurements of human reproductive health.80 However, taking only sperm production and time to pregnancy into consideration as a measurement of reproductive health may not be comprehensively enough. Sperm morphology may be a more sensitive tool to measure reproductive health and testicular stress, as illustrated n this review. The problem is that despite the WHO25, 26 and NASA-ESHRE guidelines,81 for the performance of semen analyses, results, especially those for sperm morphology, from around the world, are not comparable due to the use of different techniques and interpretations of the guidelines. Another method, to obtain more information about a male's potential fertility and overall health may be to look at sperm morphology patterns. There are genetically determined sperm morphology abnormalities and those caused by stress (physiological, mental and environment), which are reversible when the stress source is taken away. As mentioned before, it may be that the testes can recuperate after a single adverse attack or two, but after repeated episodes of stress or continuous stress, the testes may not be able to repair itself and permanent lower normal sperm morphology values may be the result.
The measurement or evaluation of sperm morphology remains therefore a very important tool in the diagnosis of a male's fertility potential and in the clinical decision making for the treatment of patients with infertility problems.
The authors declare no competing financial interests.
References
- Cary WH. Sterility diagnosis: the study of sperm cell migration in female secretions and interpretation of findings. NY State J Med. 1930;30:131–6. [Google Scholar]
- Moench GL, Holt H. Sperm morphology in relation to fertility. Am J Obstet Gyncol. 1931;22:199–210. [Google Scholar]
- Williams WW. Spermatic abnormalities. N Engl J Med. 1937;217:946–51. [Google Scholar]
- Hammen R. Studies on impaired fertility in man with special reference to the male. Acta Obstet Gynecol Scand. 1944;24 Suppl 3:1–205. [Google Scholar]
- MacLeod J. Gold RZ. The male factor in fertility and infertility. IV. Sperm morphology in fertile and infertile marriage. Fertil Steril. 1952;2:394–414. doi: 10.1016/s0015-0282(16)30661-6. [DOI] [PubMed] [Google Scholar]
- Eliasson R. Standards for investigation of human semen. Andrologie. 1971;3:49–64. [Google Scholar]
- Eliasson R.Analysis of semenIn: Behrman SJ, Kistner SW, editors.Progress in Infertility.2nd ed.Boston, CA: Little, Brown & Co.1975691–713. [Google Scholar]
- Freund M. Standards for the rating of human sperm morphology. Int J Fertil. 1966;11:97–118. [PubMed] [Google Scholar]
- Katz DF, Overstreet JW, Samuals SJ, Niswander PW, Bloom TD, et al. Morphometric analysis of spermatozoa in the assessment of human male infertility. J Androl. 1986;7:203–10. doi: 10.1002/j.1939-4640.1986.tb00913.x. [DOI] [PubMed] [Google Scholar]
- Eliasson R.Analysis of semenIn: Burger H, de Kretser D, editors.The Testis. New York: Raven Press; 1981381–99. [Google Scholar]
- Menkveld R, Kruger TF.Basic semen analysisIn: Acosta AA, Swanson RJ, Ackerman SB, Kruger TF, van Zyl JA, Menkveld R, editors.Human Spermatozoa in Assisted Reproduction. Baltimore, MD: Williams & Wilkins; 199068–84. [Google Scholar]
- Cary WH, Hotchkiss RS. Semen appraisal. A differential stain that advances the study of cell morphology. JAMA. 1934;102:587–90. [Google Scholar]
- Fredricsson B, Sennerstam R. Morphology of live seminal and postcoital cervical spermatozoa and its bearing on human fertility. Acta Obstet Gynecol Scand. 1984;63:329–33. doi: 10.3109/00016348409155526. [DOI] [PubMed] [Google Scholar]
- Fredricsson B, Björk G. Morphology of postcoital spermatozoa in the cervical secretion and its clinical significance. Fertil Steril. 1977;28:841–5. doi: 10.1016/s0015-0282(16)42738-x. [DOI] [PubMed] [Google Scholar]
