Abstract
TOMM40 SNP rs157580 has been associated with triglyceride levels in Genome-wide association studies (GWAS). Chronic caregiving stress moderates the association between triglyceride levels and a nearby SNP rs439401 that is associated with triglyceride levels in GWAS. Here, we report data from two independent Caucasian samples (242 U.S. women and men; 466 Danish men) testing the hypothesis that chronic family stress also moderates the association between rs157580 and triglyceride levels. The interaction of rs157580 and family stress in predicting triglyceride levels was statistically significant in the U.S. sample (p = 0.004) and marginally significant (p = 0.075) in the Danish sample. The G allele of rs157580 was associated with increased triglyceride levels among family stressed cases in both samples compared with A/A cases, but not among controls. Chronic family stress moderates the association of rs157580 variants with triglyceride levels and should be taken into account for disease risk assessment and potential intervention.
Introduction
Genome-wide association studies (GWAS) demonstrate evidence for genetic association with individual traits but this evidence varies in consistency across different variants (SNPS) in different GWAS studies. One potential explanation for this inconsistency among studies is the presence of gene-environment interactions where the environmental variable has different distributions in the studies. The apolipoprotein cluster on chromosome 19 including TOMM40 and APOE is known to have an important role in determining inter-individual differences in lipid metabolism and a cluster of related metabolic traits (Ken-Dror et al., 2010; Talmud et al., 2009). One SNP, rs439401 near the APOE gene, was found to be associated with triglyceride levels (p = 1.8 × 10 −9) in a GWAS among a large sample of Europeans (Aulchenko et al., 2009). Individuals homozygous for the rs439401 minor allele (T) had decreased levels of triglyceride compared to the major allele (C) carriers. However, when we examined the association between the rs439401 genotype and a panel of metabolic traits in a sample of stressed caregivers, caring for a relative with Alzheimer’s disease, and age-, race-, sex- and SES-matched non-caregiver controls (Kring et al., 2010), among stressed caregivers the rs439401 T/T genotype was associated with an adverse metabolic profile (e.g., higher triglyceride levels, higher waist circumference and lower HDL-cholesterol); in contrast, T/T controls exhibited a favorable metabolic profile. The effect of genotype in controls was consistent with the finding in the GWAS sample (Aulchenko et al., 2009) in which the majority of participants were not likely exposed to a level of chronic stress comparable to that of caregiving for a relative with Alzheimer’s disease.
In the same GWAS, the G allele on another SNP, rs157580 in the TOMM40 gene, was found associated with lower levels of triglyceride (p = 1.2 ×10 −8) and a favorable profile for LDL-cholesterol and total cholesterol (Aulchenko et al., 2009). This was also supported by another GWAS (Sabatti et al., 2009) in which the G allele was associated with lower LDL-cholesterol in a Finnish sample. The effect of the rs439401 C allele to increase triglyceride levels in non-stressed controls in our previous study (Kring et al., 2010) is consistent with higher levels of triglycerides in rs439401 C allele carriers in the GWAS (Aulchenko et al., 2009). These findings led us to examine whether the association between triglyceride levels and rs157580 is also moderated by chronic family related stress. Caregiving for a relative with dementia has been regarded as a significant chronic family stressor, a fact supported by elevated levels of depressive symptomatology observed among caregivers (Schulz et al., 1995; Schulz and Williamson, 1991). We hypothesize that, similar to rs439401, chronic family stress has the potential to moderate the association between rs157580 variants and triglyceride levels. Importantly, we have the opportunity to test this hypothesis in two independent samples - one is a U.S. sample where the chronic family stress is caregiving for a relative with Alzheimer’s disease or other dementia (U.S. sample); and the other is a Danish sample in which the family stressor is operationalized as having a child or relative with long term illness (Iqbal Kring et al., 2011). In addition to our primary focus on triglyceride levels, we also did additional exploratory analyses (results reported in Supplemental Material) assessing associations between rs157580 genotypes and metabolic traits relevant to cardiovascular disease (CVD) in both samples.
In addition to the complexity introduced by the gene-by-stress interaction there is genomic complexity in this region with two SNPs showing similar patterns of association with triglyceride levels. Specifically, moderate linkage disequilibrium (LD) exists between rs439401 and rs157580 (SNP correlation coefficient r2 = 0.33 based on the HAPMAP CEU panel) suggesting that the observed results may not be independent observations. Thus, we further investigated joint effects of rs157580 and rs439401 or their haplotypes in determining triglyceride levels, and how chronic family stress impacts the association between genetic variants and triglyceride levels.
