Abstract
During mammalian fertilisation, the zona pellucida (ZP) matrix surrounding the oocyte is responsible for the binding of the spermatozoa to the oocyte and induction of the acrosome reaction (AR) in the ZP-bound spermatozoon. The AR is crucial for the penetration of the ZP matrix by spermatozoa. The ZP matrix in mice is composed of three glycoproteins designated ZP1, ZP2 and ZP3, whereas in humans, it is composed of four (ZP1, ZP2, ZP3 and ZP4). ZP3 acts as the putative primary sperm receptor and is responsible for AR induction in mice, whereas in humans (in addition to ZP3), ZP1 and ZP4 also induce the AR. The ability of ZP3 to induce the AR resides in its C-terminal fragment. O-linked glycans are critical for the murine ZP3-mediated AR. However, N-linked glycans of human ZP1, ZP3 and ZP4 have important roles in the induction of the AR. Studies with pharmacological inhibitors showed that the ZP3-induced AR involves the activation of the Gi-coupled receptor pathway, whereas ZP1- and ZP4-mediated ARs are independent of this pathway. The ZP3-induced AR involves the activation of T-type voltage-operated calcium channels (VOCCs), whereas ZP1- and ZP4-induced ARs involve both T- and L-type VOCCs. To conclude, in mice, ZP3 is primarily responsible for the binding of capacitated spermatozoa to the ZP matrix and induction of the AR, whereas in humans (in addition to ZP3), ZP1 and ZP4 also participate in these stages of fertilisation.
Keywords: acrosome reaction, fertilisation, oocyte, signalling pathways, spermatozoa, zona pellucida glycoproteins
Introduction
Mammalian fertilisation is a highly synchronized process that involves a complex series of interactions between the spermatozoon and the egg, culminating in their unison. The initial steps in fertilisation involves the binding of the spermatozoon to the zona pellucida (ZP) matrix surrounding the egg, followed by induction of the acrosome reaction (AR) in the zona-bound spermatozoon, a pre-requisite for penetration of the ZP matrix by the spermatozoon. The spermatozoon acrosome is a Golgi-derived organelle that forms a cap over the anterior two-thirds of its nucleus. The AR involves the fusion of the sperm membrane with the outer acrosomal membrane, resulting in release of the acrosomal contents and exposure of the inner acrosomal membrane on the anterior head of the spermatozoon. Various physiological agents, such as progesterone, serum albumin, follicular fluid, hormones (including biogenic amines), hydrolytic enzymes (particularly proteases), hyaluronic acid and ZP glycoproteins, have been implicated in the induction of the AR.1, 2, 3 In the present paper, we review the role of the ZP matrix and its constituents in AR induction. Various downstream signalling pathways involved in the ZP glycoprotein-induced AR will also be discussed. On the basis of the current literature and studies from our group, the salient differences in the ZP glycoprotein-mediated induction of the AR in mouse versus human will be highlighted.
Induction of AR by the ZP matrix
Composition of the ZP matrix
The mammalian ZP is composed of either three or four glycoproteins (Figure 1). The murine ZP matrix is composed of three glycoproteins designated ZP1 (623 amino acids (aa)), ZP2 (713 aa) and ZP3 (424 aa).4 Pig,5 cow6 and dog7 also have three glycoproteins, but instead of ZP1, ZP4 is present (Figure 1). However, the ZP matrices of rats, hamsters, bonnet monkeys and humans are composed of four glycoproteins: ZP1, ZP2, ZP3 and ZP4.8, 9, 10, 11, 12, 13 In humans, ZP1 has a 638-aa polypeptide backbone; ZP2 has 745 aa; ZP3 has 424 aa and ZP4 has 540 aa. The ZP glycoproteins are heavily glycosylated and have N- as well as O-linked glycans, which have crucial roles in the spermatozoon–ZP interaction and AR induction.14, 15 The orthologue of the human Zp4 gene is present in the mouse genome as a pseudogene and is, therefore, not expressed in the murine ZP matrix.7 In non-mammalian species, more than four ZP genes have been detected; for example, the chicken genome contains six genes (Zp1, Zp2, Zp3, Zp4, ZpAX and ZpD),16 and the Xenopus genome contains five genes (Zp2, Zp3, Zp4, ZpD and ZpAX).7
Figure 1.
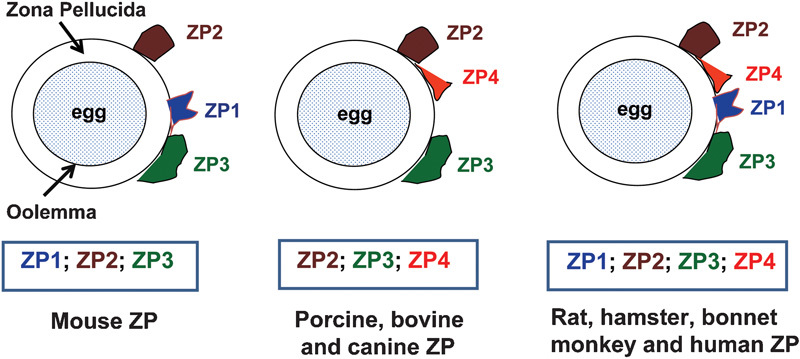
Schematic representation of the composition of the ZP in various mammals: The ZP matrix of the mammalian oocyte is composed of either three or four glycoproteins. The mouse ZP matrix is composed of three glycoproteins, namely, ZP1 (blue), ZP2 (brown) and ZP3 (green). The rat, hamster, bonnet monkey and human ZP matrices are composed of four glycoproteins: ZP1, ZP2, ZP3 and ZP4 (red). The bovine, porcine and canine ZP matrices contain three glycoproteins, with ZP4 replacing ZP1. ZP, zona pellucida.
Induction of the AR by the ZP matrix
Pioneering work by Paul Wassarman's group established that the binding of mouse sperm to the egg ZP is followed by AR induction.17 Solubilized ZPs isolated from unfertilized mouse eggs induce the AR, whereas those isolated from embryos fail to do so.17 As observed in murine models, incubation of capacitated human sperm with intact zonae or acid-disaggregated zonae also leads to a significant increase in the AR.18, 19 Progesterone and follicular fluid have priming effects on the ZP-induced AR.20 In contrast to the mouse ZP, the human ZP of fertilized oocytes retains its ability to bind sperm and also induce the AR.21 However, the rate of penetration of the human ZP matrix by such acrosome-reacted sperm is much lower than that of human sperm that has reacted with the ZP of unfertilized oocytes. These observations suggest that during fertilisation in humans, the block in polyspermy may also occur at the level of sperm penetration through the ZP matrix.21
Signalling events during ZP-mediated acrosomal exocytosis
Binding of a capacitated spermatozoon to the ZP matrix activates transmembrane signals that trigger cellular cascades resulting in the AR in the zona-bound sperm (Figure 2). At least two different receptor-mediated signalling pathways in the sperm plasma membrane are responsible for ZP-induced acrosomal exocytosis. One is a Gi protein-coupled receptor that activates the phospholipase Cβ1 (PLCβ1)-mediated signalling pathway, and the other is a tyrosine kinase receptor coupled to PLCγ (Figure 2).22 Incubation of mouse sperm membrane preparations with heat solubilized ZP prepared from unfertilized mouse eggs leads to a dose-dependent increase in guanosine triphosphate γ-S binding, as well as GTPase activity, suggesting that the Gi-coupled receptor pathway is involved in the ZP-mediated induction of the AR.23 The ZP may selectively activate Gi1 and Gi2 subtypes of Gi in the sperm.24 The participation of a second G protein, Gαq/11, has also been suggested.25
Figure 2.
