Abstract
The cysteine-rich secretory proteins (CRISPs) are a subgroup of the CRISP, antigen 5 and Pr-1 (CAP) protein superfamily, and are found only in vertebrates. They show a strong expression bias to the mammalian male reproductive tract and the venom of poisonous reptiles. Within the male reproductive tract CRISPs have been implicated in many aspects of male germ cell biology spanning haploid germ cell development, epididymal maturation, capacitation, motility and the actual processes of fertilization. At a structural level, CRISPs are composed of two domains, a CAP domain, which has been implicated in cell–cell adhesion, and a CRISP domain, which has been shown to regulate several classes of ion channels across multiple species. Herein, we will review the current literature on the role of CRISPs in male fertility, and by inference to related non-mammalian protein, infer potential biochemical functions.
Keywords: CRISPs, epididymis, fertility, spermatozoa, testis
Introduction
Mammalian fertilization encompasses a series of complex events required to achieve pregnancy. Morphologically complete spermatozoa are produced during spermatogenesis in the testis; however, the potential for fertilization cannot be achieved until spermatozoa undergo two post-testicular maturation events: epididymal maturation and capacitation. Epididymal maturation is a progressive process that occurs during passage through the epididymis that ultimately confers on sperm the capacity for motility, and the full suite of proteins required for oocyte recognition and fertilization.1, 2 Capacitation, on the other hand, occurs in the female reproductive tract and includes the release of suppressive molecules called ‘decapacitation factors', the translocation of molecules within the membrane and the initiation of various signal transduction processes. Capacitation leads to sperm hyperactivated motility, their ability to interact with the zona pellucida of the oocyte and undergo the acrosome reaction.
The cysteine-rich secretory proteins (CRISPs) are a group of proteins found in vertebrates that show a strong expression bias in the male reproductive tract in mammals, to the venom of poisonous reptiles and the saliva of the lamprey.3 Most mammals produce three CRISPs; however, mice produce four CRISPs. Within mammals, CRISPs are also expressed in lower levels within non-reproductive tissues, including secretory glands, skeletal muscle, the spleen and the thymus.4
Within mammals, male germ cells encounter CRISPs at virtually every phase of development and maturation. CRISP2 forms an integral component of the sperm head and tail; sperm are bathed in CRISPs within the epididymis, then later mixed with CRISPs from both the seminal vesicles and the prostate and finally at least two CRISPs remain present at, and have been implicated in, the actual processes of fertilization. Not surprisingly, therefore, the CRISP genes have been nominated as infertility candidate genes and proposed as targets for contraceptive action.5, 6, 7 This article will review current literature on the role of CRISPs in male fertility; it will draw upon the function of related non-mammalian CRISPs and other related proteins to infer function and finally will summarize the actual evidence for the role of CRISPs in mammalian male fertility.
CRISP structure
CRISPs are proteins defined by the presence of 16 absolutely conserved cysteines that fold into two domains: a N-terminal CRISP, antigen 5 and Pr-1 (CAP) domain (∼21 kDa) that contains six conserved cysteine residues, and the smaller C-terminal CRISP (or cysteine-rich) domain (∼6 kDa) that contains 10 conserved cysteine residues.8 In turn, the CRISP domain is composed of two regions: a hinge and an ion channel regulatory (ICR) region. Structural data indicate that all cysteines are involved in intradomain disulfide bonding9, 10, 11, 12 and that the CAP domain contains an active site-like pocket.13 Although the hinge region is rigidly attached to the CAP domain,9 there is a relatively high degree of rotational freedom between the hinge region and the ICR region in the CRISP domain.14
From an evolutionary perspective, CRISPs are only found in vertebrates.15 The CAP protein superfamily, within which the CRISPs form one of nine subfamilies, includes 437 known proteins across the Kingdoms Archaea, Bacteria and Eukaryota.8 Humans express 31 CAP proteins, and mice 33, of which 3 and 4, respectively, are CRISPs. As the name suggests, all CAPs contain a related N-terminal CAP domain. Despite this wide and evolutionarily conserved expression, no single unifying biochemical role for the CAP domain has been elucidated. CAP domains have, however, been implicated in a range of functions spanning immunity, chemoattraction, fertility, carcinogenesis, as a protease and as a protease inhibitor.13, 16, 17, 18 For a detailed review of CAP expression and function, readers are referred to Gibbs et al.8
A consensus on the function of the CRISP domain is, however, beginning to form, in which data strongly suggest that most (if not all) CRISP domains function to regulate ion channels. ICR activity has been most extensively explored using reptile venoms, but emerging data suggest that this is also likely to be the case for non-reptile CRISPs. The ability to regulate ion channels was first demonstrated by Mochca-Morales et al.19 using the Mexican Bearded Lizard CRISP helothermine. Since then reptilian CRISPs have been shown to regulate many classes of ion channels, including voltage-gated ion channels, ryanodine receptors (RyRs) and cyclic nucleotide-gated ion channels A1–3.20, 21, 22, 23, 24, 25
Of note, it has been shown that the ability of the CRISP domain to regulate ion channel gating, for the King Brown snake CRISP pseudechetoxin, is enhanced by the presence of the CAP domain.26 Specifically full-length pseudechetoxin at 100 nmol l−1 inhibited the cyclic nucleotide-gated ion channel A2, 30 times more potently than the ICR region of pseudechetoxin at 12 µmol l−1.26
CRISP nomenclature
CRISP nomenclature can be very confusing. The reasons for this are related to differences in the order within which CRISPs were discovered between species, multiple groups bestowing different names on the same protein (Table 1) and the mouse containing four CRISPs as opposed to the usual three observed in other mammals. This will be briefly clarified to allow an effective assessment of the literature.
Table 1. Summary of CRISP nomenclature.
| Mouse CRISP name | Other known names |
|---|---|
| CRISP1 | Protein DE,47, 49, 81 AEG,82 sialoprotein,83 SEP,48 protein IV,84 32-kDa protein,85 MEP786 |
| CRISP2 | AA1,87 TPX188 |
| CRISP3 | Specific granule protein 28,89 horse seminal plasma protein-360 |
| CRISP4 | No other known names |
Abbreviations: AA1, autoantigen 1; AEG, acidic epididymal glycoprotein; CRISP, cysteine-rich secretory protein; MEP7, mouse epididymal protein 7; SEP, specific epididymal protein.
The inconsistencies within the CRISP nomenclature are highlighted in the phylogenetic tree constructed by Gibbs et al.,8 which in some instances reveals as much interspecies variation between apparent orthlogous, as within a species between homologs of CRISPs.8 Therefore, care must be taken when comparing inter- and intraspecies CRISP data; for example, the mouse CRISP1 primary sequence is more closely related to human CRISP3 than human CRISP1 (Table 2). Similarly, comparisons reveal that mouse CRISP4, rather than mouse CRISP1, is the likely orthologous protein to human CRISP1.27, 28 To aid data interpretation, a table comparing CRISP identity and similarity (homology), including the human, mouse, rat and horse, is shown in Table 2.
