Abstract
Objective
Severe respiratory failure is a well recognized complication of pandemic H1N1 influenza infection. Limited data regarding the efficacy of rescue therapies including high frequency oscillatory ventilation (HFOV) and extracorporeal membranous oxygenation (ECMO) have been previously reported in the setting of H1N1 influenza infection in the United States.
Design
Retrospective, single center cohort study.
Setting
Pediatric, cardiac, surgical, and medical intensive care units in a single tertiary care center in the United States.
Patients
127 consecutive patients with confirmed Influenza A infection requiring hospitalization between April 1, 2009 and October 31, 2009.
Interventions
Electronic medical records were reviewed for demographic and clinical data.
Measurements and main results
The number of ICU admissions appears inversely related to age with 69% of admissions less than 20 years of age. Median duration of ICU care was 10.0 days [4.0, 24.0], and median duration of mechanical ventilation was 8.0 days [0.0, 23.5]. Rescue therapy (HFOV or ECMO) was utilized in 36% (12/33) of ICU patients. The severity of respiratory impairment was determined by PaO2/FiO2 ratio (P/F) and oxygenation index (OI). HFOV at 24-hours resulted in improvements in median P/F (71 [58, 93] vs. 145 [126, 185]; P<0.001), OI (27 [20, 30] vs. 18 [12, 25]; P=0.016), and FiO2 (100 [70, 100] vs. 45 [40, 55]; P<0.001). ECMO resulted in anticipated improvement in parameters of oxygenation at both 2- hours and 24-hours after initiation of therapy. Despite the severity of oxygenation impairment, overall survival for both rescue therapies was 75% (9/12), 80% (4/5) for HFOV alone, and 71% (5/7) for HFOV + ECMO.
Conclusion
In critically ill adult and pediatric patients with H1N1 infection and severe lung injury, the utilization of HFOV and ECMO can result in significant improvements in P/F ratio, OI, and FiO2. However, the impact on mortality is less certain.
Keywords: influenza, acute respiratory distress syndrome, H1N1, mechanical ventilation, high frequency oscillatory ventilation, extracorporeal membrane oxygenation (ECMO), hypoxemia, respiratory failure, lung injury
INTRODUCTION
The novel H1N1 influenza A virus was first noted in the North America in March, 2009, and by July, 2009, 43,677 cases were reported in the United States [1]. WHO data indicate an estimate of 16,713 deaths worldwide [2]. Estimates suggest that 7–31% of patients with H1N1 requiring hospitalization require care in an intensive care unit (ICU) [3–6]. Mortality in patients infected with H1N1 who require ICU care has been estimated to be 7–41% [3–6]. Previous studies have identified several independent risk factors for severe disease requiring ICU admission including: underlying lung disease [7–10], obesity [7, 10, 11], pregnancy [8] and race [7, 10].
Management guidelines for acute respiratory distress syndrome (ARDS) associated with H1N1 have been focused on lung protective ventilation and are not necessarily specific to the H1N1 patient population. The Australian and New Zealand Intensive Care Influenza Investigators (ANZIC) reported their experience with extracorporeal membranous oxygenation (ECMO) as rescue therapy in H1N1 patients with severe ARDS [11]. Survival with the utilization of ECMO in the ANZIC population was 79%. Regional differences in utilization of ECMO likely exist and, to date, reports of the efficacy of ECMO in North America during the H1N1 epidemic are very limited. In one publication from Canada, the ECMO survival rate for refractory hypoxemia for H1N1 influenza infection was 67%. However, this report described only six patients across four centers [12]. Similar reports of limited patient populations, including early data from the University of Michigan in July, 2009 [13] demonstrated mortality of 30% for ten patients who required surgical ICU admission for consideration of ECMO. Additionally, it remains unclear whether other rescue therapies, including high frequency oscillatory ventilation (HFOV), offer significant benefit. Therefore, we report our experience with ECMO and HFOV in the treatment of adults and children with H1N1 influenza infection.
PATIENTS AND METHODS
Study Design
This was a single center retrospective study of infants, children, and adults conducted at a tertiary care center in the United States. The study was reviewed and approved by the Duke University Institutional Review Board with waiver of informed consent.
