Abstract
Distal tibial fractures tend towards delayed- or nonunion. The purpose of this study was to evaluate the safety and efficacy of early minimally invasive intervention (MII) in the treatment of these fractures. A total 24 consecutive patients who underwent operative treatment for distal tibial fractures were randomized into a control and an intervention group. MII entailed aspirating iliac crest bone marrow and peripheral blood, yielding mesenchymal stem cells (MSCs) and platelet-rich plasma (PRP) respectively, that were mixed with demineralized bone matrix (DBM) and injected under fluoroscopic control into the fracture site. No complications occurred in either group. The median time to union was 1.5 months in the MII group and 3 months in the control group. MII was found to be a safe and efficient procedure.
Introduction
The US Food and Drug Administration defines a nonunion as a fracture that does not heal within 9 months, nonunion occurring in 1 out of every 40 fractures.1
The clinical definition of delayed union is a lack of healing progression within 3 consecutive months. The prevalence of both nonunion and delayed union fractures is much higher in long bone fractures. Fractures of the distal third of the tibia are especially prone to nonunion and delayed union owing to a limited soft tissue envelope and a poor blood supply in close to 15% of such fractures.2,3 This can result in significant morbidity and is an economic burden. The treatment of these conditions usually involves both biological stimulation and alteration of the mechanical environment to promote healing. An autologous iliac crest bone graft is considered the gold standard therapy, combining the three significant qualities of the graft, namely osteoinduction, osteoconduction and osteogenesis.4 However, although the amount of graft material is limited, iliac crest bone graft involves significant morbidity at the donor site and a high rate of complications and is, therefore, performed only when strictly indicated.5,6
Advancements in our understanding of the biology of bone healing led to the development of other, less invasive, forms of grafts, aimed at enhancing bone regeneration. Whether injected or applied in an open technique, these strategies are aimed at facilitating osteoinduction and osteoconduction (at different levels), and to lead to osteogenesis.
Whereas an osteoconductive scaffold can be provided by various types of matrices and osteoinduction can be promoted by bone morphogenetic proteins, osteogenesis is the exclusive result of the activity of progenitor cells at the fracture site.7
Mesenchymal stem cells (MSCs) are multipotent adult stem cells. Obtained from an adult rather than an embryo, they harbor the potential for proliferation, although their potential for differentiation is limited to the various cell lines of the mesenchyme.8 These unique cells, though quite rare, can be found in very low concentrations in all mesenchymal tissues, including bone marrow.9
Obtaining a high number of MSCs is a major obstacle in their clinical use, as ex vivo culture expansion is associated with loss of potency and phenotypic modifications of the cells, and a relatively long period of incubation with associated contamination risks.10 Recently, we developed a fast and effective method for isolating large numbers of MSCs from bone marrow aspirates that can be used clinically in selected cases.11
The purpose of the present investigation was to evaluate the safety of early prophylactic and minimally invasive intervention (MII) in the treatment of distal tibial fractures by the percutaneous introduction of an aspirated bone marrow–enriched MSCs-graft into the fracture site, as compared with nontreatment, in a randomized, controlled trial. The study was designed as a Phase I study, the hypothesis being that injection of an MSCs-based composite graft at 3–6 weeks into surgically treated distal tibial fractures would be safe and might provide a biological advantage.12,13,14 This was expected to be clinically evident as a reduction in delayed- or nonunion and reduced healing time versus conventionally treated fractures.
Results
Patients
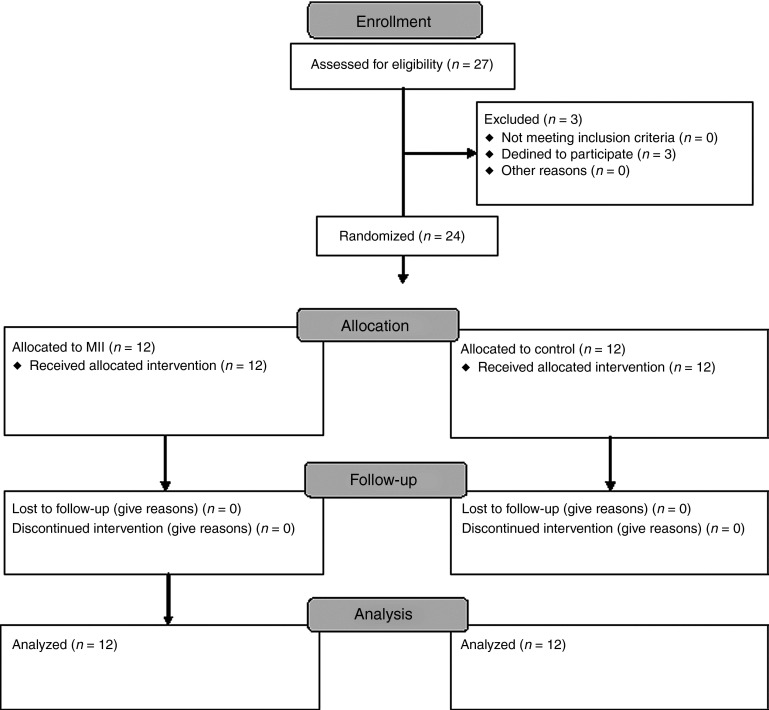
A total 12 patients were enrolled in the intervention group and 12 in the control group. All the patients were classified as low risk, and all reached the primary endpoint (Figure 1). All the patients presented with extra-articular distal tibial fractures, AO fracture classification 43.15
Figure 1.