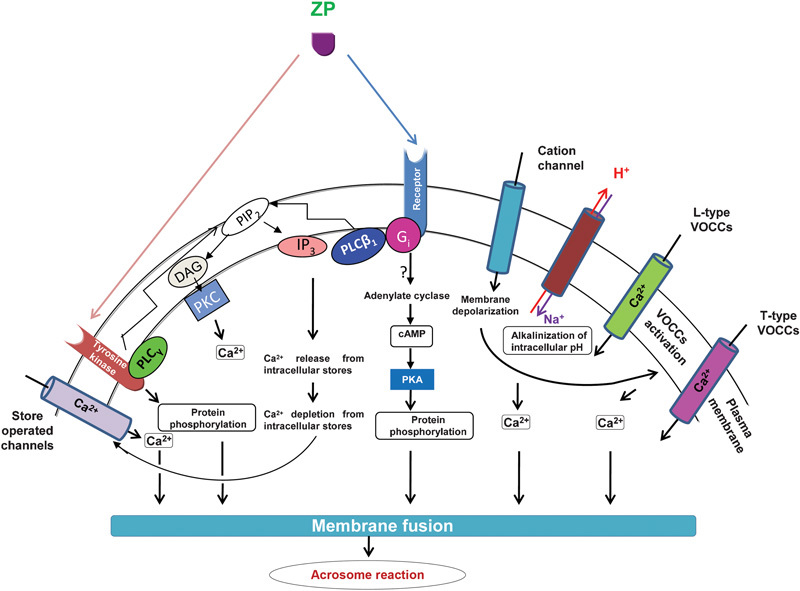
Schematic representation of various signalling pathways involved in ZP-mediated acrosomal exocytosis. Two prominent signalling pathways are known to operate in the sperm membrane upon ZP binding. One is the pertussis toxin sensitive Gi protein-coupled receptor linked to PLCβ1. The other is a putative tyrosine kinase receptor coupled to PLCγ. Receptor activation also induces adenylate cyclase activation, leading to the generation of cAMP and activation of PKA, which phosphorylates and activates downstream effector proteins. Agonist binding also activates cation channels present on the sperm plasma membrane, leading to membrane depolarisation and activation of L- and T-type VOCCs. An increase in intracellular alkalinisation also occurs due to activation of sodium/proton exchange pump that probably increases or amplifies calcium signals. PLCβ1 and PLCγ hydrolyse PIP2 in the membrane, leading to the generation of IP3 and DAG. DAG mediates PKC translocation to the plasma membrane and its activation, whereas IP3 mediates calcium entry into the sperm cytosol from intracellular stores. Depletion of calcium from internal stores leads to activation of voltage insensitive SOCs on the sperm cell surface by an undefined mechanism. This mediates another round of calcium entry, which leads to activation of components involved in the fusion of the outer acrosomal membrane with the sperm plasma membrane resulting in the AR. AR, acrosome reaction; cAMP, cyclic adenosine monophosphate; DAG, 1,2-diacylglycerol; PKA, protein kinase A; IP3, 1,4,5-inositol triphosphate; PIP2, phosphatidylinositol 4,5-bisphosphate; PLC, phospholipase C; SOC, store-operated channel; VOCC, voltage-operated calcium channel; ZP, zona pellucida.
Gi acts as a signal transducing element downstream of the ZP3–receptor interactions and couples receptor occupancy to changes in the ionic conductance and/or a variety of intracellular second messenger system cascades whose activation in turn results in the release of acrosomal contents.26 One such cascade is likely to be the activation of sodium/proton (Na+/H+) exchange pumps, resulting in intracellular alkalinisation.26, 27 Second messengers include the adenylate cyclase–cyclic adenosine monophosphate system, which activates protein kinase A (PKA), leading to the phosphorylation of specific putative proteins involved in acrosomal exocytosis. In addition, the activation of PLCβ1 and/or PLCγ leads to an increase in the levels of 1,2-diacylglycerol (DAG) and inositol 1,4,5-triphosphate (IP3). DAG may stimulate protein phosphorylation through PKC, whereas IP3 may activate intracellular calcium ([Ca2+]i) release through the modulation of IP3-sensitive intracellular calcium stores.25, 26, 27 Studies with the mouse ZP solubilized by acid disaggregation have shown that the ZP-induced AR is a Ca2+-dependent exocytotic event involving an increase in [Ca2+]i mediated primarily by T-type voltage-operated calcium channels (VOCCs).28, 29, 30 A role for L-type VOCCs has also been proposed during induction of the AR.31, 32 Inhibition of solubilized ZP-mediated AR induction by 3-quinuclidinyl benzilate (an antagonist of muscarinic receptors), tyrphostin A-48 (a tyrosine kinase inhibitor) and pertussis toxin (an inhibitor of Gi protein signalling) suggests that the binding of the ZP to sperm plasma membrane receptors involves several downstream signalling pathways.28
Spermatozoa maintain an inwardly negative membrane potential and conductance through cation channels, producing a depolarizing current. Binding of mouse ZP3 to sperm activates a cation channel (impermeable to anions) that conducts monovalent and divalent cations and leads to sperm membrane depolarisation from about −60 to −30 mV. Depolarisation of the sperm membrane potential opens the T-type VOCCs. However, the voltage-dependent inactivation of T currents occurs within 50–100 ms during depolarisation,29, 33, 34 thereby terminating the ZP3-induced calcium influx. The T-type channels may also be modulated by their state of tyrosine phosphorylation during capacitation and ZP3 stimulation.35 However, a sustained release of calcium is an absolute requirement for an induction of the AR.
After depletion of calcium from internal stores, store-operated channels, which are voltage-insensitive calcium channels in the plasma membrane, are activated and mediate the second phase of calcium entry, referred to as capacitative calcium entry.36 Mammalian transient receptor potential proteins, which are homologues of the Drosophila melanogaster photoreceptor cell transient receptor potential protein, are involved in the ZP3-mediated capacitative calcium entry in mice.36 Transient receptor potential homologues have also been located in human sperm.37, 38 In addition, members of soluble N-ethyl maleimide-sensitive factor attachment protein receptor proteins present in the acrosome region of mammalian sperm may also facilitate calcium entry, thereby leading to the AR.39, 40 The high intracellular free calcium concentration together with DAG leads to membrane fusion and finally acrosomal exocytosis.2, 41
Induction of the AR by the solubilized human ZP depends on extracellular Ca2+20, 42 and involves activation of Gi protein-coupled receptor pathway signalling,20, 42, 43, 44, 45 tyrosine kinases,42 PKA, PKC, phosphoinositide-3 kinase,42, 46 T-type VOCCs and gamma aminobutyric acid-A receptor-associated chloride channels.42
Roles of ZP constituent glycoproteins in induction of the AR
It seems that the composition and, consequently, the structure of the mammalian ZP is more complicated than expected because, depending on the species: (i) it is formed by three or four ZP glycoproteins (Figure 1); (ii) in the three-glycoprotein model, it can be formed by ZP1, ZP2 and ZP3 or ZP2, ZP3 and ZP4 (Figure 1); and (iii) the protein responsible for sperm binding and AR induction may vary across species. To delineate the roles of individual zona proteins, various groups have either used the purified protein from a native source (which makes it difficult to rule out minor contamination from other egg-associated or zona proteins) or the recombinant protein. Using recombinant protein ensures that it is not contaminated by other zona proteins. However, the recombinant proteins may not have the conformation and glycosylation of its native counterpart. Nonetheless, both approaches have been used to delineate the role of individual zona proteins in binding sperm and inducing the AR. Table 1 summarizes the role of individual zona proteins in sperm binding and AR induction.
Table 1. Zona pellucida glycoproteins involved in sperm binding and induction of the acrosome reaction in mice and humans.
| Species | ZP protein | Function | Reference | |
|---|---|---|---|---|
| Binding to capacitated spermatozoa | Induction of acrosome reaction | |||
| Mice | Native ZP3 | Yes | Yes | 17, 47 |
| Recombinant ZP3 | Yes | Yes | 58 | |
| Humans | ZP1 | |||
| Baculovirus-expressed recombinant protein | Yes | Yes | 31 | |
| ZP3 | ||||
| Escherichia coli-expressed recombinant ZP3 | ND | Yes | 65 | |
| Yes | No | 53 | ||
| Baculovirus-expressed recombinant ZP3 | Yes | Yes | 52, 53 | |
| ND | Yes | 61 | ||
| ND | Yes | 32 | ||
| Mammalian-expressed recombinant ZP3 | ND | Yes | 62 | |
| Yes | Yes | 63 | ||
| ND | Yes | 64 | ||
| Native ZP3 | Yes | Yes | 55,60 | |
| ZP4 | ||||
| E. coli-expressed recombinant ZP4 | Yes | No | 53 | |
| Baculovirus-expressed recombinant ZP4 | Yes | Yes | 52,53 | |
| ND | Yes | 61 | ||
| ND | Yes | 32 | ||
| Native ZP4 | Yes | Yes | 55, 60 | |
Abbreviations: ND, not done; ZP, zona pellucida.