Patient Selection and Data Collection
All patients admitted to Duke University Medical Center between April 1, 2009 and October 31, 2009 with either confirmed or probable H1N1 were included in the analysis. Confirmed cases include patients with influenza A with confirmation of H1N1 genotype by polymerase chain reaction performed at the North Carolina State Reference Laboratory. Probable case of H1N1 was defined as confirmed influenza A not further sub-typed. However, > 99% of tested influenza A isolates in North Carolina during the period of enrollment were H1N1.
We identified 127 pediatric (0–17 yrs) and adult (18–70 yrs) patients with confirmed or probable H1N1. APACHE II score was calculated using the worst values for the 24-hour period after initial admission to an intensive care unit. For patients who required vasopressor support, the mean arterial pressure was defined to be less than or equal to 40 mmHg. Patients who had previously been awake but who required heavy sedation or paralysis for mechanical ventilation were judged to have a Glasgow Coma Score of 10. Mean airway pressure, FiO2, and PaO2 were additionally documented at the time of initiation of ECMO or HFOV.
Criteria for HFOV included a plateau pressure greater than or equal to 30 cm H2O and FiO2 greater than or equal to 0.50 on conventional ventilation in the setting of bilateral infiltrates consistent with ARDS and/or the presence of significant air leak. Patients were considered candidates for ECMO when their OI during HFOV exceeded 40 for greater than 2–4 hours for the adult patients and 4–6 hours for the pediatric patients. Contraindications for ECMO included documented irreversible brain injury or multi-system organ failure, underlying malignancy with limited life expectancy and/or unresponsive septic shock (the latter being a relative contraindication only). There is no policy regarding specific clinical contraindications to HFOV at our facility. After initiation of HFOV, all persons entering the patient room utilized N-95 or surgical masks. We are not aware of any transmission of H1N1 in our hospital with this protocol.
Some patients received multiple modalities of rescue therapy during their hospitalization. Data from each initiation of rescue modality were included in the analysis. If a patient did not have data available at the specific time point, data were collected closest to the goal time point within the following parameters: 2-hours before (1–6 hour), 2-hours after (1–6 hour), and 24-hours after (12–36 hour). If multiple values existed, the value closest to the goal time point was reported. All ECMO patients were cannulated as venovenous (VV) with exception of one pediatric patient (child with underlying congenital heart disease) who received venoarterial (VA) ECMO.
Statistical Analysis
Descriptive statistics are presented as median [IQR]. Significant differences between groups were identified using the Wilcoxon rank-sum test using SPSS (Chicago, IL) and GraphPad (San Diego, CA) software. A two-tailed P value of less than 0.05 was considered statistically significant.
RESULTS
Characteristics of H1N1 (confirmed or probable) patients requiring ICU care
We identified 127 patients hospitalized with H1N1 infection between April 1, 2009 and October 31, 2009. 46% (58/127) of the patients admitted to the hospital with H1N1 were under the age of 18. ICU care was required in 26% (33/127). During the enrollment period, 33 patients with H1N1 infection required ICU care with a peak in ICU admissions and mortality during the month of September (Figure 1). There was a relatively high prevalence of African-American patients who required ICU care [57% (19/33) as compared to a 38% African-American distribution in the local community] (Table 1). The majority of the patients who required ICU care had significant co-morbid medical conditions [94% (31/33)]. There was an inverse relationship between decade of life and number of patients admitted to the ICU (Figure 2). 39% (13/33) of patients admitted to the ICU were < 20 years of age. The median APACHE II score for all patients with influenza A and/or confirmed H1N1 infection admitted to the ICU was 16.0 [13.0, 24.0]. The duration of care for subjects in our cohort include hospitalization 18.0 days [7.3, 30.5], ICU care 10.0 days [4.0, 24.0], and mechanical ventilation 8.0 days [0, 23.5].
Figure 1. Epidemic curve in patients with confirmed or probable H1N1 admitted to the hospital, ICU, or who died during the enrollment period.
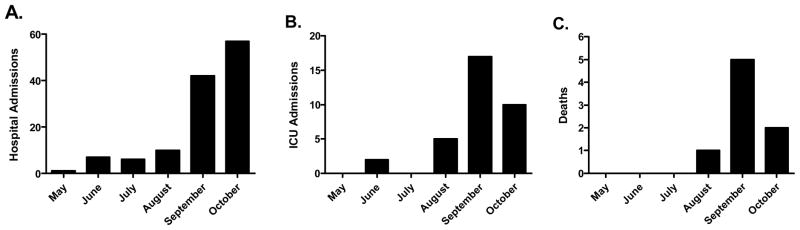
Patients at our institution with H1N1 had an increase in hospital admissions (A), ICU admissions (B), and death (C) in September 2009.