Table 2. Sequence identity (white boxes) and similarity (homology) (gray boxes) between human, horse, mouse and rat CRISPs expressed as percentages.
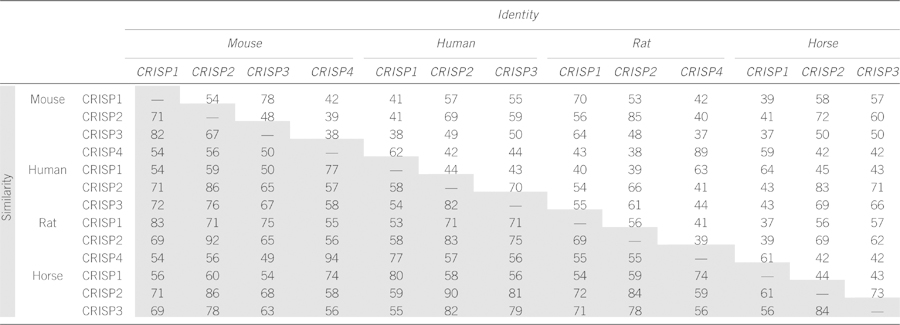
Of potential relevance, several years ago reproductive proteins were identified as among the 10% most evolutionary divergent proteins.29 The high level of divergence and interspecies variation is an indicator of rapid evolution, and as proposed by Swanson and Vacquire30 is a means to drive speciation, that is, biochemical barriers blocking interspecies fertilization. The list of most divergent proteins included CRISP2.
CRISPs in male fertility
Developing germ cells come into contact with CRISPs during every phase of development in the adult male mammal (Figure 1). They are incorporated into the developing sperm acrosome and tail during spermatogenesis, they bathe sperm within the epididymis, and they are mixed with sperm upon ejaculation and present and potentially involved, at the time of fertilization. Although much of the precise biochemistry surrounding CRISP function during these critical periods remains to be elucidated, their presence is surely more than coincidence. Herein, we will summarize the current state of knowledge regarding CRISP localization and where possible speculate on function. A reoccurring theme within this description will be the potential for functional redundancy between CRISPs. It should also be remembered that there are several non-CRISP CAP proteins produced in the male reproductive tract. These include GLIPR1, GLIPR1L1 and GLIPR1L2.31, 32 As such, there is also the potential for functional redundancy between CAP domains. Because of the relatively low state of knowledge on these proteins, however, this potential will not be considered in depth herein.
Figure 1.
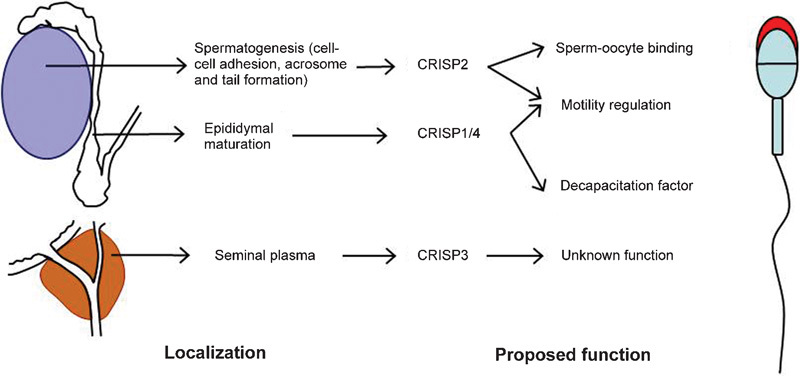
Diagrammatic representation of CRISP expression and function in male fertility. CRISP2 is expressed in haploid germ cells, wherein it is incorporated into the growing acrosome and sperm tail. Within the testis, it has been proposed that CRISP2 is involved in germ cell–Sertoli cell adhesion, and in the tail, it has been proposed to be involved in regulating flagellar beating via its ability to regulate ryanodine receptors. CRISP2 remains associated with the fusogenic region of the sperm head after the acrosome reaction. CRISP1 and 4 are both expressed by the principal cells of the epididymis and become incorporated into the maturing spermatozoa and have been implicated as a decapacitation factor. CRISP3 is excreted from the prostate and seminal vesicles and forms part of the seminal plasma. Although variations in CRISP3 sperm content and sequence have been correlated with fertility, no defined role is currently known. CRISP, cysteine-rich secretory protein.
CRISPs during spermatogenesis
On the basis of published data, Crisp2 is the only CRISP normally expressed in the mammalian testis. Crisp2 transcription initiates in pachytene spermatocytes; however, via the activity of the RNA-binding protein DAZL, Crisp2 mRNA undergoes a period of translational delay before the initiation of translation in round spermatids.33, 34, 35, 36, 37 As shown by the staining (Figure 2), mouse CRISP2 was localized in round spermatids through to elongated spermatids, wherein the protein becomes included into the developing acrosome and the connecting piece and outer dense fibers of the sperm tail.34, 35, 36, 38 The potential function of CRISP2 in the connecting piece is further discussed in relation to sperm motility later in this review. Unlike the other CRISP genes, Crisp2 is not induced by androgens.39
Figure 2.
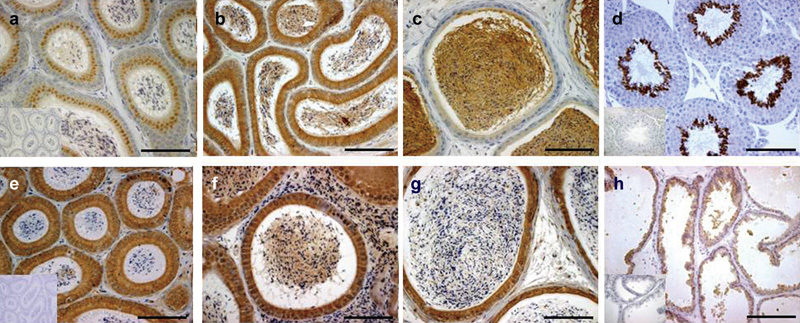
Localization of murine CRISP proteins in male reproductive tissues. (a–c) CRISP1 staining of the caput, corpus and cauda epididymis, respectively. Staining present in the cytoplasm and stereocilia of the principal cells and spermatozoa within the epididymal lumen. (d) CRISP2 staining of the testis shows protein present within the round and elongated spermatids. (e–g) CRISP4 staining of the caput, corpus and cauda epididymis, respectively. Staining present within the cytoplasm of the principal cells, within the stereocilia and in the epididymal lumen, including within epididymosomes. (h) CRISP3 staining of the prostate gland within the apical aspect of the epithelium and in luminal secretory products. Scale bars=100 µm. CRISP, cysteine-rich secretory protein.
