Abstract
To bind and fertilize the egg, the spermatozoon should undergo few biochemical and motility changes in the female reproductive tract collectively called capacitation. The capacitated spermatozoon binds to the egg zona pellucida, and then undergoes the acrosome reaction (AR), which allows its penetration into the egg. The mechanisms regulating sperm capacitation and the AR are not completely understood. In the present review, we summarize some data regarding the role and regulation of the epidermal growth factor receptor (EGFR) in these processes. In the capacitation process, the EGFR is partially activated by protein kinase A (PKA), resulting in phospholipase D (PLD) activation and actin polymerization. Protein kinase C alpha (PKCα), which is already activated at the beginning of the capacitation, also participates in PLD activation. Further activation of the EGFR at the end of the capacitation enhances intracellular Ca2+ concentration leading to F-actin breakdown and allows the AR to take place. Under in vivo conditions, the EGFR can be directly activated by its known ligand epidermal growth factor (EGF), and indirectly by activating PKA or by transactivation mediated by G protein-coupled receptors (GPCRs) activation or by ouabain. Under physiological conditions, sperm PKA is activated mainly by bicarbonate, which activates the soluble adenylyl cyclase to produce cyclic adenosine monophosphate (cAMP), the activator of PKA. The GPCR activators angiotensin II or lysophosphatidic acid, as well as ouabain and EGF are physiological components present in the female reproductive tract.
Keywords: acrosome reaction, capacitation, PI3K, PKA, PKC, spermatozoa
Introduction
Ejaculated mammalian spermatozoa should reside in the female genital tract for several hours before gaining the ability to fertilize the egg. In human sperm, however, sperm must move out of the seminal plasma immediately after ejaculation and appear in the fallopian tube within minutes. As soon as sperm are moving out of the ejaculate and passing the cervical mucus, they undergo several biochemical changes collectively called capacitation (reviewed in Florman and Ducibella1 and Gadella and Visconti2). These changes are still not clear, but it seems certain to involve molecules absorbed on, or integrated into, the sperm plasma membrane during epididymal maturation, and on contact of spermatozoa with the seminal plasma they render the spermatozoa capable of fertilization. The removal or alteration of these molecules prepares the sperm toward successful binding to the egg and fertilization. It was shown by Yanagimachi and Chang3 that capacitation can be mimicked in vitro, making the analysis of capacitation mechanisms considerably easier.
During mammalian fertilization, the capacitated spermatozoon penetrates the cumulus oophrous of the ovum, and then binds to the zona pellucida with its plasma membrane intact. After binding to the egg zona pellucida, the spermatozoon undergoes an exocytotic process called the acrosome reaction (AR) (reviewed in Yanagimachi et al.,4 Roldan and Shi,5 Florman et al.6 and Breitbart7). This event is required for fertilization, because it enables passage of the spermatozoon through the zona pellucida and its subsequent fusion with the egg oolema. Therefore, elucidation of the mechanisms regulating the AR is important for understanding the process of mammalian fertilization. In our laboratory and others it was shown that variety of agonists can trigger the AR via receptor-mediated mechanisms.1, 6, 8, 9, 10 Although zona pellucida-derived glycoproteins are thought to be the physiological inducers of the AR,11, 12 the reaction can be induced in vitro by various constituents of the female reproductive tract including progesterone,13, 14 prostaglandins,15 atrial natriuretic peptide,16 epidermal growth factor (EGF),9, 10, 17 ouabain10 and other ligands. These agonists may have a direct and/or synergistic effect with other constituents of the female reproductive10 or on the zona pellucida.14 The question regarding the physiological role of these factors under in vivo conditions is still an open question. Assuming that acrosome-reacted sperm cannot bind and fertilize the egg, we suggest that premature AR before reaching the egg zona pellucida, might be a way of selection in which the ‘bad' sperm will undergo the so-called non-specific AR and would not be able to fertilize, whereas the ‘best' selected sperm will reach the egg in its intact morphology and will fertilize it. Thus, to study the selection mechanism, it is very important to understand the mechanism of action of the various physiological factors that induce the AR. One of these mechanisms, the EGF receptor (EGFR) system is described in this review.
Actin remodeling in sperm capacitation and before the AR
In recent years, our laboratory focused on the formation of actin filaments during mammalian sperm capacitation and the depolymerization of these filaments before the AR.18 The formation of F-actin during capacitation was observed mainly in the sperm head and also in the tail.18, 19 It was shown almost 30 years ago that in echinoderm sperm, actin can be polymerized and that actin is localized in the microfilaments in the acrosomal process.20 Later, it was suggested that sperm motility is affected by the rapid polymerization of actin.21 In our early studies with isolated bovine sperm membranes, we suggested that F-actin network located between the plasma membrane and the outer acrosomal membrane forms a scaffold that immobilizes phospholipase C-γ1, which is involved in the AR (reviewed in Breitbart and Spungin22) The observation that both actin depolymerization23 and membrane fusion24 require relatively high calcium concentration (in the mmol l−1 range) supports the notion that actin filaments constitute the final barrier to fusion (reviewed in Breitbart and Spungin22). We have recently suggested that translation of nuclear-encoded proteins occurs in sperm mitochondria during capacitation,25 and this finding was later confirmed by sperm proteomics approach.26 In other cell types, it was shown that mRNA can be translocated on actin filaments to the translation location in the cell; thus we suggested that the formation of F-actin during sperm capacitation might be important for the translocation of nuclear mRNA to the sperm mid-piece where the mitochondria are located.