Study flow chart.
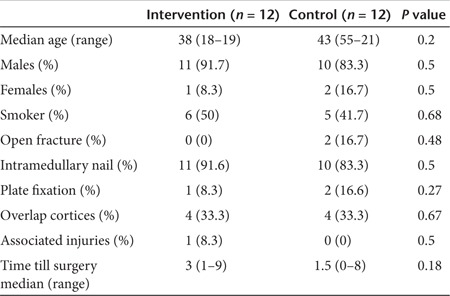
No significant differences were found between the two groups in demographic characteristics or fracture parameters following primary stabilization surgery at the baseline time point (Table 1).
Table 1. Demographics of Intervention and control groups at baseline.
Intervention group (composite graft)
The average time between index surgery and intervention was 30.2 days (SD = 8.7 days). The mean number of stem cells isolated for injection was 1.03 × 108 MSCs (SD = 79,500), well over our set minimum for grafting. Up to, but not >1.0 × 108 MSCs were incorporated into the graft. The mean number of platelets in the platelet-rich plasma (PRP) was 1.10 × 109 per concentrate (SD = 664).
Safety
There were no procedure-related adverse events in the intervention group. There were two such events in the control group. One patient suffered dehiscence of the proximal end of the surgical wound following fixation of the fracture with an anatomic plate. Empiric antibiotic treatment with gentamycin and cefazolin was switched to cefuroxime following culture of Enterobacter cloacae from the wound. Significant clinical improvement under intravenous treatment within 4 days resulted in a switch to oral treatment until completion of the 4 week regimen. A second patient showed a clinically significant delayed union of the fracture, manifested by ongoing rest pain at the fracture site and no radiographic progress in any of the four cortices after 6 months. The patient was successfully treated by removal of the distal locking screw of the intramedullary nail (dynamization) and healing was evident at the 9-month follow-up.
Fracture healing
All fractures healed within the 12-month follow-up, There were no nonunions. However, three of the patients in the control group (25%) experienced delayed union as their fractures did not unite by the 3-month follow-up (Table 2). All three underwent primary stabilization of their fractures using an intramedullary nail. One patient had no specific complaints, whereas the other two, including the patient mentioned above experienced significant resting pain. No other adverse events were noted with regard to these three patients.
Table 2. Delayed union—demographic and clinical data.
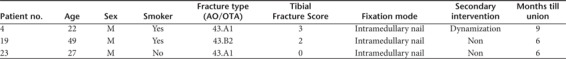
As two of the patients experienced fracture healing by 6 months, they were not assigned to undergo dynamization or any other secondary intervention.
In addition, radiologic evaluation showed a significant decrease in fusion time in the intervention group, with a reduction in median time to union from 3 to 1.5 months according to unblinded and blinded evaluation. A reduction in mean time from 4.0 to 2.2 months according to the unblinded evaluation and a reduction of 3.4–2.3 months according to the blinded evaluation (P < 0.03 and P < 0.06, respectively), (Figure 2) was recorded.
Figure 2.
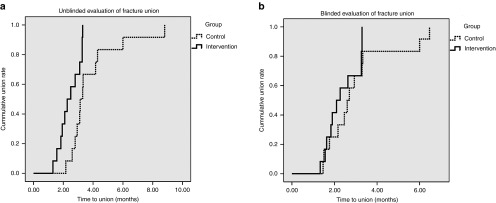
Blinded and unblinded evaluation of radiographic analysis: Kaplan Meier Curve. Time to radiographic union of the fractures in the intervention (straight line) and the control (dotted line) groups as determined by (a) unblinded and (b) blinded evaluators. The data are presented in Kaplan Meier curves as cumulative union (ratio) over time (months).
It is noteworthy that at the 6-month time point, when all fractures in the intervention group had already healed, an ossified mass was seen adjacent to the fracture site, indicating the site of graft injection (Figure 3).
