Abstract
A genetic predisposition for thoracic aortic aneurysms and dissections (TAAD) can be inherited in an autosomal dominant manner with decreased penetrance and variable expression. Four genes identified to date for familial TAAD account for approximately 20% of the heritable predisposition. In a cohort of 514 families with two or more members with presumed autosomal dominant TAAD, 48 (9.3%) families have one or more members who were at 50% risk to inherit the presumptive gene causing TAAD had an intracranial vascular event. In these families, gender is significantly associated with disease presentation (p <0.001), with intracranial events being more common in women (65.4%) while TAAD events occurred more in men (64.2%,). Twenty-nine of these families had intracranial aneurysms (ICA) that could not be designated as saccular or fusiform due to incomplete data. TGFBR1, TGFBR2, and ACTA2 mutations were found in 4 families with TAAD and predominantly fusiform ICAs. In 15 families, of which 14 tested negative for 3 known TAAD genes, 17 family members who were at risk for inheriting TAAD had saccular ICAs. In 2 families, women who harbored the genetic mutation causing TAAD had ICAs. In 2 additional families, intracranial, thoracic and abdominal aortic aneurysms were observed. This study documents the autosomal dominant inheritance of TAADs with saccular ICAs, a previously recognized association that has not been adequately characterized as heritable.I these families, routine cerebral and aortic imaging for at risk members could prove beneficial for timely medical and surgical management to prevent a cerebral hemorrhage or aortic dissection.
Keywords: thoracic aortic aneurysm, aortic dissection, fusiform intracranial aneurysms, saccular intracranial aneurysms, abdominal aortic aneurysm, genetic counseling
Introduction
An inherited predisposition to thoracic aortic aneurysms leading to aortic dissections (TAAD) can occur in association with genetic syndromes, such as Marfan syndrome (MFS) and Loeys-Dietz syndrome (LDS), or it can be inherited in families in the absence of syndromic features. In families without syndromic features, TAAD is primarily inherited in an autosomal dominant manner with variable expression and decreased penetrance, and is designated familial TAAD [Milewicz et al., 1998]. These families show considerable variability in disease presentation, including the location of aortic disease in the ascending aorta, penetrance of the disease, and the presence or absence of associated features, such as patent ductus arteriosus (PDA), bicuspid aortic valve (BAV), and livedo reticularis [Khau et al., 2004; Loscalzo et al., 2007; Guo et al., 2007]. Studies have also confirmed significant genetic heterogeneity for familial TAAD. Genetic mapping studies have identified 6 chromosomal loci and the genes at TAAD2, TAAD4 and 16p have been identified as TGFBR2, ACTA2 and MYH11, respectively [Guo et al., 2001; Hasham et al., 2003; Vaughan et al., 2001; Guo et al., 2007; Pannu et al., 2005; Zhu et al., 2006]. In families with TAAD associated with either PDA or livedo reticularis, the associated features served as phenotypic markers of the disease gene in family members who were not penetrant for aortic disease, which helped map the defective gene [Zhu et al., 2006; Guo et al., 2007]. Therefore, clinical manifestations not previously recognized as part of a heritable syndrome may contribute to the identification of novel genes for familial TAAD. In addition, such features can direct genetic counseling and clinical management of these families [Loscalzo et al., 2007].
A genetic predisposition for intracranial saccular aneurysms (ICA) leading to subarachnoid hemorrhages (SAH) is heritable in some families. Genetic syndromes due to single gene mutations can predispose to ICAs, such as vascular Ehlers Danlos syndrome and polycystic kidney disease [Schievink, 2004; Tang and Spiegelhalther, 2007]. About 9-14% of patients with ICAs have a first-degree relative with an ICA compared to 3-6% of individuals who do not have an ICA [Kissela et al., 2002; De et al., 1996; Wang et al., 1995]. Linkage analysis of ICA families has been used to map multiple loci for genes predisposing to ICAs or SAH (1p36, 5q31, 5p15, 7q11, 14q22, 17cen, 19q13, and Xp22) and establish genetic heterogeneity for this vascular disease [Foroud et al., 2008; Verlaan et al., 2006]. In contrast to familial TAAD, no Mendelian genes have been identified for familial ICAs. It is notable that ICAs have been reported to be associated with abdominal aortic aneurysms, which can both occur in a single patient and aggregate in families [Kim et al., 2005].