Methods
Study Samples
U.S. Sample: The U.S. participants were enrolled in 2001 to 2004 and detailed study procedures are described elsewhere (Kring et al., 2010). Briefly, family stressed cases, defined as having the primary responsibility for care of a spouse or relative with diagnosed Alzheimer’s disease or other major dementia, were recruited using flyers, advertisements in the local media, and community outreach efforts. Controls were identified by asking cases to nominate two to five friends with similar demographic factors (e.g., gender, age, and race) who lived in their neighborhood. The U.S. study was approved by Duke University Medical Center (DUMC) Institutional Review Board (IRB), and the informed written consent forms were obtained from all subjects in U.S. sample. A questionnaire battery was given to participants during the home visit by a nurse and returned on their visit to the General Clinical Research Center at DUMC. The clinic visit was scheduled during the same week as the in-home visit, and consisted of a general physical examination, and a blood sample was drawn for genotyping and measurement of lipids and other biomarkers. Due to the low numbers of African Americans and to allow comparisons between the two samples only the 242 Caucasians enrolled and with sufficient DNA were included in the present study, consisting of 124 cases and 118 controls.
Danish Sample: The Danish sample was a follow-up survey in 1998-2000 of the initial sample consisted of obese (BMI ≥ 31) cases (n = 1930) and non-obese controls (n=3601), selected from Caucasian men examined at the draft boards in Copenhagen and its surroundings during 1943-1977 (Iqbal Kring et al., 2011). All obese cases and half of the controls, still living the region, were invited to a follow-up survey in 1992-1994 at the mean age of 46 years (S46) and in 1998-2000 at the mean age of 49 years (S49). The criteria for invitation to the follow-up surveys and the participation have been described previously (Black et al., 2005; Kring et al., 2008; Sonne-Holm et al., 1989). In total, 1458 participants were genotyped using the DNA extracted from blood samples at S46. Among these, only 466 (197 obese and 269 non-obese) had been assessed in S49 with triglyceride levels. The Danish Data Protection Agency and Ethical Committees of Copenhagen and Frederiksberg municipalities approved the study, which was in accordance with the Helsinki Declaration II. All participants signed written consent before participating. In total, 466 men with triglyceride levels were assessed in this present study.
Genotyping
The genotyping of rs157580 and rs439401 for the U.S. sample was conducted using ABI 7900 Taqman system (Applied Biosystems, Carlsbad, California, USA) using standard genotyping protocols implemented at the Center of Human Genetics at DUMC. Genotyping of rs157580 and rs439401 for the Danish sample was from genome-wide genotyping results on the Illumina 610k quad chip, which was carried out at the Centre National de Génotypage (CNG), (Evry, France). Genotypes for both assays met quality control standards established in both labs. The genotype distributions were consistent with Hardy-Weinberg equilibrium (P > 0.05) for both samples.
Endophenotypes and Covariates
Fasting serum triglyceride levels were assayed by the Center for Disease Control and Prevention approved laboratory facility at LabCorp in Burlington, North Carolina for the U.S. sample. Serum triglyceride levels in Danish sample were assessed using colorimetric test kits (Roche TG, Roche Diagnostics GmbH, Mannheim, Germany) on a COBAS MIRA Plus (Roche Diagnostic Systems Inc., Mannheim, Germany) and the intra-assay CV was 0.9%. Triglyceride levels were log-transformed to achieve a more normal distribution. Use of medications commonly prescribed to treat diabetes, hypertension or to lower blood lipids was collected by self-report in both samples. Medication use was coded 1 if any of these medications were used, and 0 for none used. The U.S. sample included both men (coded as 1) and women (coded as 2) in contrast to Danish cohort that only included men. Other endophenotypes were also examined in both samples (see Supplementary Material).
Family Stress
In the U.S. sample, family stress was defined by being a caregiver for a relative with Alzheimer’s disease. Cases and controls were based on the sampling design of the study (Schulz and Beach, 1999; Vitaliano et al., 2002; Vitaliano et al., 2003). Compared to controls, cases in the present study have been reported to have significantly more adverse levels of depressive symptoms, hopelessness, anxiety, perceived stress, social support, and sleep quality (Brummett et al., 2006; Kring et al., 2010). Thirty-nine subjects (22 cases and 17 controls) were classified as obese (BMI ≥ 31 kg/m2).
In the Danish sample, family stress was assessed by a self-administered questionnaire verified with participants by trained staff. The two questions were “Do you have long-term illness of children?” and “Do you have long-term illness of family member?”(Iqbal Kring et al., 2011) Participants who responded “yes” to either question were assigned to the stressed group (N=239), and participants who responded “no” to both questions were grouped into the control group (N=227). In the Danish sample, of those who were obese (BMI > 31 kg/m2) 98 were classified as cases (coded as 1 in analyses), and the remaining 99 were classified as controls (coded as 2).