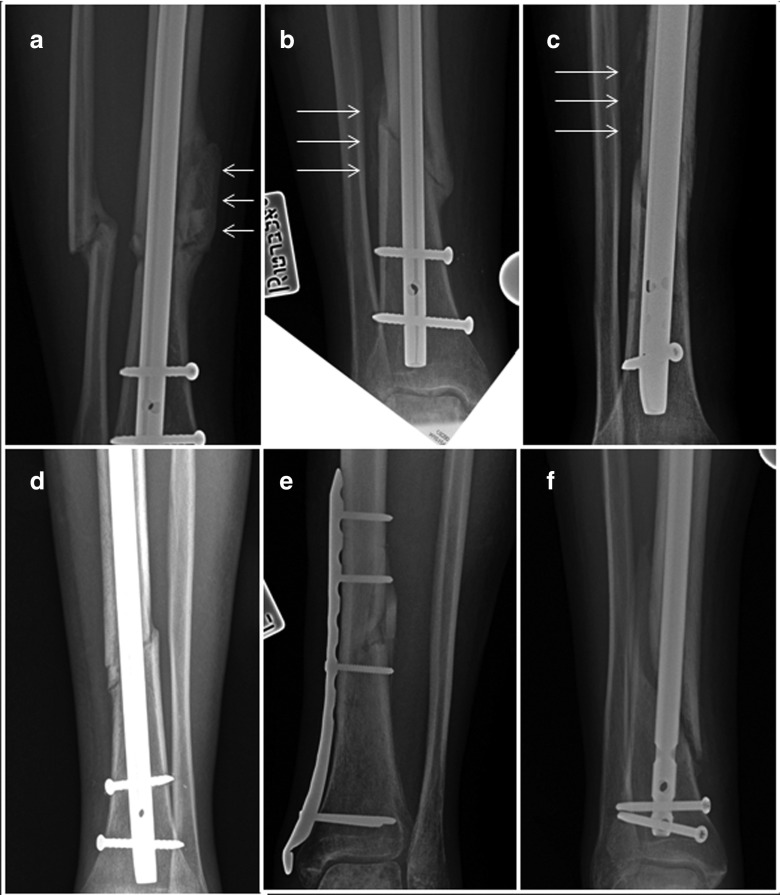
Figure 3.
Representative radiographs of healed fractures at 3 months follow-up. Anterio-posterior (AP) radiographs of the fracture area of three representative patients (a–c) from the intervention (MII) group and (d–f) from the control group taken at the 3 months follow-up. Note early calcifying callus (white arrows) where graft was placed.
Pain and quality of life
Neither of the clinical assessments (Short Form-12 (SF-12) and Visual Analogue Scale) indicated a significant difference between the two groups.
Biologic graft control
For the patients in the intervention group, a small portion of the mixed composite graft (500 µl volume) was subcutaneously transplanted into immune-deficient mice. These grafts served as biological controls, allowing assessment of bone tissue formation in the animal model.
Histological analysis of the retrieved grafts at sacrifice revealed bone formation in all samples. Immuno-histochemical analysis was not performed due to technical difficulties and therefore determination of the origin of the cells within the ossified tissue was not possible.
Discussion
We performed a prospective, single center randomized and controlled clinical study aimed at assessing the safety of implanting human MSCs for the repair of distal tibial fractures. There were no complications related to the intervention, and healing time was significantly shorter. Our results show that isolation and grafting of stem cells can be a safe and quick procedure with minimal discomfort or risk to the patient.
All the 24 patients sustained a fracture that was classified as having a low risk for nonunion and fixed with a reamed intramedullary nail or a plate. We documented three cases of delayed union in the control group (n = 12) but no delayed union in the intervention group (n = 12). The rate of delayed union documented in the control group is in line with previously published data.2,3
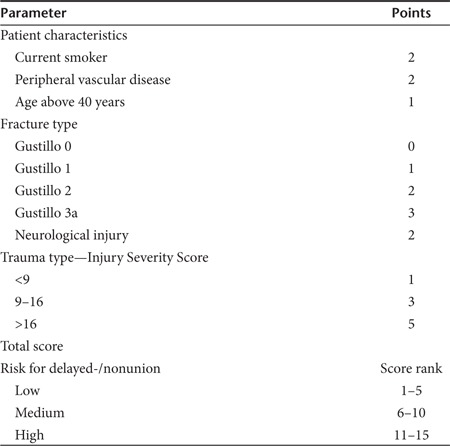
A potential for bias between the groups in such a study may be uneven distribution of the more severe (i.e., high grade open fractures) injuries. We formulated the Tibial Fracture Score to address this potential problem (Table 3). However, despite the fact that all patients originated in our Level One Trauma Center, unexpectedly all of them had fractures that were rated as low risk for nonunion or delayed union.