- Mortimer D, Leslie EE, Kelley RW, Templeton AA. Morphological selection of human spermatozoa in vivo and in vitro. J Reprod Fertil. 1982;64:391–9. doi: 10.1530/jrf.0.0640391. [DOI] [PubMed] [Google Scholar]
- Katz DF, Diel l, Overstreet JW. Differences in the movement of morphologically normal and abnormal human seminal spermatozoa. Biol Reprod. 1982;26:566–70. doi: 10.1095/biolreprod26.4.566. [DOI] [PubMed] [Google Scholar]
- Menkveld R, Stander FS, Kotze TJ, Kruger TF, van Zyl JA. The evaluation of morphological characteristics of human spermatozoa according to stricter criteria. Hum Reprod. 1990;5:586–92. doi: 10.1093/oxfordjournals.humrep.a137150. [DOI] [PubMed] [Google Scholar]
- Menkveld R, Franken DR, Kruger TF, Oehninger S. Sperm selection capacity of the human zona pellucida. Mol Reprod Dev. 1991;30:346–52. doi: 10.1002/mrd.1080300409. [DOI] [PubMed] [Google Scholar]
- Liu DY, Baker HW. Morphology of spermatozoa bound to the zona pellucida of human oocytes that failed to fertilized in vitro. J Fertil Reprod. 1992;94:71–84. doi: 10.1530/jrf.0.0940071. [DOI] [PubMed] [Google Scholar]
- Garrett C, Baker HW. A new fully automated system for the morphometric analysis of human sperm heads. Fertil Steril. 1995;63:1306–17. [PubMed] [Google Scholar]
- Comhaire F, Schoonjans F, Vermeulen L, de Clercq N. Methodological aspects of sperm morphology evaluation: comparison between strict and liberal criteria. Fertil Steril. 1994;62:857–61. doi: 10.1016/s0015-0282(16)57016-2. [DOI] [PubMed] [Google Scholar]
- World Health Organization WHO Laboratory Manual for the Examination of Human Semen and Semen–Cervical Mucus Interaction1st ed.Singapore: Press Concern; 1980 [Google Scholar]
- World Health Organization WHO Laboratory Manual for the Examination of Human Semen and Semen–Cervical Mucus Interaction2nd ed.Cambridge: Cambridge University Press; 1987 [Google Scholar]
- World Health Organization WHO Laboratory Manual for the Examination of Human Semen and Sperm–Cervical Mucus Interaction3rd ed.Cambridge: Cambridge University Press; 1992 [Google Scholar]
- World Health Organization WHO Laboratory Manual for the Examination of Human Semen and Sperm–Cervical Mucus Interaction4th ed.Cambridge: Cambridge University Press; 1999 [Google Scholar]
- World Health Organization WHO Laboratory Manual for the Examination and Processing of Human Semen5th ed.Geneva: World Health Organisation Press; 2010 [Google Scholar]
- Morgentaler A, Fung MY, Harris DH, Powers RD, Alper MM. Sperm morphology and in vitro fertilization outcome: a direct comparison of World Health Organization and strict criteria methodologies. Fertil Steril. 1995;64:1177–82. doi: 10.1016/s0015-0282(16)57981-3. [DOI] [PubMed] [Google Scholar]
- Eliasson R.Basic semen analysisIn: Matson P, editor.Current Topics in Andrology. Perth, WA: Ladybrook Publishing; 200335–89. [Google Scholar]
- Eliasson R. Semen analysis with regard to sperm number, sperm morphology and functional aspects. Asian J Androl. 2010;12:26–32. doi: 10.1038/aja.2008.58. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Menkveld R, Kruger TF.Basic semen analysisIn: Acosta AA, Kruger TF, editors.Human Spermatozoa in Assisted Reproduction.2nd ed.Carnforth: Parthenon Publishing; 199653–71. [Google Scholar]
- Kruger TF, Acosta AA, Simmons KF, Swanson RJ, Matta JF, et al. Predictive value of abnormal sperm morphology in in vitro fertilization. Fertil Steril. 1988;49:112–7. doi: 10.1016/s0015-0282(16)59660-5. [DOI] [PubMed] [Google Scholar]
- Kruger TF, Menkveld R, Stander FS, Lombard CJ, van der Merwe JP, et al. Sperm morphological features as a prognostic factor in in vitro fertilization. Fertil Steril. 1986;46:1118–23. doi: 10.1016/s0015-0282(16)49891-2. [DOI] [PubMed] [Google Scholar]