Table 1.
Characteristics of 33 patients admitted to intensive care between April 1, 2009 and October 31, 2009 with the diagnosis of Influenza A, including those with confirmed H1N1.
| Characteristic | Number of patients (%) | |
|---|---|---|
| Age | 0–17 | 12/33 (36%) |
| 18–40 | 11/33 (33%) | |
| 41–64 | 8/33 (24%) | |
| 65+ | 2/33 (6%) | |
| Gender | Female | 16/33 (48%) |
| Male | 17/33 (52%) | |
| Ethnicity | Black/African-American | 19/33 (57%) |
| White/Caucasian | 10/33 (30%) | |
| Hispanic | 1/33 (3%) | |
| Other/Not Reported | 3/33 (9%) | |
| APACHE II Score | 0–9 | 3/33 (9%) |
| 10–14 | 8/33 (24%) | |
| 15–19 | 12/33 (36%) | |
| 20–24 | 5/33 (15%) | |
| 24–29 | 2/33 (6%) | |
| 30+ | 3/33 (9%) | |
| Co-Morbidities | Autoimmune disease | 4/33 (12%) |
| Cardiovascular Disease | 6/33 (18%) | |
| Diabetes | 6/33 (18%) | |
| Hypertension | 5/33 (15%) | |
| Obstructive Lung Disease | 13/33 (39%) | |
| Pregnancy | 2/33 (6%) | |
| Malignancy | 5/33 (15%) | |
| Chronic Renal Insufficiency/ESRD | 2/33 (6%) | |
| Seizure Disorder | 4/33 (12%) |
Figure 2. Age distribution of critically ill patients with confirmed of probable H1N1.
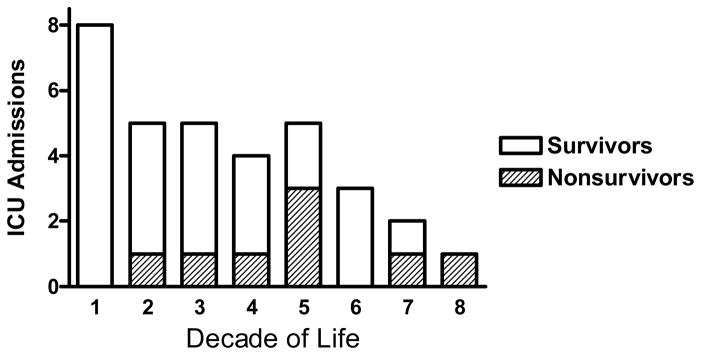
The number of ICU admissions in subjects with H1N1 are reported for each decade of life. There is an inverse relationship between decade of life and number of ICU admissions.
Patients with H1N1 on ECMO required significantly more blood products when compared to other ICU patients with H1N1. During the enrollment period, patients on ECMO received more units of packed red blood cells with a median of 21 units [16, 29] versus 0 units [0, 3] (p<0.05), more fresh frozen plasma 3 [3, 7] versus 0 [0, 0] (P<0.001), and more platelets 13 [3, 39] versus 0 [0, 0] (P<0.001). One patient had active pulmonary hemorrhage requiring multiple transfusions of red blood cells. The remainder received transfusions as needed to maintain a goal hemoglobin of 10 g/dL and a platelet count greater than 75,000 as per center protocol while on ECMO.
H1N1 patients with ARDS and rescue therapy
All patients requiring mechanical ventilation were ventilated with a low tidal-volumes strategy as per the ARDS Network recommendations [13]. In terms of other adjunct therapies, 58% (19/33) of all patients who required ICU care received steroids; while 27% (9/33) of the study population received inhaled nitric oxide (iNO). In patients who were eventually cannulated for ECMO, 86% (6/7) received steroids and/or inhaled nitric oxide; while 40% of the patients who received only HFOV as rescue therapy received steroids and/or iNO. 43% (3/7) of patients on ECMO developed acute renal failure and required hemodialysis. Hemoconcentration was not otherwise utilized in management of these patients. Recruitment maneuvers and prone positioning were not utilized for patients on conventional ventilation, although recruitment maneuvers were performed via a standardized protocol for patients requiring HFOV.