It is important to note that the status of being a caregiver or having a child or relative with long-term illness is an objective indicator of stress in contrast to subjective self-reports of stress. Prior research has shown that G×E interaction findings are more consistently replicated when an objective indicator of stress is used (Jonassaint et al., 2012; Karg et al., 2011; Uher and McGuffin, 2010). Subjective experience data were also available in the U.S. sample and consisted of the Perceived Stress Scale (PSS), the Spielberger Trait Anxiety Scale (STAI-trait), the Center for Epidemiologic Studies Depression Scale (CES-D), feelings of hopelessness (4-item scale), and sleep quality measured using the Pittsburgh Sleep Quality Index (PSQI). The detailed methods for these measures are described elsewhere (Brummett et al., 2006). In addition to our primary analysis of rs157580 × caregiving stress as a predictor of triglyceride levels, we also conducted exploratory analyses of the rs157580 interaction with these measures of the subjective experience of caregiver stress to evaluate the robustness of our findings using the objective caregiver stress measure in the analysis. No such subjective experience data were available in the Danish sample.
Statistical analyses
LD was measured with the SNP correlation coefficient (r2) for rs157580 and rs439401 as implemented in Haploview (Barrett et al., 2005). Linear regression models were used to assess associations of SNP variants / haplotypes, stress condition (case versus control), and the interaction of SNP variants / haplotypes and stress status with triglyceride levels in both U.S. and Danish samples. Analyses were adjusted for age, gender, and the use of medication (yes or no) in the U.S. sample, and for age and medication use in the Danish all male samples. An additive genetic model was used. Regression analyses were performed using SAS 9.3 (SAS Institute Inc., Cary, North Carolina). Haplotype association tests were conducted in HAPSTAT (version 3.0) (Lin and Zeng, 2006; Lin et al., 2005). A global test of the haplotype interaction was performed using a standard likelihood ratio test. A significance level of 0.05 was used for all analyses.
Results
Descriptive Characteristics
Table 1 provides the characteristics of the two samples by cases and controls. Genotype frequencies and minor allele frequencies (MAFs) of SNP rs157580 did not differ significantly between cases and controls across the two samples. The U.S. participants were older than Danish participants. Triglyceride levels were higher in the Danish sample, as compared to the U.S. sample. The U.S. study included more females than males, whereas the Danish sample was comprised of only men. In the U.S. sample, age was significantly higher (p = 0.034) in cases than controls, but the range was similar (24 – 84 years for cases and 22 – 86 for controls). In the Danish sample, individuals with family stressed cases and controls were of the similar age. Triglyceride levels were not different between cases with family stress versus and controls in both U.S. and Danish samples.
Table 1.
Study population characteristics by family stress status.
| U.S. Sample |
Danish Sample |
|||
|---|---|---|---|---|
| Case | Control | Case | Control | |
| Sample size n (%) | 124 (51.2) | 118 (48.8) | 239 (51.3) | 227 (48.7) |
| Rs157580 A/A (%) | 50 (40.3) | 45 (38.1) | 108(45.2) | 100 (44.1) |
| Rs157580 A/G (%) | 58 (46.8) | 59 (50.0) | 96 (40.2) | 97 (42.7) |
| Rs157580 G/G (%) | 16 (12.9) | 14 (11.9) | 35 (14.6) | 30 (13.2) |
| MAFs | 0.36 | 0.37 | 0.35 | 0.35 |
| Age (years)a | 63.2 ± 13.1b | 59.4 ± 14.3b | 48.8 ± 5.7 | 48.7 ± 6.1 |
| Females/males (n) | 89/35 | 88/30 | 0/239 | 0/227 |
| Medication use (n) | 58 | 45 | 12 | 4 |
| TG (mg/dL) | 121.8 ± 69.1 | 126.4 ± 85.4 | 146.0 ± 99.1 | 142.7 ± 110.4 |
MAFs = minor allele frequencies, TG = triglycerides.
Shown as mean ± standard deviation.
Significantly different between caregiver and controls at a level of 0.05.