We previously demonstrated that the process of actin polymerization depends on phospholipase D (PLD) activity.27 We have shown that this activity is regulated by the crosstalk between the protein kinases A and C (PKA/PKC).27 In a more recent publication, we demonstrated that phosphatidylinositol 4-kinase (PI4K) regulate the activity of PLD by its activity product phosphatidylinositol 4,5-bisphosphate (PIP2(4,5)) that is required as a cofactor for the activation of PLD in many cell types.19, 28, 29, 30, 31 It was shown that PIP2 is produced gradually during sperm capacitation and, in parallel, PLD activity and F-actin levels are increased. We also show that spermine (10 µmol l−1), a constituents of the semen, can enhance the activity of PI4K, leading to increase in the production of PIP2(4,5). This enhancement in PI4K and PLD activity is accompanied by elevation in actin polymerization.19, 27 Furthermore PIP2(4,5) serves as a precursor for two well-defined second messengers produced by its phospholipase C-catalyzed hydrolysis;32 diacylglycerol, which activates PKC,33 and inositol 1,4,5-triphosphate, which mobilizes Ca2+ from intracellular stores.32 PKC is involved in sperm AR and actin polymerization19, 27 and inositol 1,4,5-triphosphate is involved in intracellular calcium regulation in sperm.34, 35, 36
It is not clear how PI4K is regulated in sperm capacitation. We show elsewhere that 10 µmol l−1 of spermine stimulates PI4K and actin polymerization, and this stimulation is abrogated by 1 mmol l−1 spermine probably because of its binding to PIP2(4,5) and preventing PLD activation.19 At the time of ejaculation, sperm are exposed to millimolar spermine.37 Spermine is taken up very fast by the sperm cells and released rapidly when incubated under capacitation conditions.38 Thus, we suggest that spermine appears to be a physiological regulator of PI4K activity.
Another phosphatidylinositol kinase is the phosphatidylinositol 3-kinase (PI3K) that can phosphorylate PIP2(4,5) to produce phosphatidylinositol-3,4,5-triphosphate (PIP3(3,4,5)).39 Although PIP2(4,5) and PIP3(3,4,5) represent less than 1% of membrane phospholipids, they function in several crucial cellular processes.40 A role for PI3K has been suggested in sperm functions during sperm capacitation and the AR.19, 41, 42 We have recently shown that PI3K is significantly activated toward the end of the incubation under capacitation conditions and involved in the AR; however, it does not mediate F-actin formation in sperm capacitation.19 We also show PI3K-dependent PIP3(3,4,5) formation in sperm capacitation that is stimulated by activation of PKA.19 Thus, we suggest that under regular in vitro capacitation conditions PLD is a central regulator of F-actin formation during sperm capacitation, whereas PI3K is a major player in the AR. However, when PKA is overactivated by adding 8Br-cyclic adenosine monophosphate (8Br-cAMP) (1 mmol l−1), we can see significant stimulation of PI3K activation, as well as PI3K-dependent F-actin formation.9, 19 This observation led us to suggest that F-actin formation can be induced under various conditions: (i) when PKA activity is relatively low and PKC activity is high, enhancement of PIP2(4,5) via activation of PI4K by spermine or PKC leads to PLD activation and actin polymerization. Under these conditions PIP3(3,4,5) is not involved in actin polymerization; and (ii) when PKA activity is relatively high and PKC activity is downregulated,27 PKA enhances PIP2(4,5) via activation of PI3K, which activates PI4K and PI4P5K indirectly, leading to PLD activation and actin polymerization.19
Role of EGFR in AR: localization of EGFR and effect on AR
It was shown by others that EGFR is involved in boar sperm motility43 and were localized at higher extent to the acrosome region than to the post-acrosome and the flagellum.43 Moreover, EGF signaling was shown to be an important pathway identified in high fertility sperm in a recent comprehensive proteomic analysis.44 We previously show that bovine sperm express EGFR that is involved in the AR and in actin polymerization during sperm capacitation.9, 17, 18 We also show that EGFR was localized to the sperm head and midpiece, but not to the principal region of the tail.9 The localization of EGFR in the head of sperm led us to investigate the role of EGFR in the AR. It was shown that EGF can induce the AR when added at the end of capacitation.9, 10, 17 It is well established that PI3K is a downstream effector of EGFR, and indeed, we have shown that inhibition of PI3K by 10 nmol l−1 wortmannin blocked the AR induced by EGF. Moreover, in a recent publication, we show that activation of G protein-coupled receptors (GPCRs), angiotensin II receptor type 1 or lysophosphatydic acid receptor causes transactivation of EGFR mediated by PKA and Src.9 Adding angiotensin II, lysophosphatydic acid or cAMP to capacitated sperm induced EGFR- and PI3K-dependent AR.9
To summarize the effect of EGF, we showed dual effects in which it enhanced F-actin formation during sperm capacitation, whereas in capacitated sperm, when F-actin level is already high, EGF causes F-actin breakdown (Figure 1). Our unpublished data revealed that the F-actin-severing protein gelsolin is inactive during sperm capacitation; therefore, we can see EGF-induced F-actin formation. However, before the AR, gelsolin can be activated by elevating intracellular Ca2+ concentration ([Ca2+]i) by EGFR activation causing F-actin breakdown.
Figure 1.