The first hint at CRISP2 function came from a study by Maeda et al.40 who used a Jurkat cell transfection assay to identify CRISP2 as having the potential to promote fusion between Jurkat and Sertoli cells. Further, they showed that CRISP2 antisera were able to interfere in the cell adhesion between germ and Sertoli cells. Using deletion studies, the adhesive sequence was narrowed to the N-terminal most 101 amino acids.33 Although this observation is at odds with reports on CRISP2 localization within the testis, it does corroborate observations on the localization of CRISP2 at the site of fertilization and the involvement of CAP domain sequences in sperm–egg fusion (see later).
In addition to the localization of CRISP2 within testis, three CRISP2-binding partners have been identified: mitogen-activating protein kinanse kinase kinase 11, gametogenetin 1 and sperm head and tail-associated protein.41, 42, 43 All three CRISP2-binding proteins require the CRISP domain for binding; however, each has a specific region of colocalization with CRISP2. Mitogen-activating protein kinanse kinase kinase 11 and CRISP2 colocalize in the developing and mature sperm acrosome,41 gametogenetin 1 and CRISP2 colocalize in the principal piece of the sperm tail,42 and sperm head and tail-associated protein and CRISP2 colocalize in the sperm head and principal piece of the sperm tail.43
CRISPs in the epididymis and during epididymal sperm maturation
On leaving the testis, sperm enter the epididymis and begin the process of epididymal maturation. During this time within the seminal plasma, spermatozoa are surrounded by huge concentrations of CRISPs produced by the principal cells of the epididymal epithelium. In the mouse, CRISP1 is produced by all regions of the epididymis, but is preferentially expressed in the cauda.4, 28 In contrast, the second mouse epididymal CRISP, CRISP4, is preferentially produced in the caput, incorporated into epididymosomes and binds to the sperm surface.4, 27, 28
Humans, however, appear to produce only one CRISP in the epididymis, CRISP1. CRISP1 is produced by principal cells throughout the epididymis, whereupon it is incorporated into epididymosomes and presumably transferred onto the sperm surface.44, 45, 46 Collectively, these data raise the possibility that at a functional level, the function of human CRISP1 may be equivalent to the sum of the activities of mouse CRISP1 plus CRISP4.
Rats also contain only one epididymal CRISP, CRISP1. However, CRISP1 is produced as two isoforms, proteins D and E, which are differentially localized within the rat epididymis47 Protein D is more abundant than E (70% of total rat CRISP1), and is synthesized by the principal cells of all regions of the epididymis and localizes to the sperm head.47, 48, 49, 50 Protein E is produced only in the corpus and proximal cauda epididymis.47 Although originally localized in the dorsal region of the sperm head, as rat spermatozoa undergo the acrosome reaction, protein E migrates to the equatorial segment.51, 52 Protein estimates indicate that CRISP1 has a concentration of 1.6 mg ml−1 within rat epididymal secretions.53 The two distinct isoforms of CRISP1 in rats has led to the hypothesis of two different functions. Most simplistically data suggest that the loosely associated D form may function as a decapacitation factor (see below) and that the E form may function during fertilization (see below).
CRISPs as a decapacitation factor
Although the precise function of the CRISPs in the epididymis remains enigmatic, data describing their properties once they are diluted in the fluids of the female reproductive tract, suggest that they may function as decapacitation factors. Decapacitation factors function to keep sperm within the epididymis in a quiescent state. Their diffusion off sperm initiates (at least some of) the pathways leading to capacitation.54
Several pieces of data suggest that CRISP1, in particular, may be a decapacitation factor. The rat D isoform of CRISP1 interacts with spermatozoa transiently and has a reversible association with the sperm surface.50 The majority of human CRISP1 is also weakly attached to ejaculated sperm, is easily removed by washing and thus likely to rapidly diffuse off the sperm in the female reproductive tract.45 Mouse CRISP1 was identified in the decapacitation factor containing fraction of mouse epididymal fluid.55 Furthermore, the addition of purified native CRISP1 to rat spermatozoa in vitro was able to inhibit the manifestation of tail protein tyrosine phosphorylation, a robust marker of capacitation, and the number of sperm undergoing the acrosome reaction in response to cholesterol removal from the sperm plasma membrane.56 Collectively, these, and other data, suggest that CRISP1 binds to sites on the sperm surface resulting in a suppressed (or quiescent) state of storage in the cauda epididymis.50 The mechanism by which CRISP1 inhibits capacitation is currently unknown, but may logically involve the inhibition of ion channels similar to that observed for reptile CRISPs or mouse CRISP2.14
In apparent conflict with the rat data, Crisp1 knockout male mice were fertile. They did, however, manifest a subtle form of subfertility characterized by lower levels of tyrosine phosphorylation after in vitro capacitation.57 Although the reason for this apparent conflict remains to be resolved, it may be the result of species variation in the function of CRISP1 between the mouse and the rat; the absence of CRISP1 may alter epididymal sperm maturation, or the presence of CRISP4 in the mouse may compensate for the absence of CRISP1.
CRISPs at the ejaculate and in the accessory sex organs
CRISP3 is produced by the normal human vas deferens and the prostate.58 CRISP3 is produced by the mouse prostatic epithelium and is present in secretions within the lumen (Figure 2).4 Similarly, CRISP3 is produced in horse seminal vesicles59 and is present in both horse and human seminal plasma at concentrations of 0.3–1.3 mg ml−1 and 14.8 µg ml−1, respectively.58, 60 Despite its presence in such high concentrations, the function of these CRISPs within the ejaculate remains virtually unknown. As mentioned below, although the localization of CRISP3 on horse sperm has been correlated with relative fertility, the crude concentration of CRISP3 in the ejaculate has no relationship with fertility status.61
Regardless of the above, we have some insight of the biochemistry of CRISP3 thanks to some excellent research on CRISP3 in the immune systems and its association with various pathological conditions.58, 62, 63 Within human serum and seminal plasma, CRISP3 binds to β-microseminoprotein and α1B-glycoprotein in a 1∶1 stoichiometry.63, 64 A three-dimensional nuclear magnetic resonance spectrum for the interaction between CRISP3 and α1B-glycoprotein has been determined.65 By analogy to snake CRISPs that become bound to snake serum proteins to limit the chances of self-envenomation,66 it has been proposed that the binding of CRISP3 to β-microseminoprotein in seminal plasma serves to limit CRISP3 bioactivity.63 The challenge remains, however, to determine the bioactivity that must be limited.