Table 3. Tibial Fracture Score.
All patients underwent primary fixation of their fractures by a team of well-experienced orthopedic trauma surgeons. The high rate of union and the relatively short time to union in both the control and intervention groups represent a better outcome than commonly published for these fractures. Improved outcome for control treatment patients enrolled in clinical trials in various medical fields has been previously reported.2,4,16,17 High performance during clinical trials may be explained by team motivation and close patient follow-up. In our study, despite an overall superior outcome, intervention reduced the healing period by about 40 days, with minimal discomfort (according to unblinded reviewers, trend only according to blinded evaluator). Such an approach could result in substantial savings for third party payers, employers, and government agencies.18
Although early intervention in tibial fractures and placement of an iliac crest bone graft has been shown to shorten healing time and increase union rate,19 such graft harvesting is associated with increased morbidity.20 The present study combines the advantages of a minimally invasive surgical procedure with novel cell isolation technology.
The identification and characterization of MSCs is a critical yet demanding and time-consuming task. In our study it was impossible to verify the surface marker profile of each transplanted sample due to time limitations and similarly it was not possible to conduct differentiation assays before transplantation. However, as this study represents the summation of a decade-long endeavor aimed at developing a novel platform of freshly isolated MSCs for clinical use, we based our procedure on our previous data.11 We showed that this method of positive selection yields cells that correlate with the currently acceptable definitions of MSCs in terms of surface marker profile, proliferation and differentiation capacity.
An animal model of composite graft ossification was used as a biologic control for the graft, paired with each patient in the intervention group. At follow-up, all treated individuals, as well as their paired animals, underwent ossification at the graft site. Interestingly, it was difficult to evaluate whether the cells within the newly formed bone were graft cells or recruited cells. This is not surprising as our understanding of the role MSCs play in the process of fracture healing focuses on maintenance of the unique pro-regenerative environment and balance of the immune response around the fracture to induce neo-angiogenesis. This means recruiting more bone-forming cells, preventing fibrous scar tissue from forming and enhancing formation of functional bone tissue.
Our study has several limitations. First, the specific contribution of each component of the composite graft in the clinical setting is not known. Although previous reports indicate that administration of PRP alone may not suffice,21 it was used in this study as a scaffold enabling MSCs to remain in the fracture site and function. Furthermore, the study alone does not allow us to evaluate whether synergistic effects played a role in the observed outcome. A larger study, probably multicentered, should be undertaken to address this issue by incorporating more study groups, where omitting one or more factors from the graft.
Second, despite the large number of cells introduced into the fracture bed, their actual role and biological activity remain unknown. A step back to the laboratory for some bench assays may extend our understanding of the actual function of stem cells in tissue regeneration.
As this was a pioneer study and no clinical data were available at the time of study conception, we based our protocol on the available data from animal studies that suggested an MSC concentration of 1 × 106 cells per ml.22 We limited the total number of MSCs so as not to exceed 1.0 × 108 cells per sample which is equivalent to 2.0 × 107 cells per ml, to avoid overpopulation and rapid depletion of nutrients from within the graft area. More recent human data suggest the use of these same concentrations.23,24
Another limitation is the potentially reactive biological environment at the fracture site during the 3–6 week healing period. According to our results, we cannot predict the effect of such treatment during later phases of delayed union or established fracture nonunion.
Our study shows that a composite graft comprised of freshly isolated autologous MSCs mixed with PRP and demineralized bone matrix (DBM) is safe in the early management of fractures of the distal tibia. Further investigation and optimization of the system may yield even better results. Future applications of freshly isolated MSCs may include delivery with active signal molecules and transient or permanent genetic and functional modifications of the cells. This graft, in turn, may be applied preventively in cases where difficulties in fracture healing are foreseen. As such technology is likely to be available primarily in referral surgical centers we foresee this technique as being adopted in regional centers that will dedicate the appropriate resources to treat the referred patients.
Materials and Methods
Study design. This was a prospective randomized (1:1 allocation ratio), open label, controlled study. The clinical trial was approved by the Institutional Review Board of our medical center as well as the Supervisory Review Board of the Ministry of Health. All participants provided written informed consent. The study was registered at http://www.clinicaltrial.gov (ID: NCT00250302; Registry Name: “Autologous Implantation of Mesenchymal Stem Cells for the Treatment of Distal Tibial Fractures”).