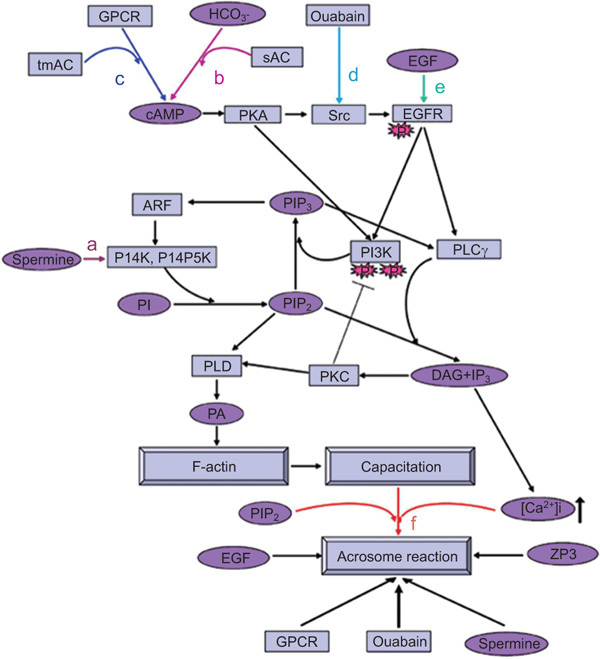
A model representing the mechanisms underlying EGFR activation during bovine sperm capacitation and AR. (a) Activation of PLD in sperm capacitation: at the beginning of the capacitation process, spermine can induce PI4K activation leading to PIP2(4,5) formation,19 a cofactor for PLD activation. The activation of PLD occurs by PKCα, which is already activated at the beginning of the capacitation.82 PLD can also be activated by activating PKA.27 Activation of PLD leads to F-actin formation during sperm capacitation.27 (b–e) Partial activation of EGFR in sperm capacitation: the EGFR can be activated via EGF (e) or by the cAMP-dependent PKA/Src system by activating GPCRs,9 which activate membrane-bound adenylyl cyclase to form cAMP, by HCO−3,7 which activates the soluble adenylyl cyclase (b, c), or by ouabain (d),10 which activates the tyrosine kinase Src. The activated EGFR can lead to PLD activation and F-actin formation via activation of PI3K/ARF/PI4K to form PIP2 and via PLC/PKC, two pathways needed for PLD activation. The activation of PI3K is upregulated by PKA and downregulated by PKC.82 Full activation of the EGFR before the acrosome reaction: further activation of the EGFR at the end of the capacitation process, by GPCR activation, ouabain, cAMP or EGF, enhances [Ca2+]i9 and PI3K activity, leading to F-actin breakdown and the occurrence of the AR (f). The addition of exogenous PIP2 or spermine, which leads to intracellular PIP2 formation (f), can induce the acrosome reaction via stimulating PIP2 hydrolysis to form IP3, leading to mobilization and increase in [Ca2+]i and activation of actin-severing proteins to depolymerize F-actin, resulting in the occurrence of the acrosome reaction (f). [Ca2+]i, intracellular Ca2+ concentration; cAMP, cyclic adenosine monophosphate; DAG, diacylglycerol; EGF, epidermal growth factor; EGFR, epidermal growth factor receptor; GPCR, G protein-coupled receptor; IP3, inositol 1,4,5-triphosphate; PA, phosphatidic acid; PI3K, phosphatidylinositol 3-kinase; PI4K, phosphatidylinositol 4-kinase; PI4P5K, phosphatidylinositol 4-phosphate 5-kinase; PIP2(4,5), phosphatidylinositol 4,5-bisphosphate; PKA, protein kinase A; PKC, protein kinase C; PLC, phospholipase C; PLD, phospholopase D; sAC, soluble adenylyl cyclase; tmAC, transmembrane adenylyl cyclase; ZP, zona pellucida.
Regulation of sperm EGFR
We have shown elsewhere that GPCR activation can lead to PKA- and Src-dependent EGFR activation in bovine sperm.9 In other cell types, EGFR can be activated by GPCR through a process called transactivation.45, 46, 47, 48, 49 The majority of receptor tyrosine kinase transactivation by GPCRs in many cell types is mediated by metalloproteinase-dependent shedding, or by release of growth factor-like substances such as heparin-binding EGF, known as triple membrane-passing signals.50 In this mechanism, the GPCR activates a Zn2+-dependent metalloproteinase to cleave pro-heparin-binding EGF, releasing an EGF-like ligand, which binds to the EGFR and activates it. In some cases, the tyrosine kinase Src mediates the GPCR–EGFR transactivation process51 by phosphorylating EGFR on Y845, known to be the Src target.52, 53, 54 This phosphorylation (Y845) in the kinase domain is implicated in stabilizing the activation loop and maintaining the active state of the receptor.55, 56 EGFR-Y845 phosphorylation can lead to the activation of the EGFR by autophosphorylation that leads to the activation of various cascades. These cascades include the mitogen-activated protein kinase cascade57 and PI3K cascade58 that are activated by phosphorylation of EGFR-Y1068 and were shown to regulate the AR.42, 59, 60 As mentioned above, EGFR in sperm can be activated by agonists of two GPCRs, angiotensin II receptor type 1 and lysophosphatydic acid receptor,9 indicating that EGFR transactivation occurs in sperm. Moreover, we have shown that these agonists induce the AR that is mediated by EGFR activation.9
