Lay summary
Animals exhibit “behavioral types” (akin to human personalities) where individuals differ consistently on traits like activity which may influence the predators it encounters and the prey it captures. Here we demonstrate that active jumping spiders are more likely to encounter and consume inactive crickets and vice versa. This presents a potential explanation for the persistence of behavioral types in natural populations, as behavioral variation in one trophic level maintains variation in the associated level.
Key words: behavioral syndrome, foraging mode, intraspecific variation, personality, predator–prey interaction.
Abstract
Consistent interindividual differences in behavior (i.e., “behavioral types”) may be a key factor in determining the outcome of species interactions. Studies that simultaneously account for the behavioral types of individuals in multiple interacting species, such as predator–prey systems, may be particularly strong predictors of ecological outcomes. Here, we test the predator–prey locomotor crossover hypothesis, which predicts that active predators are more likely to encounter and consume prey with the opposing locomotor tendency. We test this hypothesis using intraspecific behavioral variation in both a predator and prey species as predictors of foraging outcomes. We use the old field jumping spider, Phidippus clarus (Araneae, Salticidae), and the house cricket, Acheta domesticus (Orthoptera, Gryllidae), as a model predator–prey system in laboratory mesocosm trials. Stable individual differences in locomotor tendencies were identified in both P. clarus and A. domesticus, and the outcome of foraging bouts depended neither on the average activity level of the predator nor on the average activity level of prey. Instead, an interaction between the activity level of spiders and crickets predicted spider foraging success and prey survivorship. Consistent with the locomotor crossover hypothesis, predators exhibiting higher activity levels consumed more prey when in an environment containing low-activity prey items and vice versa. This study highlights 1) the importance of intraspecific variation in determining the outcome of predator–prey interactions and 2) that acknowledging behavioral variation in only a single species may be insufficient to characterize the performance consequences of intraspecific trait variants.
INTRODUCTION
The factors that influence the outcome of species interactions are numerous, including species identity (Burns 2004), environmental contingencies (Kordas et al. 2011), body state (Pierce et al. 2000), and even the traits of the specific individuals involved in the interaction. One of the primary goals of ecology is to understand how the outcomes of species interactions are determined, and how these interactions collectively beget higher order ecological phenomena (e.g., species coexistence, community stable states, and cyclical patterns in species’ abundance). In recent years, behavioral ecologists have begun to focus more and more on behavioral variation occurring at the level of the individual and how individual differences in behavior can dictate the magnitude (Réale and Festa-Bianchet 2003; Biro et al. 2004), nature (Pruitt and Ferrari 2011; Pruitt, Cote, et al. 2012), and diversity (Riechert 1991; Tinker et al. 2008) of species interactions that individuals will experience (Sih et al. 2012).
Consistent individual differences in behavior are referred to variably as behavioral tendencies, behavioral syndromes, temperament, personality, and/or behavioral types (Gosling 2001; Sih, Bell, and Johnson 2004; Reale et al. 2007). In the study presented here, we will refer to correlations among functionally dissimilar aspects of behavior as behavioral syndromes (Sih, Bell, and Johnson 2004; Sih, Bell, Johnson, and Ziemba 2004) and consist individual differences in behavior as behavioral types or “BTs” (Sih and Bell 2008). Individual differences in behavior have been documented in countless vertebrates (Dingemanse et al. 2002; Cote et al. 2008, 2012; Carter et al. 2010) and invertebrates (Wilson et al. 2010; Berning et al. 2012), and aspects of behavior commonly studied within this literature include boldness, aggressiveness, activity levels, and, to a lesser extent, social behavior (Carter et al. 2010; Wilson et al. 2010; Cote et al. 2011, 2012). Although our understanding of the proximate causes of BTs continues to grow at an impressive rate (McElreath and Strimling 2006; Kempenaers et al. 2008; Biro and Stamps 2010; Stamps and Groothuis 2010; Jones et al. 2011; van Oers et al. 2011), our knowledge of how BTs impact higher order ecological phenomenon has lagged behind significantly (argued in Sih et al. 2012; Wolf and Weissing 2012). This lag is unfortunate because of the potential synergies between the behavioral syndromes literature and the rapidly moving literatures devoted to intraspecific variation and individual specialization within general ecology (lamented in Dall et al. 2012).