Rescue therapy (defined as HFOV or ECMO) was utilized in 36% (12/33) of the ICU patients in our study population. Patients receiving rescue therapy were evenly distributed between pediatrics and adult populations. For all patients receiving HFOV, there were 5 pediatric (0–17 yrs) and 7 adult (18–51 yrs) patients. For the subset of patients on HFOV whom eventually required ECMO, there were 3 pediatric (0–17) and 4 adult (18–33 yrs) patients. Patients in our institution who received rescue therapy had a median APACHE II of 17.5 [13.5, 22.3] compared to 15.0 [12.0, 20.5] in ICU patients not requiring rescue therapy (P=0.43). 21% (7/33) of ICU patients received ECMO as a mode of rescue treatment. Three patients (25%) received HFOV more than once during the course of their hospital course, and all patients who were placed on ECMO received HFOV. 58% (7/12) of patients requiring HFOV support ultimately required ECMO. HFOV at 2-hours did not result in objective clinical improvement as measured by P/F, OI, or FiO2 (Figure 3). However, HFOV at 24-hours resulted in improvements in median P/F (71 [58, 93] vs. 145 [126, 185]; P<0.001), OI (27 [20, 30] vs. 18 [12, 25]; P=0.016), and FiO2 (100 [70, 100] vs. 45 [40, 55]; P<0.001).
Figure 3. HFOV and impact on oxygenation impairment.
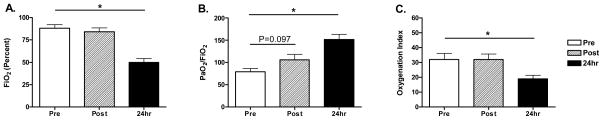
Patients receiving HFOV required high FiO2 (A) with low P/F (B) and high OI (C). Response to HFOV was reported at both 2-hours and 24-hours after induction of HFOV (N=12, data presented as mean +/−SEM, *P<0.05).
As predicted by the nature of the therapy, ECMO at 2-hours resulted in improvements in OI (55 [36, 62] vs. 14 [10, 28]; P=0.018) and FiO2 (100 [80, 100] vs. 55 [40, 80]; P=0.031), and P/F (58 [41, 83] vs. 100 [91, 223]; P=0.035) (Figure 4). ECMO at 24-hours maintained improvement in OI (55 [36, 62] vs. 11 [9, 14]; P=0.009), FiO2 (100 [80, 100] vs. 40 [30, 40]; P=0.015), and we observed improvement in P/F (58 [41, 83] vs. 182 [135, 217]; P=0.025).
Figure 4. ECMO and impact on oxygenation impairment.
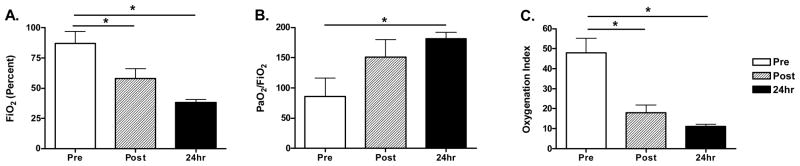
Patients receiving ECMO required high FiO2 (A) with low P/F (B) and high OI (C). Response to ECMO was reported at both 2-hours and 24-hours after induction of ECMO (N=7, data presented as mean +/−SEM, *P<0.05).
The median duration of rescue therapy was 4.5 days [2.0, 17] for HFOV and 10.0 days [8.0, 28.0] for ECMO. The seven patients who were placed on ECMO underwent HFOV for a median duration of 6 days (range 0–15 days) prior to cannulation. While on ECMO, all patients were transitioned to conventional ventilation as tolerated using lung-protective strategies including low tidal volume ventilation (6 ml/kg). Patients receiving rescue therapy had a median length of hospital stay of 35.0 days [22.0, 39.0] while ICU patients who received standard supportive care remained in the hospital a median of 10.0 days [5.0, 22.0], (P=0.001). The median length of ICU stay for patients receiving rescue therapy was 24.0 days [14.0, 38.0] compared to 5.0 days [3.0, 9.0] for ICU patients receiving standard supportive care (P<0.001).