SNP Association Analyses
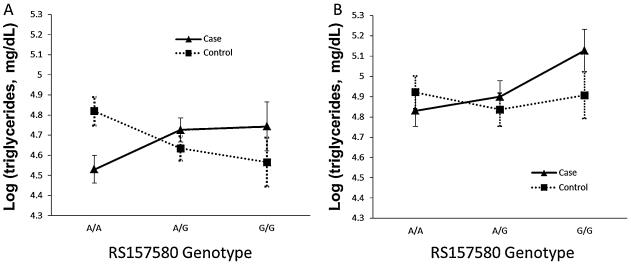
In U.S. participants, linear regression analyses did not show statistically significant main effects for rs157580 or family stress group status, however, the interaction term for rs157580 and stress group was statistically significant (interaction p = 0.004). The mean of the predicted log triglyceride levels by rs157580 genotypes in family stressed cases and controls is shown in Figure 1A. The predicted geometric mean level of triglyceride was 18.7 ± 4.4 mg / dL higher for cases with rs157580 G/G (114.9 ± 2.2 mg / dL) than controls (96.1 ± 2.2 mg / dL) with the same genotype, and 31.2 ± 4.2 mg / dL lower for A/A cases (92.7 ± 2.1 mg / dL) than A/A controls (123.9 ± 2.1mg / dL). In family stressed cases, those with the G/G (114.9 ± 2.2 mg / dL) and A/G genotypes (112.8 ± 2.1 mg / dL) had similar (p = 0.892) triglyceride levels and higher levels (p = 0.110 and 0.029, Cohen’s d = 0.29 and 0.26, respectively) than those with the A/A genotype (92.7 ± 2.1 mg / dL). In contrast, among controls triglyceride levels were similar (p = 0.614) in those with G/G (96.1 ± 2.2 mg / dL) and A/G (102.9 ± 2.1 mg / dL) genotypes, who had lower levels (p = 0.070 and 0.040, Cohen’s d = -0.36 and -0.27, respectively) than controls with the A/A genotype (123.9 ± 2.1 mg / dL). We also performed a conditional analysis by including both rs439401 and rs157580 and their interactions with stress group in the model to test if the statistically significant association between triglyceride levels and rs157580 × stress group was independent of rs439401 × stress group. The results of a conditional test of the interaction terms of both SNPs showed a trend of association with triglyceride levels (p = 0.064 for rs157580 × stress group; and p = 0.082 for rs439401 × stress group).
Figure 1. Predicted log(triglycerides) in relation to rs157580 genotypes by stress group.
Predicted log(triglyceride) with 95% confidence intervals in relation to rs157580 genotypes by stress group (cases vs. controls) adjusted for age, gender (U.S. samples) and medication use with an additive model: A) in U.S. sample, rs157580 × Stress group interaction p = 0.004; and B) in Danish sample, rs157580 × Stress group interaction p = 0.075.
Since there was oversampling of obese men in Danish sample compared to the U.S. sample, in order to investigate the effect of obesity we also analyzed the association of rs157580 × stress group and triglyceride levels adjusting for BMI as an additional covariate in the model, and the interaction term remained significant in the U.S. sample (p = 0.033).
We also conducted analyses using measures of the subjective experience of caregiver stress -- PSS, STAI-Trait, CES-D, feelings of hopelessness and PSQI in place of objective caregiver status. The p values of the interaction of rs157580 with these subjective measures were 0.225 for PSS, 0.025 for STAI-Trait, 0.184 for CES-D, 0.313 for hopelessness, and 0.055 for PSQI, respectively. Only rs157580 × STAI-trait was significant, and rs157580 × PSQI was marginally significant. When the rs157580 × caregiver status interaction was included in these models, however, the rs157580 interactions with these subjective measures were no longer significant (p = 0.239 for STAI-Trait and p = 0.216 for PSQI), while the rs157580 × caregiver status remained significant (ps = 0.001 - 0.04).
In the Danish sample, the main effect terms for rs157580 or stress group were not statistically significant, however, the stress by genotype interaction for triglyceride levels was marginally significant (p = 0.075). Figure 1B shows the mean of predicted log triglyceride levels versus rs157580 genotypes in family stressed cases and controls for Danish samples. The predicted geometric mean level of triglyceride was 33.5 ± 2.2 mg / dL higher for G/G cases (168.7 ± 1.1 mg / dL) than G/G controls (135.2 ± 1.1 mg / dL), and 11.9 ± 2.2 mg / dL lower for A/A cases (125.3 ± 1.1 mg / dL) than A/A controls (137.2 ± 1.1 mg / dL). Cases with the G/G genotype (168.7 ± 1.1 mg / dL) had higher triglyceride levels than those with A/G (134.3 ± 1.1 mg / dL, p = 0.025, Cohen’s d = 0.33) or A/A (125.3 ± 1.1 mg / dL, p = 0.003, Cohen’s d = 0.41) genotypes, while there were no statistically significant or trend differences in triglyceride levels across genotypes among the controls. The results of the conditional test of the interaction terms of both SNPs showed that no interaction of SNP and stress group was associated with triglyceride levels in the Danish sample.
Again we analyzed the association of rs157580 × stress group and triglyceride level adjusting for BMI as an additional covariate in the model, and the interaction term which was previously marginally significant in the Danish samples became statistically significant (p = 0.039). There were no measures of subjective stress available in the Danish sample, eliminating the possibility to conduct parallel analyses in this sample.