ZP1
In a murine model, ZP1 purified from ZPs of unfertilized eggs does not interfere significantly with the binding of sperm to eggs in vitro, suggesting that ZP1 does not bind to sperm.47 Furthermore, ZP1 purified from mouse eggs has no significant effect on AR compared with the respective control.17 However, it has been postulated that cross-linking by ZP1, the filaments formed by ZP2–ZP3 heterodimers, may provide stability and structural integrity to the ZP matrix.48 Studies in quail and chicken have shown that ZP1 (dimeric in chicken) is capable of inducing the AR.49, 50 Recent studies from our group have shown that both Escherichia coli- and baculovirus-expressed recombinant human ZP1 conjugated to fluorescein isothiocyanate bind to the anterior head of capacitated human spermatozoa (Table 1).31 Baculovirus-expressed recombinant ZP1 also generates a dose-dependent increase in acrosomal exocytosis, which involves activation of both T- and L-type VOCCs. The failure of E. coli-expressed recombinant human ZP1 to induce the AR suggests that glycosylation of ZP1 is critical for its ability to induce the AR. Induction of the AR by ZP1 does not depend on activation of the Gi protein-coupled receptor pathway, whereas human solubilized ZP- as well as ZP3- (described below) mediated ARs involve activation of the Gi protein. Inhibition of PKA and PKC significantly reduces the ZP1-mediated induction of the AR.31
ZP2
Mouse ZP2 purified from ZPs of unfertilized eggs does not interfere with sperm–egg binding or with induction of the AR.17, 51 Monoclonal and polyclonal antibodies against mouse ZP2 do not affect the initial binding of the sperm to the egg but do significantly inhibit the binding of acrosome-reacted sperm to the ZP, suggesting that ZP2 serves as a secondary receptor for sperm during fertilisation.51 In humans, neither E. coli- nor baculovirus-expressed recombinant ZP2 binds to the capacitated acrosome-intact human spermatozoa or induces any significant increase in AR.52, 53 The fluorescein isothiocyanate-coupled recombinant human ZP2 has shown binding to the equatorial region of acrosome-reacted spermatozoa, suggesting that as in mice, human ZP2 is not involved in the induction of the AR and primarily acts as a secondary sperm receptor.53 Employing a highly specific monoclonal antibody (MA-1615) generated against baculovirus-expressed recombinant human ZP2 that is devoid of reactivity in ELISA and western blots with recombinant human ZP3 and ZP4,54 purification of human ZP2 from ZPs of unfertilized human oocytes from the assisted reproduction program has been reported.55 Purified native human ZP2 binds to the acrosomal region of only acrosome-reacted human spermatozoa, corroborating the findings observed using recombinant ZP2.55
ZP3
The initial adhesion event between the mouse sperm and the ZP is a high affinity event involving about 30 000 binding sites (300 molecules/µm2) ascribed to ZP3, which are sufficient to tether a spermatozoon to the extracellular matrix prior to AR induction.56 The contact subsequently becomes more tenacious, and the bound spermatozoon undergoes the AR. Among the various physiological and pharmacological inducers of the AR, ZP3 has been accepted as the natural agonist (except in guinea pig) that initiates the AR upon binding of the acrosome-intact mammalian spermatozoa to the ZP.57 Purified mouse ZP3 binds to the anterior head region of the capacitated acrosome-intact spermatozoon, thus acting as a putative primary sperm receptor.47 Further, recombinant mouse ZP3 expressed in mammalian cells also decreases sperm–ZP binding and triggers acrosomal exocytosis in capacitated mouse sperm.58 In hamsters and humans, ZP3 performs the function of primary sperm receptor.52, 53, 55, 59 Studies employing purified native human ZP3,60 as well as baculovirus-expressed recombinant ZP3,32, 52, 53, 61 have shown dose-dependent increases in acrosomal exocytosis. Moreover, human ZP3 expressed in mammalian cells also leads to an increase in the AR.62, 63, 64 However, there are conflicting observations with respect to the efficacy of E. coli-expressed recombinant human ZP3 in inducing the AR. According to one report, E. coli-expressed recombinant ZP3 induces the AR, but a significant increase in the AR is observed only after 18 h of incubation of the capacitated sperm with the recombinant protein.65 Our group has shown that E. coli-expressed recombinant human ZP3, though binding to the anterior head of the capacitated spermatozoon, fails to induce the AR, suggesting that glycosylation of ZP3 is critical for AR induction (Table 1).52, 53
Delineation of the domain of ZP3 involved in induction of the AR
To understand the role of ZP3 during fertilisation, it is imperative to delineate the region(s) responsible for its functional activity. Studies with insoluble pronase-digested mouse ZP3 revealed that small glycopeptides (about 1.5–6.0 kDa) are capable of inhibiting the binding of sperm to eggs; however, they did not induce the sperm to complete acrosomal exocytosis.66 Further, mouse ZP3 was digested with either papain or V8 protease to yield a 55-kDa glycoprotein.67, 68 The ∼55 kDa glycopeptide was derived from the carboxy-terminal half of ZP3 and possessed four or five potential N-linked glycosylation sites, and after removal of N-linked oligosaccharides by treating with N-glycanase, a 25-kDa glycopeptide was generated. Both untreated and N-glycanase treated glycopeptides inhibited the binding of sperm to eggs and induced sperm to complete the AR in vitro to about the same extent as intact ZP3. These findings suggest that the sperm-binding site of mouse ZP3 is located in the carboxy-terminal half of ZP3 and does not involve N-linked oligosaccharides. In addition to the biochemical approaches, several molecular genetic approaches have been used to identify the location of the sperm-binding site of ZP3. These approaches were made possible by the successful cloning and sequencing of the mouse Zp3 gene and polypeptide in the late 1980s.69, 70, 71 Exon swapping and site-directed mutagenesis studies with recombinant mouse ZP3 expressed in an embryonal carcinoma (EC) cell line revealed that the sperm combining site is located in the carboxy-terminal region of ZP3, encoded by exon 7 of the Zp3 gene,72, 73, 74 which corroborates the biochemical approaches described above.
Recombinant hamster ZP3 expressed in EC cells failed to inhibit in vitro binding of mouse sperm to eggs. However, substitution of the hamster Zp3 exon 7 with mouse Zp3 exon 7 of the recombinant hamster ZP3 led to inhibition of the binding of mouse gametes.75 In this context, a fusion construct consisting of human IgG (Fc) and either exon 7 or 8 of mouse Zp3 were prepared. An EC cell line carrying the recombinant gene was produced that secreted chimeric glycoproteins designated either EC-huIgG (Fc)/mouse ZP3 (7) or EC-huIgG (Fc)/mouse ZP3 (8). It was observed that only EC-huIgG (Fc)/mouse ZP3 (7) bound specifically to the plasma membrane overlying the sperm head to a similar extent as mouse ZP3 isolated from eggs, and at nanomolar concentrations EC-huIgG (Fc)/mouse ZP3 (7) inhibited the binding of mouse sperm to eggs in vitro. Collectively, these observations provide evidence that sperm recognize and bind to a region of mouse ZP3 that is encoded by exon 7 and is immediately downstream of its ‘ZP domain'. This conclusion is supported by another recent report on the inhibitory effects of the carboxy-terminal region of recombinant mouse ZP3 in vitro.76 It is of interest that ZP3 is among the 10% most different proteins between rodents and humans.77 The region of ZP3 encoded by exon 7 has undergone a relatively large number of changes during evolution compared with the remainder of the polypeptide and is a proposed site of positive Darwinian selection.78, 79
Human ZP3 has a polypeptide backbone of 424 aa, with a signal peptide (SP) at 1–22 aa that facilitates its secretion (Figure 2a). A tetra basic consensus furin cleavage site (349–352 aa) is present upstream of a hydrophobic transmembrane-like domain (387–409 aa). In mature human ZP3, both the SP and the transmembrane-like domain are cleaved off. Of 12 cysteine (Cys) residues, eight are conserved in various species. The disulphide linkages of the first four Cys residues form a loop-within-loop motif (Cys46/Cys140 and Cys78/Cys99), and the second four form a crossover motif (Cys217/Cys282 and Cys239/Cys300).80 The remaining four Cys residues (Cys319, Cys321, Cys322 and Cys327) lying within a tight cluster towards the C-terminus are linked by two unassigned disulfide linkages.80 Human ZP3 has a conserved domain designated the ‘ZP domain' (45–304 aa), which is also present in other zona proteins and several extracellular proteins, such as Tamm–Horsefall protein and α- and β-tectorin.81, 82 The human ZP3 ‘ZP domain' consists of two conserved subdomains, the N-terminal (45–175 aa) and C-terminal (214–304 aa), separated by a short protease sensitive hinge (Figure 3a). To delineate the functional domain of human ZP3, cDNAs encoding various fragments of human ZP3 were cloned and expressed using a baculovirus expression system (Figure 3b). Significant induction of the AR was observed when capacitated human sperm were incubated with recombinant human ZP3 fragments corresponding to 214–348 and 214–305 aa.83 A recombinant ZP3 N-terminal fragment (23–175 aa) failed to induce any significant increase in the AR, suggesting that the functional activity of human ZP3 also resides in its C-terminal domain (Figure 3b).