Here, we propose to test how variation in BTs within multiple species impacts the net outcome of their interaction. We focus on a predator–prey interaction and how the BTs of the specific individuals involved in the interaction unite to shape performance outcomes. Although numerous studies have considered how variation in the BTs of either predator (Riechert 1991; Kobler et al. 2009; Exnerová et al. 2010; Pruitt and Krauel 2010) or prey (Réale and Festa-Bianchet 2003; Carter et al. 2010; Jones and Godin 2010; Smith and Blumstein 2010) each individually impact individuals’ performance within a trophic level, only once has a study considered the effects of BTs in both predators and prey simultaneously in a unified test system (Pruitt, Stachowicz, et al. 2012). Notably, this weakness is not specific to the behavioral syndromes literature. Throughout general ecology, numerous reviews have criticized that, although species interacts are inherently bidirectional in nature, they are rarely ever studied as such (Lima 2002; Agrawal et al. 2007; Sih et al. 2012). Additionally, among the behavioral syndromes studies that have considered the effects of BTs in multispecies interactions, results have demonstrated that the outcome of species interactions can be contingent on the BTs of the specific individuals involved (Webster et al. 2009; Pruitt, Stachowicz, et al. 2012). Taken together, we argue that if it is our goal to understand how individual differences in behavior shape animal’s ecology, then graduating to multispecies investigations is one of the unavoidable frontiers/challenges for behavioral syndromes studies.
Here, we test the predictions of the predator–prey locomotor crossover hypothesis using stable BTs in a predator and its prey. The locomotor crossover hypothesis, champion by Huey and Pianka (1981), predicts that active predators will tend to consume sedentary prey, whereas sedentary predators will tend to capture active prey. Put another way, predators that have active BTs (i.e., that go out and search for prey) are more likely to encounter prey with the opposing locomotor tendency and vice versa. By extension, the locomotor hypothesis predicts that a shift in the presentation of active versus sedentary BTs in either predator or prey will have an impact on the performance of BTs in the interacting trophic level: 1) in prey populations dominated by sedentary BTs, predators possessing active BTs are predicted to have greater foraging success than sedentary predators, whereas 2) in prey populations dominated by active BTs, predators possessing sedentary BTs are predicted to have greater foraging success than those exhibiting active BTs. From the perspective of prey: in predator populations dominated by active BTs, prey possessing active BTs are predicted to enjoy greater survivorship; in contrast, in predator populations dominated by sedentary BTs, prey possessing sedentary BTs are predicted to enjoy higher survivorship. Thus, the performance of either predator or prey will be contingent on the BTs expressed in the interacting trophic level. If present, such effects could help stabilize species interactions and maintain phenotypic variation in multiple trophic levels by ensuring that no strategy (in predator or prey) will consistently yield superior performance (Schreiber et al. 2011). In contrast, it could be that a single BT will outperform all others concerning foraging regardless of prey type and availability, as is the prevailing, familiar hypothesis in the literature.
In the study presented here, we explored the predictions of the locomotor crossover hypothesis using differences in BT in the old field jumping spider, Phidippus clarus (Araneae, Salticidae), and the house cricket, Acheta domesticus (Orthoptera, Gryllidae). First, we tested for the presence of stable individual differences in the locomotor behavior in P. clarus and A. domesticus. Second, using laboratory mesocosms, we staged interactions between predators of known BT and prey groups with different average BT compositions, from sedentary to highly active. We predicted higher prey consumption rates (i.e., lower prey survivorship) when active P. clarus were paired with sedentary A. domesticus or when sedentary P. clarus were paired with active A. domesticus. In contrast, we predicted lower prey consumption rates (aka higher prey survivorship) when active P. clarus were paired with active A. domesticus or when sedentary P. clarus were paired with sedentary A. domesticus. Such context-dependent performance trade-offs are important because they can help to maintain phenotypic variation in multiple trophic levels (Rosenheim et al. 2004; Scharf et al. 2006; Perry 2007; Avgar et al. 2008; Schreiber et al. 2011).
METHODS
Spider collection
A population of P. clarus was haphazardly collected in old fields and agricultural plots near the University of Pittsburgh’s Pymatuning Laboratory of Ecology (Linesville, PA) in June and July 2012. Spiders were collected by sweep-netting through golden rods (Solidago canadensis) and other herbaceous plant during daylight hours (700–1800 hours). All adult female P. clarus were collected (n = 81) and transported to the laboratory at the University of Pittsburgh. Spiders were housed individually in clear plastic containers (diameter = 11cm, height = 10cm) at room temperature. Housing containers contained a single cardboard bridge, which served as a retreat, and a cotton ball soaked with water. The spiders were fed a maintenance diet of 4 two-week-old A. domesticus weekly.
Experimental crickets, A. domesticus, were obtained commercially (Fluker’s Farm, Port Allen, LA) and stored individually in clear plastic containers described above. Crickets were fed ad libitum with chick feed (Kalmbach Feeds, Upper Sandusky, OH) and provided water with a soaked cotton ball.