Haplotype Association Analysis
The r2 of 0.31 in the U.S. sample and 0.56 in the Danish sample shows rs439401 and rs157580 are in modest LD in our two samples. The estimated frequencies of four haplotypes (AC, AT, GC, and GT) are shown in Table 2. The haplotype frequencies were similar in U.S. and Danish samples. We first tested for haplotype main effect in the model, and, as observed in the single SNP model, no haplotype was statistically significant at a level of 0.05 in both samples. Next we tested the global null hypothesis for the interaction of haplotypes and stress group using a likelihood ratio test and results (df = 3, p = 0.027, in U.S. sample and p = 0.088 in Danish sample, respectively) indicated that at least one of the haplotype by stress group interactions is associated with triglyceride levels in the U.S. sample and a similar trend in the Danish sample. We then examined haplotype (GT, AT and GC; reference haplotype = AC) main effects and their interaction with stress group in the model (Table 2). In both samples, the significant global test for the haplotype interaction appears to arise from the GT haplotype by stress group. The haplotype GT × stress group and haplotype GC × stress group were associated with triglyceride levels in U.S. sample (p = 0.001 for haplotype GT and p = 0.033 for haplotype GC), and the haplotype AT × stress group showed suggestion association with triglyceride levels (p = 0.074). In the Danish sample, haplotype GT × stress group was associated with triglyceride levels (p = 0.011), while haplotype GC × stress group (p = 0.462), or haplotype AT × stress group (p = 0.763) was not statistically significant. The model coefficients are similar in the two datasets. The family stressed cases (stress group = 1) with both minor alleles (haplotype GT) had higher triglyceride levels than controls (stress group = 2) with the same haplotype, indicating the direction of effect of haplotype by stress group was consistent with that of SNPs by stress group.
Table 2.
The estimated haplotype frequency and the results of haplotype association test with log(triglyceride) in both samples (Reference haplotype is AC).
| Variable | U.S. Sample | Danish Sample | ||||||
|---|---|---|---|---|---|---|---|---|
|
|
||||||||
| Frequency | Estimate | SE | P | Frequency | Estimate | SE | P | |
| AC | 0.528 | - | - | - | 0.547 | - | - | - |
| GT | 0.268 | 0.480 | 0.155 | 0.002 | 0.237 | 0.339 | 0.122 | 0.005 |
| AT | 0.106 | 0.371 | 0.264 | 0.160 | 0.107 | 0.017 | 0.187 | 0.926 |
| GC | 0.098 | 0.452 | 0.253 | 0.074 | 0.109 | 0.174 | 0.186 | 0.349 |
| Intercept | - | 3.515 | 0.284 | 0.000 | - | 4.998 | 0.239 | 0.000 |
| Age | - | 0.005 | 0.003 | 0.041 | - | -0.007 | 0.004 | 0.068 |
| Medication | - | 0.236 | 0.064 | 0.000 | - | 0.160 | 0.131 | 0.221 |
| Gender | - | 0.143 | 0.073 | 0.051 | - | - | - | - |
| Stress group | - | 0.363 | 0.102 | 0.000 | - | 0.102 | 0.079 | 0.195 |
| GT×Stress group | - | -0.335 | 0.101 | 0.001 | - | -0.200 | 0.079 | 0.011 |
| AT×Stress group | - | -0.288 | 0.161 | 0.074 | - | -0.037 | 0.122 | 0.763 |
| GC×Stress group | - | -0.337 | 0.158 | 0.033 | - | -0.085 | 0.115 | 0.462 |
SE = Standard error
Discussion
The results of our study show a consistent pattern, in two independent samples, of stress-by-SNP genotype and stress-by-haplotype interactions with triglyceride levels for the TOMM40 SNP rs157580 and the APOE SNP rs439401. In the U.S. sample, the rs157580 G allele was significantly associated with an adverse triglyceride profile among stressed cases compared with A/A cases but was not significantly associated with triglyceride profile among controls. Similarly, in the independent Danish sample, the G allele was associated with increased triglyceride levels among cases with family stress but not in controls. These similar patterns of findings in two independent samples provide strong support for our hypothesis that chronic family stress moderates the association between SNP rs157580 and triglyceride levels (see Supplementary Material for other metabolic traits).
Rs157580 and rs439401 are not in strong LD in our samples based on the r2 values. The conditional test in the U.S. sample showed the interaction of rs157580 × stress group is marginally significantly associated with triglyceride levels independent of rs439401, and the interaction of rs439401 × stress group is also marginally significantly associated with triglyceride levels independent of rs157580. This suggested that rs157580 × stress group association with triglyceride level is not due to the correlation of rs157580 and rs439401, at least in the U.S. sample. The association was further weakened in the Danish sample. This may be explained by r2 = 0.56 in Danish sample that indicated the two SNPs were more correlated in Danish sample compared with r2 = 0.31 in the U.S. sample. Haplotype association tests showed an interaction of haplotype GT × stress group on triglyceride levels in both samples. The haplotype association confirmed the SNP rs157580 × stress group interaction in determining triglyceride levels. This consistency in the direction of effect and in the effect sizes in the model including the stress-by-haplotype interaction is quite remarkable, especially when considering the differences in the two datasets. The association between rs157580 × stress group and triglyceride level was an extension and replication of rs439401 × stress group association with triglyceride level.