We have identified TAAD families in which one or more members also had a cerebrovascular event, such as cerebral aneurysm, dissection or hemorrhage. A number of factors made it difficult to determine if the same mutant gene causing TAAD was also responsible for the cerebrovascular events. First, ICAs affect 2% of the population [Rinkel et al., 1998], so families with multiple members with ICAs were needed to assess the probability that TAAD and ICA could be part of a single-gene disorder. Second, the genetic basis of fusiform aneurysms appears to be distinct from the genetic etiologies of intracranial saccular aneurysms. Two reports suggest that TGFBR1, TGFBR2 and ACTA2 gene mutations lead to fusiform cerebral aneurysms [Guo et al., 2009; Tran-Fadulu et al., 2008]. Herein, we describe families with unique clinical presentations in which TAAD and saccular ICAs segregate in families as a single-gene disorder inherited in an autosomal dominant manner. This phenotype is not due to mutations in known TAAD genes, which suggests that single, yet-to-be identified, gene defects can cause both types of aneurysms.
Materials and Methods
Patients
This study has been approved by the Institutional Review Board at the University of Texas Health Science Center at Houston. Families with inheritance of both TAAD and ICAs were identified by screening 514 families with two or more members diagnosed with TAAD and known syndromes causing TAAD excluded. Family histories including diagnosis of ICA and TAAD were obtained from the proband or other family member and confirmed by medical records when possible. Measurement of the thoracic aortic diameters was performed using 2-D echocardiography, CT or MRI. Cross-sectional aortic diameter was evaluated at the aortic annulus, the sinuses of Valsalva, the sino-tubular junction, and the ascending aorta. Nomograms comparing body surface area to aortic diameter at the sinuses of Valsalva were used to determine whether the patient had a dilated aorta compared to individuals of similar age and body size. ICAs were confirmed by cerebrovascular imaging (MRA or CT angiography). Alternatively, past vascular events were classified as saccular aneurysms based on a presentation of SAH or if procedures used to repair saccular ICAs, such as endovascular coiling or surgical clipping were used.
Statistical Analysis
Chi square test of independence was performed to test the association of gender and disease presentation.
DNA Sequencing Protocol
Bidirectional sequencing of ACTA2, TGFBR1, and TGFBR2 exons was done using intron-based, exon-specific primers. PCR amplifications were carried out using HotStar Taq™ DNA polymerase (Qiagen Inc.Valencia, CA). PCR products were treated using Exo_SAP (USB corporation, OH) to digest primers and followed with sequencing PCR using the BigDye™ sequencing reaction mix (Applied Biosystems, CA). The sequencing PCR products were purified using the BigDye XTerminator kit (Applied Biosystems, CA) and then loaded on an ABI3730xl sequencing instrument using the Rapid36 run module. The DNA sequencing results were analyzed using the Mutation Surveyor software (SoftGenetics, PA).
Results
We have recruited 514 families with two or more members with TAAD for our research studies. The majority of these families demonstrate potential autosomal dominant inheritance of TAAD with decreased penetrance and variable clinical expression [Guo et al., 2007; Milewicz et al., 1998]. Forty-eight (9.3%) of these families included at least one member who was at risk to inherit a gene mutation causing TAAD and had an intracranial vascular event, such as cerebral aneurysm, rupture or hemorrhage. In these families, gender is significantly associated with disease presentation (p <0.001) and intracranial events are more common in women (65.4%) and TAAD occurred more commonly in men (64.2%). Fifteen individuals from 12 unrelated families had both TAAD and an intracranial vascular event. It is interesting to note that 3 men from families TAA071, TAA090, and TAA108 (pedigrees not shown) presented initially with TAAD, and later with ICAs. In addition, one woman presented with an ICA prior to developing TAAD (TAA287). Note that no family had significant hypertension segregating with the vascular diseases.
DNA from an affected family member in 47 of 48 families was sequenced for the TGFBR1, TGFBR2 and ACTA2 genes. MYH11 was not sequenced because mutations have only been identified in families with TAAD associated with PDA [Pannu et al., 2007]. Four of these 47 families were found to have either a TGFBR1, TGFBR2 or ACTA2 mutation. Two TAAD families (TAA090 and TAA067) have the TGFBR2 R460H mutation, and include members with either saccular or fusiform aneurysms [Tran-Fadulu et al., 2008]. In family TAA090, an affected man survived an ascending aortic dissection but died from a ruptured intracranial aneurysm [Pannu et al., 2005]. In a family with a TGFBR1 R487W mutation (TAA009), two affected individuals were identified with asymptomatic fusiform aneurysms of the basilar artery and no enlargement of the aorta [Tran-Fadulu et al., 2008]. Family TAA377 included two members with fusiform aneurysms of the internal carotid arteries resulting from an ACTA2 R258C mutation [Guo et al., 2009]. Studies on the remaining families were negative for mutations in these genes. It appears that TGFBR1, TGFBR2 and ACTA2 mutations, albeit rare in this cohort of TAAD plus intracranial vascular event families, can be associated with fusiform cerebral aneurysms.