Figure 3.
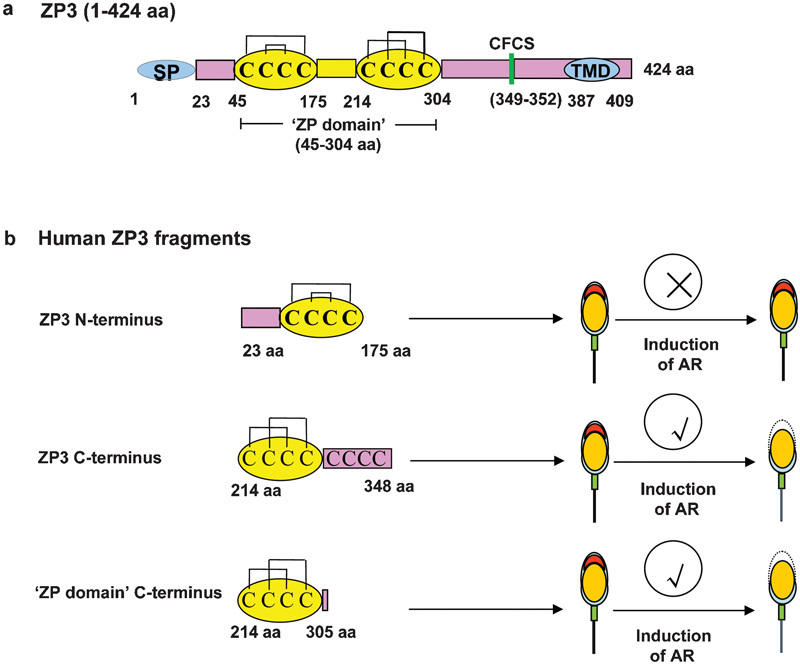
Schematic diagram showing the different domains of human ZP3 and their ability to induce the AR. (a) Human ZP3 has a 424-aa polypeptide backbone comprising an SP (1–22 aa), ZP domain (45–304 aa, yellow), CFCS (349–352 aa, green vertical bar) and TMD (387–409 aa). The ZP domain of ZP3 is composed of N- (45–175 aa) and C-terminal (214–304 aa) subdomains. Mapped disulfide linkages between different Cys residues are shown in black lines. (b) An N-terminal ZP3 fragment without the SP (23–175 aa), a C-terminal fragment excluding the CFCS (214–348 aa) and the ZP domain C-terminal subdomain (214–305 aa) were expressed in insect cells and purified recombinant proteins evaluated for AR induction in capacitated human spermatozoa.83 The N-terminal fragment of ZP3 failed to induce the AR, whereas both C-terminal fragments induced the AR. AR, acrosome reaction; CFCS, consensus furin cleavage site; C, cysteine; SP, signal peptide; TMD, transmembrane-like domain; ZP, zona pellucida.
ZP4
The mouse ZP matrix is composed of ZP1, ZP2 and ZP3, but lacks a ZP4. Our group, along with others, has investigated the role of ZP4 in induction of the AR in humans. E. coli-expressed recombinant human ZP4 binds to the anterior head of capacitated acrosome-intact human spermatozoa but does not induce the AR.53 On the other hand, baculovirus-expressed recombinant human ZP4 not only binds to the anterior head of capacitated acrosome-intact spermatozoa, but also induces a dose-dependent increase in the AR32, 52, 53, 61 (Table 1). These observations were further confirmed by employing immunoaffinity purified native human ZP4 from solubilized human ZP.55, 60 However, it may be noted that the purified human ZP4 fractions from eggs were contaminated with ZP1, and thus its ability to induce the AR may have been due to the combined effect of both ZP1 and ZP4. The importance of ZP4 either alone or as a hetero-oligomer complex with ZP3 during sperm binding and subsequent induction of the AR has also been demonstrated in Xenopus,84 rabbits,85 pigs86 and non-human primates.87 Hence, in humans, ZP4 also acts in conjunction with ZP1 and ZP3 to induce the AR.
Do different human zona proteins use the same downstream signalling pathway?
As discussed above, in humans (in addition to ZP3), ZP1 and ZP4 also mediate the induction of the AR.31, 32, 52, 53, 60, 61 Using pharmacological inhibitors, subtle differences in the downstream signalling pathways used by the ZP glycoproteins were observed, which are summarized in Table 2. ZP3-mediated induction of the AR in humans is inhibited by pertussis toxin, whereas pertussis toxin does not inhibit ZP1- or ZP4-mediated acrosomal exocytosis, which indicates that ZP1/ZP4 act through a Gi protein-independent pathway.31, 52, 60
Table 2. Downstream signalling pathways associated with human ZP glycoprotein mediated induction of the acrosome reaction.
| Pathway | Inhibitor | Inhibition of induction of AR mediated by | ||
|---|---|---|---|---|
| ZP3 | ZP1 | ZP4 | ||
| Extracellular Ca2+ | Ethylene glycol tetraacetic acid | Yes | Yes | Yes |
| Gi protein-coupled receptor | Pertussis toxin | Yes | No | No |
| T-type VOCCs | Pimozide, amiloride | Yes | Yes | Yes |
| L-type VOCCs | Verapamil, nifedipine | No | Yes | Yes |
| PKA | H-89 | No | Yes | Yes |
| PKC | Chelerythrine | Yes | Yes | Yes |
Abbreviations: AR, acrosome reaction; PKA, protein kinase A; PKC, protein kinase C; VOCC, voltage-operated calcium channel; ZP, zona pellucida.
T-type VOCC inhibitors (mibefradil and pimozide) inhibit acrosomal exocytosis mediated by ZP3 and a C-terminal fragment of recombinant ZP3, whereas L-type VOCC inhibitors do not.60, 83 However, ZP1- and ZP4-mediated increases in the AR involve both L- and T-type VOCCs.31, 60 (Table 2). Though ZP3 involves activation of adenylate cyclase, PKA is not critical in ZP3 downstream signalling, suggesting redundancy of PKA and supplementation by parallel signalling pathways. Activation of PKA, however, is crucial for ZP1-/ZP4-mediated signalling, as its pharmacological inhibitor, H-89, specifically inhibits the ZP1-/ZP4-induced AR.31, 60 These studies suggest that the downstream signalling pathways involved in the ZP1- and ZP4-induced ARs are very similar but are different from that employed by ZP3 (Table 2). Human ZP1 and ZP4 are paralogues that may have arisen from a common ancestral gene either by gene duplication or exon swapping.11, 78, 79 The aa sequence identity of human ZP1 and ZP4 is 47%, which further supports the notion that AR induction mediated by ZP1/ZP4 is likely to follow similar downstream signalling events.