We note that status of being a caregiver or having a child or relative with long-term illness is an objective indicator of stress in contrast to subjective self-reports of stress, such as PSS, STAI-Trait, CES-D, feelings of hopelessness, and PSQI. As we reported in the U.S. sample, the interactions of rs157580 with these subjective experience measures were no longer significant, but rs157580 × caregiving status remained significant in relation to triglyceride levels. This further highlights the importance of using an objective indicator of stress like caregiving. It is also likely that the consistency in findings in both samples stems from our use of objective measures of stress.
In the GWAS using a large cohort of 16 European populations, the rs157580 G allele was found associated with lower levels of triglycerides (p = 1.2 ×10 −8) and other lipids (Aulchenko et al., 2009). It is likely that, like controls in the current study, most participants in the large European GWAS were not exposed to a chronic stressor as severe as being the caregiver of a relative with Alzheimer’s disease or an ill child or a family member. Therefore, the pattern of association between rs157580 genotype and triglyceride levels among controls in the current study might be expected to be similar to the pattern in GWAS. The results showed that controls carrying the G allele indeed did have lower triglyceride levels (Figure 1A) compared to those with the A/A genotype in U.S. sample, which is consistent with the results in GWAS.
There are several limitations in our work that need to be considered - with the most obvious being that the Danish sample included only men. Although participants in both samples were Caucasians, there might be more genetic heterogeneity in the U.S. compared to the Danish sample. Despite this difference, which would make it harder to replicate a G×E finding in both studies, we found similar rs157580 × stress effects in both samples, highlighting the robustness of the finding. There was also an oversampling of obese men in Danish sample. Among 466 Danish men, 197 men had BMI ≥ 31.0 kg/m2 compared with 39 participants out of 242 U.S. samples. However, when we analyzed the association of rs157580 × stress and triglyceride level adjusting for BMI as an additional covariate in the model, the interaction terms became significant in both samples. The stressor – having a child or relative with a long term illness – in the Danish sample may have been different from the stress of being the primary caregiver for a relative with Alzheimer’s disease or other major dementia in the U.S. sample. Despite these differences between the two samples, it is remarkable how similar the findings were – particularly in those who were exposed to family stress in both samples, in whom the minor allele haplotype was uniformly associated with higher triglyceride levels (see Supplementary Material for other metabolic traits).
A major implication of the present findings is that, in research aimed at identifying genetic variants involved in the pathogenesis and course of complex diseases, it will be essential to evaluate environmental factors like psychological stress that have the potential to moderate the impact of genetic variants on the development and course of complex diseases. With respect to clinical implications, those carrying the G/G genotypes in the stressed groups in both samples had geometric mean levels of triglyceride that were 20-45 mg/dL higher than those with the A/A genotype – differences that have been shown to be associated, independently of total cholesterol and high density lipoprotein levels, with increased coronary heart disease (CHD) risk (Iso et al., 2001; McBride, 2007). The risk of diseases like type 2 diabetes (T2D) and CHD, for example, that are associated with these metabolic traits could be increased among individuals with rs157580 G allele who experienced chronic stress, but might be reduced for G carriers who are not exposed to chronic stress. Early monitoring or preventative intervention for the CHD risk might be conducted for susceptible G allele individuals with family stress. Training in stress coping skills has been shown to reduce both psychological (depression, anxiety and perceived stress) and physiological (blood pressure) indicators of stress in persons who are caregivers for a relative with Alzheimer’s disease (Williams et al., 2010). Without taking stress exposure into account, the high disease risk among stressed G carriers would be missed, along with the opportunity to reduce their risk using, for example, an approach that begins with training in stress coping skills, diet and exercise, adding pharmacologic treatment if these approaches do not suffice to reduce risk levels.
In conclusion, the current study found in two independent samples that the association of TOMM40 rs157580 genotype with triglyceride levels is moderated by family stress (at a significant level in the U.S. sample and a trend level in the Danish sample). The G allele is associated with an adverse profile in cases with family stress in both samples, while among controls the G allele may be associated with a more positive metabolic profile. Further replication in larger samples will be required to confirm the association of the G allele not only with pre-disease endophenotypes, but also with the development and course of clinical disease related to these endophenotypes in stressed persons. The results could lead to the rs157580 G allele being used to identify individuals at increased risk of developing T2D and/or or CHD when exposed to chronic stress, in whom preventative interventions – behavioral and/or pharmacological -- might then be shown to reduce risk of disease. Further investigation will be required to identify the underlying biological mechanism(s) for the observed interaction between SNP and family stress status on triglyceride levels.
Supplementary Material
Highlights.
The interaction of rs157580 genotype and chronic family stress as a predictor of triglyceride levels was investigated in two independent Caucasian samples.
Chronic family stress moderates the association between rs157580 genotype and triglyceride levels.