Spider activity level assay
To determine activity levels, each spider (n = 52) was removed from its housing containers and placed into clean plastic cylindrical vials (diameter = 3cm and height = 8.5cm). These vials were housed in clear plastic containers identical to housing containers. Each spider was placed in the bottom of the vial, and we measured the time taken for the spider to climb to the top of the vials with a stopwatch. Phidippus clarus regularly respond to novel environments by climbing up (Sweeney K, Pruitt JN, personal observation), and thus, ascension is a common locomotor pattern for this species (Hoefler 2007). The spiders were allowed a maximum of 5min to complete their assay. Between each assay, vials were cleaned with isopropanol and the experimental spider was replaced into its housing container. To ensure that these values were repeatable and consistent across time, this assay was repeated for each spider weekly for 4 weeks.
Cricket activity level assay
Similarly, to determine the activity level for individual crickets (n = 192), we measured crickets’ latency to initiate movement and general activity level in a novel, open field environment (e.g., Wilson et al. 2010). Individual crickets were removed from their housing containers and placed in the center of a clear circular plastic chamber (diameter = 17cm and height = 7.8cm). The assay chamber sat atop a grid separated into 8 wedges of identical area. For 3min prior to experimentation, the crickets were allowed an acclimation period inside an opaque black dish (diameter = 7.1cm and height = 2.8cm). The latency for initial movement and the number of wedge lines crossed on the grid in a period of 5min were recorded. After each assay, the cricket was replaced back into its housing container. To test whether crickets’ activity scores were repeatable measures, we assayed another set of crickets (N = 39) 5 times, once every other day for 10 days. Crickets used to determine repeatability were excluded from other assays/experiments.
Staged predator–prey encounters
An individual spider and 6 crickets were placed into 12 in. × 12 in. × 12 in. mesocosm chambers (n = 28). Two sides of the container were composed of cloth screens, which allowed natural air flow but did not allow the organisms to escape (Bioquip 1450 BCV). In order to distribute a stratified random allotment of crickets with different activity levels in each mesocosm, crickets were sorted ordinally from least active to most active. Groups of 6 crickets with the closest activity levels were combined and assigned a randomly chosen P. clarus (i.e., researchers were blind to spider activity level) and placed into a mesocosm along with 2 cardboard bridges, several water-soaked cotton balls, and haphazardly dispersed chick feed for the crickets. These environments were left alone for 1 week, at which point we recorded how many crickets were killed by the spiders over the course of 7 days. To confirm that cricket mortality was the result of spiders and not other causes, we ran no-spider controls (n = 8) concurrently and compared cricket mortality in the presence versus absence of spiders.
Statistical analyses
To test for repeatability in spider and cricket behavioral tendencies, we used a nested Anova to partition variance into within-individual versus between-individual variability. The resulting intraclass correlation coefficient score was used as our estimate of repeatability (Boake 1989; Falconer and Mackay 1996). Once these consistent differences were established, we assigned each individual (spiders and crickets, analyzed separately) its average activity level across all 4 measurements to be used in further analysis. To test for associations between individuals’ behavioral tendencies and their morphology and/or body state, we constructed general linear models, 1 for spiders and 1 for crickets. In the model predicting spider behavior, we included prosoma width, cephalothorax length, individuals’ mass (g), and body condition (residuals of mass on prosoma width) (Jakob et al. 1996) as predictor variables and spiders’ latency to ascend a vial (s) as our response variable. In our model predicting cricket behavior, we included crickets’ thorax width, body mass (g), and body condition (residuals of mass on thorax width) as predictor variables and crickets’ average activity level as our response variable. To test the influence of predator and prey activity on spider foraging performance (and cricket mortality rate), we performed an ordinal logistic regression. We included spiders’ body condition, spiders’ cephalothorax width, spiders’ activity scores, the average activity scores of the 6 crickets within the mesocosm (hereafter “average cricket activity score”), and the interaction term spider activity score × average cricket activity score as predictor variables. The response variable for this model was the number of crickets killed over the duration of the 1-week trial. Descriptive statistics will be provided as means ± standard errors.