Although CRISP3 is expressed by the normal prostate, several studies have revealed an association between increased CRISP3 production and malignant prostate disease.67 CRISP3 expression is increased more than 50-fold in pre-malignant prostate lesions and in primary tumors compared with normal prostatic epithelium.68, 69 The significance of this observation is yet to be realized; however, CRISP3 shows promise as a marker for prostate disease.68, 69
CRISP2 in ion channel regulation and sperm motility
A role for CRISP2 in ICR activity was first intimated through the nuclear magnetic resonance structure of the CRISP2 CRISP domain, and then proven using recombinant CRISP2 in in vitro assays.14 This study showed that the CRISP2 CRISP domain was capable of inhibiting Ca2+ flow through RyR2 in a non-voltage-dependent manner and activated RyR1 opening in a weakly voltage-dependent manner.14 As an extension of this work, studies in humans have shown that RyRs are localized over the sperm head, including the head/mid-piece junction (connecting piece), in which they are hypothesized to mediate Ca2+ release from internal store and to modulate flagellar activity.70, 71
CRISPs in sperm–oocyte binding
Several lines of evidence suggest a role for CRISPs in the interaction between the sperm and the oocyte at fertilization. Specifically, rat (protein E) and mouse CRISP1 irreversibly associate with the sperm plasma membrane during epididymal transit.56, 72, 73 and remain localized to the fusogenic region of the sperm head after the acrosome reaction.52 A similar localization was observed on human sperm,44, 74 and CRISP1 antibodies were capable of significantly reducing the number of penetrating human sperm in the hamster oocyte penetration assay.75 CRISP1 antisera have been shown to interfere with sperm–oocyte binding76 and the coincubation of peptides derived from the CAP signature motifs in the CAP domain (amino acids 114–158) of rat CRISP1-inhibited sperm–zona pellucida binding and sperm–egg fusion in in vitro fertilization.74 Furthermore, although Crisp1 knockout mice produced litters of normal size, their sperm showed a reduced ability to penetrate the zona pellucida and to adhere to the oocyte plasma membrane.57
It also has been shown that mouse CRISP2 remains associated in the equatorial region of the head after the acrosome reaction and that the equivalent CRISP2 CAP domain peptides to those mentioned above, reduces sperm–oocyte binding.77 In vitro sperm competition assays suggested that both CRISP1 and CRISP2 have common binding sites on the oocyte surface.77 These data, and associated data showing the presence of at least one other CAP at the site of fertilization,31 leave little doubt that CAP proteins are involved in fertilization. The precise number of CAPs and their mode of action, however, remain to be elucidated.
CRISPs and male infertility
Not surprisingly, given the widespread and apparently multilayered production of CRISPs in the male reproductive tract, aberrant CRISP function has been proposed as a cause of both human and animal infertility. As implied by in the evolutionary study from Swanson and Vacquier,30 CRISPs tend to have a high degree of intraspecies sequence variation. To date, however, only variations in the horse Crisp3 gene have been identified as a statistically significant cause of reduced fertility. Specifically, Hamann et al.78 identified a single polymorphism in Crisp3 of the Hanoverian warm blood horse that were related to stallion fertility.78 The E208K polymorphisms when present in a heterozygous state compromised fertility by an average of 7% compared with animals carrying the more common allele in a homozygous state. The E208K polymorphism sits within the hinge region of the CRISP domain.
A similar study using human samples identified a large number of single nucleotide polymorphisms in human CRISP2.79 Although none of the single nucleotide polymorphisms were definitively associated within infertility, at least one (C196R) is of biochemical significance by virtue of its ability to interfere with binding to gametogenetin 1. This substitution also occurs within the hinge region and is proposed to destabilize the cross-disulfide bonds.79 Similarly, several studies have shown that the human CRISP2 gene occurs in a region frequently associated with translocations and male infertility.80
Novel contraceptive target
Equally obviously, CRISPs, by virtue of the reproductive tract enriched expression, have been proposed as potential contraceptive targets, and considerable resources have been devoted to testing this hypothesis. The use of CRISP1 for immunocontraception has been shown to result in the production of anti-CRISP1 immunoglobulinin and their adherence to sperm in the rat and the macaque. Although some studies show reduced sperm–zona binding in vitro, males retained fertility.5, 6, 7 Although these studies, and the Crisp1 knockout mouse, suggest that the collective inhibition of all CRISPs may form the basis of a contraceptive, immunocontraceptives are unlikely to be an effective and acceptable mode of action.
Conclusion
Collectively, the CRISPs are a fascinating group of proteins. They show a notable expression bias to the male reproductive tract and are present at virtually every phase of adult germ cell development and maturation. It is hard, therefore, to believe that they do not have an important role. Defining these roles has, however, proved elusive and is exacerbated by a number of factors, including their protein structure and the limited availability of precise analytical tools. Considerable progress has, however, been made in defining their function within the last few years and a picture of function in cell–cell adhesion and ion channel regulation is beginning to emerge.
Acknowledgments
We thank Dr Gerard Gibbs for assistance with Table 2 and helpful discussions over many years, Jo Merriner for the immunohistochemistry figures and Anne O'Connor for review of this paper.
The authors declare no competing financial interests.