Role of oligosaccharide moieties in sperm–ZP interactions and induction of the AR
An understanding of the molecular basis of sperm–egg interactions is still elusive. Various models suggest that it depends on: (i) carbohydrate moieties present on the opposing gamete surfaces; (ii) protein–protein interactions; and (iii) protein–carbohydrate interactions. The protein–carbohydrate interactions are responsible for 75–80% of sperm binding to the ZP, and remaining sperm bind by protein–protein interactions.88, 89
Murine models
Chemically deglycosylated forms of mouse ZP3 fail to induce the AR, suggesting that glycosylation of ZP3 is critical for its functional activity. However, selective removal of N-linked oligosaccharides from mouse ZP3 by endo-β-N-acetyl-𝒹-glucosamine treatment has no effect on the induction of the AR, whereas removal of O-linked oligosaccharides by alkaline hydrolysis abrogates its ability to induce the AR.90 Initial studies implicated galactose in α- or β-linkages at the non-reducing terminus of O-linked oligosaccharides and N-acetylglucosamine (GlcNAc) in β-linkages as the sugar determinants on mouse ZP3 that are responsible for the binding of sperm to the ZP.91 However, mice deficient in glycosyl transferase, which amends terminal galactose in an α-linkage, are fully fertile92, 93 implicating galactose in β-linkages or GlcNAc or both as critical residues.57 Mannose has also been suggested to have an important role in murine sperm receptor activity.94 Subsequently, site-directed mutagenesis revealed that glycosylation of serine residues at positions 332 and 334 is critical for the sperm receptor activity of ZP3.73
Human
Binding studies with various lectins suggest that the human ZP matrix has a high concentration of 𝒹-mannose.95, 96 The presence of mannose-binding sites has been reported on human sperm.97, 98 Several oligosaccharide moieties, such as mannose, GlcNAc, fucose and galactose, along with complex glycoconjugates bearing selectin-like ligands, are involved in human sperm–egg binding.99, 100 On the contrary, Chapman and colleagues showed that E. coli-expressed recombinant human ZP3, presumably lacking glycosylation, induced the AR, suggesting that glycosylation of ZP3 may not be an absolute requirement for AR induction.65 Our group has expressed recombinant human ZP1, ZP2, ZP3 and ZP4 using E. coli and baculovirus expression systems.31, 52, 53 Both E. coli- and baculovirus-expressed recombinant human ZP1, ZP3 and ZP4 conjugated with fluorescein isothiocyanate bind to the anterior head of capacitated acrosome-intact human spermatozoa.31, 53 The binding patterns of ZP1 and ZP4 revealed that a higher percentage of sperm show binding of these proteins to the acrosomal cap as opposed to that seen with ZP3, where equatorial binding predominates in the acrosome-intact spermatozoa.31, 53 The binding profiles of E. coli- and baculovirus-expressed recombinant human ZP1, ZP3 and ZP4 are comparable, suggesting that glycosylation is not critical for binding per se. These results are corroborated by similar findings that the E. coli-expressed bonnet monkey ZP3 and ZP4 bind to monkey sperm.87, 101
E. coli-expressed recombinant human ZP1, ZP3 and ZP4 fail to induce any significant increase in the AR, whereas baculovirus-expressed recombinant ZP1, ZP3 and ZP4 induce dose-dependent increases in the AR.31, 32, 52, 53, 61 These studies suggest that glycosylation of human zona proteins is critical for induction of the AR. Expression of recombinant human ZP3 and ZP4 using a baculovirus expression system in the presence of tunicamycin made available these proteins with reduced N-linked oligosaccharides.53 Incubation of capacitated human sperm with the above recombinant proteins significantly reduces the proteins ability to induce the AR, suggesting that N-linked glycosylation of human zona proteins are critical for AR induction.53 The importance of N-linked glycosylations has been further confirmed using immunoaffinity-purified human ZP3 and ZP4 from solubilized human ZP. Removal of N-linked glycosides from human ZP3 and ZP4 by treatment with N-glycosidase F significantly decreases their respective abilities to induce the AR.60. Removal of O-linked glycans by alkali hydrolysis (β-elimination) from either baculovirus-expressed recombinant human ZP3 and ZP4 or native human ZP3 and ZP4 purified from human eggs has no significant effect on their AR induction ability.53, 60 Hence, in contrast to mouse, where O-linked glycosylation of ZP3 is critical for AR induction, in humans, N-linked glycosylation of ZP1, ZP3 and ZP4 are critical for mediating the AR.
Conclusion
The mouse model for the roles of individual ZP glycoproteins in binding capacitated acrosome-intact spermatozoa and subsequent induction of the AR is not tenable in other species. In mice, ZP3 is primarily responsible for AR induction. In humans (in addition to ZP3), ZP1 and ZP4 may also be involved in AR induction. In mouse, O-linked glycans of ZP3 are involved in the AR, whereas in humans, N-linked glycans of ZP1, ZP3 and ZP4 are critical for AR induction. Hence, it is imperative that each species be investigated in detail to determine the roles of zona proteins during fertilisation.
Acknowledgments
We acknowledge the scientific work of the present and past members of Reproductive Cell Biology Laboratory, National Institute of Immunology, New Delhi, which enabled us to write this review. Financial support from the National Institute of Immunology, New Delhi, Department of Biotechnology, Government of India, and the Indian Council of Medical Research, Government of India, is gratefully acknowledged.
The authors declare no competing financial interests.
References
- Meizel S. Molecules that initiates or help stimulate the acrosome reaction by their interaction with the mammalian sperm surface. Am J Anat. 1985;174:285–302. doi: 10.1002/aja.1001740309. [DOI] [PubMed] [Google Scholar]
- Roldan ER, Murase T, Shi QX. Exocytosis in spermatozoa in response to progesterone and zona pellucida. Science. 1994;266:1578–81. doi: 10.1126/science.7985030. [DOI] [PubMed] [Google Scholar]
- Gupta SK, Bansal P, Ganguly A, Bhandari B, Chakrabarti K. Human zona pellucida glycoproteins: functional relevance during fertilization. J Reprod Immunol. 2009;83:50–5. doi: 10.1016/j.jri.2009.07.008. [DOI] [PubMed] [Google Scholar]
- Bleil JD, Wassarman PM. Structure and function of the zona pellucida: identification and characterization of the proteins of the mouse oocyte's zona pellucida. Dev Biol. 1980;76:185–202. doi: 10.1016/0012-1606(80)90371-1. [DOI] [PubMed] [Google Scholar]
- Hedrick JL, Wardrip NJ. On the macromolecular composition of the zona pellucida from porcine oocytes. Dev Biol. 1987;121:478–88. doi: 10.1016/0012-1606(87)90184-9. [DOI] [PubMed] [Google Scholar]
- Noguchi S, Yonezawa N, Katsumata T, Hashizume K, Kuwayama M, et al. Characterization of the zona pellucida glycoproteins from bovine ovarian and fertilized eggs. Biochim Biophys Acta. 1994;1201:7–14. doi: 10.1016/0304-4165(94)90143-0. [DOI] [PubMed] [Google Scholar]
- Goudet G, Mugnier S, Callebaut I, Monget P. Phylogenetic analysis and identification of pseudogenes reveal a progressive loss of zona pellucida genes during evolution of vertebrates. Biol Reprod. 2008;78:796–806. doi: 10.1095/biolreprod.107.064568. [DOI] [PubMed] [Google Scholar]
- Hoodbhoy T, Joshi S, Boja ES, Williams SA, Stanley P, et al. Human sperm do not bind to rat zonae pellucidae despite the presence of four homologous glycoproteins. J Biol Chem. 2005;280:12721–31. doi: 10.1074/jbc.M413569200. [DOI] [PubMed] [Google Scholar]
- Izquierdo-Rico MJ, Jimenez-Movilla M, Llop E, Perez-Oliva AB, Ballesta J, et al. Hamster zona pellucida is formed by four glycoproteins: ZP1, ZP2, ZP3, and ZP4. J Proteome Res. 2009;8:926–41. doi: 10.1021/pr800568x. [DOI] [PubMed] [Google Scholar]