RESULTS
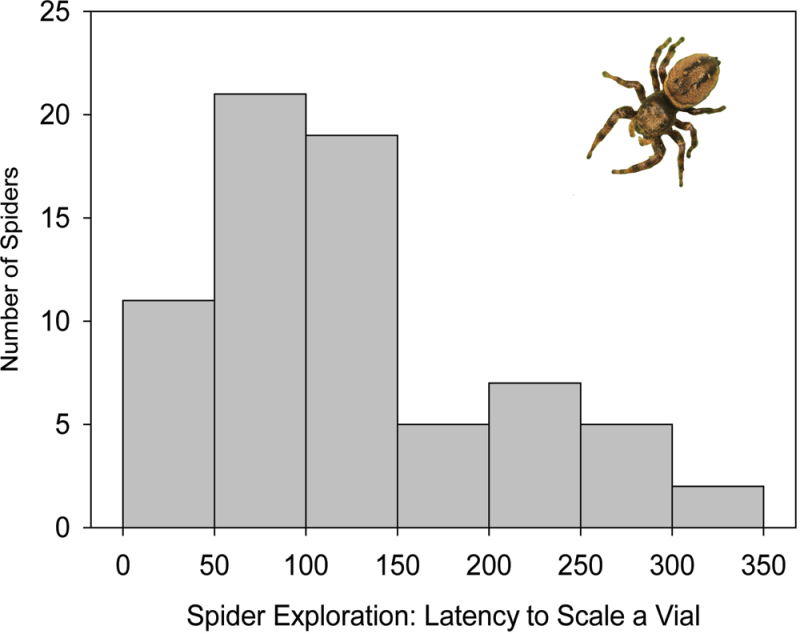
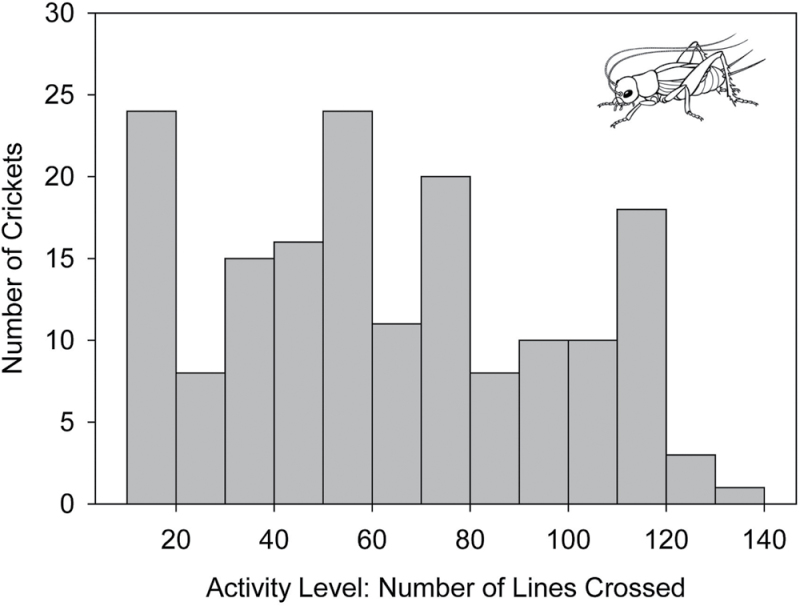
The spiders in this study exhibited consistent interindividual differences in activity level (i.e., exploration speed, F 37,114 = 1.90, P = 0.0054, repeatability = 0.62; Figure 1), and these differences were not predicted by any morphological characteristics measured (F 4,36 = 0.11, P = 0.98; Table 1). Similarly, crickets used in this study exhibited consistent individual differences in activity level (i.e., distance travelled; F 37,140 = 4.45, P < 0.001, repeatability = 0.74; Figure 2). Because all crickets were at the same life stage, and no morphological character measured predicted these differences (F 3,35 = 0.29, P = 0.83; Table 1), we confirm that individual A. domesticus exhibit BTs for activity.
Figure 1.
Frequency histogram of individual jumping spiders’ (Phidippus clarus) latency to scale (s) a clear plastic vial (height = 8.5cm).
Table 1.
None of the morphological characteristics measured explained a significant portion of the between-individual variation in activity for both species
| Morphological character | F-ratio1,1 | P value |
|---|---|---|
| Spiders | ||
| Body condition | 0.0006 | 0.98 |
| Mass | 0.016 | 0.90 |
| Cephalothorax length | 0.18 | 0.67 |
| Cephalothorax width | 0.005 | 0.95 |
| Crickets | ||
| Body condition | 0.71 | 0.41 |
| Mass | 0.60 | 0.45 |
| Thorax width | 0.68 | 0.41 |
Figure 2.
Frequency histogram of the activity levels of individual domestic crickets (Acheta domesticus), as estimated by the number of lines crossed over 5min in a novel experimental arena.