G allele of rs157580 was associated with increased triglyceride levels among cases with family stress in both samples compared with A/A caregivers, but not among controls.
Chronic family stress should be taken into account for disease risk assessment and intervention.
Acknowledgments
Funding Sources: This study was supported by NHLBI grant P01-HL36587, the Behavioral Medicine Research Center, Duke University Medical Center, Durham, NC, USA, and Center for Pharmacogenomics at the University of Copenhagen, Denmark.
Footnotes
Disclosure: Redford Williams is a founder and major stockholder in Williams LifeSkills, Inc., a company that develops, tests and markets behavioral products for stress and anger management.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Aulchenko YS, Ripatti S, Lindqvist I, Boomsma D, Heid IM, Pramstaller PP, Penninx BW, Janssens AC, Wilson JF, Spector T, Martin NG, Pedersen NL, Kyvik KO, Kaprio J, Hofman A, Freimer NB, Jarvelin MR, Gyllensten U, Campbell H, Rudan I, Johansson A, Marroni F, Hayward C, Vitart V, Jonasson I, Pattaro C, Wright A, Hastie N, Pichler I, Hicks AA, Falchi M, Willemsen G, Hottenga JJ, de Geus EJ, Montgomery GW, Whitfield J, Magnusson P, Saharinen J, Perola M, Silander K, Isaacs A, Sijbrands EJ, Uitterlinden AG, Witteman JC, Oostra BA, Elliott P, Ruokonen A, Sabatti C, Gieger C, Meitinger T, Kronenberg F, Doring A, Wichmann HE, Smit JH, McCarthy MI, van Duijn CM, Peltonen L. Loci influencing lipid levels and coronary heart disease risk in 16 European population cohorts. Nat Genet. 2009;41:47–55. doi: 10.1038/ng.269. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barrett JC, Fry B, Maller J, Daly MJ. Haploview: analysis and visualization of LD and haplotype maps. Bioinformatics. 2005;21:263–265. doi: 10.1093/bioinformatics/bth457. [DOI] [PubMed] [Google Scholar]
- Black E, Holst C, Astrup A, Toubro S, Echwald S, Pedersen O, Sorensen TI. Long-term influences of body-weight changes, independent of the attained weight, on risk of impaired glucose tolerance and Type 2 diabetes. Diabet Med. 2005;22:1199–1205. doi: 10.1111/j.1464-5491.2005.01615.x. [DOI] [PubMed] [Google Scholar]
- Brummett BH, Babyak MA, Siegler IC, Vitaliano PP, Ballard EL, Gwyther LP, Williams RB. Associations among perceptions of social support, negative affect, and quality of sleep in caregivers and noncaregivers. Health Psychol. 2006;25:220–225. doi: 10.1037/0278-6133.25.2.220. [DOI] [PubMed] [Google Scholar]
- Iqbal Kring SI, Barefoot J, Brummett BH, Boyle SH, Siegler IC, Toubro S, Hansen T, Astrup A, Pedersen O, Williams RB, Sorensen TI. Associations between APOE variants and metabolic traits and the impact of psychological stress. PLoS One. 2011;6:e15745. doi: 10.1371/journal.pone.0015745. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Iso H, Naito Y, Sato S, Kitamura A, Okamura T, Sankai T, Shimamoto T, Iida M, Komachi Y. Serum triglycerides and risk of coronary heart disease among Japanese men and women. American journal of epidemiology. 2001;153:490–499. doi: 10.1093/aje/153.5.490. [DOI] [PubMed] [Google Scholar]
- Jonassaint CR, Ashley-Koch A, Whitfield KE, Hoyle RH, Richman LS, Siegler IC, Royal CD, Williams R. The serotonin transporter gene polymorphism (5HTTLPR) moderates the effect of adolescent environmental conditions on self-esteem in young adulthood: a structural equation modeling approach. Biological psychology. 2012;91:111–119. doi: 10.1016/j.biopsycho.2012.05.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Karg K, Burmeister M, Shedden K, Sen S. The serotonin transporter promoter variant (5-HTTLPR), stress, and depression meta-analysis revisited: evidence of genetic moderation. Archives of general psychiatry. 2011;68:444–454. doi: 10.1001/archgenpsychiatry.2010.189. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ken-Dror G, Talmud PJ, Humphries SE, Drenos F. APOE/C1/C4/C2 gene cluster genotypes, haplotypes and lipid levels in prospective coronary heart disease risk among UK healthy men. Mol Med. 2010;16:389–399. doi: 10.2119/molmed.2010.00044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kring SI, Brummett BH, Barefoot J, Garrett ME, Ashley-Koch AE, Boyle SH, Siegler IC, Sorensen TI, Williams RB. Impact of psychological stress on the associations between apolipoprotein E variants and metabolic traits: findings in an American sample of caregivers and controls. Psychosomatic medicine. 