References
- Austin CR. Observations on the penetration of the sperm in the mammalian egg. Aust J Sci Res B. 1951;4:581–96. doi: 10.1071/bi9510581. [DOI] [PubMed] [Google Scholar]
- Chang MC. Fertilizing capacity of spermatozoa deposited into the fallopian tubes. Nature. 1951;168:697–8. doi: 10.1038/168697b0. [DOI] [PubMed] [Google Scholar]
- Ito N, Mita M, Takahashi Y, Matsushima A, Watanabe YG, et al. Novel cysteine-rich secretory protein in the buccal gland secretion of the parasitic lamprey, Lethenteron japonicum. Biochem Biophys Res Commun. 2007;358:35–40. doi: 10.1016/j.bbrc.2007.04.065. [DOI] [PubMed] [Google Scholar]
- Reddy T, Gibbs GM, Merriner DJ, Kerr JB, O'Bryan MK. Cysteine-rich secretory proteins are not exclusively expressed in the male reproductive tract. Dev Dyn. 2008;237:3313–23. doi: 10.1002/dvdy.21738. [DOI] [PubMed] [Google Scholar]
- Ellerman DA, Brantua VS, Martinez SP, Cohen DJ, Conesa D, et al. Potential contraceptive use of epididymal proteins: immunization of male rats with epididymal protein DE inhibits sperm fusion ability. Biol Reprod. 1998;59:1029–36. doi: 10.1095/biolreprod59.5.1029. [DOI] [PubMed] [Google Scholar]
- Cuasnicu PS, Gonzalez Echeverria F, Piazza AD, Cameo MS, Blaquier JA. Antibodies against epididymal glycoproteins block fertilizing ability in rat. J Reprod Fertil. 1984;72:467–71. doi: 10.1530/jrf.0.0720467. [DOI] [PubMed] [Google Scholar]
- Perez Martinez S, Conesa D, Cuasnicu PS. Potential contraceptive use of epididymal proteins: evidence for the participation of specific antibodies against rat epididymal protein DE in male and female fertility inhibition. J Reprod Immunol. 1995;29:31–45. doi: 10.1016/0165-0378(95)00927-d. [DOI] [PubMed] [Google Scholar]
- Gibbs GM, Roelants K, O'Bryan MK. The CAP superfamily: cysteine-rich secretory proteins, antigen 5, and pathogenesis-related 1 proteins—roles in reproduction, cancer, and immune defense. Endocr Rev. 2008;29:865–97. doi: 10.1210/er.2008-0032. [DOI] [PubMed] [Google Scholar]
- Guo M, Teng M, Niu L, Liu Q, Huang Q, et al. Crystal structure of the cysteine-rich secretory protein stecrisp reveals that the cysteine-rich domain has a K+ channel inhibitor-like fold. J Biol Chem. 2005;280:12405–12. doi: 10.1074/jbc.M413566200. [DOI] [PubMed] [Google Scholar]
- Shikamoto Y, Suto K, Yamazaki Y, Morita T, Mizuno H. Crystal structure of a CRISP family Ca2+-channel blocker derived from snake venom. J Mol Biol. 2005;350:735–43. doi: 10.1016/j.jmb.2005.05.020. [DOI] [PubMed] [Google Scholar]
- Wang J, Shen B, Guo M, Lou X, Duan Y, et al. Blocking effect and crystal structure of natrin toxin, a cysteine-rich secretory protein from Naja atra venom that targets the BKCa channel. Biochemistry. 2005;44:10145–52. doi: 10.1021/bi050614m. [DOI] [PubMed] [Google Scholar]
- Eberspaecher U, Roosterman D, Kratzschmar J, Haendler B, Habenicht UF, et al. Mouse androgen-dependent epididymal glycoprotein CRISP-1 (DE/AEG): isolation, biochemical characterization, and expression in recombinant form. Mol Reprod Dev. 1995;42:157–72. doi: 10.1002/mrd.1080420205. [DOI] [PubMed] [Google Scholar]
- Milne TJ, Abbenante G, Tyndall JD, Halliday J, Lewis RJ. Isolation and characterization of a cone snail protease with homology to CRISP proteins of the pathogenesis-related protein superfamily. J Biol Chem. 2003;278:31105–10. doi: 10.1074/jbc.M304843200. [DOI] [PubMed] [Google Scholar]
- Gibbs GM, Scanlon MJ, Swarbrick J, Curtis S, Gallant E, et al. The cysteine-rich secretory protein domain of Tpx-1 is related to ion channel toxins and regulates ryanodine receptor Ca2+ signaling. J Biol Chem. 2006;281:4156–63. doi: 10.1074/jbc.M506849200. [DOI] [PubMed] [Google Scholar]
- Gibbs GM, O'Bryan MK. Cysteine rich secretory proteins in reproduction and venom. Soc Reprod Fertil Suppl. 2007;65:261–7. [PubMed] [Google Scholar]
- Fernandez C, Szyperski T, Bruyere T, Ramage P, Mosinger E, et al. NMR solution structure of the pathogenesis-related protein P14a. J Mol Biol. 1997;266:576–93. doi: 10.1006/jmbi.1996.0772. [DOI] [PubMed] [Google Scholar]
- al-Anzi B, Chandler DE. A sperm chemoattractant is released from Xenopus egg jelly during spawning. Dev Biol. 1998;198:366–75. [PubMed] [Google Scholar]
- Yamakawa T, Miyata S, Ogawa N, Koshikawa N, Yasumitsu H, et al. cDNA cloning of a novel trypsin inhibitor with similarity to pathogenesis-related proteins, and its frequent expression in human brain cancer cells. Biochim Biophys Acta. 1998;1395:202–8. doi: 10.1016/s0167-4781(97)00149-8. [DOI] [PubMed] [Google Scholar]
- Mochca-Morales J, Martin BM, Possani LD. Isolation and characterization of Helothermine, a novel toxin from Heloderma horridum horridum (Mexican beaded lizard) venom. Toxicon. 1990;28:299–309. doi: 10.1016/0041-0101(90)90065-f. [DOI] [PubMed] [Google Scholar]
- Nobile M, Magnelli V, Lagostena L, Mochca-Morales J, Possani LD, Prestipino G. The toxin helothermine affects potassium currents in newborn rat cerebellar granule cells. J Membr Biol. 1994;139:49–55. doi: 10.1007/BF00232674. [DOI] [PubMed] [Google Scholar]
- Nobile M, Noceti F, Prestipino G, Possani LD. Helothermine, a lizard venom toxin, inhibits calcium current in cerebellar granules. Exp Brain Res. 1996;110:15–20. doi: 10.1007/BF00241369. [DOI] [PubMed] [Google Scholar]
- Morrissette J, Kratzschmar J, Haendler B, el-Hayek R, Mochca-Morales J, et al. Primary structure and properties of helothermine, a peptide toxin that blocks ryanodine receptors. Biophys J. 1995;68:2280–8. doi: 10.1016/S0006-3495(95)80410-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhou Q, Wang QL, Meng X, Shu Y, Jiang T, et al. Structural and functional characterization of ryanodine receptor–natrin toxin interaction. Biophys J. 2008;95:4289–99. doi: 10.1529/biophysj.108.137224. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yamazaki Y, Brown RL, Morita T. Purification and cloning of toxins from elapid venoms that target cyclic nucleotide-gated ion channels. Biochemistry. 2002;41:11331–7. doi: 10.1021/bi026132h. [DOI] [PubMed] [Google Scholar]