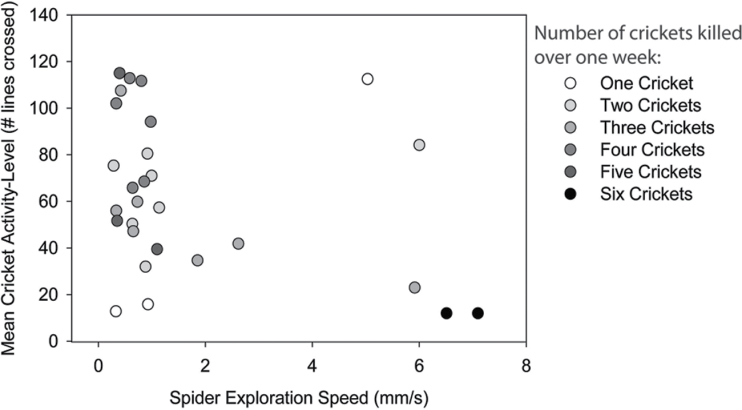
The foraging performance of individual spiders depended neither on the average activity level of the spider (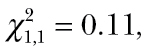 P = 0.74) nor on the average group activity level of the prey (
P = 0.74) nor on the average group activity level of the prey (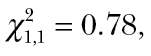 P = 0.38), but by an interaction between the activity level of predators and prey (
P = 0.38), but by an interaction between the activity level of predators and prey (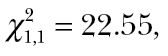 P < 0.0001; Figure 3). Spider foraging performance is augmented when it falls on the negative relationship between spider and cricket activity levels. This trend is not driven by the few spiders that had the highest activity. We performed 2 additional statistical models where 2 groups of outliers were removed, respectively, and the interaction between predator and prey activity remained significant (Supplementary Table S1). Thus, spiders with higher activity levels consumed more prey when in an environment containing a group of low-activity crickets and vice versa. In addition, spider foraging performance was not associated with any observed morphological state (body condition:
P < 0.0001; Figure 3). Spider foraging performance is augmented when it falls on the negative relationship between spider and cricket activity levels. This trend is not driven by the few spiders that had the highest activity. We performed 2 additional statistical models where 2 groups of outliers were removed, respectively, and the interaction between predator and prey activity remained significant (Supplementary Table S1). Thus, spiders with higher activity levels consumed more prey when in an environment containing a group of low-activity crickets and vice versa. In addition, spider foraging performance was not associated with any observed morphological state (body condition: 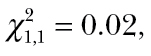 P = 0.89; cephalothorax width:
P = 0.89; cephalothorax width: 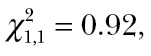 P = 0.34). Finally, we detected large differences in the number of surviving crickets between trials containing spiders and no-spider controls (t = 4.96, df = 34, P < 0.001): no-spider controls had an average mortality of 0.62±0.52, whereas trials containing spiders had an average mortality of 3.18±1.42.
P = 0.34). Finally, we detected large differences in the number of surviving crickets between trials containing spiders and no-spider controls (t = 4.96, df = 34, P < 0.001): no-spider controls had an average mortality of 0.62±0.52, whereas trials containing spiders had an average mortality of 3.18±1.42.
Figure 3.
An interaction plot depicting the simultaneous effects of cricket and spider locomotor behavior on the number of prey killed over a 1-week mesocosm experiment. Cricket behavior (y axis) is an activity level measure, as estimated by the number of lines individuals’ crossed over a 5-min period in an experimental arena. Individual data points represent the average activity levels of 6 crickets that constituted the prey population in each mesocosm. Spider behavior (x axis) is an estimate of exploration speed, as estimated by individuals’ time to scale an 8.5-cm vial. Data points represent the behavior of 1 spider that served as the single predator in each mesocosm trial.
DISCUSSION
In this study, we sought to test the locomotor crossover hypothesis using intraspecific trait variants in predators and prey. Specifically, we tested how shifts in the average activity level of prey populations impacted the mortality rate of prey (and conversely) and the foraging rate of predators, using the spider P. clarus as a model predator and A. domesticus as its prey. The locomotor crossover hypothesis predicts high prey mortality rate when sedentary prey interact with active predators and when active prey interact with sedentary predators. Consistent with this hypothesis, we found a high incidence of prey mortality when sedentary crickets were paired with active spiders and when active crickets were paired with sedentary spiders (Figure 3). Thus, our study adds to the small, albeit growing, number of studies that have experimentally documented a negative association between the locomotor tendencies of predators and prey. Importantly, the existing studies are derived from taxonomically divergent test systems spanning both vertebrate and invertebrate systems, as well as terrestrial and marine habitats. Taken together, the preponderance of existing evidence suggests the subtle differences in the behavioral (i.e., locomotor) tendencies of both predators and prey can be instrumental in determining the frequency and intensity of their trophic interactions.
Our data have demonstrated the presence of stable individual differences in behavior in both A. domesticus and P. clarus. Although conventional views of behavior have marveled at its seemingly infinite plasticity, a vast contemporary literature has documented behavioral consistency in countless animals. The majority of such studies have focused on a few key axes of behavior, including boldness, aggressiveness, sociability/sociality, exploration, and activity level. Similar to many other studies, we detected consistent individual differences in the activity levels of both the domestic cricket (A. domesticus) and old field jumping spider (P. clarus). Notably, our study is actually the second study to document individual differences in the locomotor behavior of A. domesticus (Wilson et al. 2010) and independently confirms the presence of individual variation in activity level. For reference, P. clarus is now the 20th spider species known to exhibit consistent individual differences in behavior (Pruitt and Riechert 2012). Documenting stable differences in the locomotor behavior of P. clarus and A. domesticus is significant for our study because it was a necessary precondition to test the locomotor crossover hypothesis using intraspecific trait variation. More broadly, the fact that individual differences in activity level have been documented in dozens (if not hundreds) of animal systems further supports the possibility that locomotor crossover may play an important role in the trophic interactions of many predator–prey systems.