Sample size. Sample size was based upon traditional Phase I trials in which assessment of safety is deemed acceptable on a sample size of 10 patients. Assuming a 10–15% drop out rate, 12 patients were enrolled in each group. To evaluate the biological effects of the treatment it was necessary to compare the intervention group with a control group that received standard treatment.
Patients. Of 27 consecutive patients who underwent surgery for extra-articular fractures of the distal third of the tibia and met the inclusion criteria (Table 4), 24 consented to the study. All patients were operated on by fellowship-trained orthopedic trauma surgeons. The primary surgical procedures consisted of subcutaneous plating or intramedullary nailing of the fracture. Informed consent was obtained within the first week after surgery.
Table 4. Inclusion and exclusion criteria.

Surgical technique. A total of 21 patients underwent a nailing procedure and three patients underwent plating. All nailing procedures were done using reamed tibial nails (Universal Tibial Nail, Synthes, Oberdorff, Switzerland) with a diameter of 11 mm or more, using either a patellar split or a medial parapatellar approach. In 15 cases, where the fracture did not allow for the placement of at least two interlocks, a third-generation nail was used (Expert Tibial Nail, Synthes, Oberdorff, Switzerland). When the surgeons considered that a plate fixation was more suitable for fracture fixation, a percutaneous plate was used, according to a minimally invasive plating osteosynthesis technique.25
In all cases, closed reduction was performed using manual traction; percutaneous bone clamps; satisfactory maintenance of axis (<5 degrees of deviation in each plane) and length (<1 cm shortening); and rotation.
To avoid confounding, which may stem from variability in the orthopedic surgical outcome, gap size in the fracture site was specifically quantified to ensure that the void would not significantly exceed graft volume and be within the acceptable limits of surgical practice.
Cases in which plates were used, percutaneous, medial, locked precontoured plates (Synthes, Oberdorff, Switzerland) were used, guided by fluoroscopy for placement. In no case was open reduction performed.
Randomization. To obtain balanced and comparable groups of patients, the severity of the fracture and the risk of delayed union was assessed using the Tibial Fracture Score (Table 3). This incorporated parameters related to the patient, the fracture, and the overall trauma. Fractures were classified according to the AO/OTA classification26 for location and configuration of the bone injury and according to the Gustilo classification for soft tissue.27
Using the Tibial Fracture Score, the patients were stratified according to risk of nonunion: high, intermediate, or low. This method was intended to ensure that randomization was balanced according to factors likely to confound the rate of fracture healing, thus creating two comparable groups of patients with similar clinical profiles.
The patients were randomized, using a four block random number generator (http://www.randomization.com) to receive standard postoperative follow-up (control group) or to undergo early intervention (treatment group).
The randomization sequence was concealed, using sequentially numbered, sealed envelopes. These were opened only after an eligible patient consented to take part in the trial.
Composite graft. The composite graft was composed of PRP, sorted MSCs in suspension and DBM that was delivered under fluoroscopic guidance into the fracture site.
DBM: The DBM used in this study was Ignite ICS injectable scaffold (Wright Medical Technology, Arlington, TN) contained in 2 ml vials.
MSCs: Bone marrow aspirates were mixed 1:1 (v/v) with a 90% normal saline/10% heparin (1,000 IU/ml) solution and transferred under sterile conditions to our Good Manufacturing Practice laboratory for processing. A positive selection technique for identifying MSCs from the bone marrow–nucleated cells using CD105 surface cell markers was developed. Anti-human CD105 antibodies (Miltenyi Biotech, Bergisch Gladbach, Germany) were conjugated to colloidal super-paramagnetic microbeads, which were then used to labeled the CD105-expressing cells from the bone marrow aspirate, as previously described.11 The cells were subsequently separated on a Magnetic-Activated Cell Sorting (CliniMACS; Miltenyi Biotech) column. The entire separation procedure was carried out using clinical grade reagents and instrumentation (Miltenyi Biotec). The result of the separation was a cell population containing a nearly pure MSC subpopulation. The selection procedure took about two hours. A minimum of 5 × 106 MSCs per sample were required for transplantation.
PRP: Peripheral blood samples were transferred under sterile conditions to the Good Manufacturing Practice laboratory where the 100 ml samples were centrifuged at 1,000 rpm for 10 minutes to separate the cellular fraction from the plasma. A 40 ml volume of the supernatant was collected and then centrifuged at 4,000 rpm for 10 minutes to concentrate the platelets. The supernatant was decanted leaving the most concentrated 5 ml of plasma for grafting. The minimal platelet concentration for clinical use was set at a minimum 900 platelets per μl for each patient. PRP also served as a mixing solution for the DBM.