- Brown RL, Haley TL, West KA, Crabb JW. Pseudechetoxin: a peptide blocker of cyclic nucleotide-gated ion channels. Proc Natl Acad Sci USA. 1999;96:754–9. doi: 10.1073/pnas.96.2.754. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Suzuki N, Yamazaki Y, Brown RL, Fujimoto Z, Morita T, et al. Structures of pseudechetoxin and pseudecin, two snake-venom cysteine-rich secretory proteins that target cyclic nucleotide-gated ion channels: implications for movement of the C-terminal cysteine-rich domain. Acta Crystallogr D Biol Crystallogr. 2008;64:1034–42. doi: 10.1107/S0907444908023512. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jalkanen J, Huhtaniemi I, Poutanen M. Mouse cysteine-rich secretory protein 4 (CRISP4): a member of the Crisp family exclusively expressed in the epididymis in an androgen-dependent manner. Biol Reprod. 2005;72:1268–74. doi: 10.1095/biolreprod.104.035758. [DOI] [PubMed] [Google Scholar]
- Nolan MA, Wu L, Bang HJ, Jelinsky SA, Roberts KP, et al. Identification of rat cysteine-rich secretory protein 4 (Crisp4) as the ortholog to human CRISP1 and mouse Crisp4. Biol Reprod. 2006;74:984–91. doi: 10.1095/biolreprod.105.048298. [DOI] [PubMed] [Google Scholar]
- Makalowski W, Boguski MS. Evolutionary parameters of the transcribed mammalian genome: an analysis of 2,820 orthologous rodent and human sequences. Proc Natl Acad Sci USA. 1998;95:9407–12. doi: 10.1073/pnas.95.16.9407. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Swanson WJ, Vacquier VD. The rapid evolution of reproductive proteins. Nat Rev Genet. 2002;3:137–44. doi: 10.1038/nrg733. [DOI] [PubMed] [Google Scholar]
- Gibbs GM, Lo JC, Nixon B, Jamsai D, O'Connor AE, et al. Glioma pathogenesis-related 1-like 1 is testis enriched, dynamically modified, and redistributed during male germ cell maturation and has a potential role in sperm–oocyte binding. Endocrinology. 2010;151:2331–42. doi: 10.1210/en.2009-1255. [DOI] [PubMed] [Google Scholar]
- Ren C, Ren CH, Li L, Goltsov AA, Thompson TC. Identification and characterization of RTVP1/GLIPR1-like genes, a novel p53 target gene cluster. Genomics. 2006;88:163–72. doi: 10.1016/j.ygeno.2006.03.021. [DOI] [PubMed] [Google Scholar]
- Maeda T, Nishida J, Nakanishi Y. Expression pattern, subcellular localization and structure–function relationship of rat Tpx-1, a spermatogenic cell adhesion molecule responsible for association with Sertoli cells. Dev Growth Differ. 1999;41:715–22. doi: 10.1046/j.1440-169x.1999.00470.x. [DOI] [PubMed] [Google Scholar]
- Foster JA, Gerton GL. Autoantigen 1 of the guinea pig sperm acrosome is the homologue of mouse Tpx-1 and human TPX1 and is a member of the cysteine-rich secretory protein (CRISP) family. Mol Reprod Dev. 1996;44:221–9. doi: 10.1002/(SICI)1098-2795(199606)44:2<221::AID-MRD11>3.0.CO;2-5. [DOI] [PubMed] [Google Scholar]
- O'Bryan MK, Loveland KL, Herszfeld D, McFarlane JR, Hearn MT, et al. Identification of a rat testis-specific gene encoding a potential rat outer dense fibre protein. Mol Reprod Dev. 1998;50:313–22. doi: 10.1002/(SICI)1098-2795(199807)50:3<313::AID-MRD7>3.0.CO;2-M. [DOI] [PubMed] [Google Scholar]
- O'Bryan MK, Sebire K, Meinhardt A, Edgar K, Keah HH, et al. Tpx-1 is a component of the outer dense fibers and acrosome of rat spermatozoa. Mol Reprod Dev. 2001;58:116–25. doi: 10.1002/1098-2795(200101)58:1<116::AID-MRD14>3.0.CO;2-8. [DOI] [PubMed] [Google Scholar]
- Jiao X, Trifillis P, Kiledjian M. Identification of target messenger RNA substrates for the murine deleted in azoospermia-like RNA-binding protein. Biol Reprod. 2002;66:475–85. doi: 10.1095/biolreprod66.2.475. [DOI] [PubMed] [Google Scholar]
- Busso D, Cohen DJ, Hayashi M, Kasahara M, Cuasnicu PS. Human testicular protein TPX1/CRISP-2: localization in spermatozoa, fate after capacitation and relevance for gamete interaction. Mol Hum Reprod. 2005;11:299–305. doi: 10.1093/molehr/gah156. [DOI] [PubMed] [Google Scholar]
- Haendler B, Habenicht UF, Schwidetzky U, Schuttke I, Schleuning WD. Differential androgen regulation of the murine genes for cysteine-rich secretory proteins (CRISP) Eur J Biochem. 1997;250:440–6. doi: 10.1111/j.1432-1033.1997.0440a.x. [DOI] [PubMed] [Google Scholar]
- Maeda T, Sakashita M, Ohba Y, Nakanishi Y. Molecular cloning of the rat Tpx-1 responsible for the interaction between spermatogenic and Sertoli cells. Biochem Biophys Res Commun. 1998;248:140–6. doi: 10.1006/bbrc.1998.8918. [DOI] [PubMed] [Google Scholar]
- Gibbs GM, Bianco DM, Jamsai D, Herlihy A, Ristevski S, et al. Cysteine-rich secretory protein 2 binds to mitogen-activated protein kinase kinase kinase 11 in mouse sperm. Biol Reprod. 2007;77:108–14. doi: 10.1095/biolreprod.106.057166. [DOI] [PubMed] [Google Scholar]
- Jamsai D, Bianco DM, Smith SJ, Merriner DJ, Ly-Huynh JD, et al. Characterization of gametogenetin 1 (GGN1) and its potential role in male fertility through the interaction with the ion channel regulator, cysteine-rich secretory protein 2 (CRISP2) in the sperm tail. Reproduction. 2008;135:751–9. doi: 10.1530/REP-07-0485. [DOI] [PubMed] [Google Scholar]
- Jamsai D, Rijal S, Bianco DM, O'Connor AE, Merriner DJ, et al. A novel protein, sperm head and tail associated protein (SHTAP), interacts with cysteine-rich secretory protein 2 (CRISP2) during spermatogenesis in the mouse. Biol Cell. 2009;102:93–106. doi: 10.1042/BC20090099. [DOI] [PubMed] [Google Scholar]
- Hayashi M, Fujimoto S, Takano H, Ushiki T, Abe K, et al. Characterization of a human glycoprotein with a potential role in sperm-egg fusion: cDNA cloning, immunohistochemical localization, and chromosomal assignment of the gene (AEGL1) Genomics. 1996;32:367–74. doi: 10.1006/geno.1996.0131. [DOI] [PubMed] [Google Scholar]