At the core of this study was the idea that the foraging success of predators and survivorship of prey would be different and predictable based on a priori knowledge of individuals’ locomotor tendencies, formalized in the locomotor crossover hypothesis. Our study tests this idea using a novel experimental approach, where we intentionally biased the average activity levels of prey populations and tested whether this impacted the foraging rate of spiders bearing different locomotor tendencies. Our results closely matched the predictions of the locomotor crossover hypothesis, as indeed the greatest foraging success of spiders and lowest survivorship of prey occurred along a negative axis (Figure 3). This result is actually fairly surprising, given the possibility that predators and/or prey could have modulated their activity levels after assessing the behavior of individuals in the opposing trophic level. Such shifts, though fascinating, would have obscured our ability to observe locomotor crossover. However, our results show that individuals in either trophic level do not perfectly and adaptively shift their behavior, and some trait combinations yield low performance for either trophic level. Thus, it seems that the performance of both predator and prey could depend on the frequencies of active versus sedentary BTs in the opposing trophic level and possibly even their own. Developing a formal, even game theoretical, model to explore the dynamics of such 2-trait systems is an exciting avenue of ongoing collaborative research.
The science of behavioral ecology has, by and large, progressed via single-species investigations, which tend to regard heterospecifics (e.g., predators, prey items, disease, and parasites) in an ancillary capacity. This paradigm is not unique to behavioral ecology; in fact, most of population biology has treated species interactions as unidirectional phenomena—which, of course, they are not. Though single-species approaches have obvious advantages (e.g., they are more tractable and allow us to consider our focal species in greater detail), a small but growing literature is demonstrating that the predicted outcomes of species interactions can vary wildly when we consider their dynamic, bidirectional nature. Toward this end, in the study reported here, we considered how behavioral variation in 2 interacting species together simultaneously influences the outcome of a simple predator–prey interaction. Specifically, we found that the success of individuals in either trophic level (predator or prey) depended not only on their own behavioral strategies but on the behavioral strategies present within the opposing trophic level. If, however, we had restricted our focus to only a single species (e.g., predators), not only would we have missed the nuanced, context-dependent trade-offs predicted by the locomotor crossover hypothesis, we would not have detected any significant effects of predator BT on their performance whatsoever. Context-dependent performance trade-offs are of ecological and evolutionary significance because they suggest that the performance of particular trait variants in one species (e.g., predators) will depend on the BTs of individuals in another species (e.g., prey) and vice versa. Consequently, no single BT will consistently yield high performance, and this could help maintain strategic variation in multiple populations. Taken together, our data emphasize that there is a value (or perhaps even a need) to broadening the focus of behavioral syndromes studies beyond single-species studies and instead begin to consider behavioral variation in multiple interacting species. Such investigations promise to increase our understanding of the ecological causes and consequences of the behavioral variation, which we observe in virtually every animal population.
SUPPLEMENTARY MATERIAL
Supplementary material can be found at http://www.beheco.oxfordjournals.org/
FUNDING
We would like to acknowledge Howard Hughes Medical Institute’s site grant to the University of Pittsburgh (52006957) and the College of Arts and Sciences for funding the undergraduate research of K.S.
Supplementary Material
Acknowledgments
Credit for the Phidippus clarus photograph in Figure 1 goes to Tom Murray.