In fifteen TAAD families, 17 family members at risk for inheriting TAAD as an autosomal dominant condition with decreased penetrance and variable clinical expressivity had documented saccular ICAs (Fig. 1). None of these families had longstanding, untreated hypertension as the cause of their aortic or cerebral aneurysms. At least one member of all 15 families, except for TAA175 (no sample was available from a surviving member for sequencing), was sequenced for TGFBR1, TGFBR2 and ACTA2; no mutation was identified in these genes. In 4 families (Fig. 1a), the TAAD and ICA phenotypes could be inherited in an autosomal dominant manner, and at least two individuals in each pedigree were identified with saccular ICAs. Of note, only women had ICAs in these families. Two of these families (TAA062 and TAA395) also had members with onset of abdominal aortic aneurysms (AAAs) under the age of 70 years.
Figure 1.
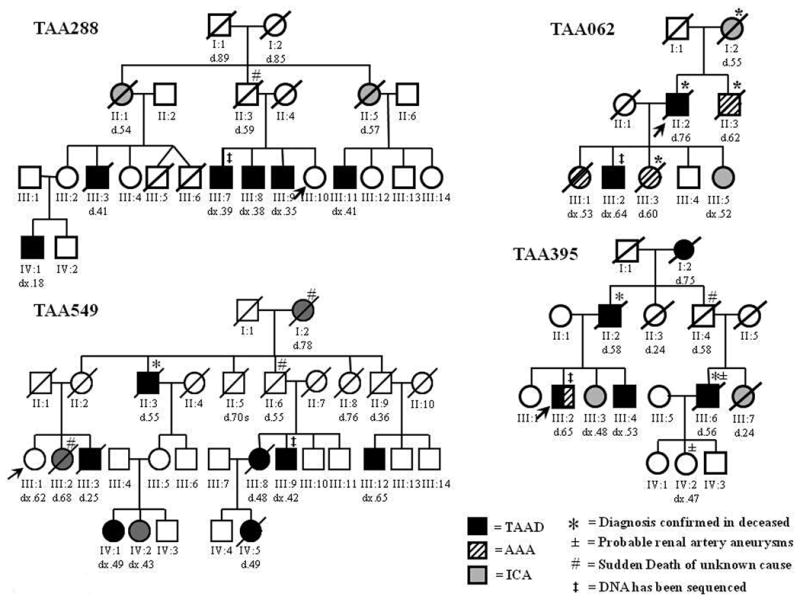
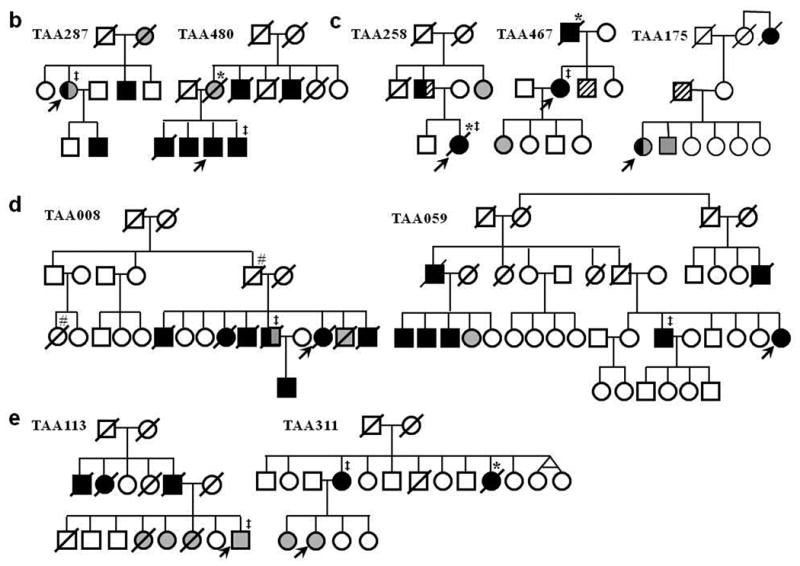
Families with multiple members with thoracic aortic aneurysms and dissections (TAAD) with one or more members with saccular intracranial aneurysms (ICAs). a. Pedigrees with multiple individuals with TAAD and at least two members at risk for inheriting the TAAD gene who had ICAs (TAA288 and TAA549), and AAAs in addition to ICAs (TAA062 and TAA395). b. Pedigrees with an inheritance pattern of TAAD and ICAs similar to family TAA288 and TAA549. c. Pedigrees with TAAD, ICAs and AAAs inherited in an autosomal dominant manner. d. Pedigrees with many members with TAAD and only one member with ICAs. e. Pedigrees with TAAD and ICAs that do not follow a Mendelian pattern for inheritance of a combined TAAD and ICA predisposition gene. Squares represent males, circles represent females and triangles represent miscarriages. A line through the symbol represents a deceased person. TAAD means thoracic aortic aneurysm/dissection, ICA means intracranial aneurysm, and AAA means abdominal aortic aneurysm. “d” indicates age at death, “dx” indicates age at diagnosis. The asterisks (*) on a pedigree symbol indicates that the cause of death was verified by medical records, autopsy report or death certificate. The legend explains the designations for the other symbols used.