This EGFR transactivation is mediated by the tyrosine kinase Src that is known to be involved in sperm capacitation and the AR.9, 10, 61, 62 In human sperm, Src was found in the flagellum and head and was localized to membrane fraction.63 Src is also involved in protein tyrosine phosphorylation and motility during sperm capacitation.62, 64 It was shown that Src forms a complex with PKA that can phosphorylate and activate Src.62, 63 Recently, Src was localized to the post-acrosomal region of the head, neck and mid-piece of human sperm.63 Src was found to be activated during human sperm capacitation and appears to be involved in regulating sperm capacitation, calcium fluxes, tyrosine phosphorylation and the AR.9, 10, 65 In a recent study, it has been suggested that the Src downregulates protein phosphatase 2, resulting in the increase of protein tyrosine phosphorylation in sperm capacitation.66 In our work, we have shown that Src is involved in bovine sperm capacitation and AR through regulation of the EGFR activation.9 Moreover, we show that the AR induced by AngII or lysophosphatydic acid is mediated by the transactivation of the EGFR via a mechanism involving Src and PKA.9
The EGFR signal transactivation can also be induced by treating cells with ouabain, a specific inhibitor of Na+/K+-ATPase.67 The Na+/K+-ATPase is a heteromeric, integral membrane Na+/K+ exchange protein. The enzyme consists of two subunits: the α-subunit contains the catalytic function and the cation, ouabain and ATP-binding sites,68 whereas the β-subunit is necessary for localization to the plasma membrane69, 70, 71, 72 and stabilization of the K+-occluded intermediate form of the protein.73, 74 The α4 isoform has been identified only in the testes of several species75, 76, 77, 78 and in bovine sperm.79 This isoform shows high affinity to ouabain, Na+ and K+, and its activity is inhibited by low concentrations of ouabain (10 µmol l−1),78, 80 which also inhibits sperm motility. In recent studies, it was shown that 100 µmol l−1 ouabain induces bovine sperm capacitation without any effect on sperm motility.79, 81 In a recent publication, we have shown that nanomolar concentration of ouabain is present in bovine semen and in vaginal fluids of the cow.10 Under in vitro conditions, ouabain at 10 nmol l−1 does not affect the AR; however, this concentration of ouabain together with a very low concentration of EGF (0.1 ng ml−1), can induce the AR that was found to be mediated by Src, PKA and EGFR activation.10
We have shown before that activation of PKA by adding 8Br-cAMP causes high activation of EGFR.3 It is well accepted that PKA is activated in sperm capacitation; thus it is expected to see some activation of the EGFR during capacitation even without adding its ligand to the incubation medium. Indeed, we found that the EGFR is partially activated in sperm incubated under capacitation conditions without any added ligand.9 This partial activation is not enough to induce the AR at the end of the capacitation, which can be induced by further activating the EGFR by its ligand EGF or by AngII, lysophosphatydic acid or ouabain.9, 10 However, as mentioned above, physiological concentrations of ouabain or EGF alone cannot induce the AR in capacitated sperm, unless they added together.4 This is a unique way of receptor regulation by which two components that exist in the female reproductive tract are needed to fully activate the EGFR.
Conclusion
In the capacitation process, the EGFR is partially activated by PKA, resulting in PLD activation and actin polymerization. PKCα, which is already activated at the beginning of the capacitation, also participates in PLD activation. Further activation of the EGFR at the end of the capacitation enhanced [Ca2+]i, leading to F-actin breakdown and allows the AR to take place. Under physiological conditions, the EGFR can be directly activated by EGF and/or indirectly by activating PKA or by transactivation mediated by GPCR activation or by ouabain. Sperm PKA is activated mainly by bicarbonate, which activates the soluble adenylyl cyclase to produce cAMP, the activator of PKA. The GPCR activators angiotensin II or lysophosphatidic acid, as well as ouabain and EGF, are physiological components present in the female reproductive tract.
The authors declare no competing financial interests.
References
- Florman HM, Ducibella T.Fertilization in mammalsIn: Neill JD, editor. Physiology of Reproduction San Diego, CA: Elsevier; 200655–112. [Google Scholar]
- Gadella BM, Visconti A.Regulation of capacitationIn: de Jonge CJ, Barratt C, editors. The Sperm Cell Cambridge: Cambridge University Press; 2006134–69. [Google Scholar]
- Yanagimachi R, Chang MC. Fertilization of hamster eggs in vitro. Nature. 1963;200:281–2. doi: 10.1038/200281b0. [DOI] [PubMed] [Google Scholar]
- Yanagimachi R, Knobil E, Neil JD. The Physiology of Reproduction. New York: Raven Press; 1994. Mammalian fertilization; pp. 189–317. [Google Scholar]
- Roldan ER, Shi QX. Sperm phospholipases and acrosomal exocytosis. Front Biosci. 2007;12:89–104. doi: 10.2741/2050. [DOI] [PubMed] [Google Scholar]