- Ganguly A, Sharma RK, Gupta SK. Bonnet monkey (Macaca radiata) ovaries, like human oocytes, express four zona pellucida glycoproteins. Mol Reprod Dev. 2008;75:156–66. doi: 10.1002/mrd.20808. [DOI] [PubMed] [Google Scholar]
- Hughes DC, Barratt CL. Identification of the true human orthologue of the mouse Zp1 gene: evidence for greater complexity in the mammalian zona pellucida. Biochim Biophys Acta. 1999;1447:303–6. doi: 10.1016/s0167-4781(99)00181-5. [DOI] [PubMed] [Google Scholar]
- Lefièvre L, Conner SJ, Salpekar A, Olufowobi O, Ashton P, et al. Four zona pellucida glycoproteins are expressed in the human. Hum Reprod. 2004;19:1580–6. doi: 10.1093/humrep/deh301. [DOI] [PubMed] [Google Scholar]
- Conner SJ, Lefievre L, Hughes DC, Barratt CL. Cracking the egg: increased complexity in the human zona pellucida. Hum Reprod. 2005;5:1148–52. doi: 10.1093/humrep/deh835. [DOI] [PubMed] [Google Scholar]
- Dell A, Morris HR, Easten RL, Patankar M, Clark GF. The glycobiology of gametes and fertilization. Biochem Biophys Acta. 1999;1473:196–203. doi: 10.1016/s0304-4165(99)00179-8. [DOI] [PubMed] [Google Scholar]
- Topfer-Petersen E. Carbohydrate-based interactions on the route of a spermatozoon to fertilization. Hum Reprod Update. 1999;5:314–9. doi: 10.1093/humupd/5.4.314. [DOI] [PubMed] [Google Scholar]
- Smith J, Paton IR, Hughes DC, Burt DW. Isolation and mapping the chicken zona pellucida genes: an insight into the evolution of orthologous genes in different species. Mol Reprod Dev. 2005;70:133–45. doi: 10.1002/mrd.20197. [DOI] [PubMed] [Google Scholar]
- Bleil JD, Wassarman PM. Sperm–egg interactions in the mouse: sequence of events and induction of the acrosome reaction by a zona pellucida glycoprotein. Dev Biol. 1983;95:317–24. doi: 10.1016/0012-1606(83)90032-5. [DOI] [PubMed] [Google Scholar]
- Cross NL, Morales P, Overstreet JW, Hanson FW. Induction of acrosome reaction by the human zona pellucida. Biol Reprod. 1988;38:235–44. doi: 10.1095/biolreprod38.1.235. [DOI] [PubMed] [Google Scholar]
- Franken DR, Bastiaan HS, Oehninger SC. Physiological induction of the acrosome reaction in human sperm: validation of a microassay using minimal volumes of solubilized, homologous zona pellucida. J Assist Reprod Genet. 2000;17:374–8. doi: 10.1023/A:1009493708268. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schuffner AA, Bastiaan HS, Duran HE, Lin ZY, Morshedi M, et al. Zona pellucida-induced acrosome reaction in human sperm: dependency on activation of pertussis toxin-sensitive Gi protein and extracellular calcium, and priming effect of progesterone and follicular fluid. Mol Hum Reprod. 2002;8:722–7. doi: 10.1093/molehr/8.8.722. [DOI] [PubMed] [Google Scholar]
- Patrat C, Auer J, Fauque P, Leandri RL, Jouannet P, et al. Zona pellucida from fertilised human oocytes induces a voltage-dependent calcium influx and the acrosome reaction in spermatozoa, but cannot be penetrated by sperm. BMC Dev Biol. 2006;6:59. doi: 10.1186/1471-213X-6-59. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Doherty CM, Tarchala SM, Radwanska E, de Jonge CJ. Characterization of two second messenger pathways and their interactions in eliciting the human sperm acrosome reaction. J Androl. 1995;16:36–46. [PubMed] [Google Scholar]
- Ward CR, Storey BT, Kopf GS. Activation of a Gi protein in mouse sperm membranes by solubilized proteins of the zona pellucida, the egg's extracellular matrix. J Biol Chem. 1992;267:14061–7. [PubMed] [Google Scholar]
- Ward CR, Storey BT, Kopf GS. Selective activation of Gi1 and Gi2 in mouse sperm by the zona pellucida, the egg's extracellular matrix. J Biol Chem. 1994;269:13254–8. [PubMed] [Google Scholar]
- Walensky LD, Snyder SH. Inositol 1,4,5-trisphosphate receptors selectively localized to the acrosomes of mammalian sperm. J Cell Biol. 1995;130:857–69. doi: 10.1083/jcb.130.4.857. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kopf GS, Wilde MW. Signal transduction processes leading to acrosomal exocytosis in mammalian spermatozoa. Trends Endocrinol Metab. 1990;1:362–8. doi: 10.1016/1043-2760(90)90085-h. [DOI] [PubMed] [Google Scholar]
- Florman HM, Arnoult C, Kazam IG, Li C, O'Toole CM. A perspective on the control of mammalian fertilization by egg-activated ion channels in sperm: a tale of two channels. Biol Reprod. 1998;59:12–6. doi: 10.1095/biolreprod59.1.12. [DOI] [PubMed] [Google Scholar]
- Bailey JL, Storey BT. Calcium influx into mouse spermatozoa activated by solubilized mouse zona pellucida, monitored with the calcium fluorescent indicator, fluo-3. Inhibition of the influx by three inhibitors of the zona pellucida induced acrosome reaction: tyrphostin A48, pertussis toxin, and 3-quinuclidinyl benzilate. Mol Reprod Dev. 1994;39:297–308. doi: 10.1002/mrd.1080390307. [DOI] [PubMed] [Google Scholar]
- Arnoult C, Cardullo RA, Lemos JR, Florman HM. Activation of mouse sperm T-type Ca2+ channels by adhesion to the egg zona pellucida. Proc Natl Acad Sci USA. 1996;93:13004–9. doi: 10.1073/pnas.93.23.13004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rockwell PL, Storey BT. Kinetics of onset of mouse sperm acrosome reaction induced by solubilized zona pellucida: fluorimetric determination of loss of pH gradient between acrosomal lumen and medium monitored by dapoxyl (2-aminoethyl) sulfonamide and of intracellular Ca2+ changes monitored by fluo-3. Mol Reprod Dev. 2000;55:335–49. doi: 10.1002/(SICI)1098-2795(200003)55:3<335::AID-MRD12>3.0.CO;2-5. [DOI] [PubMed] [Google Scholar]
- Ganguly A, Bukovsky A, Sharma RK, Bansal P, Bhandari B, Gupta SK. In humans, zona pellucida glycoprotein-1 binds to spermatozoa and induces acrosomal exocytosis. Hum Reprod. 2010;25:1643–56. doi: 10.1093/humrep/deq105. [DOI] [PubMed] [Google Scholar]
- Jose O, Hernandez-Hernandez O, Chirinos M, Gonzalez-Gonzalez ME, Larrea F, et al. Recombinant human ZP3-induced sperm acrosome reaction: evidence for the involvement of T- and L-type voltage-gated calcium channels. Biochem Biophys Res Commun. 2010;395:530–4. doi: 10.1016/j.bbrc.2010.04.059. [DOI] [PubMed] [Google Scholar]
- Lievano A, Santi CM, Serrano CJ, Treviño CL, Bellvé AR, et al. T-type Ca2+ channels and α1E expression in spermatogenic cells, and their possible relevance to the sperm acrosome reaction. FEBS Lett. 1996;388:150–4. doi: 10.1016/0014-5793(96)00515-7. [DOI] [PubMed] [Google Scholar]
- Santi CM, Darszon A, Hernandez-Cruz A. A dihydropyridine-sensitive T-type Ca2+ current is the main Ca2+ current carrier in mouse primary spermatocytes. Am J Physiol. 1996;271:C1583–93. doi: 10.1152/ajpcell.1996.271.5.C1583. [DOI] [PubMed] [Google Scholar]
- Arnoult C, Lemos JR, Florman HM. Voltage-dependent modulation of T-type calcium channels by protein tyrosine phosphorylation. EMBO J. 1997;16:1593–9. doi: 10.1093/emboj/16.7.1593. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jungnickel MK, Marrero H, Birnbaumer L, Lémos JR, Florman HM. Trp2 regulates entry of Ca2+ into mouse sperm triggered by egg ZP3. Nat Cell Biol. 2001;3:499–502. doi: 10.1038/35074570. [DOI] [PubMed] [Google Scholar]
- Wes PD, Chevesich J, Jeromin A, Rosenberg C, Stetten G. TRPC1, a human homolog of a Drosophila store-operated channel. Proc Natl Acad Sci USA. 1995;92:9652–6. doi: 10.1073/pnas.92.21.9652. [DOI] [PMC free article] [PubMed] [Google Scholar]
- De Blas GA, Darszon A, Ocampo AY, Serrano CJ, Castellano LE, et al. TRPM8, a versatile channel in human sperm. PLoS One. 2009;4:e6095. doi: 10.1371/journal.pone.0006095. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tomes CN, Michaut M, De Blas G, Visconti P, Matti U, et al. SNARE complex assembly is required for human sperm acrosome reaction. Dev Biol. 2002;243:326–38. doi: 10.1006/dbio.2002.0567. [DOI] [PubMed] [Google Scholar]