- Kratzschmar J, Haendler B, Eberspaecher U, Roosterman D, Donner P, et al. The human cysteine-rich secretory protein (CRISP) family. Primary structure and tissue distribution of CRISP-1, CRISP-2 and CRISP-3. Eur J Biochem. 1996;236:827–36. doi: 10.1111/j.1432-1033.1996.t01-1-00827.x. [DOI] [PubMed] [Google Scholar]
- Sullivan R, Frenette G, Girouard J. Epididymosomes are involved in the acquisition of new sperm proteins during epididymal transit. Asian J Androl. 2007;9:483–91. doi: 10.1111/j.1745-7262.2007.00281.x. [DOI] [PubMed] [Google Scholar]
- Brooks DE. Purification of rat epididymal proteins ‘D' and ‘E', demonstration of shared immunological determinants, and identification of regional synthesis and secretion. Int J Androl. 1982;5:513–24. doi: 10.1111/j.1365-2605.1982.tb00283.x. [DOI] [PubMed] [Google Scholar]
- Kohane AC, Cameo MS, Pineiro L, Garberi JC, Blaquier JA. Distribution and site of production of specific proteins in the rat epididymis. Biol Reprod. 1980;23:181–7. doi: 10.1095/biolreprod23.1.181. [DOI] [PubMed] [Google Scholar]
- Roberts KP, Ensrud KM, Hamilton DW. A comparative analysis of expression and processing of the rat epididymal fluid and sperm-bound forms of proteins D and E. Biol Reprod. 2002;67:525–33. doi: 10.1095/biolreprod67.2.525. [DOI] [PubMed] [Google Scholar]
- Roberts KP, Ensrud-Bowlin KM, Piehl LB, Parent KR, Bernhardt ML, et al. Association of the protein D and protein E forms of rat CRISP1 with epididymal sperm. Biol Reprod. 2008;79:1046–53. doi: 10.1095/biolreprod.108.070664. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cohen DJ, Da Ros VG, Busso D, Ellerman DA, Maldera JA, et al. Participation of epididymal cysteine-rich secretory proteins in sperm–egg fusion and their potential use for male fertility regulation. Asian J Androl. 2007;9:528–32. doi: 10.1111/j.1745-7262.2007.00283.x. [DOI] [PubMed] [Google Scholar]
- Rochwerger L, Cuasnicu PS. Redistribution of a rat sperm epididymal glycoprotein after in vitro and in vivo capacitation. Mol Reprod Dev. 1992;31:34–41. doi: 10.1002/mrd.1080310107. [DOI] [PubMed] [Google Scholar]
- Roberts KP, Ensrud KM, Wooters JL, Nolan MA, Johnston DS, et al. Epididymal secreted protein Crisp-1 and sperm function. Mol Cell Endocrinol. 2006;250:122–7. doi: 10.1016/j.mce.2005.12.034. [DOI] [PubMed] [Google Scholar]
- Fraser LR. Mouse sperm capacitation in vitro involves loss of a surface-associated inhibitory component. J Reprod Fertil. 1984;72:373–84. doi: 10.1530/jrf.0.0720373. [DOI] [PubMed] [Google Scholar]
- Nixon B, MacIntyre DA, Mitchell LA, Gibbs GM, O'Bryan M, et al. The identification of mouse sperm-surface-associated proteins and characterization of their ability to act as decapacitation factors. Biol Reprod. 2006;74:275–87. doi: 10.1095/biolreprod.105.044644. [DOI] [PubMed] [Google Scholar]
- Roberts KP, Wamstad JA, Ensrud KM, Hamilton DW. Inhibition of capacitation-associated tyrosine phosphorylation signaling in rat sperm by epididymal protein Crisp-1. Biol Reprod. 2003;69:572–81. doi: 10.1095/biolreprod.102.013771. [DOI] [PubMed] [Google Scholar]
- Da Ros VG, Maldera JA, Willis WD, Cohen DJ, Goulding EH, et al. Impaired sperm fertilizing ability in mice lacking cysteine-rich secretory protein 1 (CRISP1) Dev Biol. 2008;320:12–8. doi: 10.1016/j.ydbio.2008.03.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Udby L, Bjartell A, Malm J, Egesten A, Lundwall A, et al. Characterization and localization of cysteine-rich secretory protein 3 (CRISP-3) in the human male reproductive tract. J Androl. 2005;26:333–42. doi: 10.2164/jandrol.04132. [DOI] [PubMed] [Google Scholar]
- Schambony A, Gentzel M, Wolfes H, Raida M, Neumann U, et al. Equine CRISP-3: primary structure and expression in the male genital tract. Biochim Biophys Acta. 1998;1387:206–16. doi: 10.1016/s0167-4838(98)00122-8. [DOI] [PubMed] [Google Scholar]
- Magdaleno L, Gasset M, Varea J, Schambony AM, Urbanke C, et al. Biochemical and conformational characterisation of HSP-3, a stallion seminal plasma protein of the cysteine-rich secretory protein (CRISP) family. FEBS Lett. 1997;420:179–85. doi: 10.1016/s0014-5793(97)01514-7. [DOI] [PubMed] [Google Scholar]
- Novak S, Smith TA, Paradis F, Burwash L, Dyck MK, et al. Biomarkers of in vivo fertility in sperm and seminal plasma of fertile stallions. Theriogenology. 2010;74:956–67. doi: 10.1016/j.theriogenology.2010.04.025. [DOI] [PubMed] [Google Scholar]
- Bjartell AS, Al-Ahmadie H, Serio AM, Eastham JA, Eggener SE, et al. Association of cysteine-rich secretory protein 3 and beta-microseminoprotein with outcome after radical prostatectomy. Clin Cancer Res. 2007;13:4130–8. doi: 10.1158/1078-0432.CCR-06-3031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Udby L, Sorensen OE, Pass J, Johnsen AH, Behrendt N, et al. Cysteine-rich secretory protein 3 is a ligand of alpha1B-glycoprotein in human plasma. Biochemistry. 2004;43:12877–86. doi: 10.1021/bi048823e. [DOI] [PubMed] [Google Scholar]
- Udby L, Lundwall A, Johnsen AH, Fernlund P, Valtonen-Andre C, et al. beta-Microseminoprotein binds CRISP-3 in human seminal plasma. Biochem Biophys Res Commun. 2005;333:555–61. doi: 10.1016/j.bbrc.2005.05.139. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ghasriani H, Fernlund P, Udby L, Drakenberg T. A model of the complex between human beta-microseminoprotein and CRISP-3 based on NMR data. Biochem Biophys Res Commun. 2009;378:235–9. doi: 10.1016/j.bbrc.2008.11.040. [DOI] [PubMed] [Google Scholar]
- Aoki N, Sakiyama A, Kuroki K, Maenaka K, Kohda D, et al. Serotriflin, a CRISP family protein with binding affinity for small serum protein-2 in snake serum. Biochim Biophys Acta. 2008;1784:621–8. doi: 10.1016/j.bbapap.2007.12.010. [DOI] [PubMed] [Google Scholar]