Mortality associated with H1N1
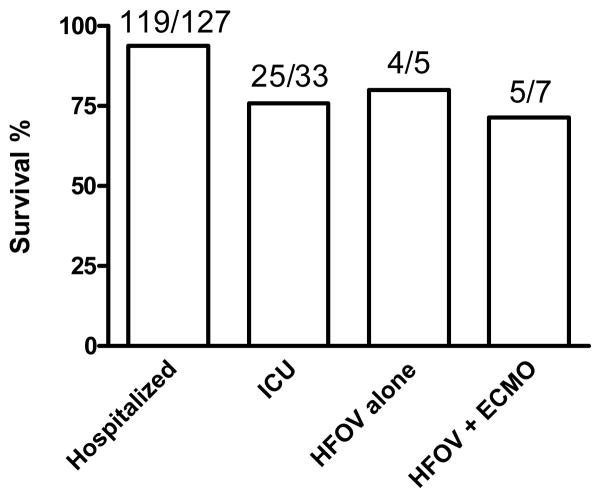
The overall survival for patients hospitalized with confirmed or probable H1N1 was 93.7% (119/127) (Figure 5). The median APACHE II score for all patients receiving ICU care was 16 [13, 20] with an overall survival of 75% (25/33) during the study period. The overall survival for ICU patients who did and did not require rescue therapy was 75% (9/12) and 76% (16/21), respectively. Patients receiving ECMO had a median APACHE II of 19.0 [13.0, 23.0] with an overall survival of 71.4% (5/7) [Table 2]. In addition, all patients receiving rescue therapy had APACHE II scores between 10 and 24 with three patients having an APACHE II between 20 and 24, all of whom received ECMO.
Figure 5. Survival in patients with confirmed or presumed H1N1.
Overall percent survival during the enrollment period for patients receiving either hospitalization, ICU care, HFOV alone, or HFOV + ECMO.
Table 2.
APACHE II scores of patients receiving rescue therapy versus standard care.
| APACHE II Score | Standard Care (n = 21) | HFOV (n = 12) | ECMO (n = 7) |
|---|---|---|---|
| 0–9 | 3 | 0 | 0 |
| 10–14 | 5 | 3 | 2 |
| 15–19 | 7 | 5 | 2 |
| 20–24 | 2 | 3 | 3 |
| 25–29 | 2 | 0 | 0 |
| 30+ | 2 | 1 | 0 |
All deaths occurred in the setting of withdrawal of care. Of the three patients who received rescue therapy and died, withdrawal occurred in the setting of: (1) massive pulmonary hemorrhage as a result of gram-negative septic shock which prompted the need for ECMO; (2) refractory hypoxemia and inability to be decannulated after a 38-day ECMO course; and (3) refractory hypoxemia on HFOV complicated by underlying hematologic malignancy and Tamiflu-resistant H1N1. Of the eight patients who died during the study period, two had an APACHE II of 12 or 13 (including both patients on ECMO), three between 17 and 21, and the remaining three 25 or greater. Two of the patients who died and were not offered rescue therapy had active malignancy, one had a CVA which was diagnosed during admission and the other two had multi-organ system failure and families who stated they would not want aggressive care.
In addition to severity of lung injury, overall mortality in this cohort was associated with presence of co-morbidities and drug resistant H1N1 genotype. The H275Y mutation, which has been associated with putative resistance to oseltamivir, was found in 12% (4/33) of the ICU patients’ H1N1 isolates. Three (75%) individuals infected with this variant of H1N1 did not survive to hospital discharge. The fourth patient with the H275Y mutation was discharged to hospice care and subsequently died at home.
DISCUSSION
H1N1 influenza infection required significant critical care resources, especially in a small subset of patients with refractory ARDS. Despite lengthy support with HFOV and/or ECMO, overall survival was quite good (Figure 5) given the extreme severity of respiratory illness. This review of our adult and pediatric data lends support to the use of these rescue approaches in patients with severe H1N1 respiratory failure. This is the first such comprehensive report in the United States, and our ECMO survival data approximate that published from the Australia/New Zealand and Canadian experiences.
Critical care resources were required in 26% (33/127) of hospitalized patients with H1N1 influenza infection (confirmed or probable). The majority of patients requiring care in an ICU setting had significant co-morbid medical conditions previously associated with severe H1N1 infection. H1N1 infection requiring any ICU care was associated with a prolonged duration of hospitalization and mechanical ventilator support. Rescue therapy was utilized in 36% (12/33) of these ICU patients with respiratory failure.