- Michaut M, Tomes CN, De Blas G, Yunes R, Mayorga LS. Calcium-triggered acrosomal exocytosis in human spermatozoa requires the coordinated activation of Rab3A and N-ethylmaleimide-sensitive factor. Proc Natl Acad Sci USA. 2000;97:9996–10001. doi: 10.1073/pnas.180206197. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harrison RA, Roldan ER. Phosphoinositides and their products in the mammalian sperm acrosome reaction. J Reprod Fertil Suppl. 1990;42:51–67. [PubMed] [Google Scholar]
- Bhandari B, Bansal P, Talwar P, Gupta SK. Delineation of downstream signalling components during acrosome reaction mediated by heat solubilized human zona pellucida. Reprod Biol Endocrinol. 2010;8:7. doi: 10.1186/1477-7827-8-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bastiaan H, Franken D, Wranz P. G-protein regulation of the solubilized human zona pellucida-mediated acrosome reaction and zona pellucida binding. J Assist Reprod Genet. 1999;16:332–6. doi: 10.1023/A:1020462201291. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Franken DR, Morales PJ, Habenicht UF. Inhibition of G protein in human sperm and its influence on acrosome reaction and zona pellucida binding. Fertil Steril. 1996;66:1009–11. [PubMed] [Google Scholar]
- Lee MA, Check JH, Kopf GS. A guanine nucleotide-binding regulatory protein in human sperm mediates acrosomal exocytosis induced by the human zona pellucida. Mol Reprod Dev. 1992;31:78–86. doi: 10.1002/mrd.1080310114. [DOI] [PubMed] [Google Scholar]
- Bielfeld P, Faridi A, Zaneveld LJ, de Jonge CJ. The zona pellucida-induced acrosome reaction of human spermatozoa is mediated by protein kinases. Fertil Steril. 1994;61:536–41. [PubMed] [Google Scholar]
- Bleil JD, Wassarman PM. Mammalian sperm–egg interaction: identification of a glycoprotein in mouse egg zonae pellucidae possessing receptor activity for sperm. Cell. 1980;20:873–82. doi: 10.1016/0092-8674(80)90334-7. [DOI] [PubMed] [Google Scholar]
- Greve JM, Wassarman PM. Mouse egg extracellular coat is a matrix of interconnected filaments possessing a structural repeat. J Mol Biol. 1985;181:253–64. doi: 10.1016/0022-2836(85)90089-0. [DOI] [PubMed] [Google Scholar]
- Sasanami T, Murata T, Ohtsuki M, Matsushima K, Hiyama G, et al. Induction of sperm acrosome reaction by perivitelline membrane glycoprotein ZP1 in Japanese quail (Coturnix japonica) Reproduction. 2007;133:41–9. doi: 10.1530/REP-06-0104. [DOI] [PubMed] [Google Scholar]
- Okumura H, Kohno Y, Iwata Y, Mori H, Aoki N, et al. A newly identified zona pellucida glycoprotein, ZPD, and dimeric ZP1 of chicken egg envelope are involved in sperm activation on sperm–egg interaction. Biochem J. 2004;384:191–9. doi: 10.1042/BJ20040299. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bleil JD, Greve JM, Wassarman PM. Identification of a secondary sperm receptor in the mouse egg zona pellucida: role in maintenance of binding of acrosome-reacted sperm to eggs. Dev Biol. 1988;128:376–85. doi: 10.1016/0012-1606(88)90299-0. [DOI] [PubMed] [Google Scholar]
- Chakravarty S, Suraj K, Gupta SK. Baculovirus-expressed recombinant human zona pellucida glycoprotein-B induces acrosomal exocytosis in capacitated spermatozoa in addition to zona pellucida glycoprotein-C. Mol Hum Reprod. 2005;11:365–72. doi: 10.1093/molehr/gah165. [DOI] [PubMed] [Google Scholar]
- Chakravarty S, Kadunganattil S, Bansal P, Sharma RK, Gupta SK. Relevance of glycosylation of human zona pellucida glycoproteins for their binding to capacitated human spermatozoa and subsequent induction of acrosomal exocytosis. Mol Reprod Dev. 2008;75:75–88. doi: 10.1002/mrd.20726. [DOI] [PubMed] [Google Scholar]
- Bukovsky A, Gupta SK, Bansal P, Chakravarty S, Chaudhary M, et al. Production of monoclonal antibodies against recombinant human zona pellucida glycoproteins: utility in immunolocalization of respective zona proteins in ovarian follicles. J Reprod Immunol. 2008;78:102–14. doi: 10.1016/j.jri.2007.10.004. [DOI] [PubMed] [Google Scholar]
- Chiu PC, Wong BS, Lee CL, Pang RT, Lee KF, et al. Native human zona pellucida glycoproteins: purification and binding properties. Hum Reprod. 2008;23:1385–93. doi: 10.1093/humrep/den047. [DOI] [PubMed] [Google Scholar]
- Thaler CD, Cardullo RA. The initial molecular interaction between mouse sperm and the zona pellucida is a complex binding event. J Biol Chem. 1996;271:23289–97. doi: 10.1074/jbc.271.38.23289. [DOI] [PubMed] [Google Scholar]
- Wassarman PM. Mammalian fertilization: molecular aspects of gamete adhesion, exocytosis, and fusion. Cell. 1999;96:175–83. doi: 10.1016/s0092-8674(00)80558-9. [DOI] [PubMed] [Google Scholar]
- Beebe SJ, Leyton L, Burks D, Ishikawa M, Fuerst T, et al. Recombinant mouse ZP3 inhibits sperm binding and induces the acrosome reaction. Dev Biol. 1992;151:48–54. doi: 10.1016/0012-1606(92)90212-y. [DOI] [PubMed] [Google Scholar]
- Moller CC, Bleil JD, Kinloch RA, Wassarman PM. Structural and functional relationships between mouse and hamster zona pellucida glycoproteins. Dev Biol. 1990;137:276–86. doi: 10.1016/0012-1606(90)90254-g. [DOI] [PubMed] [Google Scholar]
- Chiu PC, Wong BS, Chung MK, Lam KK, Pang RT, et al. Effects of native human zona pellucida glycoproteins 3 and 4 on acrosome reaction and zona pellucida binding of human spermatozoa. Biol Reprod. 2008;79:869–77. doi: 10.1095/biolreprod.108.069344. [DOI] [PubMed] [Google Scholar]
- Caballero-Campo P, Chirinos M, Fan XJ, González-González ME, Galicia-Chavarría M, et al. Biological effects of recombinant human zona pellucida proteins on sperm function. Biol Reprod. 2006;74:760–8. doi: 10.1095/biolreprod.105.047522. [DOI] [PubMed] [Google Scholar]
- van Duin M, Polman JE, de Breet IT, van Ginneken K, Bunschoten H, et al. Recombinant human zona pellucida protein ZP3 produced by chinese hamster ovary cells induces the human sperm acrosome reaction and promotes sperm–egg fusion. Biol Reprod. 1994;51:607–17. doi: 10.1095/biolreprod51.4.607. [DOI] [PubMed] [Google Scholar]
- Dong KW, Chi TF, Juan YW, Chen CW, Lin Z, et al. Characterization of the biologic activities of a recombinant human zona pellucida protein 3 expressed in human ovarian teratocarcinoma (PA-1) cells. Am J Obstet Gynecol. 2001;184:835–43. doi: 10.1067/mob.2001.113849. [DOI] [PubMed] [Google Scholar]
- Bray C, Son JH, Kumar P, Harris JD, Meizel S. A role for the human sperm glycine receptor/Cl− channel in acrosome reaction initiated by recombinant ZP3. Biol Reprod. 2002;66:91–7. doi: 10.1095/biolreprod66.1.91. [DOI] [PubMed] [Google Scholar]
- Chapman N, Kessopoulou E, Andrews P, Hornby D, Barratt CR. The polypeptide backbone of recombinant human zona pellucida glycoprotein-3 initiates acrosomal exocytosis in human spermatozoa in vitro. Biochem J. 1998;330:839–45. doi: 10.1042/bj3300839. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Florman HM, Bechtol KB, Wassarman PM. Enzymatic dissection of the functions of the mouse egg's receptor for sperm. Dev Biol. 1984;106:243–55. doi: 10.1016/0012-1606(84)90079-4. [DOI] [PubMed] [Google Scholar]
- Rosiere TK, Wassarman PM. Identification of a region of mouse zona pellucida glycoprotein mZP3 that possesses sperm receptor activity. Dev Biol. 1992;154:309–17. doi: 10.1016/0012-1606(92)90070-w. [DOI] [PubMed] [Google Scholar]
- Litscher ES, Wassarman PM. Characterization of mouse ZP3-derived glycopeptide, gp55, that exhibits sperm receptor and acrosome reaction-inducing activity in vitro. Biochemistry. 1996;35:3980–5. doi: 10.1021/bi952722m. [DOI] [PubMed] [Google Scholar]
- Kinloch RA, Roller RJ, Fimiani CM, Wassarman DA, Wassarman PM. Primary structure of the mouse sperm receptor polypeptide determined by genomic cloning. Proc Natl Acad Sci USA. 1988;85:6409–13. doi: 10.1073/pnas.85.17.6409. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ringuette MJ, Chamberlin ME, Baur AW, Sobieski DA, Dean J. Molecular analysis of cDNA coding for ZP3, a sperm binding protein of the mouse zona pellucida. Dev Biol. 1988;127:287–95. doi: 10.1016/0012-1606(88)90315-6. [DOI] [PubMed] [Google Scholar]