- Grow DR, Oehninger S, Seltman HJ, Toner JP, Swanson RJ, et al. Sperm morphology as diagnosed by strict criteria: probing the impact of teratozoospermia on fertilization rate and pregnancy outcome in a large in vitro fertilization population. Fertil Steril. 1994;62:559–67. doi: 10.1016/s0015-0282(16)56946-5. [DOI] [PubMed] [Google Scholar]
- Oehninger S, Acosta AA, Kruger TF, Veeck LL, Flood J, et al. Failure of fertilization in in vitro fertilization: the “occult” male factor. J In Vitro Fert Embryo Transf. 1988;5:181–7. doi: 10.1007/BF01131119. [DOI] [PubMed] [Google Scholar]
- Menkveld R. Clinical significance of the normal sperm morphology value as proposed in the 5th WHO Laboratory Manual for the Examination and Processing of Human Semen. Asian J Androl. 2010;12:47–58. doi: 10.1038/aja.2009.14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Handelsman DJ, Cooper TG. Afterword to Semen Analysis in 21st Century Medical special issue in Asian Journal of Andrology. Asian J Androl. 2010;12:118–23. doi: 10.1038/aja.2009.50. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Auger J. Assessing human sperm morphology: top models, underdogs or biometrics. Asian J Androl. 2010;12:36–46. doi: 10.1038/aja.2009.8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nelson CM, Bunge RG. Semen analysis: evidence for changing parameters of male fertility potential. Fertil Steril. 1974;25:503–7. doi: 10.1016/s0015-0282(16)40454-1. [DOI] [PubMed] [Google Scholar]
- MacLeod J, Gold RZ. The male factor in fertility and infertility. II. Spermatozoon counts in 1000 men of known fertility and in 1000 cases of infertile marriage. J Urol. 1951;66:436–9. doi: 10.1016/S0022-5347(17)74358-3. [DOI] [PubMed] [Google Scholar]
- Menkveld R, van Zyl JA, Kotze TJ, Joubert G. Possible changes in male fertility over a 15-year period. Arch Androl. 1986;17:143–4. doi: 10.3109/01485018608990186. [DOI] [PubMed] [Google Scholar]
- Auger J, Kunstmann JM, Czyglik F, Jouannet P. Decline in semen quality among fertile men in Paris during the past 20 years. N Eng J Med. 1995;332:281–5. doi: 10.1056/NEJM199502023320501. [DOI] [PubMed] [Google Scholar]
- Benshushan A, Shoshani O, Paltiel O, Schenker JG, Lewin A. Is there really a decrease in sperm parameters among healthy young men? A survey of sperm donations during 15 years. J Assist Reprod Genet. 1997;14:347–53. doi: 10.1007/BF02765840. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen Z, Isaacson KB, Toth TL, Godfrey-Bailey L, Schiff I, et al. Temporal trends in human semen parameters in New England in the United Stated, 1989–2000. Arch Androl. 2003;49:369–74. doi: 10.1080/0145010390219700. [DOI] [PubMed] [Google Scholar]
- Menkveld R, Wong WY, Lombard CJ, Wetzels AM, Thomas CM, et al. Semen parameters including WHO and strict criteria morphology, in a fertile and subfertile population: an effort towards standardisation of in vivo thresholds. Hum Reprod. 2001;16:1165–71. doi: 10.1093/humrep/16.6.1165. [DOI] [PubMed] [Google Scholar]
- Haugen TB, Egeland T, Magnus Ø. Semen parameters in Norwegian fertile men. Int J Androl. 2006;27:66–71. doi: 10.2164/jandrol.05010. [DOI] [PubMed] [Google Scholar]
- Ombelet W, Bosmans E, Janssen M, Cox A, Vlasselaer J, et al. Semen parameters in a fertile versus subfertile population: a need for a change in the interpretation of semen testing. Hum Reprod. 1997;12:987–93. doi: 10.1093/humrep/12.5.987. [DOI] [PubMed] [Google Scholar]
- Zinaman MJ, Brown CC, Selevan SG, Clegg ED. Semen quality and human fertility: a prospective study with health couples. J Androl. 2000;21:145–53. [PubMed] [Google Scholar]