Although HFOV has been used in pediatric populations since the 1980’s, studies of HFOV use in ARDS have failed to demonstrate statistically significant benefit over conventional ventilation to-date [15,16]. Results from the Canadian trial evaluating HFOV in ARDS, although not yet available, may provide more insight. Recent literature regarding management of ARDS in H1N1 notes that consideration should be given to the use of HFOV for refractory hypoxemia when available [17]. At our center, where HFOV is utilized routinely in both the adult and pediatric populations, the implementation of HFOV resulted in significant improvements in P/F, OI, and FiO2. Overall survival with the utilization of HFOV alone was 80% (4/5) and with HFOV followed by ECMO was 71% (5/7). These findings echo the results of the recent CESAR publication in terms of the success of ECMO for adult refractory hypoxemia [18].
Recent experience in Utah [7] demonstrated overall survival of 83% of patients requiring ICU care (73% in adult patients with ARDS), none of whom received HFOV or ECMO. Although it is not indicated whether such modalities were available at the centers involved, it is noted that all 8 deaths occurred in those patients with ARDS with mention of the fact that approximately 50% (14/30) of ARDS patients had significant hypoxemia requiring substantial ventilator support (positive-end expiratory pressure greater than or equal to 20 cm H2O with FiO2 1.0). As additional information regarding mortality within this group is not available, it is unclear whether rescue therapies, had they been available, might have altered outcome.
The majority of the patients who required ICU care had APACHE II scores between 10 and 24, including all those who received rescue therapy (Table 2). Of the patients who died during the study period, only two qualified for and went on to receive ECMO; the remaining six, including one who received HFOV, had contraindications to ECMO. Interestingly, both patients who died despite ECMO therapy had the lowest APACHE II scores of their cohort (12 and 13 respectively). Neither of these patients had the H275Y mutation and one death was associated with an overwhelming secondary bacterial infection.
Conclusions from this study should be interpreted with some caution. This is a retrospective analysis of a single academic center with a high level of experience utilizing HFOV and ECMO. Additionally, while our data clearly demonstrate benefits in oxygenation with rescue therapy, it remains unclear whether these rescue modalities actually impacted survival, although we speculate that they in fact did. Finally, rescue modalities of therapy were associated with a prolonged duration of hospitalization and ICU care and very significant resource utilization in terms of equipment, bed space, and staffing. The ethical consideration of these rescue therapies in the face of the limited resources of a pandemic are worthy of discussion but are beyond the scope of this report.
CONCLUSIONS
In critically ill patients with confirmed or probable H1N1 and severe ARDS, the utilization of HFOV and ECMO can result in significant improvements in oxygenation and may improve mortality in this very high risk population. Thus, we feel that it is reasonable to consider these rescue modalities in refractory ARDS related to H1N1 influenza infection in both pediatric and adult patients. The potential role of rescue modalities for severe H1N should be critically examined in a randomized, controlled trial.
Acknowledgments
Financial Support: Support was provided by the National Institutes of Health (ES016126, HL007538).
We greatly appreciate the insightful comments and critical statistical review provided by Brian Smith, MD, MPH, MHS. We additionally appreciate the tremendous care that was provided to our critically ill patients infected with H1N1 by the physicians, nurses, respiratory therapists, and other members of our clinical care teams.
References
- 1.Reed C, Angulo FJ, Swerdlow DL, Lipsitch M, Meltzer MI, Jernigan D, et al. Estimates of the prevalence of pandemic (H1N1) 2009, United States, April–July 2009. Emerg Infect Dis [serial on the Internet] 2009 Dec; doi: 10.3201/eid1512.091413. [Epub ahead of print] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Pandemic (H1N1) 2009 – update 91. http://www.who.int/csr/don/2010_3_12/en/print.html.