- Kinloch RA, Wassarman PM. Nucleotide sequence of the gene encoding zona pellucida glycoprotein ZP3—the mouse sperm receptor. Nucleic Acids Res. 1989;17:2861–3. doi: 10.1093/nar/17.7.2861. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kinloch RA, Sakai Y, Wassarman PM. Mapping the mouse ZP3 combining site for sperm by exon swapping and site-directed mutagenesis. Proc Natl Acad Sci USA. 1995;92:263–7. doi: 10.1073/pnas.92.1.263. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen J, Litscher ES, Wassarman PM. Inactivation of the mouse sperm receptor, mZP3, by site-directed mutagenesis of individual serine residues located at the combining site for sperm. Proc Natl Acad Sci USA. 1998;95:6193–7. doi: 10.1073/pnas.95.11.6193. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kinloch RA, Mortillo S, Stewart CL, Wassarman PM. Embryonal carcinoma cells transfected with ZP3 genes differentially glycosylate similar polypeptides and secrete active mouse sperm receptor. J Cell Biol. 1991;115:655–64. doi: 10.1083/jcb.115.3.655. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Williams Z, Litscher ES, Jovine L, Wassarman PM. Polypeptide encoded by mouse ZP3 exon-7 is necessary and sufficient for binding of mouse sperm in vitro. J Cell Physiol. 2006;207:30–9. doi: 10.1002/jcp.20532. [DOI] [PubMed] [Google Scholar]
- Li D, Cao S, Xu C. Polypeptide backbone derived from carboxyl terminal of mouse ZP3 inhibits sperm–zona binding. Mol Reprod Dev. 2007;74:1327–36. doi: 10.1002/mrd.20705. [DOI] [PubMed] [Google Scholar]
- Aagaard JE, Yi X, MacCoss MJ, Swanson WJ. Rapidly evolving zona pellucida domain proteins are a major component of the vitelline envelope of abalone eggs. Proc Natl Acad Sci USA. 2006;103:17302–7. doi: 10.1073/pnas.0603125103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Swanson WJ, Yang Z, Wolfner MF, Aquadro CF. Positive Darwinian selection drives the evolution of several female reproductive proteins in mammals. Proc Natl Acad Sci USA. 2001;98:2509–14. doi: 10.1073/pnas.051605998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Swanson WJ, Nielsen R, Yang Q. Pervasive adaptive evolution in mammalian fertilization proteins. Mol Biol Evol. 2003;20:18–20. doi: 10.1093/oxfordjournals.molbev.a004233. [DOI] [PubMed] [Google Scholar]
- Zhao M, Boja ES, Hoodbhoy T, Nawrocki J, Kaufman JB, et al. Mass spectrometry analysis of recombinant human ZP3 expressed in glycosylation deficient CHO cells. Biochemistry. 2004;43:12090–104. doi: 10.1021/bi048958k. [DOI] [PubMed] [Google Scholar]
- Jovine L, Qi H, Williams Z, Litscher ES, Wassarman PM. The ZP domain is a conserved module for polymerization of extracellular proteins. Nat Cell Biol. 2002;4:457–61. doi: 10.1038/ncb802. [DOI] [PubMed] [Google Scholar]
- Jovine L, Darie CC, Litscher ES, Wassarman PM. Zona pellucida domain proteins. Annu Rev Biochem. 2005;74:83–114. doi: 10.1146/annurev.biochem.74.082803.133039. [DOI] [PubMed] [Google Scholar]
- Bansal P, Chakrabarti K, Gupta SK. Functional activity of human ZP3 primary sperm receptor resides toward its C-terminus. Biol Reprod. 2009;81:7–15. doi: 10.1095/biolreprod.108.074716. [DOI] [PubMed] [Google Scholar]
- Vo LH, Hedrick JL. Independent and hetero-oligomeric-dependent sperm binding to egg envelope glycoprotein ZPC in Xenopus laevis. Biol Reprod. 2000;62:766–74. doi: 10.1095/biolreprod62.3.766. [DOI] [PubMed] [Google Scholar]
- Prasad SV, Wilkins B, Skinner SM, Dunbar BS. Evaluating zona pellucida structure and function using antibodies to rabbit 55 kDa ZP protein expressed in baculovirus expression system. Mol Reprod Dev. 1996;43:519–29. doi: 10.1002/(SICI)1098-2795(199604)43:4<519::AID-MRD15>3.0.CO;2-U. [DOI] [PubMed] [Google Scholar]
- Yurewicz EC, Sacco AG, Gupta SK, Xu N, Gage DA. Hetero oligomerization-dependent binding of pig oocyte zona pellucida glycoproteins ZPB and ZPC to boar sperm membrane vesicles. J Biol Chem. 1998;273:7488–94. doi: 10.1074/jbc.273.13.7488. [DOI] [PubMed] [Google Scholar]
- Govind CK, Gahlay GK, Choudhury S, Gupta SK. Purified and refolded recombinant bonnet monkey (Macaca radiata) zona pellucida glycoprotein-B expressed in Escherichia coli binds to spermatozoa. Biol Reprod. 2001;64:1147–52. doi: 10.1095/biolreprod64.4.1147. [DOI] [PubMed] [Google Scholar]
- Macek MB, Shur BD. Protein–carbohydrate complementarity in mammalian gamete recognition. Gamete Res. 1988;20:93–109. doi: 10.1002/mrd.1120200109. [DOI] [PubMed] [Google Scholar]
- Clark GF, Dell A. Molecular models for murine sperm–egg binding. J Biol Chem. 2006;281:13853–6. doi: 10.1074/jbc.R600001200. [DOI] [PubMed] [Google Scholar]
- Florman HM, Wassarman PM. O-linked oligosaccharides of mouse egg ZP3 account for its sperm receptor activity. Cell. 1985;41:313–24. doi: 10.1016/0092-8674(85)90084-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bleil JD, Wassarman PM. Galactose at the nonreducing terminus of O-linked oligosaccharides of mouse egg zona pellucida glycoprotein ZP3 is essential for the glycoprotein's sperm receptor activity. Proc Natl Acad Sci USA. 1988;85:6778–82. doi: 10.1073/pnas.85.18.6778. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu C, Litscher ES, Wassarman PM. Zona pellucida glycoprotein mZP3 bioactivity is not dependent on the extent of glycosylation of its polypeptide or on sulfation and sialylation of its oligosaccharides. J Cell Sci. 1997;110:745–52. doi: 10.1242/jcs.110.6.745. [DOI] [PubMed] [Google Scholar]
- Thall AD, Maly P, Lowe JB. Oocyte Galα1,3Gal epitopes implicated in sperm adhesion to the zona pellucida glycoprotein ZP3 are not required for fertilization in the mouse. J Biol Chem. 1995;270:21437–40. doi: 10.1074/jbc.270.37.21437. [DOI] [PubMed] [Google Scholar]
- Cornwall GA, Tulsiani DR, Orgebin-Crist MC. Inhibition of the mouse sperm surface alpha-𝒹-mannosidase inhibits sperm–egg binding in vitro. Biol Reprod. 1991;44:913–21. doi: 10.1095/biolreprod44.5.913. [DOI] [PubMed] [Google Scholar]
- Maymon BB, Maymon R, Ben Nun I, Ghetler Y, Shalgi R, et al. Distribution of carbohydrates in the zona pellucida of human oocytes. J Reprod Fertil. 1994;102:81–6. doi: 10.1530/jrf.0.1020081. [DOI] [PubMed] [Google Scholar]
- Jimenez-Movilla M, Aviles M, Gomez-Torres MJ, Fernández-Colom PJ, Castells MT. Carbohydrate analysis of the zona pellucida and cortical granules of human oocytes by means of ultrastructural cytochemistry. Hum Reprod. 2004;19:1842–55. doi: 10.1093/humrep/deh311. [DOI] [PubMed] [Google Scholar]
- Youssef HM, Doncel GF, Bassiouni BA, Acosta AA. Mannose-binding sites on human spermatozoa and sperm morphology. Fertil Steril. 1996;66:640–5. [PubMed] [Google Scholar]
- Furlong LI, Veaute C, Vazquez-Levin MH. Binding of recombinant human proacrosin/acrosin to zona pellucida glycoproteins. II. Participation of mannose residues in the interaction. Fertil Steril. 2005;83:1791–6. doi: 10.1016/j.fertnstert.2004.12.043. [DOI] [PubMed] [Google Scholar]
- Miranda PV, Gonzalez-Echeverria F, Marin-Briggiler CI, Brandelli A, Blaquier JA, et al. Glycosidic residues involved in human sperm-zona pellucida binding in vitro. Mol Hum Reprod. 1997;3:399–404. doi: 10.1093/molehr/3.5.399. [DOI] [PubMed] [Google Scholar]
- Oehninger S, Patankar M, Seppala M, Clark GF. Involvement of selectin-like carbohydrate binding specificity in human gamete interaction. Andrologia. 1998;30:269–74. doi: 10.1111/j.1439-0272.1998.tb01170.x. [DOI] [PubMed] [Google Scholar]
- Gahlay GK, Srivastava N, Govind CK, Gupta SK. Primate recombinant zona pellucida proteins expressed in Escherichia coli bind to spermatozoa. J Reprod Immunol. 2002;53:67–77. doi: 10.1016/s0165-0378(01)00083-3. [DOI] [PubMed] [Google Scholar]