- Florman HM, Jungnickel MK, Sutton KA. Regulating the acrosome reaction. Int J Dev Biol. 2008;52:503–10. doi: 10.1387/ijdb.082696hf. [DOI] [PubMed] [Google Scholar]
- Breitbart H. Signaling pathways in sperm capacitation and acrosome reaction. Cell Mol Biol. 2003;49:321–7. [PubMed] [Google Scholar]
- Wassarman PM, Jovine L, Litscher ES. A profile of fertilization in mammals. Nat Cell Biol. 2001;3:E59–64. doi: 10.1038/35055178. [DOI] [PubMed] [Google Scholar]
- Etkovitz N, Tirosh Y, Chazan R, Jaldety Y, Daniel L, et al. Bovine sperm acrosome reaction induced by G protein-coupled receptor agonists is mediated by epidermal growth factor receptor transactivation. Dev Biol. 2009;334:447–57. doi: 10.1016/j.ydbio.2009.08.002. [DOI] [PubMed] [Google Scholar]
- Daniel L, Etkovitz N, Weiss SR, Rubinstein S, Ickowicz D, et al. Regulation of the sperm EGF teceptor by ouabain leads to initiation of the acrosome reaction. Dev Biol. 2010;344:650–7. doi: 10.1016/j.ydbio.2010.05.490. [DOI] [PubMed] [Google Scholar]
- Arnoult C, Zeng Y, Florman H. ZP3-dependent activation of sperm cation channels regulates acrosomal secretion during mammalian fertilization. J Cell Biol. 1996;134:637–45. doi: 10.1083/jcb.134.3.637. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wassarman PM. Early events in mammalian fertilization. Annu Rev Cell Biol. 1987;3:109–42. doi: 10.1146/annurev.cb.03.110187.000545. [DOI] [PubMed] [Google Scholar]
- Baldi E, Krausz C, Forti G. Nongenomic actions of progesterone on human spermatozoa. Trends Endocrinol Metab. 1995;6:198–205. doi: 10.1016/1043-2760(95)00083-t. [DOI] [PubMed] [Google Scholar]
- Roldan ERS, Murase T, Shi QX. Exocytosis in spermatozoa in response to progesterone and zona pellucida. Science. 1994;266:1578–81. doi: 10.1126/science.7985030. [DOI] [PubMed] [Google Scholar]
- Joyce CL, Nuzzo NA, Wilson L, Zaneveld LJD. Evidence for the role of cyclooxygenase (prostaglandin synthetase) and prostaglandins in the sperm acrosome reaction and fertilization. J Androl. 1987;8:74–82. doi: 10.1002/j.1939-4640.1987.tb00953.x. [DOI] [PubMed] [Google Scholar]
- Rotem R, Zamir N, Keynan N, Barkan H, Breitbart H, et al. Atrial natriuretic peptide induces acrosomal exocytosis of human sperm. Am J Physiol. 1998;274:E218–23. doi: 10.1152/ajpendo.1998.274.2.E218. [DOI] [PubMed] [Google Scholar]
- Lax Y, Rubinstein S, Breitbart H. Epidermal growth factor induces acrosomal exocytosis in bovine sperm. FEBS Lett. 1994;339:234–8. doi: 10.1016/0014-5793(94)80422-2. [DOI] [PubMed] [Google Scholar]
- Brener E, Rubinstein S, Cohen G, Shternall K, Rivlin J, et al. Remodeling of the actin cytoskeleton during mammalian sperm capacitation and acrosome reaction. Biol Reprod. 2003;68:837–45. doi: 10.1095/biolreprod.102.009233. [DOI] [PubMed] [Google Scholar]
- Etkovitz N, Rubinstein S, Daniel L, Breitbart H. Role of PI3-kinase and PI4-kinase in actin polymerization during bovine sperm capacitation. Biol Reprod. 2007;77:263–73. doi: 10.1095/biolreprod.106.056705. [DOI] [PubMed] [Google Scholar]
- Tilney LG, Hatano S, Ishikawa H, Mooseker MS. The polymerization of actin: its role in the generation of the acrosomal process of certain echinoderm sperm. J Cell Biol. 1973;59:109–26. doi: 10.1083/jcb.59.1.109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tilney LG. The role of actin in nonmuscle cell motility. Soc Gen Physiol Ser. 1975;30:339–88. [PubMed] [Google Scholar]
- Breitbart H, Spungin B. The biochemistry of the acrosome reaction. Mol Hum Reprod. 1997;3:195–202. doi: 10.1093/molehr/3.3.195. [DOI] [PubMed] [Google Scholar]
- Spungin B, Breitbart H. Calcium mobilization and influx during sperm exocytosis. J Cell Sci. 1996;109:1947–55. doi: 10.1242/jcs.109.7.1947. [DOI] [PubMed] [Google Scholar]
- Spungin B, Margalit I, Breitbart H. Sperm exocytosis reconstructed in a cell-free system: evidence for the involvement of phospholipase C and actin filaments in membrane fusion. J Cell Sci. 1995;108 Pt 6:2525–35. doi: 10.1242/jcs.108.6.2525. [DOI] [PubMed] [Google Scholar]
- Gur Y, Breitbart H. Mammalian sperm translate nuclear-encoded proteins by mitochondrial-type ribosomes. Genes Dev. 2006;20:411–6. doi: 10.1101/gad.367606. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhao C, Guo XJ, Shi ZH, Wang FQ, Huang XY, et al. Role of translation by mitochondrial-type ribosomes during sperm capacitation: an analysis based on a proteomic approach. Proteomics. 2009;9:1385–99. doi: 10.1002/pmic.200800353. [DOI] [PubMed] [Google Scholar]