- 3.Perez-Padilla R, de la Rosa-Zamboni D, Ponce de Leon S, et al. Pneumonia and Respiratory Failure from Swine-Origin Influenza A (H1N1) in Mexico. N Engl J Med. 2009;361:680–689. doi: 10.1056/NEJMoa0904252. [DOI] [PubMed] [Google Scholar]
- 4.Jain S, Kamimoto L, Bramley AM, et al. Hospitalized Patients with 2009 H1N1 Influenza in the United States, April-June 2009. N Engl J Med. 2009;361:1935–1944. doi: 10.1056/NEJMoa0906695. [DOI] [PubMed] [Google Scholar]
- 5.Louie JK, Acosta M, Winter K, et al. Factors Associated With Death or Hospitalization Due to Pandemic 2009 Influenza A (H1N1) Infection in California. JAMA. 2009;302(17):1896–1902. doi: 10.1001/jama.2009.1583. [DOI] [PubMed] [Google Scholar]
- 6.Domìnguez-Cherit G, Lapinsky SE, Macias AE, et al. Critically Ill Patients With 2009 Influenza A (H1N1) in Mexico. JAMA. 2009;302(17):1880–1887. doi: 10.1001/jama.2009.1536. [DOI] [PubMed] [Google Scholar]
- 7.Miller RR, Markewitz BA, Rolfs RT, et al. Clinical Findings and Demographic Factors Associated With ICU Admission in Utah Due to Novel 2009 Influenza A(H1N1) Infection. Chest. 2010;137:752–758. doi: 10.1378/chest.09-2517. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Kumar A, Zarychanksi R, Pinto R, et al. Critically Ill Patients With 2009 Influenza A (H1N1) Infection in Canada. JAMA. 2009;302(17):1872–1879. doi: 10.1001/jama.2009.1496. [DOI] [PubMed] [Google Scholar]
- 9.Rello J, Rodriguez A, Ibañez P, et al. Intensive care adult patients with severe respiratory failure caused by Influenza A (H1N1) v in Spain. Critical Care. 2009;13:R148. doi: 10.1186/cc8044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.The ANZIC Investigators. Critical Care Services and 2009 H1N1 Influenza in Australia and New Zealand. N Engl J Med. 2009;361:1925–1934. doi: 10.1056/NEJMoa0908481. [DOI] [PubMed] [Google Scholar]
- 11.The Australia and New Zealand Extracorporeal Membrane Oxygenation (ANZ ECMO) Influenza Investigators. Extracorporeal Membrane Oxygenation for 2009 Influenza A (H1N1) Acute Respiratory Distress Syndrome. JAMA. 2009;302(17):1888–1895. doi: 10.1001/jama.2009.1535. [DOI] [PubMed] [Google Scholar]
- 12.Freed DH, Henzler D, White CW, et al. Extracorporeal lung support for patients who had severe respiratory failure secondary to influenza A (H1N1) 2009 infection in Canada. Can J Anesth. 2010 doi: 10.1007/s12630-009-9253-0. ePub ahead of print. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.CDC. Intensive-Care Patients with Severe Novel Influenza A (H1N1) Virus Infection – Michigan, June 2009. MMWR Morb Mortal Wkly Rep. 2009 Jul 17;58(27):749–52. [PubMed] [Google Scholar]
- 14.The Acute Respiratory Distress Syndrome Network. Ventilation with lower tidal volumes as compared with traditional tidal volumes for acute lung injury and the acute respiratory distress syndrome. N Engl J Med. 2000;432:301–8. doi: 10.1056/NEJM200005043421801. [DOI] [PubMed] [Google Scholar]
- 15.Wunsch H, Mapstone J, Takala j. High-frequency ventilation versus conventional ventilation for the treatment of acute lung injury and acute respiratory distress syndrome: a systematic review and Cochrane analysis. Anesth Analg. 2005;100:1765–1772. doi: 10.1213/01.ANE.0000145070.52315.F2. [DOI] [PubMed] [Google Scholar]
- 16.Sevransky JE, Levy MM, Marini JJ. Mechanical Ventilation in sepsis-induced acute lung injury/acute respiratory distress syndrome: an evidence-based review. Crit Care Med. 2004;32 (suppl):S548–553. doi: 10.1097/01.ccm.0000145947.19077.25. [DOI] [PubMed] [Google Scholar]
- 17.Ramsey CD, Funk D, Miller RR, et al. Ventilator management for hypoxemic respiratory failure attributable to H1N1 novel swine flu influenza virus. Crit Care Med. 2010;38(supp):e58–65. doi: 10.1097/CCM.0b013e3181cde600. [DOI] [PubMed] [Google Scholar]
- 18.Peek GJ, Mugford M, Tiruvoipati R, et al. Efficacy and economic assessment of conventional ventilatory support versus extracorporeal membrane oxygenation for severe adult respiratory failure (CESAR): a multicentre randomised control trial. Lancet. 2009;374:1351–63. doi: 10.1016/S0140-6736(09)61069-2. [DOI] [PubMed] [Google Scholar]