REFERENCES
- Agrawal AA, Ackerly DD, Adler F, Arnold AE, Caceres C, Doak DF, Post E, Hudson PJ, Maron J, Mooney KA, et al. 2007. Filling key gaps in population and community ecology. Front Ecol Environ. 5: 145–152 [Google Scholar]
- Avgar T, Horvitz N, Broitman L, Nathan R. 2008. How movement properties affect prey encounter rates of ambush versus active predators: a comment on Scharf et al. Am Nat. 172: 593–595 [DOI] [PubMed] [Google Scholar]
- Berning AW, Gadd RDH, Sweeney K, MacDonald L, Eng RYY, Hess ZL, Pruitt JN. 2012. Sexual cannibalism is associated with female behavioural type, hunger state and increased hatching success. Anim Behav. 84: 715–721 [Google Scholar]
- Biro PA, Abrahams MV, Post JR, Parkinson EA. 2004. Predators select against high growth rates and risk-taking behaviour in domestic trout populations. Proc R Soc Lond Ser B Biol Sci. 271: 2233–2237 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Biro PA, Stamps JA. 2010. Do consistent individual differences in metabolic rate promote consistent individual differences in behavior? Trends Ecol Evol. 25: 653–659 [DOI] [PubMed] [Google Scholar]
- Boake CB. 1989. Repeatability: its role in evolutionary studies of mating behavior. Evol Ecol. 3: 173–182 [Google Scholar]
- Burns JH. 2004. A comparison of invasive and non-invasive dayflowers (Commelinaceae) across experimental nutrient and water gradients. Divers Distrib. 10: 387–397 [Google Scholar]
- Carter AJ, Goldizen AW, Tromp SA. 2010. Agamas exhibit behavioral syndromes: bolder males bask and feed more but may suffer higher predation. Behav Ecol. 21: 655–661 [Google Scholar]
- Cote J, Dreiss A, Clobert J. 2008. Social personality trait and fitness. Proc R Soc B Biol Sci. 275: 2851–2858 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cote J, Fogarty S, Brodin T, Weinersmith K, Sih A. 2011. Personality-dependent dispersal in the invasive mosquitofish: group composition matters. Proc R Soc B Biol Sci. 278: 1670–1678 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cote J, Fogarty S, Sih A. 2012. Individual sociability and choosiness between shoal types. Anim Behav. 83: 1469–1476 [Google Scholar]
- Dall SRX, Bell AM, Bolnick DI, Ratnieks FLW. 2012. An evolutionary ecology of individual differences. Ecol Lett. 15: 1189–1198 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dingemanse NJ, Both C, Drent PJ, Van Oers K, Van Noordwijk AJ. 2002. Repeatability and heritability of exploratory behaviour in great tits from the wild. Anim Behav. 64: 929–938 [Google Scholar]
- Exnerová A, Svádová KH, Fučíková E, Drent P, Štys P. 2010. Personality matters: individual variation in reactions of naive bird predators to aposematic prey. Proc R Soc B Biol Sci. 277: 723–728 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Falconer D, Mackay T. 1996. Introduction to quantitative genetics. San Francisco (CA): Benjamin Cummings; [Google Scholar]
- Gosling SD. 2001. From mice to men: what can we learn about personality from animal research? Psychol Bull. 127: 45–86 [DOI] [PubMed] [Google Scholar]
- Hoefler CD. 2007. Male mate choice and size-assortative pairing in a jumping spider, Phidippus clarus . Anim Behav. 73: 943–954 [Google Scholar]
- Huey RB, Pianka ER. 1981. Ecological consequences of foraging mode. Ecology. 62: 991–999 [Google Scholar]
- Jakob EM, Marshall SD, Uetz GW. 1996. Estimating fitness: a comparison of body condition indices. Oikos. 77: 61–67 [Google Scholar]
- Jones KA, Godin J-GJ. 2010. Are fast explorers slow reactors? Linking personality type and anti-predator behaviour. Proc R Soc B Biol Sci. 277: 625–632 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jones TC, Akoury TS, Hauser CK, Neblett MF, Linville BJ, Edge AA, Weber NO. 2011. Octopamine and serotonin have opposite effects on antipredator behavior in the orb-weaving spider, Larinioides cornutus . J Comp Physiol A Neuroethol Sens Neural Behav Physiol. 197: 819–825 [DOI] [PubMed] [Google Scholar]
- Kempenaers B, Peters A, Foerster K. 2008. Sources of individual variation in plasma testosterone levels. Philos Trans R Soc Lond B Biol Sci. 363: 1711–1723 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kobler A, Klefoth T, Mehner T, Arlinghaus R. 2009. Coexistence of behavioural types in an aquatic top predator: a response to resource limitation? Oecologia. 161: 837–847 [DOI] [PubMed] [Google Scholar]
- Kordas RL, Harley CDG, O’Connor MI. 2011. Community ecology in a warming world: the influence of temperature on interspecific interactions in marine systems. J Exp Mar Biol Ecol. 400: 218–226 [Google Scholar]