Family TAA288 demonstrated a dominant inheritance of a phenotype of TAAD in the men and ICAs in the women. Based on the pedigree structure, we hypothesize that the women with the ICAs were heterozygous for a putative gene inherited in the family causing TAAD. The proband of family TAA288 (III:10) was a 29-year-old woman who had marked ectasia of the extracranial portions of the internal carotid arteries on imaging and a small infundibula at the origins of both posterior communicating arteries. Her ascending aortic diameter at the sinuses of Valsalva was normal (2.7 cm, BSA=1.61 m2). Two of the proband's brothers, 39 and 35 years of age (III:7 and III:9), had ascending aortic aneurysms involving the sinuses of Valsalva with a maximum diameter of 4.5 cm (BSA = 2.29 m2) and 4.6 cm (BSA=2.50 m2) by echocardiography, respectively. The proband's 38-year-old brother (III:8) underwent surgery of his aortic root at a diameter of 5.5 cm (BSA = 2.15 m2). The proband's father (II:3) died suddenly of an unknown cause at age 59.The proband's younger paternal aunt (II:5) died at age 57 of a ruptured intracranial aneurysm. One of this aunt's sons (III:11) has a thoracic aortic aneurysm with a diameter of 4.5 cm diagnosed by echocardiography at age 41 years (BSA=1.85 m2). The proband's older paternal aunt (II:1) died of a ruptured intracranial aneurysm at age 54. A CT scan prior to her death showed a left frontal hematoma with subarachnoid hemorrhage suggestive of rupture of an anterior communicating artery aneurysm. This woman's son (III:3) passed away at the age of 41 of an acute type A aortic dissection. At the time of his death, the aortic root measured 8.9 cm; however, an echocardiogram performed 3 years prior to his death showed an aortic root diameter of 3.3 cm (BSA = 2.06). In addition, this woman's daughter (III:2) had a normal echocardiogram and cerebrovascular imaging, but her 18-year-old son (IV:1) has an enlarged aortic root of 4.15 cm (BSA = 1.7 m2).
Family TAA549 consists of 4 generations affected with thoracic aortic aneurysm and dissection and intracranial aneurysms, primarily affecting the women. The proband (III:1) is a 67-year-old woman with mild dilatation of the ascending aorta measuring 3.8 cm (BSA = 1.9 m2) and normal cerebral vessels. Her brother (III:3) died of a thoracic aortic dissection at age 25, and her sister (III:2) had a history of cerebral aneurysm repair at age 50 and died at age 68 from a suspected aortic aneurysm. Her mother (II:2) died at age 28 of unrelated causes. A maternal uncle (II:3) died of a dissecting aneurysm at age 55 and another uncle (II:6) died suddenly of a suspected aortic dissection; however, an autopsy was not performed. Individual II:3 has a daughter who is 71 years old with a normal thoracic aorta, but mild dilatation of the common iliac arteries. Her daughter (IV:1) had a Type B dissection that was repaired at age 49, and another daughter (IV:2) had a right internal carotid artery saccular aneurysm that was embolized at age 43. Individual II:6 has a 52-year-old-son (III:9) who had a thoracic aorta repair, a daughter (III:8) who died of an aortic dissection at age 48, and a granddaughter (IV:5) who died suddenly at age 49 from a Type A aortic dissection. Another maternal uncle who died in an accident at age 36 had a son (III:12) with an abdominal aortic aneurysm . The proband's maternal grandmother died at age 78 from a suspected aortic disease and reported to have a history of intracranial aneurysm and stroke, and likely carried the inherited predisposition to both TAAD and ICA.