Assembly and delivery of the graft. Once the MSC separation was completed and the number of cells verified, the patient was taken back to the operating theater, anesthetized, and the three components: DBM, PRP, and the MSC suspension were mixed to a volume of 8 ml and delivered by percutaneous injection under fluoroscopic guidance into the fracture site. The leg was placed in a protective cast or brace for 1 week.
Operative procedure. Patients in the intervention group were electively scheduled for a composite graft procedure at 3–6 weeks following the index procedure. The patients were taken to the operating room where an iliac crest bone marrow aspirate (50 ml) using an 8GA Jamshidi needle and peripheral blood (100 ml) samples were obtained under sedation. The sterile samples were labeled and transferred to the Good Manufacturing Practice grade laboratory where the PRP was processed from peripheral venous blood by low-speed (1,000 rpm,10 minutes) followed by high-speed (4,000 rpm,10 minutes) centrifugation. The bone marrow aspirate was used as a source for MSCs. The patients were transferred to the postanesthesia care unit. After 3–4 hours, they were returned to the operating room for the second stage of the procedure. Following sterile preparation and draping of the affected extremity, PRP was mixed with autologous MSCs and DBM carrier, and under short general anesthesia or sedation the graft was injected into the fracture site under fluoroscopic control that confirmed graft location. An 8 ml graft was located in a single site that was defined by the surgical team as the most significant and accessible, in a way that would ensure positioning of the whole volume of the graft in and around the fracture gap, under the soft tissue envelope. This would provide blood supply and prevent leakage of the graft while enabling a direct approach with the needle without endangering neurovascular structures. The graft was injected into the area of the largest visible gap using an 11 GA Jamshidi needle (Medical Device Technologies, Gainesville, FL) through a stab wound. The needle was removed 3 minutes after injection was completed to allow clot formation. Before the injection, cultures were obtained from the fracture site followed by one dose of prophylactic antibiotic (intravenous cefazolin 1 g). The patients were monitored for 24 hours and then discharged.
Follow-up. Patients from both the control and the intervention groups were followed up in the outpatient clinic at 2 weeks, 6 weeks, 3 months, 6 months, 9 months, and 12 months after the index (fracture fixation) procedure. Routine anteroposterior and lateral tibial x-rays were performed at all visits except for the first one, and the patients were assessed for pain (Visual Analogue Scale), and a general quality of life measures (SF-12 questionnaire). Fracture healing was assessed by lack of pain during weight bearing and bridging of three out of four cortices in both anteroposterior and lateral radiographic views.28 Evaluation of the radiographs as part of the clinical follow-up was performed by the non-blinded surgeons. To ascertain unbiased evaluation all radiographs were assessed independently by a blinded evaluator.
The study endpoints were defined as:
Safety: Safety was evaluated in a descriptive manner by recording all adverse events in the patient population by number and severity. The study evaluated safety by assessing:
A.Procedure related to adverse events: local and systemic effects of the composite graft injection, including: donor site morbidity, local infection at the fracture site, postoperative fever or other complications.
B.General adverse events: Adverse events not directly related to the surgical procedure, such as complications related to anesthesia, hospitalization, or other general adverse events of unknown cause.
Additional observations:
A.The number of patients in each group who achieved clinical and radiological union at 3 and 6 months. Clinical union was defined as an asymptomatic patient who was able to fully bear weight on the injured leg. In addition, radiolographic fracture healing was defined by bone continuity (bony bridging) present in three out of four cortices in two projections (anterior–posterior & lateral x-rays), assessed by a blinded senior orthopedic surgeon.
B.The number of patients undergoing further procedural intervention for delayed union. Such intervention was indicated only when no clinical or radiological progress was documented for 3 consecutive months after primary stabilization surgery.
C.Pain and quality of life: Patients were asked to assess the severity of their pain using a Visual Analogue Score. The health survey SF-12 was used to assess quality of life.29 The scores are weighted averages of the physical and mental components of the 12 questions constituting the SF-12 instrument for measuring quality of life. The scores were transformed to produce a normally distributed population score. For each of the patients, the SF-12 scores were calculated both for a physical component summary and a mental component summary, using the scoring method provided.30
Statistical analysis. Demographic factors and clinical characteristics were summarized as counts and percentages for categorical variables. The mean and the standard deviation were calculated for continuous variables with normal distribution, the median and ranges were calculated for continuous variables with abnormal distribution.
The groups were compared using the χ2 test or Fisher's exact test for categorical variables and the Mann–Whitney U test for continuous variables. The time to union was assessed using the Kaplan–Meier life-table method as well as the Mantel–Cox test. All P values were two-tailed. We analyzed our results with SPSS 18 software (IBM).