- Günalp S, Onculoglu C, Gurgan T, Kruger TF, Lombard CJ. A study of semen parameters with emphasis on sperm morphology in a fertile population: an attempt to develop clinical thresholds. Hum Reprod. 2001;16:110–4. doi: 10.1093/humrep/16.1.110. [DOI] [PubMed] [Google Scholar]
- Guzick DS, Overstreet JW, Factor-Litvak P, Brazil CK, Nakajima ST, et al. Sperm morphology, motility and concentration in fertile and infertile men. N Engl J Med. 2001;345:1388–93. doi: 10.1056/NEJMoa003005. [DOI] [PubMed] [Google Scholar]
- Jedrzejczak P, Taszarek-Hauke G, Hauke J, Pawelczyk l, Duleda AJ. Prediction of spontaneous conception based on semen parameters. Int J Androl. 2007;31:499–507. doi: 10.1111/j.1365-2605.2007.00799.x. [DOI] [PubMed] [Google Scholar]
- Ombelet W, Bosmans E, Cox A, Janssen M, Mestdagh G, et al. In search for the general population's semen profile: the study of sperm parameters in partners of women with chronic anovulation. F V V Obstet Gyn. 2009;1:18–26. [PMC free article] [PubMed] [Google Scholar]
- Hotchkiss RS. Factors in stability and variability of semen specimens—observations on 640 successive samples from 23 men. J Urol. 1941;45:875–88. [Google Scholar]
- Hartmann GC, Schoenfeld C, Copelande E. Individualism in the seminal picture of infertile men. Fertil Steril. 1964;15:231–53. doi: 10.1016/s0015-0282(16)35220-7. [DOI] [PubMed] [Google Scholar]
- MacLeod J. Human seminal cytology as a sensitive indicator of the germinal epithelium. Int J Fertil. 1964;9:281–95. [PubMed] [Google Scholar]
- Kihaile P, Hirotsuru K, Kumasako Y, Misumi J, Utsunomiya T. Fertilization rrates of small-headed sperm in conventional IVF and ICSI. Arch Androl. 2003;49:327–9. doi: 10.1080/01485010390219692. [DOI] [PubMed] [Google Scholar]
- Poland ML, Moghissi KS, Giblin PT, Ager JW, Olson JM. Stability of basic semen measures and abnormal morphology within individuals. J Androl. 1986;7:211–4. doi: 10.1002/j.1939-4640.1986.tb00915.x. [DOI] [PubMed] [Google Scholar]
- Yeung CH, Tüttelmann F, Bergmann M, Nordhoff V, Vorona E, et al. Coiled sperm from infertile patients: characteristics, associated factors and biological implications. Hum Reprod. 2009;24:1288–95. doi: 10.1093/humrep/dep017. [DOI] [PubMed] [Google Scholar]
- Williams WW, Salvage A. Observations on the seminal micro-pathology of bulls. Cornell Vet. 1925;15:353–75. [Google Scholar]
- Moench GL, Holt H. Sperm morphology in relation to fertility. Am J Obstet Gyncol. 1931;22:199–210. [Google Scholar]
- Menkveld R.The basic semen analysisIn: Oehninger S, Kruger TF, editors.Male Infertility: Diagnosis and Treatment. Oxon: Informa Healthcare; 2007141–70. [Google Scholar]
- Menkveld R, Rhemrev JP, Franken DR, Vermeiden JP, Kruger TF. Acrosomal morphology as a novel criterion for male fertility diagnosis: relation with acrosin activity, morphology (strict criteria) and fertilization in vitro. Fertil Steril. 1996;65:637–4. doi: 10.1016/s0015-0282(16)58167-9. [DOI] [PubMed] [Google Scholar]
- Maree L, du Plessis SS, Menkveld R, van der Horst G. Morphometric dimensions of the human sperm head depend on the staining method used. Hum Reprod. 2010;25:1369–82. doi: 10.1093/humrep/deq075. [DOI] [PubMed] [Google Scholar]
- Menkveld R, El-Garem Y, Schill WB, Henkel R. Relationship between human sperm acrosomal morphology and acrosomal function. J Assist Reprod Genet. 2003;20:432–8. doi: 10.1023/A:1026288710638. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gandini L, Lombardo F, Paoli D, Caponecchia L, Familiari G, et al. Study of apoptotic fragmentation in human spermatozoa. Hum Reprod. 2000;15:830–9. doi: 10.1093/humrep/15.4.830. [DOI] [PubMed] [Google Scholar]