- Lima SL. 2002. Putting predators back into behavioral predator-prey interactions. Trends Ecol Evol. 17: 70–75 [Google Scholar]
- McElreath R, Strimling P. 2006. How noisy information and individual asymmetries can make ‘personality’ an adaptation: a simple model. Anim Behav. 72: 1135–1139 [Google Scholar]
- van Oers K, Buchanan KL, Thomas TE, Drent PJ. 2011. Correlated response to selection of testosterone levels and immunocompetence in lines selected for avian personality. Anim Behav. 81: 1055–1061 [Google Scholar]
- Perry G. 2007. Movement patterns in lizards. In: Reilly SM, McBrayer LB, Miles DB, editors. Lizard ecology. Cambridge: Cambridge University Press; p. 13–48 [Google Scholar]
- Pierce BM, Bleich VC, Terry Bowyer R. 2000. Selection of mule deer by mountain lions and coyotes: effects of hunting style, body size, and reproductive status. J Mammal. 81: 462–472 [Google Scholar]
- Pruitt JN, Cote J, Ferrari MCO. 2012. Behavioural trait variants in a habitat-forming species dictate the nature of its interactions with and among heterospecifics. Funct Ecol. 26: 29–36 [Google Scholar]
- Pruitt JN, Ferrari MC. 2011. Intraspecific trait variants determine the nature of interspecific interactions in a habitat-forming species. Ecology. 92: 1902–1908 [DOI] [PubMed] [Google Scholar]
- Pruitt JN, Krauel JJ. 2010. The adaptive value of gluttony: predators mediate the life history trade-offs of satiation threshold. J Evol Biol. 23: 2104–2111 [DOI] [PubMed] [Google Scholar]
- Pruitt JN, Riechert SE. 2012. The ecological consequences of temperament in spiders. Curr Zool. 58: 8 [Google Scholar]
- Pruitt JN, Stachowicz JJ, Sih A. 2012. Behavioral types of predators and prey jointly determine prey survival: potential implications for the maintenance of within-species behavioral variation. Am Nat. 179: 217–227 [DOI] [PubMed] [Google Scholar]
- Réale D, Festa-Bianchet M. 2003. Predator-induced natural selection on temperament in bighorn ewes. Anim Behav. 65: 463–470 [DOI] [PubMed] [Google Scholar]
- Reale D, Reader SM, Sol D, McDougall PT, Dingemanse NJ. 2007. Integrating animal temperament within ecology and evolution. Biol Rev. 82: 291–318 [DOI] [PubMed] [Google Scholar]
- Riechert SE. 1991. Prey abundance vs diet breadth in a spider test system. Evol Ecol. 5: 327–338 [Google Scholar]
- Rosenheim JA, Glik TE, Goeriz RE, Ramert B. 2004. Linking a predator’s foraging behavior with its effects on herbivore population suppression. Ecology. 85: 3362–3372 [Google Scholar]
- Scharf I, Nulman E, Ovadia O, Bouskila A. 2006. Efficiency evaluation of two competing foraging modes under different conditions. Am Nat. 168: 350–357 [DOI] [PubMed] [Google Scholar]
- Schreiber SJ, Bürger R, Bolnick DI. 2011. The community effects of phenotypic and genetic variation within a predator population. Ecology. 92: 1582–1593 [DOI] [PubMed] [Google Scholar]
- Sih A, Bell A, Johnson JC. 2004. Behavioral syndromes: an ecological and evolutionary overview. Trends Ecol Evol. 19: 372–378 [DOI] [PubMed] [Google Scholar]
- Sih A, Bell AM. 2008. Insights for behavioral ecology from behavioral syndromes. Adv Study Behav. 38: 227–281 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sih A, Bell AM, Johnson JC, Ziemba RE. 2004. Behavioral syndromes: an integrative overview. Q Rev Biol. 79: 241–277 [DOI] [PubMed] [Google Scholar]
- Sih A, Cote J, Evans M, Fogarty S, Pruitt J. 2012. Ecological implications of behavioural syndromes. Ecol Lett. 15: 278–289 [DOI] [PubMed] [Google Scholar]
- Smith BR, Blumstein DT. 2010. Behavioral types as predictors of survival in Trinidadian guppies (Poecilia reticulata). Behav Ecol. 21: 919–926 [Google Scholar]
- Stamps J, Groothuis TGG. 2010. The development of animal personality: relevance, concepts and perspectives. Biol Rev. 85: 301–325 [DOI] [PubMed] [Google Scholar]
- Tinker MT, Bentall G, Estes JA. 2008. Food limitation leads to behavioral diversification and dietary specialization in sea otters. Proc Natl Acad Sci USA. 105: 560–565 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Webster MM, Ward AJW, Hart PJB. 2009. Individual boldness affects interspecific interactions in sticklebacks. Behav Ecol Sociobiol. 63: 511–520 [Google Scholar]
- Wilson ADM, Whattam EM, Bennett R, Visanuvimol L, Lauzon C, Bertram SM. 2010. Behavioral correlations across activity, mating, exploration, aggression, and antipredator contexts in the European house cricket, Acheta domesticus . Behav Ecol Sociobiol. 64: 703–715 [Google Scholar]
- Wolf M, Weissing FJ. 2012. Animal personalities: consequences for ecology and evolution. Trends Ecol Evol. 27: 452–461 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.