- Cohen G, Rubinstein S, Gur Y, Breitbart H. Crosstalk between protein kinase A and C regulates phospholipase D and F-actin formation during sperm capacitation. Dev Biol. 2004;267:230–41. doi: 10.1016/j.ydbio.2003.10.034. [DOI] [PubMed] [Google Scholar]
- Brown HA, Gutowski S, Moomaw CR, Slaughter C, Sternweis PC. ADP-ribosylation factor, a small GTP-dependent regulatory protein, stimulates phospholipase D activity. Cell. 1993;75:1137–44. doi: 10.1016/0092-8674(93)90323-i. [DOI] [PubMed] [Google Scholar]
- Colley WC, Sung TC, Roll R, Jenco J, Hammond SM, et al. Phospholipase D2, a distinct phospholipase D isoform with novel regulatory properties that provokes cytoskeletal reorganization. Curr Biol. 1997;7:191–201. doi: 10.1016/s0960-9822(97)70090-3. [DOI] [PubMed] [Google Scholar]
- Hammond SM, Altshuller YM, Sung TC, Rudge SA, Rose K, et al. Human ADP-ribosylation factor-activated phosphatidylcholine-specific phospholipase D defines a new and highly conserved gene family. J Biol Chem. 1995;270:29640–3. doi: 10.1074/jbc.270.50.29640. [DOI] [PubMed] [Google Scholar]
- Hammond SM, Jenco JM, Nakashima S, Cadwallader K, Gu Q, et al. Characterization of two alternately spliced forms of phospholipase D1. Activation of the purified enzymes by phosphatidylinositol 4,5-bisphosphate, ADP-ribosylation factor, and Rho family monomeric GTP-binding proteins and protein kinase C-alpha. J Biol Chem. 1997;272:3860–8. doi: 10.1074/jbc.272.6.3860. [DOI] [PubMed] [Google Scholar]
- Berridge MJ. Inositol trisphosphate and diacylglycerol: two interacting second messengers. Annu Rev Biochem. 1987;56:159–93. doi: 10.1146/annurev.bi.56.070187.001111. [DOI] [PubMed] [Google Scholar]
- Nishizuka Y. The role of protein kinase C in cell surface signal transduction and tumour promotion. Nature. 1984;308:693–8. doi: 10.1038/308693a0. [DOI] [PubMed] [Google Scholar]
- Breitbart H, Lax Y, Rotem R, Naor Z. Role of protein kinase C in the acrosome reaction of mammalian spermatozoa. Biochem J. 1992;281:473–6. doi: 10.1042/bj2810473. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dragileva E, Rubinstein S, Breitbart H. Intracellular Ca2+–Mg2+-ATPase regulates calcium influx and acrosomal exocytosis in bull and ram spermatozoa. Biol Reprod. 1999;61:1226–34. doi: 10.1095/biolreprod61.5.1226. [DOI] [PubMed] [Google Scholar]
- Walensky LD, Snyder SH. Inositol 1,4,5-trisphosphate receptors selectively localized to the acrosomes of mammalian sperm. J Cell Biol. 1995;130:857–69. doi: 10.1083/jcb.130.4.857. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bamberg E, Weiser M, Desser H. Polyamines in bovine epididymal spermatozoa. J Reprod Fertil. 1975;45:363–5. doi: 10.1530/jrf.0.0450363. [DOI] [PubMed] [Google Scholar]
- Rubinstein S, Breitbart H. Role of spermine in mammalian sperm capacitation and acrosome reaction. Biochem J. 1991;278:25–8. doi: 10.1042/bj2780025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Auger KR, Serunian LA, Soltoff SP, Libby P, Cantley LC. PDGF-dependent tyrosine phosphorylation stimulates production of novel polyphosphoinositides in intact cells. Cell. 1989;57:167–75. doi: 10.1016/0092-8674(89)90182-7. [DOI] [PubMed] [Google Scholar]
- Czech MP. PIP2 and PIP3: complex roles at the cell surface. Cell. 2000;100:603–6. doi: 10.1016/s0092-8674(00)80696-0. [DOI] [PubMed] [Google Scholar]
- Fisher D, Abrieu A, Simon MN, Keyse S, Verge V, et al. MAP kinase inactivation is required only for G2-M phase transition in early embryogenesis cell cycles of the starfishes Marthasterias glacialis and Astropecten aranciacus. Dev Biol. 1998;202:1–13. doi: 10.1006/dbio.1998.8981. [DOI] [PubMed] [Google Scholar]
- Jungnickel MK, Sutton KA, Wang Y, Florman HM. Phosphoinositide-dependent pathways in mouse sperm are regulated by egg ZP3 and drive the acrosome reaction. Dev Biol. 2007;304:116–26. doi: 10.1016/j.ydbio.2006.12.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Oliva-Hernandez J, Perez-Gutierrez JF. Localization of the epidermal growth factor (EGF) in the epididymis and accessory genital glands of the boar and functional effects on spermatozoa. Theriogenology. 2008;70:1159–69. doi: 10.1016/j.theriogenology.2008.06.090. [DOI] [PubMed] [Google Scholar]
- Peddinti D, Nanduri B, Kaya A, Feugang JM, Burgess SC, et al. Comprehensive proteomic analysis of bovine spermatozoa of varying fertility rates and identification of biomarkers associated with fertility. BMC Syst Biol. 2008;2:19. doi: 10.1186/1752-0509-2-19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jorissen RN, Walker F, Pouliot N, Garrett TP, Ward CW, et al. Epidermal growth factor receptor: mechanisms of activation and signalling. Exp Cell Res. 2003;284:31–53. doi: 10.1016/s0014-4827(02)00098-8. [DOI] [PubMed] [Google Scholar]