- Asmann YW, Kosari F, Wang K, Cheville JC, Vasmatzis G. Identification of differentially expressed genes in normal and malignant prostate by electronic profiling of expressed sequence tags. Cancer Res. 2002;62:3308–14. [PubMed] [Google Scholar]
- Bjartell A, Johansson R, Bjork T, Gadaleanu V, Lundwall A, et al. Immunohistochemical detection of cysteine-rich secretory protein 3 in tissue and in serum from men with cancer or benign enlargement of the prostate gland. Prostate. 2006;66:591–603. doi: 10.1002/pros.20342. [DOI] [PubMed] [Google Scholar]
- Kosari F, Asmann YW, Cheville JC, Vasmatzis G. Cysteine-rich secretory protein-3: a potential biomarker for prostate cancer. Cancer Epidemiol Biomarkers Prev. 2002;11:1419–26. [PubMed] [Google Scholar]
- Harper CV, Barratt CL, Publicover SJ. Stimulation of human spermatozoa with progesterone gradients to simulate approach to the oocyte. Induction of [Ca2+]i oscillations and cyclical transitions in flagellar beating. J Biol Chem. 2004;279:46315–25. doi: 10.1074/jbc.M401194200. [DOI] [PubMed] [Google Scholar]
- Publicover S, Harper CV, Barratt C. [Ca2+]i signalling in sperm—making the most of what you've got. Nat Cell Biol. 2007;9:235–42. doi: 10.1038/ncb0307-235. [DOI] [PubMed] [Google Scholar]
- Moore A, Ensrud KM, White TW, Frethem CD, Hamilton DW. Rat epididymis-specific sperm maturation antigens. I. Evidence that the 26 kD 4E9 antigen found on rat caudal epididymal sperm tail is derived from a protein secreted by the epididymis. Mol Reprod Dev. 1994;37:181–94. doi: 10.1002/mrd.1080370209. [DOI] [PubMed] [Google Scholar]
- Roberts KP, Johnston DS, Nolan MA, Wooters JL, Waxmonsky NC, et al. Structure and function of epididymal protein cysteine-rich secretory protein-1. Asian J Androl. 2007;9:508–14. doi: 10.1111/j.1745-7262.2007.00318.x. [DOI] [PubMed] [Google Scholar]
- Ellerman DA, Cohen DJ, Da Ros VG, Morgenfeld MM, Busso D, et al. Sperm protein “DE” mediates gamete fusion through an evolutionarily conserved site of the CRISP family. Dev Biol. 2006;297:228–37. doi: 10.1016/j.ydbio.2006.05.013. [DOI] [PubMed] [Google Scholar]
- Cohen DJ, Ellerman DA, Busso D, Morgenfeld MM, Piazza AD, et al. Evidence that human epididymal protein ARP plays a role in gamete fusion through complementary sites on the surface of the human egg. Biol Reprod. 2001;65:1000–5. doi: 10.1095/biolreprod65.4.1000. [DOI] [PubMed] [Google Scholar]
- Busso D, Cohen DJ, Da Ros V, Fissore R, Cuasnicu PS. Studies on the participation of epididymal sperm protein DE/CRISP-1 in egg activation. Cell Mol Biol (Noisy-le-grand) 2003;49:407–12. [PubMed] [Google Scholar]
- Busso D, Goldweic NM, Hayashi M, Kasahara M, Cuasnicu PS. Evidence for the involvement of testicular protein CRISP2 in mouse sperm–egg fusion. Biol Reprod. 2007;76:701–8. doi: 10.1095/biolreprod.106.056770. [DOI] [PubMed] [Google Scholar]
- Hamann H, Jude R, Sieme H, Mertens U, Topfer-Petersen E, et al. A polymorphism within the equine CRISP3 gene is associated with stallion fertility in Hanoverian warmblood horses. Anim Genet. 2007;38:259–64. doi: 10.1111/j.1365-2052.2007.01594.x. [DOI] [PubMed] [Google Scholar]
- Jamsai D, Reilly A, Smith SJ, Gibbs GM, Baker HW, et al. Polymorphisms in the human cysteine-rich secretory protein 2 (CRISP2) gene in Australian men. Hum Reprod. 2008;23:2151–9. doi: 10.1093/humrep/den191. [DOI] [PubMed] [Google Scholar]
- Paoloni-Giacobino A, Kern I, Rumpler Y, Djlelati R, Morris MA, et al. Familial t(6;21)(p21.1;p13) translocation associated with male-only sterility. Clin Genet. 2000;58:324–8. doi: 10.1034/j.1399-0004.2000.580411.x. [DOI] [PubMed] [Google Scholar]
- Cameo MS, Blaquier JA. Androgen-controlled specific proteins in rat epididymis. J Endocrinol. 1976;69:47–55. doi: 10.1677/joe.0.0690047. [DOI] [PubMed] [Google Scholar]
- Lea OA, Pertrusz P, French FS. Purification and localization of acidic epididymal glycoprotein (AEG): a sperm coating protein secreted by the rat epididymis. Int J Androl (Suppl 2) 1978. pp. 592–607.
- Faye JC, Duguet L, Mazzuca M, Bayard F. Purification, radioimmunoassay, and immunohistochemical localization of a glycoprotein produced by the rat epididymis. Biol Reprod. 1980;23:423–32. doi: 10.1095/biolreprod23.2.423. [DOI] [PubMed] [Google Scholar]
- Jones R, Brown CR. Association of epididymal secretory proteins showing alpha-lactalbumin-like activity with the plasma membrane of rat spermatozoa. Biochem J. 1982;206:161–4. doi: 10.1042/bj2060161. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wong PY, Tsang AY. Studies on the binding of a 32K rat epididymal protein to rat epididymal spermatozoa. Biol Reprod. 1982;27:1239–46. doi: 10.1095/biolreprod27.5.1239. [DOI] [PubMed] [Google Scholar]
- Rankin TL, Tsuruta KJ, Holland MK, Griswold MD, Orgebin-Crist MC. Isolation, immunolocalization, and sperm-association of three proteins of 18, 25, and 29 kilodaltons secreted by the mouse epididymis. Biol Reprod. 1992;46:747–66. doi: 10.1095/biolreprod46.5.747. [DOI] [PubMed] [Google Scholar]
- Hardy DM, Huang TT, Jr, Driscoll WJ, Tung KK, Wild GC. Purification and characterization of the primary acrosomal autoantigen of guinea pig epididymal spermatozoa. Biol Reprod. 1988;38:423–37. doi: 10.1095/biolreprod38.2.423. [DOI] [PubMed] [Google Scholar]
- Kasahara M, Passmore HC, Klein J. A testis-specific gene Tpx-1 maps between Pgk-2 and Mep-1 on mouse chromosome 17. Immunogenetics. 1989;29:61–3. doi: 10.1007/BF02341616. [DOI] [PubMed] [Google Scholar]
- Kjeldsen L, Cowland JB, Johnsen AH, Borregaard N. SGP28, a novel matrix glycoprotein in specific granules of human neutrophils with similarity to a human testis-specific gene product and a rodent sperm-coating glycoprotein. FEBS Lett. 1996;380:246–50. doi: 10.1016/0014-5793(96)00030-0. [DOI] [PubMed] [Google Scholar]