Biologic control. To assess the bone-forming potential of the composite graft, 0.5 ml of graft material from each graft was subcutaneously implanted in immune compromised mice. Six weeks after implantation, the animals were sacrificed and their grafts were harvested. The biologic activity of the graft was assessed by micro-CT imaging and histopathology evaluation of bone formation.
Acknowledgments
We thank Alexandra Mahler and Shoshanah Kahn for their assistance in editing and formatting the manuscript. The study was supported by a grant from Teva Pharmaceutical Industries. This company is the recipient of a special grant from the Magnet Program of the Israel Ministry of Industry, Trade and Labor. This program is designated to support the formation of consortia made up of industrial companies and academic institutions. Our collaborating partner for this project was Teva Pharmaceutical Industries. Neither Teva nor the Israel Ministry of Industry played a role in writing either the study protocol or the investigators' brochure. They did not analyze or interpret the data. The work was done at Jerusalem, Israel. The authors declare no conflict of interest.
References
- Megas P, Panagiotis M. Classification of non-union. Injury. 2005;36 Suppl 4:S30–S37. doi: 10.1016/j.injury.2005.10.008. [DOI] [PubMed] [Google Scholar]
- Clancey GJ, Hansen ST., Jr Open fractures of the tibia: a review of one hundred and two cases. J Bone Joint Surg Am. 1978;60:118–122. [PubMed] [Google Scholar]
- Zelle BA, Bhandari M, Espiritu M, Koval KJ, Zlowodzki M, Evidence-Based Orthopaedic Trauma Working Group Treatment of distal tibia fractures without articular involvement: a systematic review of 1125 fractures. J Orthop Trauma. 2006;20:76–79. doi: 10.1097/01.bot.0000202997.45274.a1. [DOI] [PubMed] [Google Scholar]
- Phieffer LS, Goulet JA. Delayed unions of the tibia. J Bone Joint Surg Am. 2006;88:206–216. doi: 10.2106/00004623-200601000-00026. [DOI] [PubMed] [Google Scholar]
- Goulet JA, Senunas LE, DeSilva GL, Greenfield ML. Autogenous iliac crest bone graft. Complications and functional assessment. Clin Orthop Relat Res. 1997. pp. 76–81. [DOI] [PubMed]
- Younger EM, Chapman MW. Morbidity at bone graft donor sites. J Orthop Trauma. 1989;3:192–195. doi: 10.1097/00005131-198909000-00002. [DOI] [PubMed] [Google Scholar]
- Sen MK, Miclau T. Autologous iliac crest bone graft: should it still be the gold standard for treating nonunions. Injury. 2007;38 Suppl 1:S75–S80. doi: 10.1016/j.injury.2007.02.012. [DOI] [PubMed] [Google Scholar]
- Pittenger MF, Mackay AM, Beck SC, Jaiswal RK, Douglas R, Mosca JD, et al. Multilineage potential of adult human mesenchymal stem cells. Science. 1999;284:143–147. doi: 10.1126/science.284.5411.143. [DOI] [PubMed] [Google Scholar]
- Kuo TK, Ho JH, Lee OK. Mesenchymal stem cell therapy for nonmusculoskeletal diseases: emerging applications. Cell Transplant. 2009;18:1013–1028. doi: 10.3727/096368909X471206. [DOI] [PubMed] [Google Scholar]
- Mauney JR, Volloch V, Kaplan DL. Role of adult mesenchymal stem cells in bone tissue engineering applications: current status and future prospects. Tissue Eng. 2005;11:787–802. doi: 10.1089/ten.2005.11.787. [DOI] [PubMed] [Google Scholar]
- Aslan H, Zilberman Y, Kandel L, Liebergall M, Oskouian RJ, Gazit D, et al. Osteogenic differentiation of noncultured immunoisolated bone marrow-derived CD105+ cells. Stem Cells. 2006;24:1728–1737. doi: 10.1634/stemcells.2005-0546. [DOI] [PubMed] [Google Scholar]
- Oestern HJ, Tscherne H. Pathophysiology and classification of soft tissue injuries associated with fractures. Springer Verlag: Berlin; 1984. [Google Scholar]
- Einhorn TA. Enhancement of fracture-healing. J Bone Joint Surg Am. 1995;77:940–956. doi: 10.2106/00004623-199506000-00016. [DOI] [PubMed] [Google Scholar]
- McKibbin B. The biology of fracture healing in long bones. J Bone Joint Surg Br. 1978;60-B:150–162. doi: 10.1302/0301-620X.60B2.350882. [DOI] [PubMed] [Google Scholar]