- Jung J, Kim M, Choi S, Kim MJ, Suh JK, et al. Molecular mechanism of cofilin dephosphorylation by ouabain. Cell Signal. 2006;18:2033–40. doi: 10.1016/j.cellsig.2006.03.014. [DOI] [PubMed] [Google Scholar]
- Prenzel N, Zwick E, Daub H, Leserer M, Abraham R, et al. EGF receptor transactivation by G-protein-coupled receptors requires metalloproteinase cleavage of proHB-EGF. Nature. 1999;402:884–8. doi: 10.1038/47260. [DOI] [PubMed] [Google Scholar]
- Shah BH, Catt KJ. A central role of EGF receptor transactivation in angiotensin II -induced cardiac hypertrophy. Trends Pharmacol Sci. 2003;24:239–44. doi: 10.1016/S0165-6147(03)00079-8. [DOI] [PubMed] [Google Scholar]
- Wetzker R, Bohmer FD. Transactivation joins multiple tracks to the ERK/MAPK cascade. Nat Rev Mol Cell Biol. 2003;4:651–7. doi: 10.1038/nrm1173. [DOI] [PubMed] [Google Scholar]
- Prenzel N, Zwick E, Leserer M, Ullrich A. Tyrosine kinase signalling in breast cancer. Epidermal growth factor receptor: convergence point for signal integration and diversification. Breast Cancer Res. 2000;2:184–90. doi: 10.1186/bcr52. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nair VD, Sealfon SC. Agonist-specific transactivation of phosphoinositide 3-kinase signaling pathway mediated by the dopamine D2 receptor. J Biol Chem. 2003;278:47053–61. doi: 10.1074/jbc.M303364200. [DOI] [PubMed] [Google Scholar]
- Tice DA, Biscardi JS, Nickles AL, Parsons SJ. Mechanism of biological synergy between cellular Src and epidermal growth factor receptor. Proc Natl Acad Sci USA. 1999;96:1415–20. doi: 10.1073/pnas.96.4.1415. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Prenzel N, Fischer OM, Streit S, Hart S, Ullrich A. The epidermal growth factor receptor family as a central element for cellular signal transduction and diversification. Endocr Relat Cancer. 2001;8:11–31. doi: 10.1677/erc.0.0080011. [DOI] [PubMed] [Google Scholar]
- Liu J, Liao Z, Camden J, Griffin KD, Garrad RC, et al. Src homology 3 binding sites in the P2Y2 nucleotide receptor interact with Src and regulate activities of Src, proline-rich tyrosine kinase 2, and growth factor receptors. J Biol Chem. 2004;279:8212–8. doi: 10.1074/jbc.M312230200. [DOI] [PubMed] [Google Scholar]
- Cooper JA, Howell B. The when and how of Src regulation. Cell. 1993;73:1051–4. doi: 10.1016/0092-8674(93)90634-3. [DOI] [PubMed] [Google Scholar]
- Hubbard SR, Wei L, Ellis L, Hendrickson WA. Crystal structure of the tyrosine kinase domain of the human insulin receptor. Nature. 1994;372:746–54. doi: 10.1038/372746a0. [DOI] [PubMed] [Google Scholar]
- Rojas M, Yao S, Lin YZ. Controlling epidermal growth factor (EGF)-stimulated Ras activation in intact cells by a cell-permeable peptide mimicking phosphorylated EGF receptor. J Biol Chem. 1996;271:27456–61. doi: 10.1074/jbc.271.44.27456. [DOI] [PubMed] [Google Scholar]
- Cao Z, Liu L, van Winkle DM. Met5-enkephalin-induced cardioprotection occurs via transactivation of EGFR and activation of PI3K. Am J Physiol Heart Circ Physiol. 2005;288:H1955–64. doi: 10.1152/ajpheart.00256.2004. [DOI] [PubMed] [Google Scholar]
- Almog T, Lazar S, Reiss N, Etkovitz N, Milch E, et al. Identification of extracellular signal-regulated kinase 1/2 and p38MAPK as regulators of human sperm motility and acrosome reaction and as predictors of poor spermatozoa quality. J Biol Chem. 2008;283:14479–89. doi: 10.1074/jbc.M710492200. [DOI] [PubMed] [Google Scholar]
- Breitbart H, Rubinstein S, Etkovitz N. Sperm capacitation is regulated by the crosstalk between protein kinase A and C. Mol Cell Endocrinol. 2006;252:247–9. doi: 10.1016/j.mce.2006.03.019. [DOI] [PubMed] [Google Scholar]
- Reinton N, Orstavik S, Haugen TB, Jahnsen T, Tasken K, et al. A novel isoform of human cyclic 3′,5′-adenosine monophosphate-dependent protein kinase, c alpha-s, localizes to sperm midpiece. Biol Reprod. 2000;63:607–11. doi: 10.1095/biolreprod63.2.607. [DOI] [PubMed] [Google Scholar]
- Baker MA, Hetherington L, Aitken RJ. Identification of SRC as a key PKA-stimulated tyrosine kinase involved in the capacitation-associated hyperactivation of murine spermatozoa. J Cell Sci. 2006;119:3182–92. doi: 10.1242/jcs.03055. [DOI] [PubMed] [Google Scholar]
- Lawson C, Goupil S, Leclerc P. Increased activity of the human sperm tyrosine kinase SRC by the cAMP-dependent pathway in the presence of calcium. Biol Reprod. 2008;79:657–66. doi: 10.1095/biolreprod.108.070367. [DOI] [PubMed] [Google Scholar]