2010;72:427–433. doi: 10.1097/PSY.0b013e3181de30ad. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kring SI, Larsen LH, Holst C, Toubro S, Hansen T, Astrup A, Pedersen O, Sorensen TI. Genotype-phenotype associations in obesity dependent on definition of the obesity phenotype. Obes Facts. 2008;1:138–145. doi: 10.1159/000137665. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lin DY, Zeng D. Likelihood-based inference on haplotype effects in genetic association studies. J Am Stat Assoc. 2006;101:89–104. [Google Scholar]
- Lin DY, Zeng D, Millikan R. Maximum likelihood estimation of haplotype effects and haplotype-environment interactions in association studies. Genet Epidemiol. 2005;29:299–312. doi: 10.1002/gepi.20098. [DOI] [PubMed] [Google Scholar]
- McBride PE. Triglycerides and risk for coronary heart disease. JAMA : the journal of the American Medical Association. 2007;298:336–338. doi: 10.1001/jama.298.3.336. [DOI] [PubMed] [Google Scholar]
- Sabatti C, Service SK, Hartikainen AL, Pouta A, Ripatti S, Brodsky J, Jones CG, Zaitlen NA, Varilo T, Kaakinen M, Sovio U, Ruokonen A, Laitinen J, Jakkula E, Coin L, Hoggart C, Collins A, Turunen H, Gabriel S, Elliot P, McCarthy MI, Daly MJ, Jarvelin MR, Freimer NB, Peltonen L. Genome-wide association analysis of metabolic traits in a birth cohort from a founder population. Nat Genet. 2009;41:35–46. doi: 10.1038/ng.271. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schulz R, Beach SR. Caregiving as a risk factor for mortality: the Caregiver Health Effects Study. JAMA : the journal of the American Medical Association. 1999;282:2215–2219. doi: 10.1001/jama.282.23.2215. [DOI] [PubMed] [Google Scholar]
- Schulz R, O’Brien AT, Bookwala J, Fleissner K. Psychiatric and physical morbidity effects of dementia caregiving: prevalence, correlates, and causes. The Gerontologist. 1995;35:771–791. doi: 10.1093/geront/35.6.771. [DOI] [PubMed] [Google Scholar]
- Schulz R, Williamson GM. A 2-year longitudinal study of depression among Alzheimer’s caregivers. Psychology and aging. 1991;6:569–578. doi: 10.1037//0882-7974.6.4.569. [DOI] [PubMed] [Google Scholar]
- Sonne-Holm S, Sorensen TI, Jensen G, Schnohr P. Independent effects of weight change and attained body weight on prevalence of arterial hypertension in obese and non-obese men. BMJ. 1989;299:767–770. doi: 10.1136/bmj.299.6702.767. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Talmud PJ, Drenos F, Shah S, Shah T, Palmen J, Verzilli C, Gaunt TR, Pallas J, Lovering R, Li K, Casas JP, Sofat R, Kumari M, Rodriguez S, Johnson T, Newhouse SJ, Dominiczak A, Samani NJ, Caulfield M, Sever P, Stanton A, Shields DC, Padmanabhan S, Melander O, Hastie C, Delles C, Ebrahim S, Marmot MG, Smith GD, Lawlor DA, Munroe PB, Day IN, Kivimaki M, Whittaker J, Humphries SE, Hingorani AD. Gene-centric association signals for lipids and apolipoproteins identified via the HumanCVD BeadChip. Am J Hum Genet. 2009;85:628–642. doi: 10.1016/j.ajhg.2009.10.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Uher R, McGuffin P. The moderation by the serotonin transporter gene of environmental adversity in the etiology of depression: 2009 update. Molecular psychiatry. 2010;15:18–22. doi: 10.1038/mp.2009.123. [DOI] [PubMed] [Google Scholar]
- Vitaliano PP, Scanlan JM, Zhang J, Savage MV, Hirsch IB, Siegler IC. A path model of chronic stress, the metabolic syndrome, and coronary heart disease. Psychosomatic medicine. 2002;64:418–435. doi: 10.1097/00006842-200205000-00006. [DOI] [PubMed] [Google Scholar]
- Vitaliano PP, Zhang J, Scanlan JM. Is caregiving hazardous to one’s physical health? A meta-analysis. Psychological bulletin. 2003;129:946–972. doi: 10.1037/0033-2909.129.6.946. [DOI] [PubMed] [Google Scholar]
- Williams VP, Bishop-Fitzpatrick L, Lane JD, Gwyther LP, Ballard EL, Vendittelli AP, Hutchins TC, Williams RB. Video-based coping skills to reduce health risk and improve psychological and physical well-being in Alzheimer’s disease family caregivers. Psychosomatic medicine. 2010;72:897–904. doi: 10.1097/PSY.0b013e3181fc2d09. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.