In family TAA062, ICAs, AAAs and TAAs occurred in an autosomal dominant pattern. The proband was a 76-year-old man (II:2) who died of an acute type A dissection. His brother (II:3) died of a ruptured AAA at age 62, and their mother (I:2) died of a ruptured ICA at age 55. Four of the proband's children had either aortic or cerebral aneurysms. The oldest daughter (III:1) had an AAA repair at age 53 and later died of a glioblastoma. The second daughter (III:3) died of a ruptured AAA at age 60. The youngest daughter (III:5) had a saccular intracranial aneurysm at age 52 that was surgically clipped. The son (III:2) had an ascending aortic aneurysm that measured 6.5 cm and surgically repaired at age 64.
Family TAA395 included ICAs in women and TAAD in men, as well as an AAA in one man. The proband (III:2) was diagnosed at age 65 with an infra-renal abdominal aneurysm that measured 3.9 cm by ultrasound and an enlarged aortic root measuring 4.2 cm by echocardiogram (BSA 2.54 m2). The proband's 61-year-old sister (III:3) had a ruptured intracranial saccular aneurysm at age 48 that was surgically clipped and a normal ascending aorta on echocardiographic imaging. The proband's 53 year-old brother (III:4) also has an enlarged aortic root of 4.5 cm (BSA 2.08 m2). MRA imaging showed no evidence of an ICA and abdominal ultrasound showed a normal abdominal aorta. The proband's older sister (III:1) has an aortic root measuring 3.8 cm (BSA 1.91 m2), which is at the upper limit of normal. The proband's father (II:2) died at age 58 of an ascending aortic dissection, and so did the proband's paternal grandmother (I:2) at age 75. The paternal uncle (II:4) died suddenly at age 58 of an unknown cause. This man's son (III:6) died at age 56 of an acute ascending aortic dissection that was confirmed by autopsy. Interestingly, he was also found to have a “string of pearls” aneurysms of his renal arteries. The daughter of this man (IV:2) had similar aneurysms of her right renal artery but has not had either aortic or cerebrovascular imaging. Finally, the sister of this man (III:7) died postpartum at age 24, and autopsy identified cerebral hemorrhage as the cause of death.
Among the other families with TAAD and saccular ICAs, 2 families (TAA 287 and TAA480) showed inheritance patterns similar to TAA288 and TAA549, namely ICAs mainly in women and TAADs in men (Fig. 1b). However, these are smaller families and are therefore not as convincing as families TAA288 and TAA549 that a single-gene mutation causes both vascular diseases. Families TAA258, TAA467, and TAA175 demonstrated a pattern of inheritance of TAAD, ICA and AAA that is similar to TAA395 and TAA062 (Fig. 1c). In two additional families (TAA008 and TAA059), many individuals had TAAD with only one individual with an ICA, so it is uncertain whether the ICA and TAAD could have resulted from a single genetic defect (Fig.1d). Finally, 2 other families (TAA113 and TAA311) show a familial aggregation of TAAD and ICA; however, the inheritance pattern is not strongly suggestive of an autosomal dominant inheritance pattern for predisposition to either TAAD or ICAs (Fig. 1e).
Discussion
The families presented here illustrate a novel phenotype of a genetic predisposition to both TAAD and saccular ICAs inherited as a single gene disorder in an autosomal dominant manner. These families are also notable for a gender-based difference in the vascular disease presentation, with ICAs occurring more often in women and TAAD occurring more often in men. Considering the known genetic heterogeneity associated with familial TAAD and ICAs, we hypothesize that the unique phenotype presented by these families could represent a novel locus or loci for these diseases. In addition, a subset of the families with ICA and TAAD also included individuals with early onset AAAs, suggesting a defective gene that leads to aneurysm formation in multiple vascular beds. Inheritance of a predisposition to both ICAs and AAAs has previously been reported, therefore the presentation of TAAD, ICA and AAA is not unexpected [Kim et al., 2005]. In addition, genome wide association studies have identified common polymorphic variants that predispose to many different vascular diseases, including AAAs and ICAs [Helgadottir et al., 2008].
The inheritance of the disease in these families reflects some general characteristics of familial TAAD and familial ICAs. There is variability in the age of onset indicating a possible role of genetic or environmental modifiers. As noted previously, there is a gender-based variability in vascular disease presentation. Decreased penetrance also is evident, as illustrated in individual III:2 in TAA288, who is an obligate carrier of the putative defective gene based on her son having ascending aortic aneurysm but has normal aortic and cerebrovascular imaging. Family TAA395 is also remarkable due to the multiple vascular disease presentation in family members. The proband presented with both a thoracic and abdominal aortic aneurysm, and two family members were noted to have “string of pearls” aneurysms of their renal arteries, further suggesting that the defective gene affects different vascular beds.