- Müller ME, Nazarian S, Koch P, Schatzker J.1990The Comprehensive Classification of Fractures of Long Bones1st edn. Springer-Verlag: Berlin and New York [Google Scholar]
- Guo JJ, Tang N, Yang HL, Tang TS. A prospective, randomised trial comparing closed intramedullary nailing with percutaneous plating in the treatment of distal metaphyseal fractures of the tibia. J Bone Joint Surg Br. 2010;92:984–988. doi: 10.1302/0301-620X.92B7.22959. [DOI] [PubMed] [Google Scholar]
- Im GI, Tae SK. Distal metaphyseal fractures of tibia: a prospective randomized trial of closed reduction and intramedullary nail versus open reduction and plate and screws fixation. J Trauma. 2005;59:1219–23; discussion 1223. doi: 10.1097/01.ta.0000188936.79798.4e. [DOI] [PubMed] [Google Scholar]
- Heckman JD, Sarasohn-Kahn J. The economics of treating tibia fractures. The cost of delayed unions. Bull Hosp Jt Dis. 1997;56:63–72. [PubMed] [Google Scholar]
- Bone LB, Kassman S, Stegemann P, France J. Prospective study of union rate of open tibial fractures treated with locked, unreamed intramedullary nails. J Orthop Trauma. 1994;8:45–49. doi: 10.1097/00005131-199402000-00010. [DOI] [PubMed] [Google Scholar]
- Banwart JC, Asher MA, Hassanein RS. Iliac crest bone graft harvest donor site morbidity. A statistical evaluation. Spine. 1995;20:1055–1060. doi: 10.1097/00007632-199505000-00012. [DOI] [PubMed] [Google Scholar]
- Carreon LY, Glassman SD, Anekstein Y, Puno RM. Platelet gel (AGF) fails to increase fusion rates in instrumented posterolateral fusions. Spine. 2005;30:E243–6; discussion E247. doi: 10.1097/01.brs.0000160846.85397.44. [DOI] [PubMed] [Google Scholar]
- Bruder SP, Kraus KH, Goldberg VM, Kadiyala S. The effect of implants loaded with autologous mesenchymal stem cells on the healing of canine segmental bone defects. J Bone Joint Surg Am. 1998;80:985–996. doi: 10.2106/00004623-199807000-00007. [DOI] [PubMed] [Google Scholar]
- Chatterjea A, Meijer G, van Blitterswijk C, de Boer J. Clinical application of human mesenchymal stromal cells for bone tissue engineering. Stem Cells Int. 2010;215:1–12. doi: 10.4061/2010/215625. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marcacci M, Kon E, Moukhachev V, Lavroukov A, Kutepov S, Quarto R, et al. Stem cells associated with macroporous bioceramics for long bone repair: 6- to 7-year outcome of a pilot clinical study. Tissue Eng. 2007;13:947–955. doi: 10.1089/ten.2006.0271. [DOI] [PubMed] [Google Scholar]
- Redfern DJ, Syed SU, Davies SJ. Fractures of the distal tibia: minimally invasive plate osteosynthesis. Injury. 2004;35:615–620. doi: 10.1016/j.injury.2003.09.005. [DOI] [PubMed] [Google Scholar]
- Marsh JL, Slongo TF, Agel J, Broderick JS, Creevey W, DeCoster TA, et al. Fracture and dislocation classification compendium - 2007: Orthopaedic Trauma Association classification, database and outcomes committee. J Orthop Trauma. 2007;21 (10 Suppl:S1–133. doi: 10.1097/00005131-200711101-00001. [DOI] [PubMed] [Google Scholar]
- Gustilo RB, Anderson JT. Prevention of infection in the treatment of one thousand and twenty-five open fractures of long bones: retrospective and prospective analyses. J Bone Joint Surg Am. 1976;58:453–458. [PubMed] [Google Scholar]
- Whelan DB, Bhandari M, McKee MD, Guyatt GH, Kreder HJ, Stephen D, et al. Interobserver and intraobserver variation in the assessment of the healing of tibial fractures after intramedullary fixation. J Bone Joint Surg Br. 2002;84:15–18. doi: 10.1302/0301-620x.84b1.11347. [DOI] [PubMed] [Google Scholar]
- Starr AJ. Fracture repair: successful advances, persistent problems, and the psychological burden of trauma. J Bone Joint Surg Am. 2008;90 Suppl 1:132–137. doi: 10.2106/JBJS.G.01217. [DOI] [PubMed] [Google Scholar]
- Ware JE, Kosinski M, Keller SD. SF-12: How to Score the SF-12 Physical and Mental Health Summary Scales. The Health Institute, New England Medical Center: Boston, MA; 1995. [Google Scholar]