- Mitchell LA, Nixon B, Baker MA, Aitken RJ. Investigation of the role of SRC in capacitation-associated tyrosine phosphorylation of human spermatozoa. Mol Hum Reprod. 2008;14:235–43. doi: 10.1093/molehr/gan007. [DOI] [PubMed] [Google Scholar]
- Varano G, Lombardi A, Cantini G, Forti G, Baldi E, et al. Src activation triggers capacitation and acrosome reaction but not motility in human spermatozoa. Hum Reprod. 2008;23:2652–62. doi: 10.1093/humrep/den314. [DOI] [PubMed] [Google Scholar]
- Krapf D, Arcelay E, Wertheimer EV, Sanjay A, Pilder SH, et al. Inhibition of Ser/Thr phosphatases induces capacitation-associated signaling in the presence of Src kinase inhibitors. J Biol Chem. 285:7977–85. doi: 10.1074/jbc.M109.085845. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Haas M, Askari A, Xie Z. Involvement of Src and epidermal growth factor receptor in the signal-transducing function of Na+/K+-ATPase. J Biol Chem. 2000;275:27832–7. doi: 10.1074/jbc.M002951200. [DOI] [PubMed] [Google Scholar]
- Lingrel JB, Kuntzweiler T. Na+,K+-ATPase. J Biol Chem. 1994;269:19659–62. [PubMed] [Google Scholar]
- Beguin P, Hasler U, Beggah A, Horisberger JD, Geering K. Membrane integration of Na,K-ATPase alpha-subunits and beta-subunit assembly. J Biol Chem. 1998;273:24921–31. doi: 10.1074/jbc.273.38.24921. [DOI] [PubMed] [Google Scholar]
- Geering K. The functional role of the beta-subunit in the maturation and intracellular transport of Na,K-ATPase. FEBS Lett. 1991;285:189–93. doi: 10.1016/0014-5793(91)80801-9. [DOI] [PubMed] [Google Scholar]
- Hasler U, Wang X, Crambert G, Beguin P, Jaisser F, et al. Role of beta-subunit domains in the assembly, stable expression, intracellular routing, and functional properties of Na,K-ATPase. J Biol Chem. 1998;273:30826–35. doi: 10.1074/jbc.273.46.30826. [DOI] [PubMed] [Google Scholar]
- McDonough AA, Geering K, Farley RA. The sodium pump needs its beta subunit. FASEB J. 1990;4:1598–605. doi: 10.1096/fasebj.4.6.2156741. [DOI] [PubMed] [Google Scholar]
- Eakle KA, Kabalin MA, Wang SG, Farley RA. The influence of beta subunit structure on the stability of Na+/K+-ATPase complexes and interaction with K+ J Biol Chem. 1994;269:6550–7. [PubMed] [Google Scholar]
- Lutsenko S, Kaplan JH. An essential role for the extracellular domain of the Na,K-ATPase beta-subunit in cation occlusion. Biochemistry (Mosc) 1993;32:6737–43. doi: 10.1021/bi00077a029. [DOI] [PubMed] [Google Scholar]
- James PF, Grupp IL, Grupp G, Woo AL, Askew GR, et al. Identification of a specific role for the Na,K-ATPase alpha 2 isoform as a regulator of calcium in the heart. Mol Cell. 1999;3:555–63. doi: 10.1016/s1097-2765(00)80349-4. [DOI] [PubMed] [Google Scholar]
- Shamraj OI, Lingrel JB. A putative fourth Na+,K+-ATPase alpha-subunit gene is expressed in testis. Proc Natl Acad Sci USA. 1994;91:12952–6. doi: 10.1073/pnas.91.26.12952. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Underhill DA, Canfield VA, Dahl JP, Gros P, Levenson R. The Na,K-ATPase alpha4 gene (Atp1a4) encodes a ouabain-resistant alpha subunit and is tightly linked to the alpha2 gene (Atp1a2) on mouse chromosome 1. Biochemistry (Mosc) 1999;38:14746–51. doi: 10.1021/bi9916168. [DOI] [PubMed] [Google Scholar]
- Woo AL, James PF, Lingrel JB. Sperm motility is dependent on a unique isoform of the Na,K-ATPase. J Biol Chem. 2000;275:20693–9. doi: 10.1074/jbc.M002323200. [DOI] [PubMed] [Google Scholar]
- Newton LD, Kastelic JP, Wong B, van der Hoorn F, Thundathil J. Elevated testicular temperature modulates expression patterns of sperm proteins in Holstein bulls. Mol Reprod Dev. 2009;76:109–18. doi: 10.1002/mrd.20934. [DOI] [PubMed] [Google Scholar]
- Blanco G, Melton RJ, Sanchez G, Mercer RW. Functional characterization of a testes-specific alpha-subunit isoform of the sodium/potassium adenosinetriphosphatase. Biochemistry (Mosc) 1999;38:13661–9. doi: 10.1021/bi991207b. [DOI] [PubMed] [Google Scholar]
- Thundathil JC, Anzar M, Buhr MM. Na+/K+-ATPase as a signaling molecule during bovine sperm capacitation. Biol Reprod. 2006;75:308–17. doi: 10.1095/biolreprod.105.047852. [DOI] [PubMed] [Google Scholar]
- Rotman T, Etkovitz N, Spiegel A, Rubinstein S, Breitbart H. Protein kinase A and protein kinase C(alpha)/PPP1CC2 play opposing roles in the regulation of phosphatidylinositol 3-kinase activation in bovine sperm. Reproduction. 2010;140:43–56. doi: 10.1530/REP-09-0314. [DOI] [PubMed] [Google Scholar]