Saccular intracranial aneurysms are more common than the fusiform type, accounting for approximately 90% of intracranial aneurysms [Rinkel et al., 1998]. Individuals with mutations in TGFBR1, TGFBR2 or ACTA2 have been documented to have fusiform aneurysms, along with saccular ICAs. Fusiform aneurysms occur in elongated, tortuous vessels and in some cases may be due to a previous dissection. In contrast, saccular intracranial aneurysms tend to occur at regions of increased hemodynamic stress, such as the junction of arteries within the anterior circulation of the brain.
Identified genes that cause familial TAAD are also known to predispose to cerebrovascular disease. Surveillance recommendations for families with TGFBR1 and TGFBR2 are established and both aortic and cerebrovascular imaging is recommended in these families [Loeys et al., 2006; Tran-Fadulu et al., 2009]. For ACTA2 mutations, the recommendations are still being developed, but some ACTA2 mutations such as the R258C and R258H mutations confer a high risk for cerebrovascular disease, including both vascular occlusive disease and fusiform aneurysms [Guo et al., 2009]. Therefore, we recommend cerebrovascular imaging for ACTA2 mutation patients if either there is a family history of cerebrovascular disease or if cerebrovascular disease has been reported to be associated with a particular mutation.
The possibility of a genetic link between ICAs and TAAD suggests that an individual in a family displaying both disease phenotypes in various members could be at risk for both types of aneurysms, although our data suggest that women are at a higher risk for ICAs and men for TAAD. Therefore, we recommend that at-risk members in these families have routine imaging of their aorta and cerebral circulation. In ICA and TAAD families that also have members with AAAs, screening for AAAs is also recommended. After successful treatment of an ascending aortic aneurysm or ICA, imaging surveillance for asymptomatic aneurysms in the unrepaired arteries needs to be continued. Early detection of aneurysms, both aortic and cerebral, allows for early treatment and reduction of risk associated with life-threatening outcomes of an acute aortic dissection or cerebral hemorrhage. Ultimately, identification of the defective genes will mean that only family members harboring the mutant gene have to undergo surveillance for these aneurysms.
Acknowledgments
We would like to thank the families for participating in our research and the physicians who referred families. The following sources provided funding for these studies: RO1 HL62594 (D.M.M.), P50HL083794-01 (D.M.M.), UL1 RR024148 (CTSA), the Vivian Smith Foundation (D.M.M.) and the Doris Duke Charitable Trust (D.M.M.).
Footnotes
Disclosures: We have no conflicts of interest to disclose.
References
- De BM, Perusse L, Cantin L, Bouchard JM, Mathieu J. A study of inbreeding and kinship in intracranial aneurysms in the Saguenay Lac-Saint-Jean region (Quebec, Canada) Ann Hum Genet. 1996;60:99–104. doi: 10.1111/j.1469-1809.1996.tb01181.x. [DOI] [PubMed] [Google Scholar]
- Foroud T, Sauerbeck L, Brown R, Anderson C, Woo D, Kleindorfer D, Flaherty ML, Deka R, Hornung R, Meissner I, Bailey-Wilson JE, Rouleau G, Connolly ES, Lai D, Koller DL, Huston J, III, Broderick JP. Genome screen to detect linkage to intracranial aneurysm susceptibility genes: the Familial Intracranial Aneurysm (FIA) study. Stroke. 2008;39:1434–1440. doi: 10.1161/STROKEAHA.107.502930. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guo D, Hasham S, Kuang SQ, Vaughan CJ, Boerwinkle E, Chen H, Abuelo D, Dietz HC, Basson CT, Shete SS, Milewicz DM. Familial thoracic aortic aneurysms and dissections: genetic heterogeneity with a major locus mapping to 5q13-14. Circulation. 2001;103:2461–2468. doi: 10.1161/01.cir.103.20.2461. [DOI] [PubMed] [Google Scholar]