- Kahraman S, Akarsu C, Cengiz G, Dirican K, Sözen E, et al. Fertility of ejaculated and testicular megalohead spermatozoa with intracytoplasmic sperm injection. Hum Reprod. 1999;14:726–30. doi: 10.1093/humrep/14.3.726. [DOI] [PubMed] [Google Scholar]
- Chelli MH, Albert M, Ray PF, Guthauser B, Izard V, et al. Can intracytoplasmic morphologically selected sperm injection be used to select normal-sized sperm heads in infertile patients with macrocephlic sperm head syndrome. Fertil Steril. 2010;93:1347.e1–7. doi: 10.1016/j.fertnstert.2008.10.059. [DOI] [PubMed] [Google Scholar]
- Bianchi PG, Manicardi GC, Urner F, Campana A, Sakkas D. Chromatin packaging and morphology in ejaculated human spermatozoa: evidence of hidden anomalies in normal spermatozoa. Mol Hum Reprod. 1996;2:139–44. doi: 10.1093/molehr/2.3.139. [DOI] [PubMed] [Google Scholar]
- Toth A. Reversible effect of salicylazosulfapyridine on semen quality. Fertil Steril. 1979;31:538–40. doi: 10.1016/s0015-0282(16)44000-8. [DOI] [PubMed] [Google Scholar]
- Cosentino MJ, Chey WY, Takihara H, Cockett AT. The effects of sulfasalazine on human male fertility potential and seminal prosdaglandins. J Urol. 1984;132:682–6. doi: 10.1016/s0022-5347(17)49824-7. [DOI] [PubMed] [Google Scholar]
- Riley SA, Lecarpentier J, Mani V, Goodman MJ, Mandal BK, et al. Sulphasalazine induced seminal abnormalities in ulcerative colitis: results of mesalazine substitution. Gut. 1987;28:1008–12. doi: 10.1136/gut.28.8.1008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Menkveld R, Kruger TF. Sperm morphology and male urogenital infections. Andrologia. 1998;30 Suppl 1:49–53. doi: 10.1111/j.1439-0272.1998.tb02826.x. [DOI] [PubMed] [Google Scholar]
- Prisant N, Escalier D, Soufir JC, Morillion M, Schoevaert D, et al. Ultrastructural nuclear defects and increased chromosome aueuploidies in spermatozoa with elongated heads. Hum Reprod. 2007;22:1052–9. doi: 10.1093/humrep/del481. [DOI] [PubMed] [Google Scholar]
- Osawa Y, Sueoka K, Iwata S, Shinohara M, Kobayashi N, et al. Assessment of the dominant abnormal form is useful for predicting the outcome if intracytoplasmic sperm injection in the case of sever teratozoospermia. J Assist Reprod Genet. 1999;16:436–42. doi: 10.1023/A:1020573609836. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Menkveld R.LeukocytospermiaIn: Daya S, Harrison RF, Kempers RD, editors.Advances in Fertility and Reproductive Medicine. Amsterdam: Elsevier BV; 2004218–24. [Google Scholar]
- Nasr-Esfahani MH, Abasi A, Razavi S, Ashrafi S, Tavalaee M. Varicocelectomy: semen parameters and protamine deficiency. Int J Androl. 2009;32:115–22. doi: 10.1111/j.1365-2605.2007.00822.x. [DOI] [PubMed] [Google Scholar]
- Toth A. Abnormal seminal cytology in a patient with prolactin-secreting pituitary adenoma. Fertil Steril. 1981;36:818–20. doi: 10.1016/s0015-0282(16)45932-7. [DOI] [PubMed] [Google Scholar]
- Blom E.The ultrastructure of some characteristic sperm defects and a proposal for a new classification of bull spermiogram Nord Vet Med 197325383–91.Danish. [PubMed] [Google Scholar]
- Rousso D, Kourtis A, Mavromatidis G, Gkoutzioulis F, Makedos G, et al. Pyriform head: a frequent but little-studied morphological abnormality of sperm. Arch Androl. 2002;48:267–72. doi: 10.1080/01485010290031574. [DOI] [PubMed] [Google Scholar]
- Fenster L, Katz DF, Wyrobek AJ, Rempel DM, Oman D, et al. Effect of psychological stress on human semen quality. J Androl. 1997;18:194–202. [PubMed] [Google Scholar]
- te Velde E, Burdorf A, Nieschlag E, Eijkemans R, Kremer JA, et al. Is human fecundity declining in Western countries. Hum Reprod. 2010;25:1348–1353. doi: 10.1093/humrep/deq085. [DOI] [PubMed] [Google Scholar]
- Kvist U, Björndahl L.editors.Manual on Basic Semen Analysis. ESHRE Monographs Oxford: Oxford University Press; 2002 [Google Scholar]