- Guo DC, Pannu H, Papke CL, Yu RK, Avidan N, Bourgeois S, Estrera AL, Safi HJ, Sparks E, Amor D, Ades L, McConnell V, Willoughby CE, Abuelo D, Willing M, Lewis RA, Kim DH, Scherer S, Tung PP, Ahn C, Buja LM, Raman CS, Shete S, Milewicz DM. Mutations in smooth muscle alpha-actin (ACTA2) lead to thoracic aortic aneurysms and dissections. Nat Genet. 2007;39:1488–1493. doi: 10.1038/ng.2007.6. [DOI] [PubMed] [Google Scholar]
- Guo DC, Papke CL, Tran-Fadulu V, Regalado ES, Avidan N, Johnson RJ, Kim DH, Pannu H, Willing MC, Sparks E, Pyeritz RE, Singh MN, Dalman RL, Grotta JC, Marian AJ, Boerwinkle EA, Frazier LQ, LeMaire SA, Coselli JS, Estrera AL, Safi HJ, Veeraraghavan S, Muzny DM, Wheeler DA, Willerson JT, Yu RK, Shete SS, Scherer SE, Raman CS, Buja LM, Milewicz DM. Mutations in smooth muscle alpha-actin (ACTA2) cause coronary artery disease, stroke, and moyamoya disease, along with thoracic aortic disease. Am J Hum Genet. 2009;84:617–627. doi: 10.1016/j.ajhg.2009.04.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hasham SN, Willing MC, Guo DC, Muilenburg A, He RM, Tran VT, Scherer SE, Shete SS, Milewicz DM. Mapping a locus for familial thoracic aortic aneurysms and dissections (TAAD2) to 3p24-25. Circulation. 2003;107:3184–3190. doi: 10.1161/01.CIR.0000078634.33124.95. [DOI] [PubMed] [Google Scholar]
- Helgadottir A, Thorleifsson G, Magnusson KP, Gretarsdottir S, Steinthorsdottir V, Manolescu A, Jones GT, Rinkel GJ, Blankensteijn JD, Ronkainen A, Jaaskelainen JE, Kyo Y, Lenk GM, Sakalihasan N, Kostulas K, Gottsater A, Flex A, Stefansson H, Hansen T, Andersen G, Weinsheimer S, Borch-Johnsen K, Jorgensen T, Shah SH, Quyyumi AA, Granger CB, Reilly MP, Austin H, Levey AI, Vaccarino V, Palsdottir E, Walters GB, Jonsdottir T, Snorradottir S, Magnusdottir D, Gudmundsson G, Ferrell RE, Sveinbjornsdottir S, Hernesniemi J, Niemela M, Limet R, Andersen K, Sigurdsson G, Benediktsson R, Verhoeven EL, Teijink JA, Grobbee DE, Rader DJ, Collier DA, Pedersen O, Pola R, Hillert J, Lindblad B, Valdimarsson EM, Magnadottir HB, Wijmenga C, Tromp G, Baas AF, Ruigrok YM, Van Rij AM, Kuivaniemi H, Powell JT, Matthiasson SE, Gulcher JR, Thorgeirsson G, Kong A, Thorsteinsdottir U, Stefansson K. The same sequence variant on 9p21 associates with myocardial infarction, abdominal aortic aneurysm and intracranial aneurysm. Nat Genet. 2008;40:217–224. doi: 10.1038/ng.72. [DOI] [PubMed] [Google Scholar]
- Khau VK, Wolf JE, Mathieu F, Zhu L, Salve N, Lalande A, Bonnet C, Lesca G, Plauchu H, Dellinger A, Nivelon-Chevallier A, Brunotte F, Jeunemaitre X. Familial thoracic aortic aneurysm/dissection with patent ductus arteriosus: genetic arguments for a particular pathophysiological entity. Eur J Hum Genet. 2004;12:173–180. doi: 10.1038/sj.ejhg.5201119. [DOI] [PubMed] [Google Scholar]
- Kim DH, Van Ginhoven G, Milewicz DM. Evidence supporting a common genetic basis for cerebral and abdominal aortic aneurysms in a subset of families. Neurosurgery. 2005;56(4):655–61. doi: 10.1227/01.neu.0000156787.55281.53. [DOI] [PubMed] [Google Scholar]
- Kissela BM, Sauerbeck L, Woo D, Khoury J, Carrozzella J, Pancioli A, Jauch E, Moomaw CJ, Shukla R, Gebel J, Fontaine R, Broderick J. Subarachnoid hemorrhage: a preventable disease with a heritable component. Stroke. 2002;33:1321–1326. doi: 10.1161/01.str.0000014773.57733.3e. [DOI] [PubMed] [Google Scholar]
- Loeys BL, Schwarze U, Holm T, Callewaert BL, Thomas GH, Pannu H, De Backer JF, Oswald GL, Symoens S, Manouvrier S, Roberts AE, Faravelli F, Greco MA, Pyeritz RE, Milewicz DM, Coucke PJ, Cameron DE, Braverman AC, Byers PH, De Paepe AM, Dietz HC. Aneurysm syndromes caused by mutations in the TGF-beta receptor. N Engl J Med. 2006;355:788–798. doi: 10.1056/NEJMoa055695. [DOI] [PubMed] [Google Scholar]
- Loscalzo ML, Goh DL, Loeys B, Kent KC, Spevak PJ, Dietz HC. Familial thoracic aortic dilation and bicommissural aortic valve: a prospective analysis of natural history and inheritance. Am J Med Genet A. 2007;143:1960–1967. doi: 10.1002/ajmg.a.31872. [DOI] [PubMed] [Google Scholar]
- Milewicz DM, Chen H, Park ES, Petty EM, Zaghi H, Shashidhar G, Willing M, Patel V. Reduced penetrance and variable expressivity of familial thoracic aortic aneurysms/dissections. Am J Cardiol. 1998;82:474–479. doi: 10.1016/s0002-9149(98)00364-6. [DOI] [PubMed] [Google Scholar]
- Pannu H, Fadulu V, Chang J, Lafont A, Hasham SN, Sparks E, Giampietro PF, Zaleski C, Estrera AL, Safi HJ, Shete S, Willing MC, Raman CS, Milewicz DM. Mutations in transforming growth factor-beta receptor type II cause familial thoracic aortic aneurysms and dissections. Circulation. 2005;112:513–520. doi: 10.1161/CIRCULATIONAHA.105.537340. [DOI] [PubMed] [Google Scholar]
- Pannu H, Tran-Fadulu V, Papke CL, Scherer S, Liu Y, Presley C, Guo D, Estrera AL, Safi HJ, Brasier AR, Vick GW, Marian AJ, Raman CS, Buja LM, Milewicz DM. MYH11 mutations result in a distinct vascular pathology driven by insulin-like growth factor 1 and angiotensin II. Hum Mol Genet. 2007;16:3453–3462. doi: 10.1093/hmg/ddm201. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ring t, Spiegelhalter D. Risk of intracranial aneurysm bleeding in autosomaldominant polycystic kidney disease. Kidney Int. 2007;(11):1400–2. doi: 10.1038/sj.ki.5002488. [DOI] [PubMed] [Google Scholar]
- Rinkel GJ, Djibuti M, Algra A, van Gijn J. Prevalence and risk of rupture ofintracranial aneurysms: a systematic review. Stroke. 29:251–256. doi: 10.1161/01.str.29.1.251. [DOI] [PubMed] [Google Scholar]
- Schievink WI. Cerebrovascular Involvement in Ehlers-Danlos Syndrome. Curr Treat Options Cardiovasc Med. 2004;6(3):231–236. doi: 10.1007/s11936-996-0018-6. [DOI] [PubMed] [Google Scholar]
- Tran-Fadulu VT, Pannu H, Kim DH, Vick GW, III, Lonsford CM, Lafont AL, Boccalandro C, Smart S, Peterson KL, Zenger-Hain J, Willing MC, Coselli J, LeMaire SA, Ahn C, Byers PH, Milewicz DM. Analysis of Multigenerational Families with Thoracic Aortic Aneurysms and Dissections Due to TGFBR1 or TGFBR2 Mutations. J Med Genet. 2009;46(9):607–13. doi: 10.1136/jmg.2008.062844. [DOI] [PubMed] [Google Scholar]
- Vaughan CJ, Casey M, He J, Veugelers M, Henderson K, Guo D, Campagna R, Roman MJ, Milewicz DM, Devereux RB, Basson CT. Identification of a chromosome 11q23.2-q24 locus for familial aortic aneurysm disease, a genetically heterogeneous disorder. Circulation. 2001;103:2469–2475. doi: 10.1161/01.cir.103.20.2469. [DOI] [PubMed] [Google Scholar]
- Verlaan DJ, Dube MP, St-Onge J, Noreau A, Roussel J, Satge N, Wallace MC, Rouleau GA. A new locus for autosomal dominant intracranial aneurysm, ANIB4, maps to chromosome 5p15.2-14.3. J Med Genet. 2006;43:e31. doi: 10.1136/jmg.2005.033209. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang PS, Longstreth WT, Jr, Koepsell TD. Subarachnoid hemorrhage and family history. A population-based case-control study. Arch Neurol. 1995;52:202–204. doi: 10.1001/archneur.1995.00540260108026. [DOI] [PubMed] [Google Scholar]
- Zhu L, Vranckx R, Khau Van KP, Lalande A, Boisset N, Mathieu F, Wegman M, Glancy L, Gasc JM, Brunotte F, Bruneval P, Wolf JE, Michel JB, Jeunemaitre X. Mutations in myosin heavy chain 11 cause a syndrome associating thoracic aortic aneurysm/aortic dissection and patent ductus arteriosus. Nat Genet. 2006;38:343–349. doi: 10.1038/ng1721. [DOI] [PubMed] [Google Scholar]


